Abstract
Purpose of the review
Compelling evidence suggests that the Th17 lineage and other IL-17 producing cells play critical roles in host defense against pathogens at mucosal sites. However, IL-17 can also contribute to inflammatory responses at mucosal sites. In this review, we will discuss the recent progress in our understanding of the role of Th17 and other IL-17-producing cells in defining the fine balance between immunity and inflammation at different mucosal sites.
Recent Findings
Recent findings have highlighted that Th17 cytokines are important for the induction of innate and adaptive host responses and contribute to host defense against pathogens at mucosal sites. More recent developments have probed how the Th17 responses are generated in vivo in response to infections and their requirement in maintaining barrier function at mucosal sites. Most importantly, it is becoming apparent that there is a fine balance between protective and pathological manifestation of Th17 responses at mucosal sites that defines immunity or inflammation.
Summary
In this review we have summarized the recent advances in our understanding of Th17 cytokines and how they contribute to immunity versus inflammation at mucosal sites.
Keywords: Th17, Infection, mucosal, inflammation
Introduction
Mucosal surfaces are constantly exposed to foreign material and the ability to protect the host from potentially harmful organisms arises from ‘immune surveillance’ carried out by several mechanisms at the host-environment interface. When pathogens come in contact with the mucosal surface, the mucosal epithelium functions both as a physical barrier as well as a first line of defense. Interaction of the microbe with epithelial cells at the mucosa as well as resident macrophages takes place by recognition of conserved pathogen associated molecular patterns [PAMPs] on pathogens, and Toll-Like Receptors [TLRs] on host cells. The plethora of cytokines induced in response to this interaction results in a cascade of inflammatory events that mediate either protection or pathology. Beside the epithelial barrier, the induction of anti-microbial peptides at the mucosal surfaces and secreted immunoglobulins [especially Immunoglobulin A] also provide important mechanisms for host defense. Subsequent to these innate mechanisms of protection, CD4+ helper cells belonging to the adaptive immune responses are recruited to mucosal surfaces and provide host resistance. The recent characterization of the T helper 17 [Th17] cells as a distinct lineage of CD4+ cells (1–2) has greatly expanded our knowledge and filled in some of the missing gaps in host immunity not fully explained by the T helper cell 1 [Th1]/T helper cell 2 [Th2] dichotomy. Th17 cells were initially identified as a key factor in the induction of inflammation and tissue destruction associated with animal models of autoimmune disease like Multiple Sclerosis, Collagen Induced Arthritis and experimental colitis [reviewed in (3–4)]. However, recent evidence supports a role for Th17 cytokines as a bridge between innate and adaptive immune responses in host defense against a variety of pathogens at the mucosal surfaces. This review will focus on recent advances in our understanding of Interleukin-23 [IL-23] and Th17 cytokines and how they fill in some of the missing gaps in mucosal immunity and inflammation.
Th17 cytokines and cellular sources
Th17 cells produce the cytokines IL-17A [IL-17] (1–2) and IL-17F (5), as well as the cytokines IL-21 (6–7) and IL-22 (8–9). IL-17A and IL-17F act through IL-17 Receptor [IL-17R - comprised of IL-17RA and IL-17RC subunits] via Act1 and TRAF6 to induce the expression of pro-inflammatory cytokines and chemokines [reviewed in (10)]. The IL-17R is a Type I transmembrane protein that is expressed on various cell types including leucocytes, epithelial cells, mesothelial cells, vascular endothelial cells, keratinocytes and fibroblasts and respond to IL-17R-mediated signaling by production of granulocyte colony-stimulating factor [G-CSF], IL-6, IL-8 and mediate granulopoiesis and neutrophil recruitment [reviewed in (11)]. More recently, it is becoming clear that IL-17 can also act on antigen-presenting cells [APCs] such as macrophages and dendritic cells and induce cytokine and chemokine production (12–13).
The differentiation of Th17 cells takes place following exposure of naive CD4+ T cells to APC-derived polarizing cytokines such as TGF-β, IL-6, IL-21, while IL-23 acts to stabilize the commitment of Th17 cells to this lineage [reviewed in (14)]. The master regulator of Th17 differentiation is transcription factor RORγt and RORα (15–16). Several of these Th17 polarizing cytokines, such as IL-23, TGF-β, IL-6 and IL-1β are induced in APCs following activation with pathogens. For example, Klebsiella pneumoniae (17–18), Mycobacterium tuberculosis (19–20), Helicobacter pylori (21), Francisella tularensis (13, 22), Salmonella enteritidis (23), Bordetella pertussis (24), Cryptococcus (25), Candida albicans (26) and Aspergillus fumigatus (27) all induce some or all of the Th17 polarizing cytokines and can drive Th17 cell differentiation. Although these responses are primarily mediated through TLR signaling (19, 28), other TLR-independent pathways such as Syk-Card-9 pathway (26) also mediate the induction of Th17 polarizing cytokines in APCs. Furthermore, endogenous lipid mediators such as prostaglandin E2 [PGE2] (29) and apoptotic signals (30–31) that are released under inflammatory conditions can also drive Th17 cell differentiation. Much of the recent focus has been on IL-17 produced by CD4+ αβ T cells. However, innate cells such as γδ T cells (32–34), NK cells expressing RORγτ+NKp46+ (35–36) and Lymphoid-tissue inducer like cells [Lti] (37) can produce IL-17 and IL-22 and impact the innate response via induction of chemokines and antimicrobial proteins(38–39), as well as cellular recruitment to mucosal infections. These studies therefore suggest that innate IL-17 and IL-22-producing cells as well as adaptive Th17cells function as a bridge between innate and adaptive immune responses at mucosal sites in the host.
Immunity and inflammation at the respiratory mucosa
The respiratory mucosa is constantly challenged with inhaled particulates and infectious agents and is thus a major port of entry for infectious diseases. Although induction of Th17 cytokines may play a protective role against pulmonary pathogens, it is also becoming apparent that these cytokines may be responsible for the pathology associated with inflammatory conditions. One of the best characterized roles for IL-17 in protection against pathogens at the respiratory mucosa is using the gram negative extracellular bacteria Klebsiella pneumoniae. Early studies by Kolls and colleagues showed that IL-17 is important for recruitment of neutrophils in response to pulmonary challenge with K. pneumoniae (40). IL-17-dependent induction of key neutrophil chemo-attractants such as macrophage inflammatory protein-2 [MIP-2] and G-CSF was required for effective recruitment of neutrophils and pathogen clearance (41) (Figure 1). Accordingly, absence of IL-17 Receptor signaling showed greater dissemination of the bacteria due to the delay in neutrophil recruitment. The recognition of IL-17-dependent induction of G-CSF for the differentiation of CD34+ progenitors into neutrophil progenitors (42) projected a compelling role for IL-17 in the accumulation of neutrophils during infections. Confirmation that IL-17 was the key mediator of the protective responses in Klebsiella infections was shown when over-expression of IL-17 led to reversal of the disease phenotype (40). Subsequently, Kolls and colleagues also identified the cellular source of IL-17 as CD4+ and CD8+ T cells, and that the induction of IL-17 was mediated by TLR4-dependent IL-23 production (18). More recent studies have also shown that IL-22 can synergize with IL-17 and induce anti-microbial peptides like defensins, S-100 Proteins, Lipocalin and chemokines such as CCL3 and CCL20 (39, 43). Other studies have suggested a role for IL-17 in recruitment of monocytes, neutrophils and clearance and colonization of another extracellular respiratory pathogen, Streptococcus pneumoniae (44). These studies suggest that the Th17 cell lineage and the effector molecules produced by these cells have evolved to contribute to host defense against extracellular pathogens at the respiratory mucosa.
Figure 1. Role of Th17 cytokines in protection versus pathology at the mucosal surfaces.
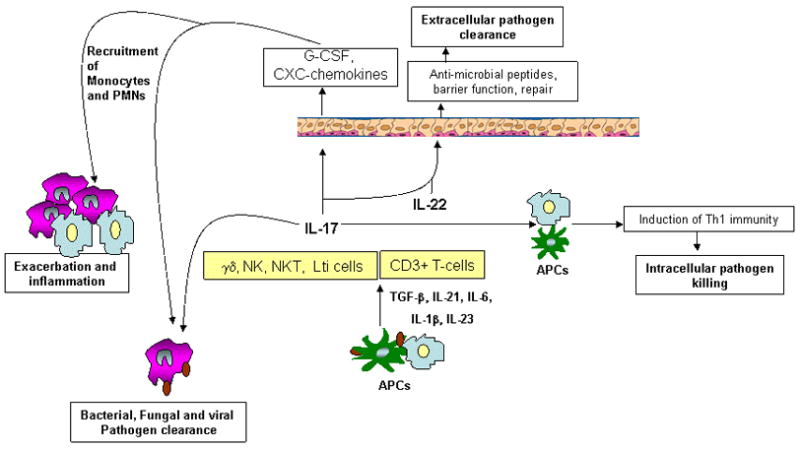
Infection-induced IL-17 and IL-22 can be produced by several immune cells found in mucosal sites. One of the targets of IL-17 and IL-22 are mucosal epithelial cells, where IL-17 augments G-CSF and CXC chemokine production resulting in recruitment of neutrophils, monocytes and other inflammatory cells that contribute to bacterial, fungal and viral clearance at mucosal sites. However, the resulting cellular infiltration can cause resulting inflammation and damage at mucosal surfaces. IL-17 can synergize with IL-22 and induce antimicrobial peptides and epithelial repair function important for control of extracellular pathogens. IL-17 can also act directly on APCs and induce cytokines such as IL-12 and drive Th1 differentiation required for intracellular pathogen clearance.
In contrast to a well described role for IL-17 in protection against respiratory extracellular pathogens, IL-17 appears to be dispensable for protection against pulmonary intracellular pathogens such as Mycobacteria. For example, IL-17R, IL-23 or IL-17 is not required for protective immunity to pulmonary challenge with M.tuberculosis (20) or M.bovis BCG (32, 45). However, the absence of IL-23/Th17 cytokines impacts the generation of granuloma and inflammation mediated as a result of the infection (20, 32). Both innate cells such as γδ T cells (32) and CD4+ T cells can produce IL-17 in response to M.tuberculosis infection (20). In contrast to Mycobacterial models, pulmonary infection with another intracellular pathogen Francisella tularensis LVS also generates a Th17 response (46) and is required for host defense (13). The protective role for IL-17 against pulmonary tularemia is mediated through IL-17-dependent induction of IL-12 and IFNγ production in macrophages and dendritic cells. IL-17−/− infected mice have lower expression of IFNγ in the lungs and exogenous delivery of IL-17 into IL-17−/− infected mice can rescue the IFNγ responses (13). A similar decrease in Th1 responses was also observed in IL-17−/− mice infected with M.bovis BCG (32), suggesting that some intracellular infections can effectively induce IFNγ responses in the host for direct pathogen control, while other pathogens require the IL-17 pathway for indirect induction of host IFNγ responses for pathogen control. It is also becoming clear that intracellular pathogens that require both T cell immunity as well as neutrophil killing for pathogen control may be dependent on IL-17 for protection. For example, the control of intracellular pathogens such as Mycoplasma pneumoniae (47) and Chlamydia muridarum (48) at the respiratory mucosa is mediated via IL-23-dependent IL-17 recruitment of neutrophils and inflammatory cells. These studies suggest that IL-17 can function directly or indirectly in protection against intracellular bacterial pathogens at the respiratory mucosa.
In models of fungal pulmonary infections such as with Aspergillus fumigatus (49) and Pneumocystis carinii (50), IL-23 and IL-17 are induced in the lungs following infection and are required for protection primarily for recruitment of neutrophils to the lung mucosa (49–50). Infection with the fungal pathogen Histoplasma capsulatum also requires the IL-17/IL-23 pathway, since absence of this pathway coincides with reduced inflammatory cell recruitment and blunted fungal clearance (51). These studies suggest that fungal pathogens at the respiratory mucosa are dependent on IL-17-mediated recruitment of inflammatory cells for fungal control.
Recent studies have addressed whether there is a protective or pathological role for IL-17 in response to viral infections such as influenza. Although some studies have suggested a protective function for IL-17 in host immunity to influenza, other studies have suggested a pathological role. For example, it has been documented that IL-17 depletion resulted in increased weight loss as well as reduced survival in mouse model of influenza (52). Furthermore, that adoptive transfer of Th17 polarized antigen-specific effectors has been shown to protect mice against a lethal influenza challenge suggesting a protective role for IL-17 that is independent on IFNγ (53). On the contrary, the IL-17R−/− mice are not more susceptible than wild type mice to influenza infection, but have reduced neutrophil influx and decreased inflammation, suggesting a pathological role for IL-17 in influenza challenge (54). These data suggest that IL-17 may be contributing to the inflammatory injury in response to infection, but that the recruitment of inflammatory cells may be required for protection.
Data from several other respiratory tract disease models suggest that the induction of IL-17 during infection is a double edged sword resulting in inflammation and damage to the respiratory tract. For example, Bacillus pertussis is a gram negative extracellular pathogen that causes whooping cough and results in pathological consequences reflected in the form of persistent cough and bronchiectasis. Infection with B.pertussis results in production of IL-23, IL-1β and IL-6 from DCs and generation of Th17 differentiation which correlates with severe respiratory pathology (24). This suggests that infection induced IL-17 responses may contribute to excessive inflammation in the lungs and destructive changes in the airways leading to infection-induced bronchiectasis. Furthermore, the persistent lung inflammation that occurs in cystic fibrosis patients due to chronic colonization and biofilm formation by Pseudomonas aeruginosa correlates with higher levels of IL-17 and IL-23 in the sputum (55–56). Human rhinoviral infections are often associated with exacerbations of asthma and chronic obstructive pulmonary disease. IL-17 has been shown to work synergistically with human respiratory rhinovirus to induce IL-8 from epithelial cells that results in induction of inflammatory cells resulting in airway disease (57). These studies clearly suggest there is a fine balance that defines a protective versus pathological role for IL-17 in respiratory tract mucosal infections (Table 1).
Table 1.
Protective versus pathological roles for Th17 cytokines in generating immunity against respiratory tract pathogens.
| Mucosal surfaces and Organisms | Type of Organism | Role of IL-17 and/or Th17 cells | Functional Significance |
|---|---|---|---|
| Klebsiella pneumoniae | Bacteria | Protective | Neutrophil recruitment and induction of key chemokines and anti-microbials (17–18, 39–40) |
| Streptococcus pneumoniae | Bacteria | Protective | Recruitment of monocytes, neutrophils (48) |
| Mycobacteria (M. tuberculosis and M.bovis BCG] | Bacteria | No effect on protection | Impacts granuloma formation (20, 32) |
| Francisella tularensis LVS | Bacteria | Protective | IL-23/Th17 pathway dependent initiation of Th1 protective immune response (46) |
| Mycoplasma pneumoniae | Bacteria | Protective | Recruitment of neutrophils (47) |
| Chlamydia muridarum | Bacteria | Protective | IL-17 induced production of chemokines by Chlamydia infected cells and neutrophils recruitment (48) |
| Aspergillus fumigatus | Fungi | Protection vs exacerbation | Reduced chemokine induction and neutrophils recruitment (27, 49) |
| Pneumocystis carinii | Fungi | Protective | Reduced recruitment of effector CD4+ T cells and chemokine induction (50) |
| Histoplasma capsulatum | Fungi | Protective | Recruitment of neutrophils (51) |
| Influenza | Virus | Protective and exacerbation | Transfer of Th17 cells is protective (53), but absence of IL-17R results in reduced inflammation due to reduced neutrophil influx (54) |
| Human rhinovirus | Virus | Exacerbation | Recruitment of neutrophils and effector T cells and pathology (57) |
Immunity and inflammation at the Oral mucosa
Commensal organisms that are present in the oral mucosal surfaces can become pathogenic due to breakdown of mucosal barrier or due to loss of host defense mechanisms. Oro-pharyngeal candidiasis [OPC] is a common infection in HIV infected individuals and immunity was thought to be mediated by Th1 responses and neutrophils (58–59). However, recent mechanistic studies using gene deficient mice has shown that the IL-17 pathway is critical for induction of CXC-chemokines and neutrophil activating factors required for recruitment of neutrophils to the oral mucosa (60–61). Also key antimicrobials such as B-defensins were suppressed in IL-17R−/− mice following OPC infection, suggesting that immunity to OPC is regulated by the Th17 pathway (60). Further, that Th17 cytokines may be good correlates of protection comes from studies showing that patients with Chronic mucocutaneous candidiasis [CMC] produce significantly lower levels of IL-17 and IL-22 mRNA and protein in vitro following antigen stimulation when compared to healthy individuals (62). Importantly, patients with autosomal dominant hyper-IgE syndrome [HIES, Job’s Syndrome] resulting from a mutation in the STAT-3 gene are extremely susceptible to bacterial infections such as S.aureus and mucocutaneous fungal infections caused by Candida species (63–65). In support of a role for STAT-3 in driving Th17 cellular responses, these patients do not generate C.albicans- and S.aureus- specific Th17 cellular responses (63). Therefore, the inability to generate Th17 responses is believed to be the mechanism underlying the susceptibility to recurrent fungal infections commonly seen in these patients (63–65). In contrast to these studies, a gastric model of C. albicans infection stimulates severe gut pathology that is exacerbated by IL-23 and IL-17 (27). Zymosan, a β-glucan- containing preparation of yeast cell walls, is a potent producer of IL-23 and can generate Th17 responses (19) via Dectin 1-Syk CARD9 signaling pathway (26). Also, Candida mannan can interact with the macrophage mannose receptor and mediate adaptive immune activation and the induction of Th17 cell differentiation (66). Therefore it is likely that Candida can induce the induction of both IL-23 and IL-17 for recruitment of neutrophils and pathogen clearance, but that in the absence of regulation may result in inflammatory responses. Peridontitis is a chronic infectious inflammatory disease characterized by cellular infiltrates resulting in alveolar bone loss and has been associated with infiltration of Th1 cells at the mucosal surface (67). However, recent evidence supports a protective role for IL-17 in chronic periodontitis induced by Porphyromonas gingivalis infection (68) and in periapical lesions induced by bacterial infections of the dental pulp (69). These studies suggest that IL-17 plays a protective role in infectious diseases at the oral mucosa, likely through their role in recruitment of neutrophils and pathogen clearance, but can also cause pathology if the Th17 responses are not regulated.
Immunity and inflammation at the gastrointestinal mucosa
The IL-23/IL-17 axis also appears to influence the fine balance between tolerance and immunity in the intestine. Accordingly, Th17 cells have been reported to be enriched in the intestine and the migration of Th17 cells in Peyer’s patches and other related tissues in the gut are dependent on expression of the chemokine receptor CCR6 in mice (70) and humans (71) and C-type lectin-like receptor CD161, in humans (72). Intestinal bacterial infections such as those caused by enterohemorrhagic Escherichia coli and enteropathogenic E.coli are a major cause of mortality worldwide (73). Citrobacter rodentium is a naturally occurring pathogen in mice and experimental studies with C.rodentium has provided a model for understanding the immunity required for protection against these attaching and effacing bacterial pathogens. Initial work by Weaver and colleagues provided evidence that IL-23 dependent Th17 responses are critical for host defense against C. rodentium, since IL-23 gene deficient mice are susceptible to C.rodentium infection (74). Furthermore, IL-17A and IL-17F gene deficient mice are also susceptible to C.rodentium infection as a result of decreased induction of antimicrobial peptides such as Beta-defensin 1, 3 and 4 (12). More recently, studies have also shown that the induction of Th17 cells during C.rodentium infection is dependent on infection-induced apoptosis, since blocking apoptosis during infection reduced the accumulation of Th17 cells in the gut (31). In addition to IL-17, IL-22 is also required for protection against C.rodentium, primarily by mediating the induction of innate antimicrobial peptides such as Reg family proteins in colonic epithelial cells (38). Accordingly, exogenous RegIIIγ fusion protein significantly protected IL-22 gene deficient mice from weight loss following C.rodentium infection. The IL-22 responses have been shown to be IL-23 dependent, since IL-23 gene deficient mice had reduced expression of IL-22 and increased susceptibility to C.rodentium infection (38). One of the major sources of innate IL-22 in the gut has been identified to be Natural killer cells [NK-22], detected in the small intestine lamina propria of mice infected with C.rodentium (35–36). That depletion of NK1.1 cells in Rag−/− mice at the early stages of infection resulted in accelerated mortality of infected mice (35–36) suggests a critical role for innate IL-22 in conferring host resistance to C.rodentium. Overall, these studies have provided novel and important insights into the role of Th17 cytokines in protective host responses against bacterial attaching and effacing pathogens in the gut.
The intracellular pathogen Salmonella enterica serotype Typhimurium, elicits an inflammatory pathway in the intestinal mucosa of humans resulting in severe pathology due to neutrophil recruitment (75). Use of streptomycin-pretreated serotype S. Typhimurium infected animal models to model this disease, has shown that IL-23 is required for the initiating the T cell-dependent amplification of inflammatory responses in the intestinal mucosa. IL-23 gene deficient infected mice exhibited decreased inflammation, decreased induction of IL-17, IL-22 and neutrophil attracting chemokines as well as reduced recruitment of neutrophils (76). The induction of the inflammatory responses is dependent on CD3+ T cells, since depletion of T cells resulted in reduction of IFNγ, IL-17, IL-22 as well as chemokines and antimicrobial peptides in the inflamed caecum (77). These studies demonstrate that IL-23 and Th17 cytokines are protective against extracellular bacteria, but drive the pathological inflammatory responses associated with intracellular pathogens at the gut mucosa.
Significant CD4+ T cell depletion occurs during acute HIV infection, especially in the Gut Associated Lymphoid Tissue [GALT] (78) and significantly impacts the ability of the intestinal mucosa to respond to other immunological challenges. Interestingly, Th17 cells of the α4+β7hi CD4+ memory subset are preferentially infected and depleted during acute SIV infection and this alters the balance between Th1 and Th17 responses (79). Highly viremic animals were found to have a predominance of Th1 response, and the frequency of Th17 cells found at mucosal sites was shown to negatively correlate with the plasma virus level (80). Furthermore, depletion of Th17 cells in the ileal mucosa of rhesus macaques impairs mucosal barrier function to SalmonellaTyphimurium dissemination (81). This may be the basis behind why Salmonella Typhimurium, which normally causes localized enteric infection in normal hosts, can lead to life threatening bacteremia in HIV-infected hosts as a result of loss of mucosal barrier function (81). Accordingly, HIV-infected individuals that receive anti-retroviral therapy undergo effective CD4+ T cell restoration and this is associated with enhanced CD4+ Th17 cell accumulation (82). These studies suggest that IL-23 can trigger a plethora of different pro-inflammatory cytokines in the gut that can impact both acute innate as well as adaptive responses leading to protection or pathological consequences in the gut mucosa (Table 2).
Table 2.
Protective versus pathological roles for Th17 cytokines in generating immunity against oral and gut pathogens.
| Mucosal surfaces and Organisms | Type of Organism | Role of IL-17 and/or Th17 cells | Functional Significance |
|---|---|---|---|
| Citrobacter rodentium | Bacteria | Protective | Induction of innate antimicrobial peptides and IL-23 dependent Th17 cell response (12, 38, 74) |
| SalmonellaTyphimurium | Bacteria | Exacerbation | IL-23 dependent initiation of T cell response and Th17 cytokines (76–77) |
| Candida [gastric] | Fungi | Exacerbation | Increased recruitment of neutrophils (27) |
| Candida [oral mucosal] | Fungi | Protective | Neutrophil recruitment with induction of CXC chemokines and antimicrobials [beta-defensins] (60) |
| Porphyromonas gingivalis [oral] | Bacterial | Protective | Neutrophil recruitment and pathogen clearance (68) |
Conclusions
Several lines of evidence suggest that Th17 cells mediate innate and adaptive immunity against a variety of pathogens at different mucosal sites. The increased incidence of mucosal infections seen in the absence of Th17 responses, as in patients with immunodeficiency [AIDS and Hyper-IgE syndrome] have opened novel avenues for immunotherapy to treat or prevent these infections. Also, the emerging evidence that Th17 cells are crucial players in generation of vaccine-induced protective responses in the respiratory tract and the gut, suggests that the targeting IL-17 has the potential to substantially impact vaccine strategies against infectious diseases. Most importantly, it is becoming apparent that there is a fine balance between protective and pathological manifestation of Th17 responses at mucosal sites. Further research for a better understanding of this fine balance will be key to defining the outcome of the infection at mucosal sites.
Acknowledgments
This work was supported by Children’s Hospital Of Pittsburgh, Pennsylvania Department of Health Formula Funding, AI075106, A1083541 from National Institute of Health, USA to SAK.
Abbreviations
- IL
Interleukin
- IFN-γ
Interferon gamma
- MIP-2
macrophage inflammatory protein-2
- G-CSF
Granulocyte colony stimulating factor
- CXCR-3
chemokine CXC motif receptor 3
- Th1
T Helper Cell 1
- Th2
T Helper Cell 2
- Th17
T Helper Cell 17
Footnotes
The authors have no conflicting financial interests.
References
- 1.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005 Nov;6(11):1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005 Nov;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 3.Dong C. IL-23/IL-17 biology and therapeutic considerations. J Immunotoxicol. 2008 Jan;5(1):43–6. doi: 10.1080/15476910801897953. [DOI] [PubMed] [Google Scholar]
- 4.Diveu C, McGeachy MJ, Cua DJ. Cytokines that regulate autoimmunity. Curr Opin Immunol. 2008 Dec;20(6):663–8. doi: 10.1016/j.coi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005 Jan 17;201(2):233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007 Jul 26;448(7152):480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 7.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007 Jul 26;448(7152):484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006 Nov;16(11):902–7. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 9.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006 Oct 2;203(10):2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine. 2009 Apr;46(1):7–11. doi: 10.1016/j.cyto.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang W, Filvaroff E, Hu Y, Grogan J. Novel therapeutic targets along the Th17 pathway. Eur J Immunol. 2009 Mar;39(3):670–5. doi: 10.1002/eji.200839105. [DOI] [PubMed] [Google Scholar]
- 12••.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009 Jan 16;30(1):108–19. doi: 10.1016/j.immuni.2008.11.009. This paper shows that IL-17A but not IL-17F, plays a major role in inflammation associated with autoimmune disorders. In contrast, both IL-17F and IL-17A were involved in host defense against mucoepithelial infection by Staphylococcus aureus and Citrobacter rodentium. This paper also shows that IL-17 can induce cyokines and chemokines in APCs such as macrophages. [DOI] [PubMed] [Google Scholar]
- 13••.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Requirement of Interleukin-17 for T helper 1 immunity and host resistance to the intracellular pathogen Francisella tularensis LVS. Immunity. 2009 doi: 10.1016/j.immuni.2009.08.025. In Press. This paper shows that IL-17 can drive IL-12 in DCs and drive Th1 differentiation required for protection against the intracellular pathogen F. tularensis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006 Sep 22;126(6):1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008 Jan;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005 Sep 19;202(6):761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003 May 1;170(9):4432–6. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008 Jun 9;205(6):1447–61. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005 Jul 15;175(2):788–95. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell P, Germain C, Fiori PL, Khamri W, Foster GR, Ghosh S, et al. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect Immun. 2007 Feb;75(2):810–9. doi: 10.1128/IAI.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butchar JP, Rajaram MV, Ganesan LP, Parsa KV, Clay CD, Schlesinger LS, et al. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007 Apr 1;178(7):4445–54. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- 23.Siegemund S, Schutze N, Freudenberg MA, Lutz MB, Straubinger RK, Alber G. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella Enteritidis. Immunobiology. 2007;212(9–10):739–50. doi: 10.1016/j.imbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Fedele G, Nasso M, Spensieri F, Palazzo R, Frasca L, Watanabe M, et al. Lipopolysaccharides from Bordetella pertussis and Bordetella parapertussis differently modulate human dendritic cell functions resulting in divergent prevalence of Th17-polarized responses. J Immunol. 2008 Jul 1;181(1):208–16. doi: 10.4049/jimmunol.181.1.208. [DOI] [PubMed] [Google Scholar]
- 25.Siegemund S, Alber G. Cryptococcus neoformans activates bone marrow-derived conventional dendritic cells rather than plasmacytoid dendritic cells and down-regulates macrophages. FEMS Immunol Med Microbiol. 2008 Apr;52(3):417–27. doi: 10.1111/j.1574-695X.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- 26.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007 Jun;8(6):630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 27.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007 Oct;37(10):2695–706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 28.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007 Oct;27(4):660–9. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008 Jul 1;181(1):721–35. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fransen JH, Hilbrands LB, Ruben J, Stoffels M, Adema GJ, van der Vlag J, et al. Mouse dendritic cells matured by ingestion of apoptotic blebs induce T cells to produce interleukin-17. Arthritis Rheum. 2009 Aug;60(8):2304–13. doi: 10.1002/art.24719. [DOI] [PubMed] [Google Scholar]
- 31••.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009 Mar 5;458(7234):78–82. doi: 10.1038/nature07781. This paper shows that the Th17 polarizing cytokines IL-6 and TGF-Beta are produced following phagocytosis of infected apoptotic cells. In contrast, phagocytosis of apoptotic cells in the absence of microbial signals induces differentiation of the T regulatory cells. Therefore this paper defines how the Th17 pathway is initiated in vivo following infection. [DOI] [PubMed] [Google Scholar]
- 32.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007 Mar 15;178(6):3786–96. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 33.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006 Oct 1;177(7):4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 34••.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009 Aug 21;31(2):321–30. doi: 10.1016/j.immuni.2009.06.020. This paper shows that TCRgd T cells share characteristics with Th17 cells [such as expression of chemokine receptor 6 (CCR6), ROR-gamma-t, aryl hydrocarbon receptor (AhR), and IL-23 receptor]. The authors also show that AhR expression is essential for IL-22 production, and that CCR6+ IL-17-producing gammadelta T cells express Toll-like receptors TLR1 and TLR2, as well as dectin-1 and can directly interact with pathogens. [DOI] [PubMed] [Google Scholar]
- 35.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009 Feb 5;457(7230):722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008 Dec 19;29(6):958–70. doi: 10.1016/j.immuni.2008.11.001. The above two papers describe the expression of the NK cell natural cytotoxicity receptor NKp46 by intestinal lymphocytes and the protective role of innate IL-22 in the immune responses to C. rodentium infection. [DOI] [PubMed] [Google Scholar]
- 37.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009 Jan 16;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008 Mar;14(3):282–9. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 39••.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008 Mar;14(3):275–81. doi: 10.1038/nm1710. The above two papers were amongst the first to show that IL-22 can induce antimicrobials invivo that are required for protection against extracellular infections at mucosal surfaces. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001 Aug 20;194(4):519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001 Sep;25(3):335–40. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 42.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996 Jun 1;183(6):2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, Berger T, et al. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009 Apr 15;182(8):4947–56. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009 Jul;119(7):1899–909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chackerian AA, Chen SJ, Brodie SJ, Mattson JD, McClanahan TK, Kastelein RA, et al. Neutralization or absence of the interleukin-23 pathway does not compromise immunity to mycobacterial infection. Infect Immun. 2006 Nov;74(11):6092–9. doi: 10.1128/IAI.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect Immun. 2008 Jun;76(6):2651–9. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007 Jan;9(1):78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, et al. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol. 2009 Jul 15;183(2):1291–300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009 Apr 15;182(8):4938–46. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun. 2007 Jun;75(6):3055–61. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deepe GS, Jr, Gibbons RS. Interleukins 17 and 23 Influence the Host Response to Histoplasma capsulatum. J Infect Dis. 2009 Jul 1;200(1):142–51. doi: 10.1086/599333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009 Mar 15;182(6):3469–81. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009 Jun 15;182(12):7353–63. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, et al. Critical Role of IL-17RA in Immunopathology of Influenza Infection. J Immunol. 2009 Sep 25; doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005 Jul 1;175(1):404–12. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubin PJ, McAllister F, Kolls JK. Is cystic fibrosis a TH17 disease? Inflamm Res. 2007 Jun;56(6):221–7. doi: 10.1007/s00011-007-6187-2. [DOI] [PubMed] [Google Scholar]
- 57.Wiehler S, Proud D. Interleukin-17A modulates human airway epithelial responses to human rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2007 Aug;293(2):L505–15. doi: 10.1152/ajplung.00066.2007. [DOI] [PubMed] [Google Scholar]
- 58.Farah CS, Elahi S, Drysdale K, Pang G, Gotjamanos T, Seymour GJ, et al. Primary role for CD4(+) T lymphocytes in recovery from oropharyngeal candidiasis. Infect Immun. 2002 Feb;70(2):724–31. doi: 10.1128/iai.70.2.724-731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fidel PL., Jr Candida-host interactions in HIV disease: relationships in oropharyngeal candidiasis. Adv Dent Res. 2006;19(1):80–4. doi: 10.1177/154407370601900116. [DOI] [PubMed] [Google Scholar]
- 60••.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009 Feb 16;206(2):299–311. doi: 10.1084/jem.20081463. This paper marks a paradigm shift in the understanding of the immune response to oropharyngeal candidiasis by highlighting the protective role of Th17 lineage in mediating recruitment of neutrophils and synthesis of anti-microbial proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farah CS, Hu Y, Riminton S, Ashman RB. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene-targeting. Oral Microbiol Immunol. 2006 Aug;21(4):252–5. doi: 10.1111/j.1399-302X.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 62.Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H, et al. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol. 2008 Nov;128(11):2640–5. doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- 63.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008 Apr 10;452(7188):773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008 Jul 7;205(7):1551–7. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65••.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008 Jul 7;205(7):1543–50. doi: 10.1084/jem.20080321. The above three important papers establish the role of STAT3 signalling in the generation of Th17 cells in the autosomal dominant hyper-IgE syndrome in humans. They also help understand the fact that increased susceptibility to the recurrent infections commonly seen in HIES may be related to the inability to produce Th17 cells in these patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009 Apr 23;5(4):329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Gemmell E, Yamazaki K, Seymour GJ. Destructive periodontitis lesions are determined by the nature of the lymphocytic response. Crit Rev Oral Biol Med. 2002;13(1):17–34. doi: 10.1177/154411130201300104. [DOI] [PubMed] [Google Scholar]
- 68.Oda T, Yoshie H, Yamazaki K. Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol Immunol. 2003 Feb;18(1):30–6. doi: 10.1034/j.1399-302x.2003.180105.x. [DOI] [PubMed] [Google Scholar]
- 69.Oseko F, Yamamoto T, Akamatsu Y, Kanamura N, Iwakura Y, Imanishi J, et al. IL-17 is involved in bone resorption in mouse periapical lesions. Microbiol Immunol. 2009 May;53(5):287–94. doi: 10.1111/j.1348-0421.2009.00123.x. [DOI] [PubMed] [Google Scholar]
- 70.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009 Mar;2(2):173–83. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007 Aug 6;204(8):1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009 Mar 16;206(3):525–34. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998 Jan;11(1):142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006 May 11;441(7090):231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 75.Gordon MA. Salmonella infections in immunocompromised adults. J Infect. 2008 Jun;56(6):413–22. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 76.Godinez I, Raffatellu M, Chu H, Paixao TA, Haneda T, Santos RL, et al. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect Immun. 2009 Jan;77(1):387–98. doi: 10.1128/IAI.00933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, et al. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect Immun. 2008 May;76(5):2008–17. doi: 10.1128/IAI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008 Jan;82(1):538–45. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79••.Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009 Sep;2(5):439–49. doi: 10.1038/mi.2009.90. The authors highlight the preferential infection of the minimally activated population of Alpha4 [+] beta7 [hi] CD4 [+] memory T cells by human immunodeficiency virus/simian immunodeficiency virus [HIV/SIV] in early stages of infection, and suggest that loss of these cells may alter the balance between Th17 and Th1 responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cecchinato V, Trindade CJ, Laurence A, Heraud JM, Brenchley JM, Ferrari MG, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008 Jul;1(4):279–88. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81••.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008 Apr;14(4):421–8. doi: 10.1038/nm1743. This paper establishes the importance of Th17 cells and their cytokines in mediating protection against Salmonella Typhimurium infection in the Simian Immunodeficiency Virus [SIV] infected macaque model. The authors show that SIV infected animals undergo depletion of ileal mucosal Th17 cells leading to systemic dissemination of Salmonella infection in these hosts. This may be the basis for the increased susceptibility of SIV infected host to severe, fulminant infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82••.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008 Nov;1(6):475–88. doi: 10.1038/mi.2008.35. This paper highlights the effect of highly active antiretroviral therapy [HAART] in restoring gut mucosal CD4+ T cells, enhancing Th17 CD4+ T-cell accumulation and generation of polyfunctional anti-HIV cellular responses. [DOI] [PubMed] [Google Scholar]


