Abstract
Despite availability of successful prevention strategies, HIV continues to spread at alarming rates, especially among women in developing countries. Vaginal microbicides offer a promising approach for blocking transmission of HIV when condom use cannot be negotiated with male partners. A major problem in the development of vaginal microbicides is chemically induced vaginal irritation, which can enhance the risk of HIV transmission. Evaluation of vaginal irritation prior to clinical trials typically uses an expensive and animal-intensive rabbit vaginal irritation model, which could be supplemented by measuring additional inflammatory biomarkers. We studied several immunological parameters as potential biomarkers of vaginal irritation, using the spermicides nonoxynol-9 and benzalkonium chloride as test microbicides. We measured amounts of cytokines, as well as inflammatory cells, in vaginal tissue lysates and on the vaginal surface. We observed that treatment with the selected microbicides increases quantities of the inflammatory cytokines interleukin-1β, CXCL8, and CCL2 in the vaginal tissue parenchyma, and of CCL2 on the vaginal surface. This observation was correlated with increases in macrophages in the vaginal parenchyma. We suggest that measurements of CCL2 and macrophages can serve as new inflammatory biomarkers to evaluate the safety of promising novel microbicides for prevention of HIV.
Keywords: cytokine, chemokine, HIV, AIDS, sexually transmitted disease, biomarker
Introduction
Better preventive measures to reduce the spread of HIV infection are urgently needed. The number of HIV-infected people worldwide increased to approximately 40 million in 2006, and the number of newly infected people increased to 4.3 million, indicating that HIV infection continues to be a major global health issue [1]. Neither a cure nor a preventive vaccine is available for HIV, and the current preventive strategies are far from satisfactory. Social and cultural issues limit the acceptance of condoms as a preventive measure. Alternative “woman controlled” preventive approaches that are socially and culturally acceptable would enable women to protect themselves from HIV infection without male cooperation.
Topical microbicides have been proposed as agents to prevent the transmission of HIV by creating chemical, biological, and/or physical barriers to infection, or by blocking or inactivating the virus at the mucosal surface where infection can occur. An ideal microbicide would need to show protection against HIV infection, as well as low toxicity after repeated use. To date, however, no effective microbicides are commercially available. More than 50 microbicide candidates are in preclinical development, and 12 are in clinical trials [2]. Pro2000 and BufferGel have advanced to Phase III clinical trials, while Tenofovir, Savvy, Lactobacilli and BZK, Praneem, Vivagel, Dapivirine/TMC-120, UC-781, cellulose sulfate, Acidform/Amphora, and Carraguard have been evaluated in Phase I and Phase II trials [3, 4]. Although several candidates appeared promising in preclinical safety studies, the results of clinical trials to date have shown that these treatments were ineffective or increased the risk of infection. In 2007, a clinical study testing the protective effects of cellulose sulfate failed for similar reasons [5, 6]. Most recently, treatment with carrageenan, a potential anti-HIV drug derived from seaweed, did not show any significant protection in a clinical trial [7]. Because of its antiviral potential, nonoxynol-9 (N-9), a nonionic detergent originally marketed as a contraceptive spermicide, was tested as a potential microbicide in the past. However, clinical trials to evaluate the efficacy of N-9 in protecting against HIV infection have generated disappointing data; repetitive use or high concentrations of N-9 resulted in genital irritations, ulcers, and either unaltered or increased infection rates for sexually transmitted diseases [8-11]. The limited success of microbicide candidates in clinical trials suggests constraints on the ability to predict the safety and efficacy of candidate microbicides. To overcome this problem, current safety testing should be supplemented with efforts to achieve a better understanding of the immunological response during microbicide treatment.
The criteria for selecting the microbicides studied to date have focused mainly on antiviral activity. Analyses of effects on the local immune response have been limited, although microbicide-induced inflammation in the vagina may have contributed to the failure of some products. A detailed study of the local immune response may be crucial in the selection of an optimal candidate because an altered environment might be more detrimental than beneficial. A local inflammatory response may not only cause discomfort, but also increase the recruitment of the potential target cells for HIV infection, such as CD4+ T lymphocytes and macrophages, to the vaginal mucosa [12, 13]; the possibility that this process might increase the risk of infection should be evaluated.
Past difficulties in microbicide development emphasize the need for a better understanding of the possible effects of these drugs on the immune system and local immune responses, such as the pro-inflammatory response and the recruitment of immune cells such as CD4+ T cells and macrophages. The current gold-standard preclinical model for assessment of microbicide safety is the rabbit vaginal irritation (RVI) model [14]. This model assesses the potential toxic effects of microbicide candidates by means of histopathology evaluations of detrimental effects on the vaginal epithelium, as well as edema and inflammation. This animal model is the standard assay for microbicide testing because the constant height of the vaginal epithelium in rabbits permits accurate histopathological assessment of epithelial damage. Damage to the vaginal epithelium results in easier access for the HIV virus, and increased inflammation may involve recruitment of additional target cells for HIV infection to the place where the virus enters the body [15]. Although the RVI model has been used for many years as the accepted standard to evaluate potential toxic effects of microbicide candidates, it is limited to observe the acute immune response that occurs within 10 days after initiation of treatment, and it does not evaluate immunological parameters or analyses the underlying cellular immune response in detail. In addition, the assay is animal intensive, requiring euthanasia of all test animals after a prescribed course of treatment (typically 10 days) to perform microscopic evaluation of vaginal tissue. Development of a model that can evaluate cytokine induction in living animals would provide a more humane and useful evaluation of vaginal irritation in the RVI model.
Cytokines and chemokines are produced during inflammatory immune responses, and they undoubtedly play a role in leukocyte recruitment to the vagina after treatment with a topical microbicide. Recent studies have shown that increased amounts of certain inflammatory cytokines are detected in vaginal lavage fluids in the RVI model [16]. Although the significance of these changes has not been fully clarified, they may be responsible for some of the not yet understood discrepancies between the results in preclinical and clinical studies. For example, the presence of elevated levels of pro-inflammatory factors may increase the proliferation of immune cells in the vaginal tissue parenchyma. Furthermore, elevated amounts of chemokines may attract additional immune cells to the vaginal tissue. Chemokines such as CXCL8/IL-8 activate neutrophils [17], while CCL2/monocyte chemotactic protein-1 recruits macrophages/monocytes [18].
In the study described here, our goal was to identify changes in pro-inflammatory cytokines and chemokines in rabbit vagina after microbicide treatment. We have shown, for the first time, that the chemokine CCL2 is produced at high levels during treatment with N-9 and benzalkonium chloride (BZK), a second spermicide tested. The high levels of CCL2 were correlated with increased amounts of inflammatory macrophages in the vagina. Furthermore, we performed flow cytometry analysis on the cells present in the vaginal tissue parenchyma after microbicide treatment and observed extensive macrophage infiltration. Our data indicate a strong relationship between the levels of soluble mediators produced and recruitment of macrophages, which may serve as the target cells for HIV-infection. Monitoring the recruitment of macrophages to the vaginal surface and monitoring of local cytokine levels may allow us to measure important predictive markers for the future development of novel microbicides.
Materials and Methods
Antibodies and Reagents
Antibodies against CD4 (clone RTH1A), CD11c (clone RT3A), CD45 (clone ISC18A), and CD138 (clone BAQ44A) were obtained from VMRD (Pullman, WA). Antibodies against CD8 (clone MCA157), CD11b (clone MCA802), CD14 (clone MCA1568), and major histocompatibility complex (MHC) II (clone MCA811) were obtained from AbD Serotec (Raleigh, NC). Antibodies against CCL2 and IL-1β, as well as recombinant rabbit CCL2, CXCL8, and IL-1β, have been described previously [19-22].
Rabbit Vaginal Irritation Model
All animal work was approved by SRI's Institutional Animal Care and Use Committee in full compliance with all regulations of the National Institutes of Health Office of Laboratory Animal Welfare. Vaginal irritation was measured essentially as described previously [14]. In brief, female nulliparous New Zealand White rabbits (2.5–4.5 kg) were dosed intravaginally for 10 consecutive days with 1 ml of actively formulated drug: 2% N-9 in carboxymethylcellulose (CMC) gel, 8% N-9 in CMC gel, 0.5% BZK, 2.0% BZK, or CMC gel as a vehicle control (CMC is widely used as a vehicle in studies of this kind)[16, 23].
Cytokine samples for immunoassay analysis were prepared by a modified version of the method described by Grillner and co-workers [24]: In brief, cotton swabs were inserted about 5 cm into the rabbit vagina, twisted gently, and transferred to a collection tube containing 1 ml sample buffer [1% HALT protease inhibitor (Pierce, Rockford, IL) and 0.1% sodium azide in phosphate buffered saline (PBS)]. Swabs were transferred to a second collection tube containing 1 ml sample buffer to recover residual cytokines from the cotton matrix, and then swabs were discarded. Both samples were pooled and centrifuged to remove debris. Supernatants were transferred to a new collection tube and stored at −80°C until further analysis.
Vaginal surface cell samples were collected by using cytology brushes (#24-2199; McKesson, Richmond, VA). The brushes were inserted about 5 cm into the vagina, gently twisted, and transferred to a collection tube containing about 5 ml wash buffer (Hank's balanced salt solution, 25 mM HEPES, and 5% calf serum). Cells were stored on ice until they were analyzed by flow cytometry.
Blood samples were collected i.v. into tubes containing EDTA as an anticoagulant. Peripheral blood mononuclear cells (PBMCs) were purified by Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ) density gradient separation according to the manufacturer's instructions. Red blood cells were lysed as described previously [25]. Cells were washed and stored on ice until they were analyzed by flow cytometry.
Animals were euthanized with sodium pentobarbitol after the last swab/brush samples had been taken. The vagina was excised and opened by a longitudinal incision. Macroscopic observations were recorded, and the vagina was cut into two halves longitudinally. One half was cut into three pieces (cranial, medial, and caudal) and fixed in formalin for histopathology. The other half was shock-frozen in liquid nitrogen and stored at −80°C until processing for cytokine analysis by enzyme-linked immunosorbent assay (ELISA), or it was stored on wet ice for inflammatory cell analysis by flow cytometry.
Histopathology
Formalin-fixed tissues were embedded in paraffin. Microtome sections were prepared, stained with Hematoxylin and Eosin (H&E), and scored by a board-certified pathologist as described previously [14]. In brief, microscopic changes were coded by the most specific topographic and morphologic diagnosis. Systematized Nomenclature of Medicine (SNOMED) and National Toxicology Program (NTP) terminology manuals were used as guidelines. Data were recorded in Labcat® Histopathology module 4.3. Gradable observations of exudate, leukocyte infiltration, erosion, hemorrhage, decreased epithelial height, edema, and fibrosis were scored by a four step grading system: 0, not observed; 1, minimal; 2, mild; 3, moderate; and 4, marked. Records of necropsy findings and changes found at tissue processing were available when evaluating the stained tissue sections.
Enzyme-linked Immunosorbent Assay
Vaginal tissues that had been frozen in liquid nitrogen and stored at −80°C were processed for cytokine analysis as follows: Tissues were thawed on ice and minced with two scalpels into small pieces. In a 2 ml microtube, approximately 0.5 ml minced tissue was mixed by vortexing with approximately 0.2 ml glass beads (Sigma-Aldrich, St. Louis, MO) and 1 ml 1% HALT protease inhibitor in PBS. Subsequently, samples were homogenized three times for 5 min by a Bullet-Blender (Next Advance, Averill Park, NY) at 4°C. Debris was removed by two sequential centrifugation steps at 4°C, and supernatants were stored at −80°C until cytokine amounts were measured by ELISA. Protein content of the samples was measured using a BCA kit (Pierce, Rockford, IL) according to the manufacturer's instructions.
Cytokine levels in vaginal swab samples and vaginal tissue lysate samples were measured in duplicate by ELISA. IL-1β and CCL2 were measured by custom ELISA as described below. CXCL8 was measured using a commercially available anti-human CXCL8 kit modified from the manufacturer's instructions (R&D systems, Minneapolis, MI). In brief, Immulon 2HB plates (Thermo Scientific, Waltham, MA) were coated with anti-CCL2, anti-CXCL8, or anti-IL-1β antibodies and incubated at 4°C overnight. Plates were washed with PBS/0.05% Tween-20 using an automated plate washer (ELx405; BioTek, Winooski, VT), and nonspecific binding was blocked with 1% bovine serum albumin in PBS for 2 h. After washing, samples or recombinant-rabbit cytokine standards were added to the plates in appropriate serial dilutions, and the plates were incubated for 2 h at room temperature. Plates were washed again, and then incubated with biotinylated detector antibodies to CCL2, CXCL8, or IL-1β for 2 h at room temperature. After another wash, streptavidin-conjugated horseradish peroxidase was added to the plates for 30 min; the plates were washed again, then developed with tetramethylbenzidine (TMB) for 30 min. OD values were read with a microplate reader (HT-340; BioTek), and cytokine concentrations were calculated by KC4 software (BioTek).
Flow Cytometry
White blood cells were extracted from vaginal tissue samples as follows: Vaginas were minced into small pieces with two scalpel blades, covered with Hank's balanced salt solution (HBSS) containing 25 mM HEPES, and stored on ice until further processing. An equal volume of a mixture of HBSS, 25 mM HEPES, 4 mU/ml Blendzyme2 (Roche, Indianapolis, IN), and 0.2 mg/ml DNAse I (Roche, Indianapolis, IN) was added to the samples, and they were stirred at 37°C for 20 min. Supernatant was filtered through a 100 μm nylon mesh, and digestion was stopped by adding wash buffer (HBSS, 25 mM HEPES, and 10% calf serum). Cells were washed with PBS. Red blood cells were lysed as described previously [25], and cells were washed with FACS buffer (PBS with 5% calf serum and 0.1% sodium azide).
Cells were stained for flow cytometry analysis in a one step staining procedure. Antibodies directly conjugated with suitable fluorophores for flow cytometry analysis were purchased, or antibodies were labeled with Alexa Fluor monoclonal antibody labeling kits or Zenon™ labeling kits according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). Cells were stained with the directly conjugated antibodies, stored on ice for 20 min, and washed. Propidium iodine was used as a viability stain, and cells were measured with a FACScalibur (BD Biosciences, San Jose, CA). Data were analyzed with FCS Express (De Novo Software, Los Angeles, CA).
Statistical Analysis
Statistical calculations were performed using Prism 4.0 (Graphpad, San Diego, CA).
Results
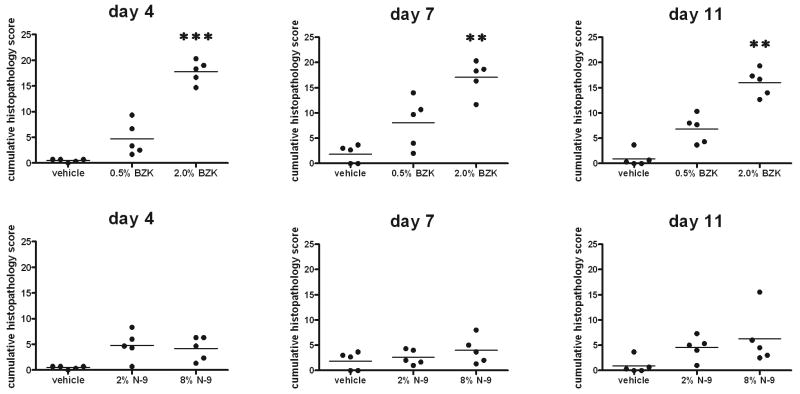
This study considered the role of inflammatory mediators produced in response to microbicide treatment to obtain an in-depth understanding of the cytokine and chemokine response that may be indicative of the acute toxicity of candidate microbicides. In our studies, we treated rabbits daily for 10 consecutive days with vehicle (CMC), N-9 (2% or 8%), or BZK (0.5% or 2%). Treatment for 10 days is sufficient to observe immediate innate immune responses, as well as early adaptive immune responses that require a minimum of about 7 days to occur. Histopathological changes were assessed after 3, 6, and 10 days of treatment (Fig. 1). Minor histopathological changes were present at all necropsy time points in the vaginal tissues of rabbits given the vehicle alone; these were considered to be the result of spontaneous conditions rather than of vehicle administration. In animals that were administered N-9 (2% or 8%) or BZK (0.5% or 2%), leukocyte infiltration, erosion, hemorrhage, edema, exudates, congestion, decreases in epithelial height, and necrosis were increased compared to observations in vehicle-treated animals. Results were not severe after 3 days of treatment, but a more prominent lesion distribution was present after 6 and 10 days of treatment. The reductions in epithelial height and the increases in inflammation after 3, 6, and 10 days of treatment with N-9 and BZK are shown in Fig. (1). To assess these results semiquantitatively, the scores obtained were summarized for each animal, and the mean for each treatment group was calculated. Changes were detectable as early as 3 days after initiation of treatment with N-9 or BZK, but the changes were only statistically significant after treatment with 2.0% BZK (Fig. 2). Although we could observe histopathologic changes after treatment with N-9, a more sensitive and quantifiable method to describe the alterations caused by N-9 would be preferable.
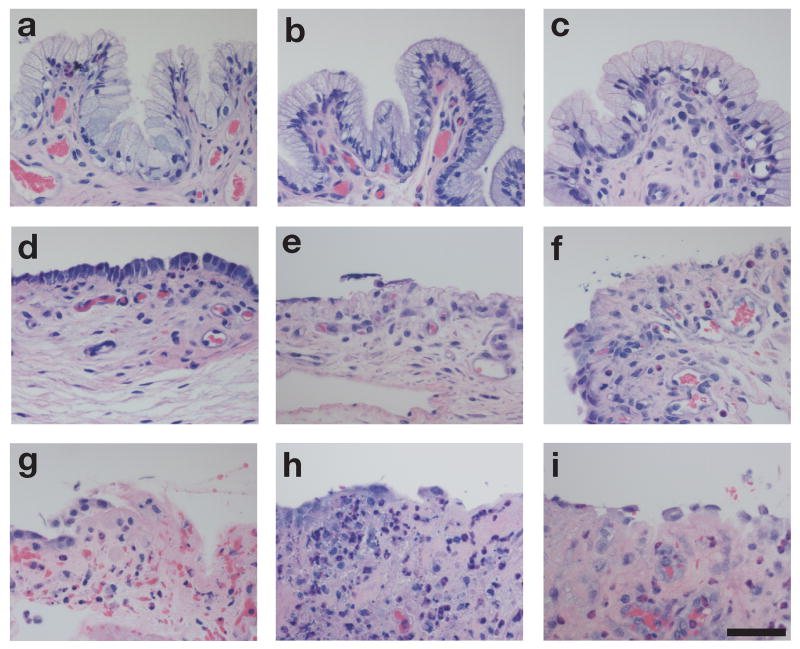
Figure 1.
Vaginal tissues of animals treated with CMC vehicle (a–c), 8.0% N-9 (d–f), or 2.0% BZK (g–i) for 3 days (a, d, g), 6 days (b, e, h), or 10 days (c, f, i). H&E staining. Bar = 50 μm.
Figure 2.
Semiquantitative pathology analysis of vaginal tissues at study days 4, 7, and 11. Rabbit vagina sections were evaluated microscopically after treatment with CMC vehicle alone, gel containing 2% N-9, gel containing 8% N-9, 0.5% BZK, or 2% BZK. Exudate, leukocyte infiltration, erosion, hemorrhage, decreased epithelial height, edema, and fibrosis were scored by a four step system: not observed, 0; minimal, 1; mild, 2; moderate, 3; and marked, 4. The cumulative scores of these parameters are shown at sacrifice days 4, 7, and 11 for one representative experiment (n=3) with 5 animals per group. Data were analyzed by the Kruskal-Wallis test (**: p<0.01; ***: p<0.001).
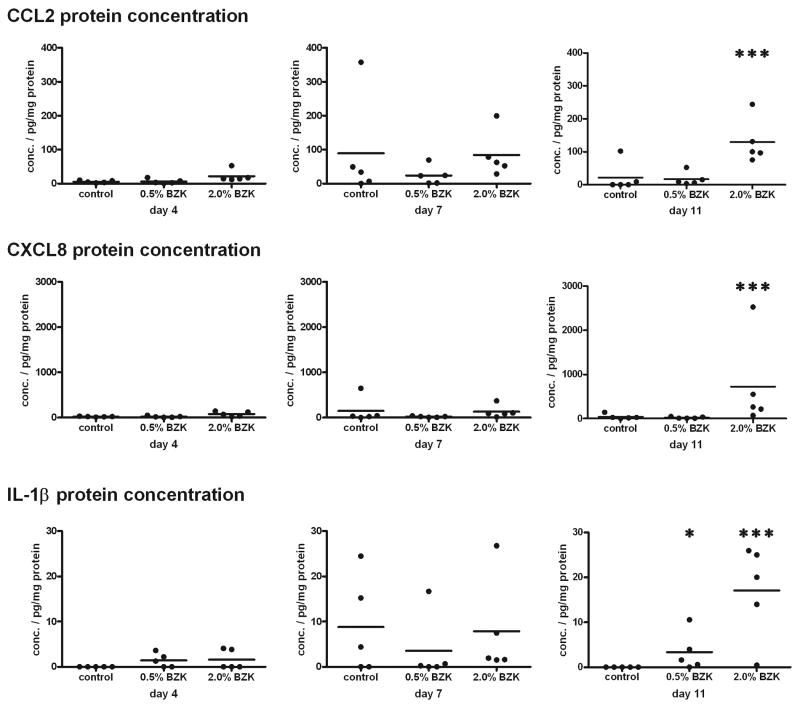
With this goal in mind, we evaluated the expression of inflammatory cytokines within the vaginal tissue, to verify our hypothesis that increased inflammation can be measured by increased cytokine production during the immune response. We extracted proteins from vaginal tissue preparations of rabbits that had been treated with N-9 (2% or 8%) or BZK (0.5% or 2%). We used ELISA to test the concentrations of various inflammatory cytokines and chemokines involved in the recruitment of leukocytes, and we normalized the amounts to the total protein concentrations. Significantly elevated amounts of CCL2, CXCL8, and IL-1β were detected in the samples from the animals treated with 2.0% BZK for 10 consecutive days (Fig. 3). However, neither treatment with 0.5% BZK (Fig. 3) nor treatment with 2.0% or 8.0% N-9 (data not shown) caused a significant increase in any of the cytokines measured. Since increases in cytokines may be the result of local secretion, potentially by epithelial cells, it is possible that the cytokines in these preparations of complete vaginal tissue parenchyma were too diluted to be detectable.
Figure 3.
Increased amounts of CCL2, CXCL8, and IL-1β were detected in vaginal tissue lysates after treatment with BZK. Animals were treated with BZK at the indicated concentrations for 3, 6, or 10 days. The day after the last treatment (day 4, 7, or 11), vaginal tissues were dissected and frozen. Frozen tissues were thawed and homogenized, and tissue lysates were prepared. Cytokine concentrations were measured by ELISA, and normalized to total protein amounts. One representative experiment is shown (n=2), with 5 animals per group. Data were analyzed by the Kruskal-Wallis test (*: p<0.05; ***: p<0.001).
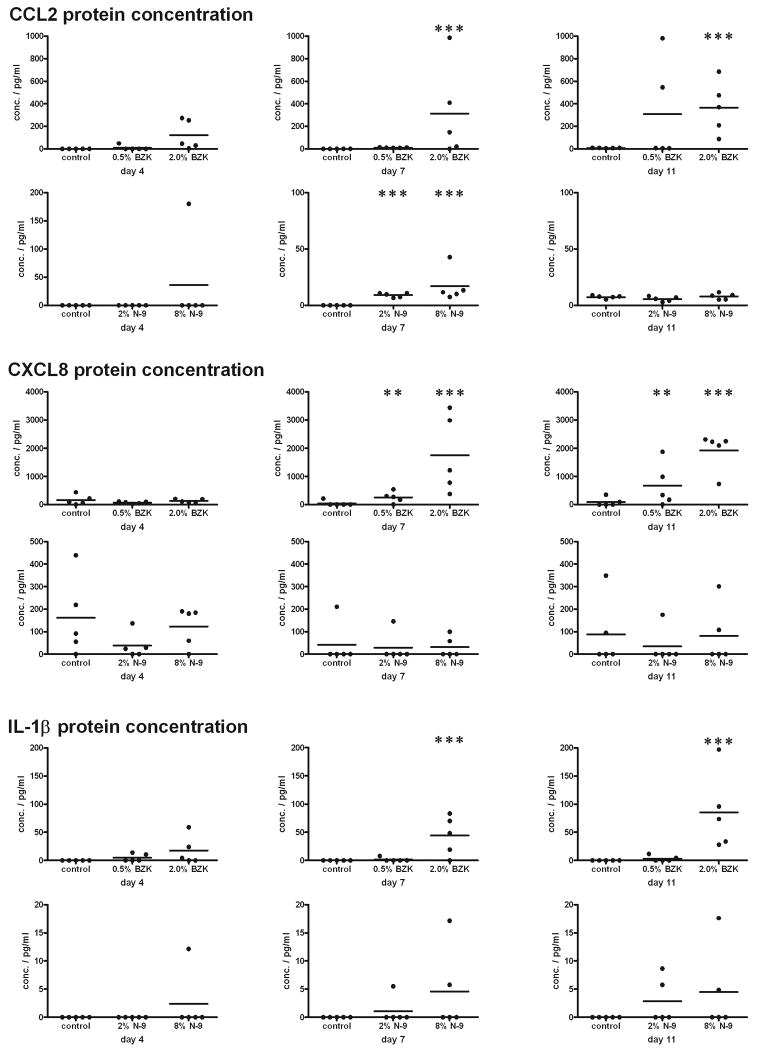
To measure the concentrations of cytokines on the vaginal epithelial surface, we collected smear samples with cotton swabs. After collection, swabs were washed two times in 1 ml collection buffer to transfer the proteins from the swab into solution. On average, we obtained 256 μg protein with each swab sample, as measured by BCA protein assay (data not shown). The concentrations of CCL2, CXCL8, and IL-1β were measured in the swab sample solutions prepared before the animals were treated, and in samples collected after 1, 3, 6, and 10 days of treatment. We found no significant increases in CCL2, CXCL8, or IL-1β before or after 1 day of treatment with N-9 or BZK (data not shown); however, as shown in Fig. (4), the concentration of CCL2 was significantly increased after 6 days of treatment with 2.0% BZK. In N-9-treated animals, the amount of CCL2 was significantly increased at earlier time points, but the concentration appeared to decrease after 10 days of treatment (Fig. 4). Increased levels of CXCL8 and IL-1β were also detected after treatment with the spermicide BZK, whereas no changes were observed after treatment with N-9 (Fig. 4). We also tested additional cytokines, including Gro, IL-1RA, and TNF-α. No significant changes in the expression of these cytokines were observed (data not shown).
Figure 4.
Increases in CCL2 were detected in vaginal swab samples from animals treated with both BZK and N-9; increases in CXCL8 and IL-1β were detected only after treatment with BZK. Animals were treated with N-9 or BZK at the indicated concentrations for 3, 6, or 10 days. Swab samples were collected the day after the treatment (day 4, 7, or 11). Cytokine concentrations were measured by ELISA. One representative experiment is shown (n=3), with 5 animals per group. Data were analyzed by the Kruskal-Wallis test (**: p<0.01; ***: p<0.001).
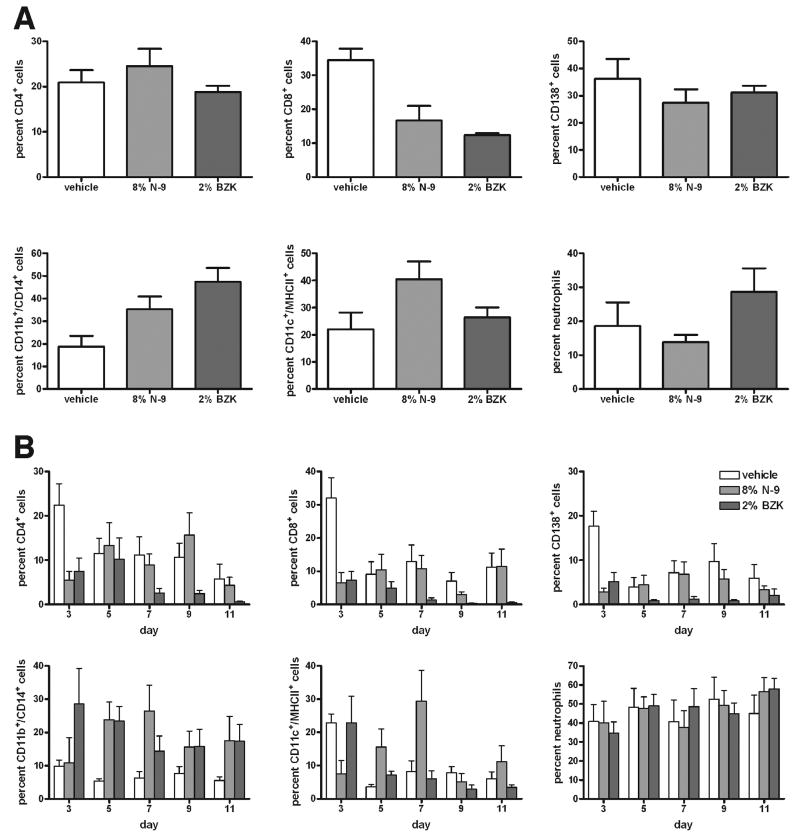
As shown in Fig. (1), our histopathology data indicate inflammation after microbicide treatment. To further elucidate the quality and quantity of this inflammatory response, we identified the types of inflammatory leukocytes within the vaginal tissue and determined the percentage of each type, by performing FACS analysis on vaginal tissue digests harvested from animals that had been treated for 10 days with 8% N-9, 2% BZK, or vehicle alone. Cells were stained to assess viability and scatter-gated for lymphocytes (low forward/low sideward scatter), large cells (high forward/low sideward scatter), and neutrophilic granulocytes (low forward/high sideward scatter). Scatter-gated lymphocytes were analyzed by flow cytometry. Although the percentages of CD4+ T lymphocytes and CD138+ B2 lymphocytes did not change, the percentage of CD8+ T lymphocytes was decreased after treatment with N-9 and BZK (Fig. 5A). Analyzing the scatter-gated large cells revealed that the percentage of CD11c+/MHCII+ dendritic cells did not change consistently after treatment with N-9 and BZK, but the percentage of CD11b+/CD14+ macrophages/monocytes increased after treatment with N-9 and BZK (Fig. 5A). The percentage of neutrophils did not change after treatment with N-9 and BZK (Fig. 5A).
Figure 5.
Flow cytometry analysis of cells prepared from the rabbit vagina by (A) enzymatic lysis of vaginal tissue samples at day 11, and (B) minimally invasive cytobrush sampling of the vaginal surface at days 3, 5, 7, 9, and 11 after study initiation. Animals had been treated intravaginally with vehicle alone, 8% N-9, or 2% BZK. Live cells were analyzed by viability staining. Lymphocytes were identified by low forward/low sideward scatter, and percentages of CD4+ T lymphocytes, CD8+ T lymphocytes, and CD138+ B2 lymphocytes are shown. Large cells were identified by high forward/low sideward scatter, and percentages of CD11b+/CD14b+ macrophages and CD11c+/MHCII+ dendritic cells are shown. Neutrophilic granulocytes were identified by their low forward/high sideward scatter, and the percentage of viable cells is shown. The percentage of CD8+ cells decreased after treatment with N-9 and BZK in the tissue lysate cells (A), as well as on the vaginal surface (B), while the percentage of CD11b+/CD14+ macrophages increased both in the vaginal tissue lysate cells (A) and on the vaginal surface (B). One of two experiments is shown (n=2), with 7 animals per group.
Although these findings enabled us to quantify the cellular immune response during N-9 and BZK treatment, they required the sacrifice of the test animals. To monitor the cellular immune response in a noninvasive manner during the study, we collected cells from the vaginal surface with a cytology brush. Animals had been treated with 8% N-9, 2% BZK, or vehicle alone for 10 days. Cells were collected every other day, and debris and dead cells were excluded by viability staining. Lymphocytes, large cells, and neutrophils were distinguished by scatter gate and by antibody staining for CD4+ T lymphocytes, CD8+ T lymphocytes, CD138+ B2 lymphocytes, CD11b+/CD14+ macrophages/monocytes, CD11c+/MHCII+ dendritic cells, and neutrophils, as described above. The results were consistent with the observations made in the vaginal digest cells: We observed decreased numbers of CD8+ T lymphocytes on the vaginal surface after treatment with BZK (Fig. 5B); the reduction after treatment with N-9 appeared to be smaller and varied more at different time points, perhaps because of the small overall number of leukocytes collected from the vaginal surface. The percentages of neutrophils and CD11c+/MHCII+ dendritic cells remained relatively unchanged. The percentage of CD11b+/CD14+ macrophages/monocytes increased after treatment with N-9 and BZK (Fig. 5B); this result is consistent with our observations in the vaginal tissue digest.
We considered whether the increase in the CD11b+/CD14+ macrophage/monocyte composition in the vaginal tissue parenchyma resulted from altered systemic leukocyte composition in the blood. To answer this question, we prepared PBMCs from each animal after 10 days of treatment with 8% N-9, 2% BZK, or vehicle alone, and used flow cytometry to compare the PBMC leukocyte composition. No significant differences in the percentages of CD4+ T lymphocytes, CD8+ T lymphocytes, CD138+ B2 lymphocytes, CD11b+/CD14+ macrophages/monocytes, CD11c+/MHCII+ dendritic cells, neutrophils, or CD11b+ monocytes were detected in the peripheral blood (data not shown). This result indicated that the increase in CD11b+/CD14+ macrophages/monocytes within the vaginal parenchyma, as well as on the vaginal surface, was a localized effect of the microbicide treatment. This local inflammatory response, as detected by flow cytometry and cytokine ELISA, may predict the safety of novel microbicides.
Discussion
Macrophages have been described as prominent target cells for HIV infection for more than 20 years [26-28]. Most compounds under consideration for use as microbicides to protect from HIV infection have been chosen for their antiviral activity; however, their effects on the host immune system in general, and on macrophages in particular, have not been adequately addressed. Major efforts have been made to identify microbicides that can disrupt viruses by means of their surfactant activity, but other, potentially harmful effects have received too little attention. For example, if antiviral activity also causes tissue damage to the host organism and leads to the recruitment of leukocytes, including CD4+ T lymphocytes and macrophages, into the vaginal tissue parenchyma, the risk of HIV infection would be increased. Therefore, even if a microbicide is capable of partly inactivating the pathogen, the presence of additional target cells susceptible to HIV-infection may result in an increased risk of viral infection.
The current model, first described by Eckstein et al. in 1969 [14], for investigating the harmful effects caused by potential new microbicides evaluates histopathological changes in the vaginal tissue parenchyma after compound treatment. Although this widely accepted method enables detection of common irritating effects in the vagina, such as exudate, erosion, hemorrhage, decreased epithelial height, edema, and fibrosis, its analysis of the underlying immunological changes in the vaginal micromilieu is limited to describing leukocyte infiltration in a general, nonspecific fashion. The model therefore fails to provide a detailed description of the immune response observed during treatment with the potential microbicide. It does not adequately evaluate changes in the immune environment, such as cytokine and chemokine secretion, and recruitment of different leukocyte subsets in response to treatment with different potential microbicides. A possible consequence of an altered cytokine environment is increased susceptibility of the host's cells to the virus due to their activation with certain cytokines [29].
In this study we compared the histopathological findings to the changes in pro-inflammatory mediators in the vaginal milieu after microbicide application. Previous preclinical safety studies considered N-9 a relatively safe product [30, 31], yet N-9 formulations failed when tested in clinical efficacy studies. In one study N-9 actually increased the risk of infection with sexually transmitted disease pathogens in some individuals [10]. These findings indicate the limitations of the current gold standard model, and suggest the need to improve it. We believe that this discrepancy between preclinical safety studies and clinical trials can be explained by an altered immune environment that is poorly detected by histopathological analysis alone.
To verify this possibility, we compared the production of inflammatory cytokines and chemokines after application of two known topical microbicides, N-9 and BZK, and we analyzed the leukocyte composition during the inflammatory response in the vaginal tissue parenchyma on a cellular level. Our approach included evaluation of tissue extracts from the rabbit vaginal tissue after treatment with N-9 or BZK. These studies showed elevated levels of IL-1β, CXCL8, and CCL2 after treatment with BZK. IL-1β is a pro-inflammatory cytokine that can be produced by activated macrophages [32, 33]. The increased production of IL-1β within the vaginal parenchyma indicates the presence of an inflammatory immune response in the tissue, possibly resulting from increases in the number of macrophages or their levels of activity within the vaginal parenchyma. We also found increased levels of CXCL8, a chemokine produced by macrophages [34] that recruits and activates neutrophils [35]; higher levels of CXCL8 support the suggestion that the microbicide treatment increases inflammation in the vaginal parenchyma.
To evaluate the safety of microbicides in a time- and cost-efficient manner, soluble mediators should be measured by means of minimally invasive procedures at the vaginal surface. Therefore, we evaluated the production of cytokines such as CXCL8 and IL-1β, as well as Gro, IL-1RA, and TNF-α, in vaginal tissue lysate and swab-smear samples. These measurements are limited by the availability of antibodies that cross-react with rabbit cytokines. The swab-smear sample approach was not only minimally invasive, but also caused less dilution of samples than the tissue lysate approach and was more successful in measuring cytokines. Swab-smear samples have been used by several research groups to collect human specimens [24, 36], suggesting that this approach may be helpful in future human clinical microbicide trials. Using the swab-smear approach, we were able to detect increased cytokine amounts after treatment with BZK, but not after treatment with N-9. Fichorova and co-workers performed studies over shorter time periods; measurements in rabbit vaginal lavage fluids showed increased amounts of CXCL8 after 2–3 days of treatment with N-9, and increases in IL-1β after 1–4 days of treatment [16]. Fichorova and co-workers used a vaginal lavage sampling approach that monitored a larger area, including the cranial vagina and the cervix, than we used in our studies. We collected vaginal lavage samples, but did not make the same observation (unpublished data). Vaginal lavage sampling may interfere with residual microbicide gel or cream still present at the cranial vagina, and the interference could easily influence the study outcome. Collection of vaginal swab samples was a more reliable approach in our laboratory, and we also consider it to be less invasive. The difference in sampling procedures may explain the differences between the results of our study and the work of Fichorova and co-workers.
The study presented here is the first report of increases in CCL2 following treatment with microbicides. Increased production of CCL2 by vaginal epithelial cells has been described in response to danger signals by TLR activation [37]. Our results indicate that certain potential microbicides may act in a similar fashion as an inflammatory stimulus. In vaginal tissue lysate samples, treatment with N-9 did not significantly increase production of CCL2. Since CCL2 production by epithelial cells has been reported [37], it is possible that the expression of CCL2 in the whole vaginal tissue parenchyma is too broadly dispersed to be detectable, but local changes at the epithelium are distinguishable. This possibility is supported by our finding that CCL2 was increased in the vaginal swab samples after treatment with either N-9 or BZK and as early as 3 days after initiation of treatment. Because no response was observed when animals were treated with vehicle alone, it is plausible to conclude that the CCL2 production was a specific response to the tested microbicide rather than a result of the mechanical treatment.
CCL2 is a chemokine that attracts lymphocytes and macrophages/monocytes that express the chemokine receptor CCR2 on their cell surfaces [38, 39]. IL-1β, a pro-inflammatory cytokine, and CXCL8, a pro-inflammatory chemokine, can both be the products of activated macrophages. Therefore, we postulate that treatment with BZK or N-9 could result in an influx of macrophages (recruited to and activated within the tissue) into the vaginal tissue parenchyma. Our flow cytometry analysis shows that the composition of leukocytes in the rabbit vaginal parenchyma of animals treated with BZK or N-9 is altered. CD11b+/CD14+ macrophages/monocytes were increased in the treated tissues, and CD8+ T lymphocytes were decreased, while the percentages of CD4+ T lymphocytes, CD138+ B2 lymphocytes, CD11c+/MHCII+ dendritic cells, and neutrophils were the same as those in the control samples. An increase in CD11b+/CD14+ macrophages/monocytes was also detectable in vaginal surface cytobrush samples. The increase in CD11b+/CD14+ macrophages/monocytes is in agreement with a finding by Milligan and co-workers, who observed larger numbers of F4/80+ macrophages in a mouse model of vaginal irritation after treatment with cholic acid [40, 41]. The histopathological score of the vaginal tissue after N-9 treatment increased insignificantly, although the number of CD11b+ macrophages was significantly increased. The difference suggests that the measurement of inflammatory cells by flow cytometry is a more sensitive approach for evaluating the inflammatory response. To ensure that the changes were not the result of an unlikely systemic change in leukocyte composition, we performed a similar flow cytometry analysis in the peripheral blood of treated animals after 10 days of local treatment with either N-9 or BZK. Despite significant changes in the vaginal parenchyma, the analysis of peripheral blood samples from the treated animals showed no changes in the leukocyte composition, indicating that the increase in macrophages is a localized effect.
In summary, our study shows that an inflammatory response takes place following treatment with microbicides such as N-9 and BZK; this response is indicated by production of cytokines and by increased levels of CCL2. Increased CCL2 may contribute to the recruitment of inflammatory macrophages, which may induce a subset of cells to produce the pro-inflammatory cytokines CXCL8 and IL-1β. The recruitment of activated macrophages, which are a major target cell population for HIV infection, may be one of the reasons for the poor efficacy of N-9 in human clinical trials. It may be possible to supplement the standard RVI studies by not only measuring cytokines that attract macrophages, such as CCL2, and cytokines that can be produced by macrophages and other hematopoietic and nonhematopoietic cells, such as CXCL8 and IL-1β, but also by measuring the macrophages in the vaginal tissue parenchyma and on the vaginal surface,. These additional measurements of the immune response may enhance the efficiency of future efforts to develop successful novel microbicides to counter HIV infection.
Acknowledgments
We would like to thank SRI's Toxicology Technical Services staff for their excellent assistance in performing this study. Additionally, we would like to thank Dr. David Fairchild for his assistance in analyzing the histopathological data. We thank Drs. Charles Litterst and Hao Zhang of NIAID for many helpful suggestions. This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No(s). N01-AI-05417 and N01-AI-70043. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
List of abbreviations
- BZK
benzalkonium chloride
- CMC
carboxymethylcellulose
- ELISA
enzyme-linked immunosorbent assay
- HBSS
Hank's balanced salt solution
- H&E
Hematoxylin and Eosin
- MHC
major histocompatibility complex
- N-9
Nonoxynol-9
- NTP
National Toxicology Program
- PBMC
peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- SNOMED
Systematized Nomenclature of Medicine
- RVI
rabbit vaginal irritation
- TMB
tetramethylbenzidine
Literature cited
- 1.UNAIDS/WHO. AIDS epidemic update. Geneva, CH: UN/WHO; 2006. pp. 1–96. [Google Scholar]
- 2.August 2008 Pipeline Update. Silver Spring, MD, USA,: Alliance for Microbicide Development; [August 04, 2008; cited]; August 04, 2008, http://www.microbicide.org]. Available from: http://www.microbicide.org.
- 3.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6:371–82. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 4.Ramjee G, Doncel GF, Mehendale S, Tolley EE, Dickson K. Microbicides 2008 Conference: From Discovery to Advocacy. AIDS Res Ther. 2008;5:19. doi: 10.1186/1742-6405-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Check E. Scientists rethink approach to HIV gels. Nature. 2007;446:12. doi: 10.1038/446012a. [DOI] [PubMed] [Google Scholar]
- 6.Honey K. Microbicide trial screeches to a halt. J Clin Invest. 2007;117:1116. doi: 10.1172/JCI32291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J. AIDS research. Microbicide fails to protect against HIV. Science. 2008;319:1026–7. doi: 10.1126/science.319.5866.1026b. [DOI] [PubMed] [Google Scholar]
- 8.Kreiss J, Ngugi E, Holmes K, et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–82. [PubMed] [Google Scholar]
- 9.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–10. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 10.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 11.Zekeng L, Feldblum PJ, Oliver RM, Kaptue L. Barrier contraceptive use and HIV infection among high-risk women in Cameroon. AIDS. 1993;7:725–31. doi: 10.1097/00002030-199305000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Koenig S, Gendelman HE, Orenstein JM, et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–93. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein G. Anti-HIV microbicides: don't forget basic immunology. Lancet. 2007;369:1429. doi: 10.1016/S0140-6736(07)60665-5. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein P, Jackson MC, Millman N, Sobrero AJ. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. J Reprod Fertil. 1969;20:85–93. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- 15.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–86. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fichorova RN, Bajpai M, Chandra N, et al. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol Reprod. 2004;71:761–9. doi: 10.1095/biolreprod.104.029603. [DOI] [PubMed] [Google Scholar]
- 17.Schroder JM, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987;139:3474–83. [PubMed] [Google Scholar]
- 18.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–90. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsukawa A, Miyazaki S, Maeda T, et al. Production and regulation of monocyte chemoattractant protein-1 in lipopolysaccharide- or monosodium urate crystal-induced arthritis in rabbits: roles of tumor necrosis factor alpha, interleukin-1, and interleukin-8. Lab Invest. 1998;78:973–85. [PubMed] [Google Scholar]
- 20.Matsukawa A, Yoshimura T, Fujiwara K, Maeda T, Ohkawara S, Yoshinaga M. Involvement of growth-related protein in lipopolysaccharide-induced rabbit arthritis: cooperation between growth-related protein and IL-8, and interrelated regulation among TNFalpha, IL-1, IL-1 receptor antagonist, IL-8, and growth-related protein. Lab Invest. 1999;79:591–600. [PubMed] [Google Scholar]
- 21.Matsukawa A, Yoshimura T, Maeda T, Takahashi T, Ohkawara S, Yoshinaga M. Analysis of the cytokine network among tumor necrosis factor alpha, interleukin-1beta, interleukin-8, and interleukin-1 receptor antagonist in monosodium urate crystal-induced rabbit arthritis. Lab Invest. 1998;78:559–69. [PubMed] [Google Scholar]
- 22.Matsukawa A, Yoshimura T, Miyamoto K, Ohkawara S, Yoshinaga M. Analysis of the inflammatory cytokine network among TNF alpha, IL-1 beta, IL-1 receptor antagonist, and IL-8 in LPS-induced rabbit arthritis. Lab Invest. 1997;76:629–38. [PubMed] [Google Scholar]
- 23.Doncel GF, Chandra N, Fichorova RN. Preclinical assessment of the proinflammatory potential of microbicide candidates. J Acquir Immune Defic Syndr. 2004;37 3:S174–80. [PubMed] [Google Scholar]
- 24.Grillner L, Landqvist M. Enzyme-linked immunosorbent assay for detection and typing of herpes simplex virus. Eur J Clin Microbiol. 1983;2:39–42. doi: 10.1007/BF02019921. [DOI] [PubMed] [Google Scholar]
- 25.Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968;6:761–4. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Gendelman HE, Orenstein JM, Baca LM, et al. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989;3:475–95. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Roy S, Wainberg MA. Role of the mononuclear phagocyte system in the development of acquired immunodeficiency syndrome (AIDS) J Leukoc Biol. 1988;43:91–7. doi: 10.1002/jlb.43.1.91. [DOI] [PubMed] [Google Scholar]
- 28.Kaul R, Pettengell C, Sheth PM, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J Reprod Immunol. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Narimatsu R, Wolday D, Patterson BK. IL-8 increases transmission of HIV type 1 in cervical explant tissue. AIDS Res Hum Retroviruses. 2005;21:228–33. doi: 10.1089/aid.2005.21.228. [DOI] [PubMed] [Google Scholar]
- 30.Miller CJ, Alexander NJ, Gettie A, Hendrickx AG, Marx PA. The effect of contraceptives containing nonoxynol-9 on the genital transmission of simian immunodeficiency virus in rhesus macaques. Fertil Steril. 1992;57:1126–8. [PubMed] [Google Scholar]
- 31.Weber J, Nunn A, O'Connor T, et al. ‘Chemical condoms’ for the prevention of HIV infection: evaluation of novel agents against SHIV(89.6PD) in vitro and in vivo. AIDS. 2001;15:1563–8. doi: 10.1097/00002030-200108170-00014. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–8. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 33.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–9. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch AE, Kunkel SL, Burrows JC, et al. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991;147:2187–95. [PubMed] [Google Scholar]
- 35.Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 36.Forslund O, Lindelof B, Hradil E, et al. High prevalence of cutaneous human papillomavirus DNA on the top of skin tumors but not in “Stripped” biopsies from the same tumors. J Invest Dermatol. 2004;123:388–94. doi: 10.1111/j.0022-202X.2004.23205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soboll G, Schaefer TM, Wira CR. Effect of toll-like receptor (TLR) agonists on TLR and microbicide expression in uterine and vaginal tissues of the mouse. Am J Reprod Immunol. 2006;55:434–46. doi: 10.1111/j.1600-0897.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 38.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- 40.Milligan GN, Dudley KL, Bourne N, Reece A, Stanberry LR. Entry of inflammatory cells into the mouse vagina following application of candidate microbicides: comparison of detergent-based and sulfated polymer-based agents. Sex Transm Dis. 2002;29:597–605. doi: 10.1097/00007435-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Milligan GN, Young CG, Meador MG, Chu CF, Stanberry LR. Effects of candidate vaginally-applied microbicide compounds on innate immune cells. J Reprod Immunol. 2005;66:103–16. doi: 10.1016/j.jri.2005.04.003. [DOI] [PubMed] [Google Scholar]