Among various sites of nitric oxide reactivity in biology,1 iron-sulfur clusters have emerged as an important class of metallocofactors targeted by NO.2 Proteins containing these clusters perform a diverse array of functions from electron transfer to small molecule sensing and substrate binding.3 Consequently, iron-sulfur clusters are potential sites for both NO-derived physiological signal transduction4 and toxicity.2a,5
The primary products of NO reactivity at iron-sulfur clusters are dinitrosyl iron complexes (DNICs).6 Identification of DNICs in proteins and tissue samples has traditionally relied upon the diagnostic g = 2.03 EPR signal,7 which is characteristic of these S = ½ species. Because iron dinitrosyls can exist in forms other than the paramagnetic mononuclear species (Chart 1), however, EPR characterization is not definitive.8 Other means of characterization include 57Fe Mössbauer,9 UV-vis,10 Raman,11 and X-ray absorption spectroscopy,12 but each of these techniques lacks diagnostic spectroscopic markers and cannot easily deconvolute spectra resulting from a mixture of species.
Chart 1.
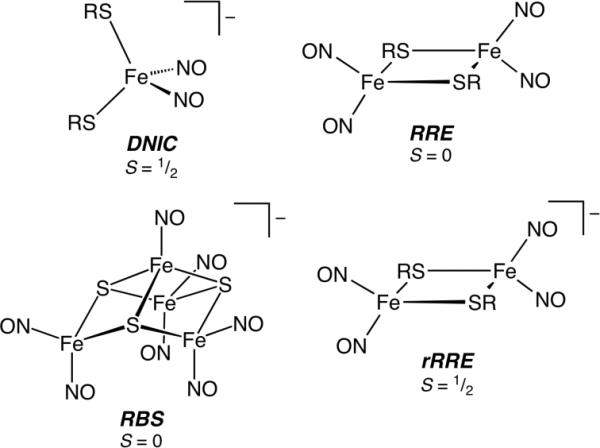
Examples of sulfur-ligated iron dinitrosyls.
In order to overcome these challenges, we have utilized 57Fe nuclear resonance vibrational spectroscopy (NRVS) to characterize the core vibrations of dinitrosyl iron complexes. This technique is selective for motion of the 57Fe nucleus, making it an ideal means of probing DNICs in protein environments.13 NRVS is not limited by the selection rules of infrared and Raman spectroscopy, ensuring that a complete set of vibrational modes involving motion of the 57Fe nucleus is observed. Here we present the first NRVS results for a nitrosylated iron-sulfur cluster protein, as well as complimentary data on several biomimetic model compounds of dinitrosyl iron complexes.
In order to provide spectroscopic benchmarks to identify dinitrosyl iron complexes in proteins, we investigated synthetic analogs comprising the four structures displayed in Chart 1. To provide maximum NRVS signal intensity, the model compounds were prepared from 57Fe-enriched Fe(OTf)2·2CH3CN (see Supporting Information, SI, for experimental details). The benzene thiolate ligand (Chart 1, R = Ph) was chosen because it facilitates the synthesis and isolation of the three different 57Fe-labeled derivatives and because the NRVS spectrum of the related Fe-S cluster, [Fe4S4(SPh)4]2−, was recorded previously.14 Additionally, (Et4N)[Fe(NO)2(SPh)2], [Fe2(μ-SPh)2(NO)4] (Roussin's red ester, RRE), and (Et4N)[Fe4S3(NO)7] (Roussin's black salt, RBS) were prepared using both 14NO and 15NO to help identify normal modes involving the nitrosyl ligands.
A representative NRVS spectrum of one of the model compounds, (Et4N)[Fe(14/15NO)2(SPh)2], is shown in Figure 1. Of note are the intense peaks above 500 cm−1, which shift to lower energy upon 15N substitution. The NRVS spectra of [Fe(μ-SPh)2(14/15NO)4] and (Et4N)[Fe4S3(14/15NO)7] display similarly shifted peaks (Figure S14), indicating that vibrations in this region most likely originate from the symmetric and asymmetric stretching modes of the N–Fe–N unit.11 In all four model compounds, the peak near 600 cm−1 appears to contain a higher energy shoulder (e.g., Figure 1). We assign this feature to Fermi resonance based on the calculated spectrum for each model compound (see SI) and the disappearance of these peaks upon 15N substitution (Figure 1). Despite these minor complexities, the region of the spectrum between 500 and 700 cm−1 provides important diagnostic NRVS signatures for each of the iron dinitrosyl complexes displayed in Chart 1 (Figure S20). Furthermore, the vibrations occur in a region of the spectrum that does not overlap with normal modes due to thiolate ligands or the {Fe4S4} core, thereby providing clear information about the nature of the Fe-NO species present. EPR spectroscopy fails in this regard because the RRE and RBS compounds are diamagnetic, and 57Fe Mössbauer spectroscopy is complicated by the fact that isomer shifts for all four dinitrosyl-containing species are very similar (Figure S25 and Table S2).
Figure 1.
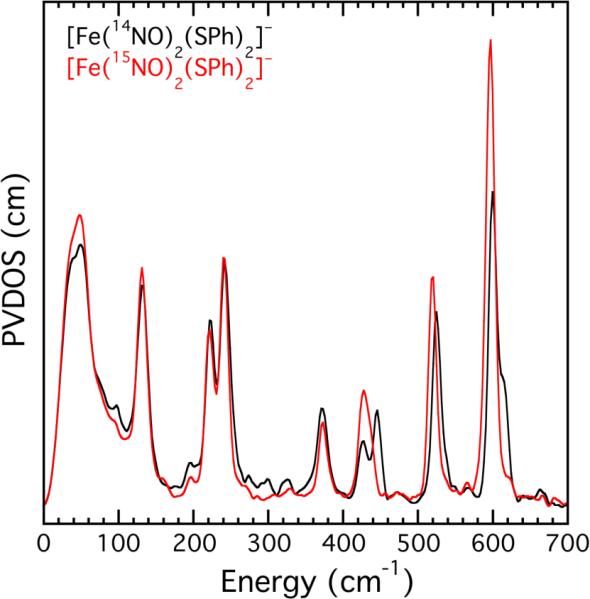
57Fe partial vibrational density of states for (Et4N)[Fe(NO)2(SPh)2].
For protein studies, a ferredoxin D14C variant from Pyroccocus furiosus was selected as a model system because of its stability at high concentrations (mM) and relatively solvent exposed Fe4S4 cluster (Figure S26).15 In the native form of the protein, the iron-sulfur cluster is bound by one aspartate (D14) and three cysteinate residues. For the present purposes, a D14C mutant was examined because it provides a more representative model of Fe4S4 clusters, which typically have four terminal cysteine thiolate ligands. Moreover, NRVS data on the untreated D14C mutant were already available,16 providing an opportunity to distinguish vibrations of the unmodified protein from those of the NO reaction product(s) (Figure S28).
Reaction of P. furiosus ferredoxin with the nitric oxide donor PAPA NONOate gave rise to a single gav = 2.03 EPR signal characteristic of a protein-bound DNIC (Figure S27). EPR spectroscopy provided no evidence for formation of rRRE (gav = 1.99),17 and spin quantitation of the DNIC signal demonstrated that this species only accounted for about 5 – 10% of the total protein concentration. Examination of the reaction mixture by NRVS, however, indicated significant formation of a dinitrosyl iron complex (Figure 2 and Figure S28). The spectral features of this species were inconsistent with either the DNIC or the diamagnetic RRE. By comparison with the model compounds, we assign this product as the tetranuclear Roussin's black salt, RBS ([Fe4S3(NO)7]−, Chart 1).
Figure 2.
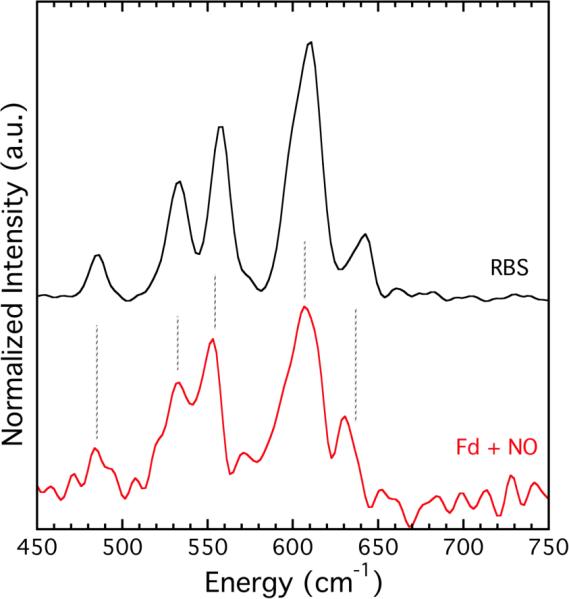
Comparison of the NRVS spectrum of RBS and the spectrum resulting from treatment of 57Fe-enriched P. furiosus Fd D14C with PAPA NONOate.
Formation of RBS by nitrosylation of a protein-bound Fe4S4 cluster has not been previously reported, although reactions of a variety of synthetic Fe4S4 clusters with NO gas generate the black salt.18 RBS formation may be related to the highly solvent-exposed nature of the Fe4S4 cluster in P. furiosus Fd, but further work is required to test this possibility as well as its potential generality. The formation of RBS, however, could help explain the results obtained in reactions of with other Fe4S4 cluster proteins with NO, where EPR spin quantitation of protein-bound DNICs accounts for only a fraction of the total iron content.19
Roussin's black salt is toxic to cells2a,20 and its formation from Fe-S clusters is a plausible mode of NO-derived toxicity in vivo. Our data provide the first evidence for the direct formation of this species from an iron-sulfur protein. The presence of RBS in addition to DNICs may have important implications for the repair of nitrosylated iron-sulfur clusters,21 the mechanism of NO signal transduction,4 and other biological consequences of nitric oxide chemistry at iron-sulfur protein cores.2
In conclusion, we provide here the first NRVS spectra of non-heme iron nitrosyl compounds and demonstrate the value of this technique in identifying the products of iron-sulfur cluster nitrosylation. In particular, the method has proved valuable in identifying diamagnetic dinitrosyl iron species that would otherwise be very difficult to observe. Moving forward, we envision extending these studies to identify the products of other biological NO targets and to help define the roles of nitric oxide in modulating the functions non-heme iron proteins.
Supplementary Material
ACKNOWLEDGMENT
This work was funded by grants from the NSF (CHE-0611944 to SJL), the NIH (GM65440 to SPC), and the DOE (DE-FG02-05ER15710 to MWA). Z.J.T. thanks NIGMS for a postdoctoral fellowship (1 F32 GM082031-02). C.E.T. received partial support under Interdepartmental Training Grant T32 GM08334 from NIGMS. We thank Drs. Yoshitaka Yoda (SPring-8) and Ilya Sergeev (ESRF) for valuable assistance with the NRVS measurements.
Footnotes
SUPPORTING INFORMATION Experimental procedures, additional figures, crystallographic data collection and refinement details, and fully labeled thermal ellipsoid diagrams for [Fe2(μ-SPh)2(NO)4] and (Et4N)[Fe2(μ-SPh)2(NO)4], as well as the corresponding CIF files. This information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).(a) Ignarro LJ, editor. Nitric Oxide: Biology and Pathobiology. Academic Press; San Diego: 2000. [Google Scholar]; (b) McCleverty JA. Chem. Rev. 2004;104:403–418. doi: 10.1021/cr020623q. [DOI] [PubMed] [Google Scholar]
- (2).(a) Butler AR, Megson IL. Chem. Rev. 2002;102:1155–1165. doi: 10.1021/cr000076d. [DOI] [PubMed] [Google Scholar]; (b) Szaciłowski K, Chmura A, Stasicka Z. Coord. Chem. Rev. 2005;249:2408–2436. [Google Scholar]
- (3).Beinert H, Holm RH, Münck E. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- (4).Ding H, Demple B. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5146–5150. doi: 10.1073/pnas.97.10.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Asanuma K, Iijima K, Ara N, Koike T, Yoshitake J, Ohara S, Shimosegawa T, Yoshimura T. Nitric Oxide. 2007;16:395–402. doi: 10.1016/j.niox.2007.02.002. [DOI] [PubMed] [Google Scholar]
- (6).Reddy D, Lancaster JR, Jr., Cornforth DP. Science. 1983;221:769–770. doi: 10.1126/science.6308761. [DOI] [PubMed] [Google Scholar]
- (7).Vanin AF, Serezhenkov VA, Mikoyan VD, Genkin MV. Nitric Oxide. 1998;2:224–234. doi: 10.1006/niox.1998.0180. [DOI] [PubMed] [Google Scholar]
- (8).Butler AR, Glidewell C, Li M. Adv. Inorg. Chem. 1988;32:335–393. [Google Scholar]
- (9).Lobysheva II, Serezhenkov VA, Stucan RA, Bowman MK, Vanin AF. Biochemistry (Moscow) 1997;62:801–808. [Google Scholar]
- (10).Foster MW, Cowan JA. J. Am. Chem. Soc. 1999;121:4093–4100. [Google Scholar]
- (11).Dai RJ, Ke SC. J. Phys. Chem. B. 2007;111:2335–2346. doi: 10.1021/jp066964f. [DOI] [PubMed] [Google Scholar]
- (12).Tsai M-C, Tsai F-T, Lu T-T, Tsai M-L, Wei Y-C, Hsu IJ, Lee J-F, Liaw W-F. Inorg. Chem. 2009;48:9579–9591. doi: 10.1021/ic901675p. [DOI] [PubMed] [Google Scholar]
- (13).Scheidt WR, Durbin SM, Sage JT. J. Inorg. Biochem. 2005;99:60–71. doi: 10.1016/j.jinorgbio.2004.11.004. [DOI] [PubMed] [Google Scholar]
- (14).Xiao Y, Koutmos M, Case DA, Coucouvanis D, Wang H, Cramer SP. Dalton. 2006:2192–2201. doi: 10.1039/b513331a. [DOI] [PubMed] [Google Scholar]
- (15).Aono S, Bryant FO, Adams MW. J. Bacteriol. 1989;171:3433–3439. doi: 10.1128/jb.171.6.3433-3439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Cramer SP, Xiao Y, Wang H, Guo Y, Smith MC. Hyperfine Interact. 2007;170:47–54. [Google Scholar]
- (17).Lu T-T, Tsou C-C, Huang H-W, Hsu I-J, Chen J-M, Kuo T-S, Wang Y, Liaw W-F. Inorg. Chem. 2008;47:6040–6050. doi: 10.1021/ic800360m. [DOI] [PubMed] [Google Scholar]
- (18).Harrop TC, Tonzetich ZJ, Reisner E, Lippard SJ. J. Am. Chem. Soc. 2008;130:15602–15610. doi: 10.1021/ja8054996. [DOI] [PubMed] [Google Scholar]
- (19).(a) Kennedy MC, Antholine WE, Beinert H. J. Biol. Chem. 1997;272:20340–20347. doi: 10.1074/jbc.272.33.20340. [DOI] [PubMed] [Google Scholar]; (b) Cruz-Ramos H, Crack J, Wu G, Hughes MN, Scott C, Thomson AJ, Green J, Poole RK. EMBO J. 2002;21:3235–3244. doi: 10.1093/emboj/cdf339. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yukl ET, Elbaz MA, Nakano MM, Moënne-Loccoz P. Biochemistry. 2008;47:13084–13092. doi: 10.1021/bi801342x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hamilton-Brehm SD, Schut GJ, Adams MWW. Appl. Environ. Microbiol. 2009;75:1820–1825. doi: 10.1128/AEM.02562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Rogers PA, Eide L, Klungland A, Ding H. DNA Repair. 2003;2:809–817. doi: 10.1016/s1568-7864(03)00065-x. [DOI] [PubMed] [Google Scholar]; (b) Tsou C-C, Lin Z-S, Lu T-T, Liaw W-F. J. Am. Chem. Soc. 2008;130:17154–17160. doi: 10.1021/ja806050x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


