Abstract
The relationship between exercise and amyotrophic lateral sclerosis (ALS), a neurodegenerative disorder characterized by motor neuron loss, rapidly progressive weakness, and early death has been controversial. We studied the effect of a high (HEX) and moderate level exercise (MEX) on body weight, motor performance, and motor neuron counts in the ventral horn of spinal cords in a transgenic mouse model of ALS (G93A-SOD1) that overexpresses a mutated form of the human SOD1 gene that is a cause of familial ALS. These transgenic mice show several similarities to the human disease, including rapid progressive motor weakness from 100 days of age and premature death at around 135 days of age. Mice were exposed to high or mid-level exercise of left sedentary (SED). At 70, 95 and 120 days of age spinal cords were processed following euthanasia. Motor neurons larger than 15 μm in diameter were counted with a design-based stereological protocol using an optical fractionator probe in the ventral horn of different regions of the cord and compared to wild-type littermates. Moderate exercise delayed the onset of motor deficit by over a week. High exercise slightly but significantly hastened the onset of motor performance deficits. Motor neuron density in the lumbar cord was significantly higher in MEX group compared to SED at 95 days of age. These results show the beneficial effects of moderate exercise on the preservation of motor performance that correlates with higher motor neuron density in the ventral horn of the lumbar spinal cord in G93A mice.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal adult-onset neurodegenerative disorder affecting upper and lower motor neurons. It is characterized by motor neuron loss, rapidly progressive weakness, and death due to respiratory failure (Brown, 1995). Most cases of ALS are sporadic but approximately 10% are familial and inherited in an autosomal dominant manner. Sporadic ALS and familial ALS (FALS) are clinically and pathologically indistinguishable. About 20% of FALS patients carry a point mutation in the superoxide dismutase 1 (SOD1) gene, which encodes for the cytosolic copper- and zinc-dependent SOD (Rosen et al., 1993). The role of SOD in ALS is not completely understood but it is thought that a toxic gain of function rather than a loss of dismutase activity is responsible for the motor neuron loss (Ripps et al., 1995). The discovery that autosomal dominant FALS is associated with point mutations in the SOD1 gene (Rosen et al., 1993) led to the development of transgenic mouse that overexpress human mutant SOD1. These transgenic mice develops an adult-onset motor neuron disease that resembles ALS with respect to motor neuron function, pathology, and biochemistry (Bruijn et al., 1997; Gurney et al., 1994) (Ripps et al., 1995) (Gruzman et al., 2007) and have proven to be an invaluable animal model of ALS.
The etiology of ALS remains elusive but the selective vulnerability of motor neurons likely arises from a combination of mechanisms, including protein misfolding, mitochondria dysfunction, oxidative damage, defective axonal transport, excitoxicity, insufficient growth factor signaling, and inflammation (Boillee et al., 2006). Modifiable risk factors for ALS have so far not being identified. Strenuous physical activity has been reported to be a risk factor but is controversial. Some epidemiological studies suggest that vigorous physical activity in the form of heavy labor or competitive athletics increases ALS risk (Scarmeas et al., 2002) (Chio et al., 2005) (Gregoire and Serratrice, 1991) (Felmus et al., 1976). Other epidemiological studies report earlier disease onset among individuals with a greater amounts of leisure time and reduced physical activity (Strickland et al., 1996) (Veldink et al., 2005). Yet others reports indicate that physical activity is not a risk factor for developing ALS (Armon, 2007) (Longstreth et al., 1991) (Qureshi et al., 2006) (Kurtzke and Beebe, 1980). Clinical trials of ALS patients have suggested that regular physical exercise may be neuroprotective, ameliorate symptoms and improve functionality (Drory et al., 2001) (Pinto et al., 1999) (Bello-Haas et al., 2007). The potential positive effect of physical activity on ALS has been tested in mouse models of ALS but conflicting results have clouded the role of physical exercise. Regular moderate intensity exercise has been reported to have neuroprotective effects delaying the onset of the disease and / or its progression with a modest increase in the survival of transgenic mice (Kirkinezos et al., 2003) (Veldink et al., 2003) (Kaspar et al., 2005). When regular moderate intensity exercise was combined with insulin-like growth factor (IGF-1) treatment, a strong synergistic effect was reported (Kaspar et al., 2005). Lifetime exposure of transgenic G93A-SOD1 mice to vigorous physical activity based on a 10h/day on a motor-driven running wheel showed that exercise did not promote hasten the progression of motor neuron degeneration (Liebetanz et al., 2004) however, in the same transgenic ALS strain, high intensity endurance treadmill exercise for 45 min/day, 5 times/week progressive increased from 9 to 22 m/min hastened the onset of weakness and death (Mahoney et al., 2004). Given these contradictory results, the relationship between different levels of exercise and motor neuron viability will require further study.
Based on previously reported protocols in the literature, we devised two exercise protocols using a motorized treadmill. G93A-SOD1 mice were treated with either a moderate-level exercise (MEX), a high-level exercise (HEX) or with a sedentary life style exempted from running (SED). The effects of exercise on motor performance and motor neurons survival were analyzed by a rotarod test and absolute motor neuron counts were determined using design-based stereology. Motor neurons were counted at 70, 95 and 120 days of age corresponding to the pre-symptomatic phase, time of disease onset, and terminal disease stage in the G93A-SOD1 strain to determine the effect of exercise on motor neuron death during disease progression.
2. Results
2.1 Body Weight
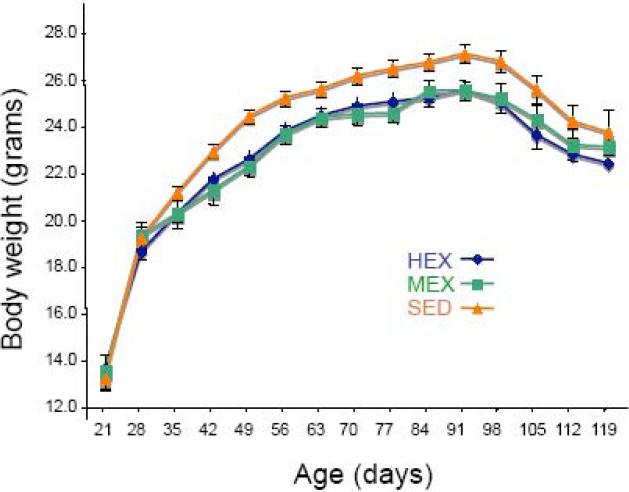
Mice from the SED, HEX and MEX groups were weighted twice a week to test whether the HEX and MEX running protocols used in the study affected the animal's body weight. Mice exposed to mid- and high-level exercise had a lower body weight (5% to 8% lower) than that of sedentary littermates. Differences in body weight were detected after 2 weeks of mice being exposed to the running protocols and the body weight differences were maintained quite steadily until terminal stage. No differences in body weight were observed between mice exposed to moderate- and high-level exercise (Figure 1).
Figure 1. Effects of mid- (MEX) and high- (HEX) level exercise on body weight in G93A transgenic mice.
G93A mice exposed to either MEX or HEX exercise maintained a 5-8% lower body weight compared to sedentary (SED) G93A transgenic mice. All values are mean ±SEM.
2.2 Motor Performance
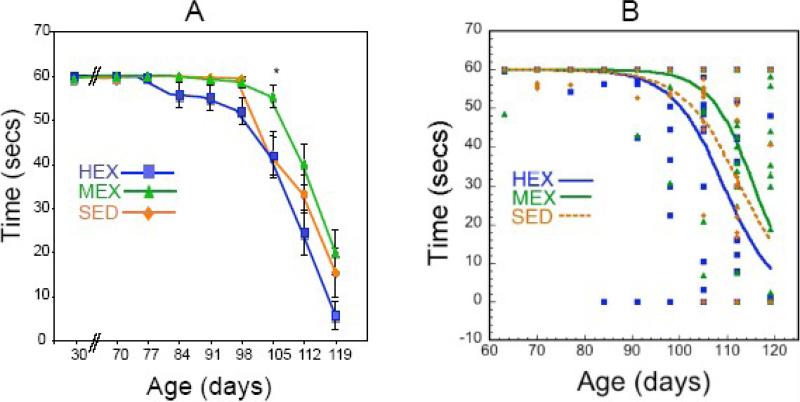
To determine if the running regimens used in the study affected the motor performance of G93ASOD1 mice, mice from the SED, HEX and MEX groups were submitted to a rotarod test twice a week. Moderate-level exercise, but not high-level exercise, had a small but significant affect on the motor performance on the rotarod. The abrupt decline in motor performance that typically occurs at around 95 days of age was delayed by over 1 week in G93A-SOD1 mice exposed to the MEX protocol (Figure 2A). We fit the data to a logistic equation as shown in Figure 2B. The fits showed that the curves were significantly different between the MEX and SED mice (R factor ratio 1.06, p<0.02) and between the MEX and HEX mice (R factor ratio 1. 24 p<0.002) and between the HEX and SED mice (R factor ratio 1.04, p<0.05). During week 15, corresponding to 105 days of age, MEX mice were able to stay on the rod 30% longer than SED mice (55.5±2.7 vs. 42.2±5.3 minutes, respectively, p<0.05) (Figure 2A). Analysis of the data using a repeated measures controlled trial approach (Hopkins, 2003) showed that there was a significant difference at week 15 between the MEX and HEX groups (p<0.01; change in means 12.4±7.5 (upper and lower 90% confidence interval)) and between the MEX and SED groups (p<0.02; change in means 15.5±9.8 (upper and lower 90% confidence interval)). The difference between HEX and SED was not significant at any of the individual weeks even though there was an overall significant difference in the fitted curves as discussed above. Finally, a standard repeated measures ANOVA showed a significant effect of time (p<0.0001) and group (HEX, MEX, SED; p<0.01) with a significant interaction between group and time (p<0.01).
Figure 2. Effects of mid- (MEX) and high- (HEX) level exercise on rotarod performance in G93A transgenic mice compared to sedentary (SED) G93A transgenic mice.
A. Starting at 30 days of age mice were placed, twice a week, on a rod at 12 rpm for 60 secs and the time remaining on the rod before falling was recorded. Not shown in the figure is the rotarod performance from 30 to 70 days of age, which was for all mice at maximum (60 s). All values are mean ±SEM. Statistical analysis was performed by ANOVA followed by post-hoc test (Tukey-Kramer), p<0.05 is considered significant. Significance (*) was reached for MEX vs. SED and HEX at 105 days of age. B. Demonstration of fitting the total data to a logistic function that combined the onset and slope of the decline. We used the R-factor ratio test, which is quite similar to an F-test to compare the fits for each set of parameters with the R-factor ratio determining the significance of the fits.
2.3 Motor Neuron Counts
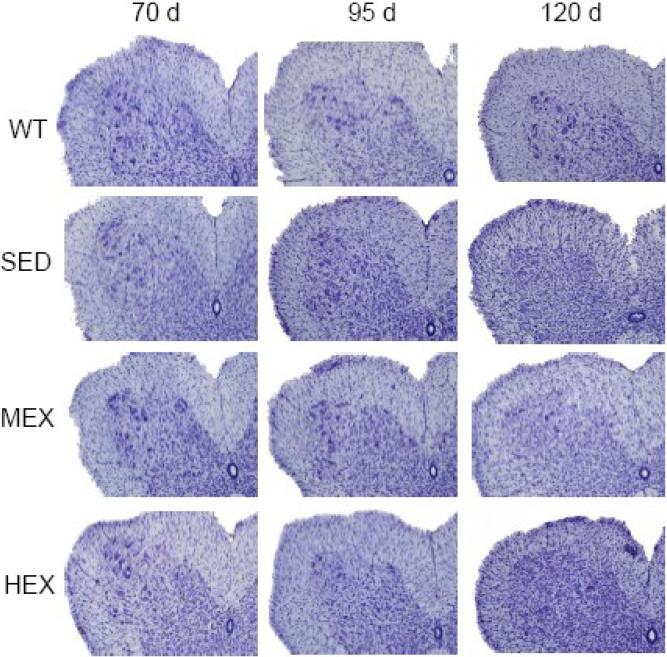
Motor neurons in the ventral horn of cervical, thoracic and lumbar sections of SED, HEX and MEX G93A-SOD1 mice and in those of age-matched sedentary wild-type (WT) mice were counted following a design-based stereological protocol (Figure 3) at the ages of 70, 95 and 120 days to determine the effect of physical activity on the motor neuron number during the progression of the disease. Figure 4 shows representative images of the ventral horn of the lumbar sections of WT, SED, MEX and HEX mice where MNs were counted. The figure illustrates the changes in cytoarchitecture and motor neuron density that occurs in this area of the spinal cord over the course of the disease. As expected, motor neuron density in the ventral horn of lumbar sections of 120 days old sedentary G93A-SOD1 mice was significantly lower than the MN density of age-matched WT mice (55% less in G93A-SOD1 mice than in WT mice). However, at 120 days of age the motor neuron density of mice exposed to moderate- and high-level of physical activity was not significantly different that the MN density of WT mice (Table I). At the age of 95 days, at the onset of the motor decline, the MN density in the lumbar section of spinal cord of MEX mice were increased by almost two-fold (p<0.05) compared to sedentary mice. This is consistent with the delay in motor performance decline detected in mice subjected to the moderate-level running exercise. At 95 days of age MN density of the MEX group in lumbar cord was also significantly different than that of the WT mice. Changes in volume does not account for the differences detected in MN density since no significant differences in the lumbar cord volume was detected at 95 day among the groups. Volume of lumbar cords of 95 days old mice were, in mm3, WT 3.07±0.33, SED 2.69±0.36, MEX 2.69±0.33, and HEX 2.76±0.27 ×109). At 70 days of age MN density of the lumbar cords of SED and HEX was significantly lower than that of WT mice. None of the MN density comparisons within an age group were significantly different in the cervical and thoracic segments of the spinal cords.
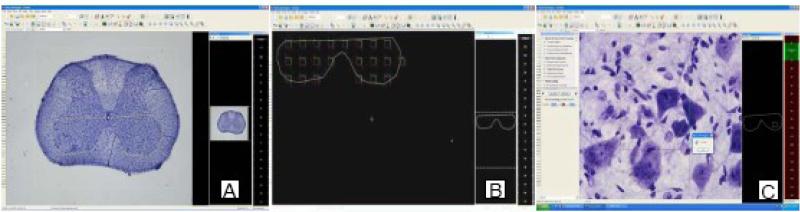
Figure 3. Quantification of motor neuron number in the ventral horn of cervical, thoracic and lumbar cord sections using an Optical Fractionator probe in the StereoInvestigator software.
A. Area of interest was traced at x4 magnification. B. The counting frame size used was 75×75 (mm) and the counting frame area was (XY)= 5625 mm2. C. Cell counting was done with a x60 N.A 1.4 oil objective. Neurons were counted only if their diameter was 15 mm or larger and only if the neuron nucleolus was inside the counting frame and the neuron was not touching the excluding borders.
Figure 4. Representative sections of the ventral horn of lumbar cords of WT, SED, MEX and HEX mice at 95 days of age stained with cresyl violet.
Original magnification x100.
Table I.
Density of motor neurons (×106 cells/mm3) in ventral horns of spinal cords of G93A mice that are sedentary (SED), exposed to high-level (HEX) or mid-level (MEX) exercise. Sedentary wild-type (WT) mice were also studied as controls. Male mice were exposed to exercise starting at 30 days of age and cords were collected at 70, 95 and 120 days of age. Neurons that are larger than 15 μm were counted.
| cervical | 70 days old thoracic | lumbar | cervical | 95 days old thoracic | lumbar | cervical | 120 days old thoracic | lumbar | |
|---|---|---|---|---|---|---|---|---|---|
| WT | 3.33±0.29 | 2.18±0.35 | 3.09±0.29 | 3.02±0.13 | 2.12±0.34 | 2.29±0.16 | 3.19±0.81 | 2.78±0.78 | 2.22±0.27 |
| SED | 2.18±0.23 | 1.80±0.11 | 1.79±0.12a | 1.95±0.09 | 1.79±0.10 | 1.82±0.16 | 2.07±0.19 | 1.94±0.21 | 1.02±0.23a |
| MEX | 2.69±0.22 | 2.58±0.14 | 2.39±0.16 | 2.31±0.16 | 2.30±0.21 | 3.62±0.27a,b | 2.22±0.16 | 2.53±0.27 | 1.67±0.23 |
| HEX | 2.36±0.20 | 1.93±0.12 | 1.70±0.14a | 1.93±0.25 | 1.84±0.14 | 2.58±0.32 | 2.23±0.23 | 2.40±0.19 | 1.43±0.15 |
All values are mean±SEM. Statistical analysis is performed by ANOVA followed by post-hoc test (Tukey-Kramer), p<0.05 is considered significant.
vs WT
vs SED within the same column.
3. Discussion
Much evidence from human research and animal studies in rodents converge to indicate that sustained moderate exercise exerts a beneficial effect to the health of the central nervous system (CNS). Exercise has a very broad effect on the brain: improves learning and memory, delays age-related cognitive decline and has neuroprotective effects against depression, brain injury and delays the onset and decline in neurodegenerative diseases. Despite controversy on the effect of exercise in ALS, a growing number of studies also indicate its beneficial effects (McCrate and Kaspar, 2008). Several clinical studies in ALS patients have demonstrated the value of moderate exercise in ameliorating disease symptoms and improving functionality (Bello-Haas et al., 2007; Drory et al., 2001; Pinto et al., 1999). Although many reports indicate the beneficial effect of moderate exercise (Kaspar et al., 2005; Kirkinezos et al., 2003), the increasing number of reports in the field appears to outline the specific effect exerted by any given exercise protocol i.e. treadmill running versus wheel running. While a report by Liebetanz (Liebetanz et al., 2004) indicated that extensive wheel running exercise is not harmful to ALS mice, a deleterious effect of high-intensity exercise was reported by Mahoney et al. (Mahoney et al., 2004) on ALS mice running on a treadmill. Similar differences on the effect of wheel running and treadmill running exercise on neuroprotection were reported. According to Veldink et al. (Veldink et al., 2003) treadmill running resulted in no significant differences in the number of MN but Kaspar et al. (Kaspar et al., 2005) reported that wheel running significantly protected motor neurons from death. Deforges et al. (Deforges et al., 2009) recently presented the differential effect of a running based and a swimming based exercise protocols in ALS mice due to the activation of different sub-population of MN.
Exercise has also been shown to be beneficial to other animal models of motor neuron diseases and injury. Grondard et al. have shown that volunteer running on a wheel increases motor neuron survival, sustained motor function, and expanded the life of a mouse model of spinal muscular atrophy (Grondard et al., 2005). The exercise induces the expression of a SMN2 transcript that translates into a more stable protein (Grondard et al., 2005). In the same model of SMA, exercise also enhances the expression of NR2A, the major activating subunit of the NMDA receptor (Biondi et al., 2008). Exercise has also have been shown to provide significant improvement to spinal cord injury models (Engesser-Cesar et al., 2005; Engesser-Cesar et al., 2007; Hutchinson et al., 2004).
Our study indicates differential effects of two intensity treadmill running protocols, on motor performance and in motor neuron counts in the ventral horn of the lumbar cords at pre-symptomatic (70 days) and at the onset of clinical symptoms (95 days). Although our study was not designed to monitor the effect of exercise on longevity, we detected premature deaths in the oldest group (120 days) of all three groups of ALS mice. The numbers of premature deaths (7 out of 22 mice in SED group, 5 of the 22 mice in MEX group and 10 of the 23 mice in HEX group died a few days before reaching 120 days) suggest a trend for higher survival rate among MEX mice that would correlate with the delayed symptoms detected at 95 days. We need to design new longevity experiments to confirm that trend since the oldest group in this paper was 120 days and all mice were euthanized for tissue collection at that age. The only significant difference at age 120 was the dramatic decline in MN density in the lumbar cord in SED group compared to WT showing the disease effect at the terminal stage. MEX and HEX groups were not different than SED or WT at that age. It is important to note that mice in exercise groups stopped running after the symptoms started (114.2±0.96 for MEX and 108.2±1.23 days for the HEX group). Mice being sedentary for 7-13 days before age 120 partially explains why the dramatic effect we see at age 95 fade at the terminal stage. Kaspar et al reported no differences in the number of MN at the terminal stage of the disease despite the neuroprotection detected by exercise + IGF-1 at the onset of the decline (Kaspar et al., 2005).
A large amount of evidence has accumulated on the role of growth factors. Increases in brain-derived neurotrophic factor (BDNF) and insulin growth factor 1 (IGF-1) levels following exercise may be central to exercise-induced benefits in the brain (Berchtold et al., 2005; Ding et al., 2006; Trejo et al., 2001). These growth factors modulate nearly all the functional end points enhanced by exercise by modulating a broad range of supporting system for brain maintenance and plasticity including neurogenesis, neuronal survival, axon outgrowth, dendritic pruning, synaptic plasticity, and angiogenesis. Enhanced hippocampal neurogenesis is one of the most reproducible and most studied effects of exercise in the rodent brain and appears to be a key mechanism mediating the exercise-related improvements in learning and memory and the exercise-induced resistance to depression (Fabel et al., 2003; Leuner et al., 2006; Trejo et al., 2001; van Praag et al., 1999; Winocur et al., 2006). In both young and old animals exercise stimulates proliferation of the neural progenitor population, increases the number of new neurons and promotes neuronal survival (Fabel et al., 2003; Trejo et al., 2001; van Praag et al., 1999). Exercise enhances both short-term potentiation and long-term potentiation in the dentate gyrus (DG) (Farmer et al., 2004; van Praag et al., 1999) and alters the DG cytoarchitecture including dendritic length and dendritic complexity, spine density and neural progenitor proliferation (Eadie et al., 2005). Increased blood flow is also seen in the hippocampus after exercise training and its increase is correlated with improved rate of learning in a hippocampus-dependent task (Pereira et al., 2007).
BDNF (Wu et al., 2008), IGF-1 (Trejo et al., 2001), and vascular endothelial growth factor (VEGF) (Fabel et al., 2003) have been reported to be involved in exercise-mediated neuroprotective actions. Exercise increases peripheral IGF and VEGF and both cross the blood brain barrier (BBB) to enter the brain (Fabel et al., 2003; Lopez-Lopez et al., 2004; Trejo et al., 2001). Exercise raises BDNF mRNA and protein levels in the neurons of hippocampus, spinal cord, cerebellum and cortex and increases brain uptake of peripheral IGF-1 (Cotman and Berchtold, 2002; Trejo et al., 2001), through an active mechanism involving specific receptors and carriers (Carro et al., 2005) and VEGF (Fabel et al., 2003). Peripheral IGF and VEGF appear to orchestrate the exercise induce angiogenesis and neurogenesis as demonstrated by using blocking antibodies to IGF (Trejo et al., 2001) or VEGF (Fabel et al., 2003). Both peripheral (Carro et al., 2005) and brain derived IGF-1 (Ding et al., 2006) influence the exercise induced plasticity in the hippocampus. Peripheral IGF-1 is necessary for exercise-induced vessel remodeling in the brain (Lopez-Lopez et al., 2004), an effect that must be in part mediated by the induction of VEGF. Exercise induced angiogenesis is associated with increase local VEGF mRNA and protein in the brain (Ding et al., 2006) which results in potent mitotic activity specific to vascular endothelial cell migration and capillary formation (Ferrara, 1996). Exercise induces BDNF mRNA and protein in neurons in several regions of the brain most robust being in the hippocampus (Cotman and Berchtold, 2002; Ding et al., 2006). Although IGF-1 expression is also induced in hippocampal neurons (Schwarz et al., 1996) peripheral increase of IGF-1 appears to be essential for neurogenesis (Trejo et al., 2001) and improved memory (Ding et al., 2006). As with IGF-1, BDNF signaling is crucial for improving learning in response to exercise (Vaynman et al., 2004; Vaynman et al., 2006) as blocking antibodies to tyrosine receptor kinase B (TrkB), the receptor for BDNF abrogates of the exercise induced hippocampus dependent learning. In addition anti-TrkB attenuates the exercise induce induction of synaptic proteins in the hippocampus (Vaynman et al., 2004; Vaynman et al., 2006). Basic fibroblast growth factor (BFGF), but not IGF directly, can facilitate long term potentiation (LTP) (von Bohlen und Halbach et al., 2008). Much evidence indicates the convergence between IGF-1 and BDNF in response to exercise as it has been shown that IGF-1 increases the effects of BDNF by enhancing BDNF signaling downstream of the TrkB receptors (Ding et al., 2006) in addition to increase expression of the TrkB receptor itself (McCusker et al., 2006).
The effects of exercise induced growth factors in the spinal cord has not been studied as intensely as in the hippocampus but BDNF, VEGF and IGF-1 have been shown to be present in the spinal cord and potentially play a role in the benefits derived from moderate exercise in ALS. The pro-survival effects of IGF-1 are specially detected on motor neurons (Dore et al., 1997). IGF-1, IGF receptors, IGF binding proteins (IGFBPs) and intracellular IGF-1 associated signaling factors (IRS-1, PI3 kinase) are all expressed in spinal cord particularly in the ventral gray matter (including motor neurons) (Bondy and Cheng, 2004). Indeed, retrogradely transported AAV-IGF-1 injected into the muscle of G93A mutant SOD1 mice slowed disease progression even when administered following disease onset (Kaspar et al., 2003). The beneficial effect of IGF-1 in ALS mice has been shown in other studies (Dobrowolny et al., 2005; Lepore et al., 2007; Nagano et al., 2005; Narai et al., 2005). VEGF therapies have also extended survival and delayed onset and progression of ALS in mouse models (Azzouz et al., 2004; Storkebaum et al., 2005). Interestingly, a deletion in the hypoxia responsive element in the promoter region of the VEGF gene causes motor neuron degeneration reminiscent to ALS (Oosthuyse et al., 2001). Based on their in vitro and in vivo ability to promote the survival of MNs, the effect of several neurotrophic factors (NFs) of different families such as BDNF and IGF-1 were tested in humans. Clinical trials have shown no benefit of systematically administered growth factors to ALS patients (Choudry and Cudkowicz, 2005; Nirmalananthan and Greensmith, 2005; Sorenson et al., 2008). Insufficient access to target MNs and the route of delivery of NFs may be responsible for the negative results. This may have been due to lack of sustained delivery, sequestration of the exogenous IGF-1 by systemic and/or CNS IGFBPs and low efficiency of protein delivery to motor neurons. Future trials using trophic factors should be performed using viral vector delivery to allow for a sustained long-term expression in the specific region of interest. Dodge et al. (Dodge et al., 2008) showed recently that a single injection of a recombinant AAV-IGF vector to the deep cerebellar nuclei, a cerebellum region with extensive brain stem and spinal cord connections, delivered sufficient IGF-1 to reduced ALS neuropathology, improve muscle strength and significantly extended the life span of systematic G93A mice.
In addition, neuronal toxicity to glutamate and to reactive oxygen species may be implicated in the effect of exercise to neuronal degeneration in ALS. Physical activity has been related to glutamate excitoxicity because high level exercise was shown to increase the vulnerability of rat hippocampal neurons to kainate lesions (Ramsden et al., 2003). Another study showed that exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle (Radak et al., 2002). In this study the effect achieved by high-level exercise may be combination of the beneficial affects of exercise together with the negative effects of stressful excursion. This needs to be taken into consideration when designing studies in mice and human.
This study demonstrates the beneficial effects of mid-level exercise (but not high-level exercise) in a transgenic model of ALS on motor performance and correlates this finding with motor neuron increases in the ventral horn of the lumbar spinal cord. Designing studies to examine (1) the size distribution of motor neurons and (2) correlating neuropathological findings with neurochemical changes (i.e. neurotrophic and vascular factors) will be the next step.
4. Experimental Procedures
4.1 Transgenic Mice, Breeding and Genotyping
Transgenic mice with the G93A human SOD1 mutation (B6SJL-TgN (SOD1-G93A)1 Gur; Jackson Laboratories, Bar Harbor, ME,USA) were bred with female B6SJL mice (Jackson Laboratories). Offspring male were genotyped by PCR on DNA extracted from tail clippings. Since G93A-SOD1 mice present gender differences in respect to the onset of the disease, life expectancy and response to exercise (Ferrante et al., 2001) (Veldink et al., 2003), only male were used in the present study. At weaning (around 30 days of age) male transgenic mice from the same “F” generation were randomly distributed in 3 different experimental groups: high-level exercise group (HEX), moderate-level exercise group (MEX) and a sedentary group (SED). Each experimental group was divided in 3 different subgroups: mice to be euthanized at 70 days of age, mice to be euthanized at 95 days of age and mice to be euthanized at 120 days of age. A total of 90 G93A-SOD1 male mice were used in this study (n=10). A total of 30 WT littermates were used as a control for motor neuron counts. Wild-type mice used in this study are the littermate controls. G93A is a heterozygous colony that is maintained by breeding a positive male (+/-) with a negative mom (-/-). The off-springs are either positive (+/-) or negative (-/-). The negative mice were used as wild-type controls. All animal experiments were carried in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the local animal care committee.
4.2 Exercise Protocols
Starting at weaning, mice in the HEX and MEX groups exercised on a motorized treadmill apparatus (Exer 6M Treadmill; Columbus Instruments, Inc., Columbus OH) following protocols adapted from previous reports (Kriz et al., 2003). All HEX and MEX mice were subjected to the same training protocol consisting of 20 min of running, 3 days a week at 5 and 10 m/min for the first and second week, respectively. After training, the actual exercise protocol for the HEX mice consisted of 60 min of exercise per day, 5 days per week at 20 m/min (1.25 Km/h). The exercise protocol for the MEX mice consisted of 30 min of exercise per day, 3 days a week at 10m/min (0.62 Km/h). Treadmill running was performed between 1:00 and 3:00 PM, at times when mice are usually sleeping, resting, or showing minimal physical activity. Mice in the control SED group were not exposed to treadmill running. Mice cages were not equipped with any device for exercising so that basal physical activity was restricted to walking around the cage and climbing the food grid.
4.3 Body Weight and Motor Performance Test
Starting at weaning, body weight and motor performance were monitored twice a week for mice in the HEX, MEX and SED groups that would be euthanized at 120 days of age. Measurements and tests were always performed at the same time of the day. Motor performance was tested on a rotarod apparatus (Columbus Instrument, Columbus, OH, USA) after two days of training to get acquainted with the apparatus. The motor performance test consisted in 3 consecutive trials of 60 seconds each on the rotarod at 12 r.p.m. The time until mice fell from the rod was recorded and the best of the three trials used as the measure of competence on the task for that day.
4.4 Tissue Preparation and Histology/Immunohistochemistry
Mice were euthanized at 70, 95 or 120 days of age by CO2 suffocation. Spinal columns were immediately removed and fixed in cold Periodate-Lysine-Paraformaldehyde solution at 4°C. Forty-eight hours later the entire spinal cord was dissected from the spinal column and cryoprotected in a graded series of 10 and 20% glycerol/2% DMSO solutions for 24 hours each. Cords were weight before being transferred to 10% glycerol (cord weights at 95 days old mice were HEX 88.4±3.1, MEX 91.9±2.1, SED 90.4±1.4, WT 93.0±2.0 mg). Spinal cords were dissected into cervical, thoracic and lumbar using the anatomical features of the cords. Frozen, coronal sections of 60 μm from the cervical, thoracic and lumbar parts of the cord were cut with a sliding microtome. Sections were serially saved in PBS containing 2 mM sodium azide at 4°C. For each cord, one every eight sections were serially mounted on a slide so that a representative group of sections for the entire cord was present in a single slide. Mounted sections were stained with 0.1% cresyl violet (CV).
4.5 Quantification of Motor Neurons
Stereological methods were employed to quantify the number of motor neuron (MN) in the ventral horn of cervical, thoracic and lumbar cord using an Optical Fractionator probe. A computer software package, StereoInvestigator (MicroBrightField, Colchester, VT) interfaced with a Nikon Eclipse 80i microscope equipped with Ludl motorized stage, Optronics Microfire color digital camera with 1600×1200 resolution and Heidenheim Z axis encoder was used to collect and analyze the stereological data.
The area of interest, the ventral horn defined as the anterior subdivision of the gray matter to the middle of the central canal, was traced in the CV stained sections at x4 magnification (figure X A). The size of the counting frame, 75×75 μm, and the area counted (XY)= 5625 μm2, was established (figure X B). Cell counting was performed using a x60 N.A 1.4 oil objective (figure X C). Neurons were only counted if their diameter was 15 μm or larger and only if the neuron nucleolus was inside the counting frame and the neuron was not touching the excluding borders. The computer cursor was set at 15 μm long for easy detection of cells that meets the 15 μm diameter criterion.
4.6 Statistical Analysis
For the rotarod performance we used three different methods of comparisons. Data were collected from sedentary mice (SED, n=22), high exercise (HEX, n=28) or moderate exercise (MEX, n= 25) as described above utilizing performance data from 70-120 days of age with measurements made at weekly intervals. First, we fit the total data to a logistic function that combined the onset and slope of the decline. Then we used the R-factor ratio test, which is quite similar to an F-test (Hamilton WC Significance tests on the crystallographic R factor. Acta Cryst. 18:502-510 (1965)) to compare the fits for each set of parameters with the R-factor ratio determining the significance of the fits. We also performed a standard repeated measures ANOVA comparing time and group. Finally we also used a method for analysis of a controlled trial with repeated measures as outlined in Hopkins WG “A spreadsheet for analysis of straightforward controlled trials” SportsScience Vol 7 sportscience.org (online journal) (2003). The motor neuron counts were analyzed using a one-way ANOVA for group including comparisons of all pairs (time, group, spinal cord region) using a Tukey-Post hoc correction. Statistical significance was defined as p<0.05.
Acknowledgements
This research is supported by grants from the Department of Veteran Affairs (Merit Award) to AD, NIH (ADCC grant P30 AG13846) to NWK. There is no conflict of interest. The authors thank to Sukru N. Kaymakcalan, Brendon Kapinos and Kerry Cormier for their technical help in establishing the exercise protocol and spinal cord processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armon C. Sports and trauma in amyotrophic lateral sclerosis revisited. J Neurol Sci. 2007;262:45–53. doi: 10.1016/j.jns.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–7. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Bello-Haas VD, Florence JM, Kloos AD, Scheirbecker J, Lopate G, Hayes SM, Pioro EP, Mitsumoto H. A randomized controlled trial of resistance exercise in individuals with ALS. Neurology. 2007;68:2003–7. doi: 10.1212/01.wnl.0000264418.92308.a4. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for BDNF protein induction in the rat hippocampus. Neuroscience. 2005;133:853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Biondi O, Grondard C, Lecolle S, Deforges S, Pariset C, Lopes P, Cifuentes-Diaz C, Li H, della Gaspera B, Chanoine C, Charbonnier F. Exercise-induced activation of NMDA receptor promotes motor unit development and survival in a type 2 spinal muscular atrophy model mouse. J Neurosci. 2008;28:953–62. doi: 10.1523/JNEUROSCI.3237-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Bondy CA, Cheng CM. Signaling by IGF-1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Brown R.H., Jr. Amyotrophic lateral sclerosis: recent insights from genetics and transgenic mice. Cell. 1995;80:687–92. doi: 10.1016/0092-8674(95)90346-1. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–38. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25:10884–93. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128:472–6. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- Choudry RB, Cudkowicz ME. Clinical trials in ALS: the tenuous past and the promising future. J Clin Pharmacol. 2005;45:1334–44. doi: 10.1177/0091270005282631. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Deforges S, Branchu J, Biondi O, Grondard C, Pariset C, Lecolle S, Lopes P, Vidal PP, Chanoine C, Charbonnier F. Motoneuron survival is promoted by specific exercise in a mouse model of amyotrophic lateral sclerosis. J Physiol. 2009;587:3561–72. doi: 10.1113/jphysiol.2009.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3:15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, Molinaro M, Rosenthal N, Musaro A. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–9. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, Roskelley EM, Treleaven CM, Rizo L, Martin H, Kim SH, Kaspar R, Taksir TV, Griffiths DA, Cheng SH, Shihabuddin LS, Kaspar BK. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. 2008;16:1056–64. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore S, Kar S, Quirion R. Rediscovering an old friend, IGF-I: potential use in the treatment of neurodegenerative diseases. Trends Neurosci. 1997;20:326–31. doi: 10.1016/s0166-2236(96)01036-3. [DOI] [PubMed] [Google Scholar]
- Drory VE, Goltsman E, Reznik JG, Mosek A, Korczyn AD. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2001;191:133–7. doi: 10.1016/s0022-510x(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22:157–71. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Cotman CW. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience. 2007;144:1033–44. doi: 10.1016/j.neuroscience.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Felmus MT, Patten BM, Swanke L. Antecedent events in amyotrophic lateral sclerosis. Neurology. 1976;26:167–72. doi: 10.1212/wnl.26.2.167. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Klein AM, Dedeoglu A, Beal MF. Therapeutic efficacy of EGb761 (Gingko biloba extract) in a transgenic mouse model of amyotrophic lateral sclerosis. J Mol Neurosci. 2001;17:89–96. doi: 10.1385/jmn:17:1:89. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor. Eur J Cancer. 1996;32A:2413–22. doi: 10.1016/s0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- Gregoire N, Serratrice G. [Risk factors in amyotrophic lateral sclerosis. Initial results apropos of 35 cases]. Rev Neurol (Paris) 1991;147:706–13. [PubMed] [Google Scholar]
- Grondard C, Biondi O, Armand AS, Lecolle S, Della Gaspera B, Pariset C, Li H, Gallien CL, Vidal PP, Chanoine C, Charbonnier F. Regular exercise prolongs survival in a type 2 spinal muscular atrophy model mouse. J Neurosci. 2005;25:7615–22. doi: 10.1523/JNEUROSCI.1245-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzman A, Wood WL, Alpert E, Prasad MD, Miller RG, Rothstein JD, Bowser R, Hamilton R, Wood TD, Cleveland DW, Lingappa VR, Liu J. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2007;104:12524–9. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–14. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–42. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57:649–55. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2003;53:804–7. doi: 10.1002/ana.10597. [DOI] [PubMed] [Google Scholar]
- Kriz J, Gowing G, Julien JP. Efficient three-drug cocktail for disease induced by mutant superoxide dismutase. Ann Neurol. 2003;53:429–36. doi: 10.1002/ana.10500. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF, Beebe GW. Epidemiology of amyotrophic lateral sclerosis: 1. A case-control comparison based on ALS deaths. Neurology. 1980;30:453–62. doi: 10.1212/wnl.30.5.453. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Haenggeli C, Gasmi M, Bishop KM, Bartus RT, Maragakis NJ, Rothstein JD. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res. 2007;1185:256–65. doi: 10.1016/j.brainres.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–24. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Hagemann K, von Lewinski F, Kahler E, Paulus W. Extensive exercise is not harmful in amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:3115–20. doi: 10.1111/j.1460-9568.2004.03769.x. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Hypotheses to explain the association between vigorous physical activity and amyotrophic lateral sclerosis. Med Hypotheses. 1991;34:144–8. doi: 10.1016/0306-9877(91)90183-y. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–8. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DJ, Rodriguez C, Devries M, Yasuda N, Tarnopolsky MA. Effects of high-intensity endurance exercise training in the G93A mouse model of amyotrophic lateral sclerosis. Muscle Nerve. 2004;29:656–62. doi: 10.1002/mus.20004. [DOI] [PubMed] [Google Scholar]
- McCrate ME, Kaspar BK. Physical activity and neuroprotection in amyotrophic lateral sclerosis. Neuromolecular Med. 2008;10:108–17. doi: 10.1007/s12017-008-8030-5. [DOI] [PubMed] [Google Scholar]
- McCusker RH, McCrea K, Zunich S, Dantzer R, Broussard SR, Johnson RW, Kelley KW. IGF-I enhances the biological activity of BDNF on cerebrocortical neurons. J Neuroimmunol. 2006;179:186–90. doi: 10.1016/j.jneuroim.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Nagano I, Ilieva H, Shiote M, Murakami T, Yokoyama M, Shoji M, Abe K. Therapeutic benefit of intrathecal injection of IGF-1 in a mouse model of ALS. J Neurol Sci. 2005;235:61–8. doi: 10.1016/j.jns.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Narai H, Nagano I, Ilieva H, Shiote M, Nagata T, Hayashi T, Shoji M, Abe K. Prevention of spinal motor neuron death by insulin-like growth factor-1 associating with the signal transduction systems in SODG93A transgenic mice. J Neurosci Res. 2005;82:452–7. doi: 10.1002/jnr.20668. [DOI] [PubMed] [Google Scholar]
- Nirmalananthan N, Greensmith L. ALS: recent advances and future therapies. Curr Opin Neurol. 2005;18:712–9. doi: 10.1097/01.wco.0000187248.21103.c5. [DOI] [PubMed] [Google Scholar]
- Oosthuyse B, Moons L, Storkebaum E. Deletion of the hypoxia-response element in the VEGF promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–8. doi: 10.1038/88842. al., e. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AC, Alves M, Nogueira A, Evangelista T, Carvalho J, Coelho A, de Carvalho M, Sales-Luis ML. Can amyotrophic lateral sclerosis patients with respiratory insufficiency exercise? J Neurol Sci. 1999;169:69–75. doi: 10.1016/s0022-510x(99)00218-x. [DOI] [PubMed] [Google Scholar]
- Qureshi MM, Hayden D, Urbinelli L, Ferrante K, Newhall K, Myers D, Hilgenberg S, Smart R, Brown RH, Cudkowicz ME. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler. 2006;7:173–82. doi: 10.1080/14660820600640596. [DOI] [PubMed] [Google Scholar]
- Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445:273–8. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Berchtold NC, Patrick Kesslak J, Cotman CW, Pike CJ. Exercise increases the vulnerability of rat hippocampal neurons to kainate lesion. Brain Res. 2003;971:239–44. doi: 10.1016/s0006-8993(03)02365-5. [DOI] [PubMed] [Google Scholar]
- Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 1995;92:689–93. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology. 2002;59:773–5. doi: 10.1212/wnl.59.5.773. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81:3492–7. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- Sorenson EJ, Windbank AJ, Mandrekar JN, Bamlet WR, Appel SH, Armon C, Barkhaus PE, Bosch P, Boylan K, David WS, Feldman E, Glass J, Gutmann L, Katz J, King W, Luciano CA, McCluskey LF, Nash S, Newman DS, Pascuzzi RM, Pioro E, Sams LJ, Scelsa S, Simpson EP, Subramony SH, Tiryaki E, Thornton CA. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008;71:1770–5. doi: 10.1212/01.wnl.0000335970.78664.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, Man WY, De Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robberecht W, Conway EM, Collen D, Moons L, Carmeliet P. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- Strickland D, Smith SA, Dolliff G, Goldman L, Roelofs RI. Physical activity, trauma, and ALS: a case-control study. Acta Neurol Scand. 1996;94:45–50. doi: 10.1111/j.1600-0404.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–34. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–30. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Veldink JH, Bar PR, Joosten EA, Otten M, Wokke JH, van den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13:737–43. doi: 10.1016/s0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- Veldink JH, Kalmijn S, Groeneveld GJ, Titulaer MJ, Wokke JH, van den Berg LH. Physical activity and the association with sporadic ALS. Neurology. 2005;64:241–5. doi: 10.1212/01.WNL.0000149513.82332.5C. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Minichiello L, Unsicker K. TrkB but not trkC receptors are necessary for postnatal maintenance of hippocampal spines. Neurobiol Aging. 2008;29:1247–55. doi: 10.1016/j.neurobiolaging.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, Lo CP, Kuo YM. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105:1585–94. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]