Abstract
Purpose
Because of differences in muscle architecture and biomechanics, the purpose of this study was to determine whether muscle contractile properties of rat hindlimb and tongue were differentially affected by aging.
Method
Deep peroneal and hypoglossal nerves were stimulated in 6 young and 7 old Fischer 344-Brown Norway rats to allow recording of muscle contractile properties of tongue and extensor digitorum longus (EDL) muscle in the hindlimb. In the same animals, the following measurements were made: (a) twitch contraction time (CT; in milliseconds), (b) half decay time (HDT; in milliseconds), (c) maximum twitch force (in grams), (d) tetanic force, and (e) fatigue index determined from repetitive stimulation of the muscles.
Results
No significant differences were observed in young versus old groups in retrusive tongue forces, whereas a significant (p < .05) decrement in EDL tetanic forces was found in old rats. Slower CT in old rats was observed only in the tongue. Old and young groups were not significantly different in fatigue index or HDT for tongue or EDL.
Conclusions
Old animals generated equivalent maximum tongue forces with stimulation, but they were slower in achieving these forces than young animals. Limb and cranial muscles were not affected equally by aging. As such, information derived from limb muscle studies may not easily generalize to the cranial motor system.
Keywords: aging, tongue, extensor digitorum longus, muscle contraction
The tongue is composed largely of fast-contracting, fatigue-resistant muscle, as studied via human cadaveric specimens (Saigusa, Niimi, Yamashita, Gotoh, & Kumada, 2001; Stal, Marklund, Thornell, DePaul, & Eriksson, 2003), primates (DePaul & Abbs, 1996), and rats (Gilliam & Goldberg, 1995; Sutlive, Shall, McClung, & Goldberg, 2000), and it has a vital role in speech and swallowing. Poor lingual control has been associated with speech and swallowing impairments in humans (Robbins et al., 2005). However, the muscles of the tongue have been understudied (Miller, Watkin, & Chen, 2002). In contrast to muscles in the limbs, tongue muscles interdigitate (Mu & Sanders, 1999), potentially to allow a variety of functional deformation profiles for different motor actions (Gilbert & Napadow, 2005). The complex, overlapping architecture of the intrinsic and extrinsic muscles of the tongue may create a technical challenge to detailed investigation and may be the cause of the relative paucity of studies concerning tongue anatomy and physiology.
The effects of aging on the structural and physiological properties of tongue muscles are not fully understood. Although most work concerning age-related changes in muscles has been performed in the limbs of human participants (cf. Carlson, 1995; Doherty, 2003; Lexell, 1995; Lexell, Taylor, & Sjostrom, 1988) or in animal hindlimb (Brown & Hasser, 1996b), a few studies have identified age-related structural and physiological alterations in the tongue in humans (McHenry, Minton, Hartley, Calhoun, & Barlow, 1999; Mortimore, Bennett, & Douglas, 2000; Nakayama, 1991; Nicosia et al., 2000) and in animal models (Hodges, Anderson, & Connor, 2004; Nagai, Russell, Jackson, & Connor, in press; Oliven, Carmi, Coleman, Majed, & Silbermann, 2001; Ota, Connor, & Konopacki, 2005). Specifically, age-related reductions have been found in the tongue muscle fiber cross-sectional area as well as in force and temporal properties of muscle contraction in humans (Mortimore et al., 2000; Nakayama, 1991) and animals (Nagai et al., in press; Oliven et al., 2001; Ota et al., 2005). Changes in neuromuscular junction morphology have also been reported in the rat tongue (Hodges et al., 2004). These basic aspects of muscle structure and physiology have implications for synaptic function, muscle fatigue, and contraction speed.
Reduced tongue strength may have clinical implications. Evidence of tongue weakness in persons with dysphagia has been found to correlate with abnormalities during the oral phase of the swallow in humans (Clark, Henson, Barber, Stierwalt, & Sherrill, 2003; Lazarus et al., 2000). These data are relevant to aging because an increased duration of the oral portion of the swallow has been identified in healthy, nondysphagic elderly persons (Robbins, Hamilton, Lot, & Kempster, 1992; Shaw et al., 1995). Accordingly, muscles of the tongue that contribute to the oral swallow may be particularly affected by aging, and tongue weakness and temporal delays in the oral phase of the swallow may be associated with swallowing impairment.
Because of the limited study of tongue muscle changes with aging, most inferences concerning the causes of age-related weakness and fatigue come from data derived from human (cf. Carlson, 1995; Doherty, 2003; Lexell, 1995; Lexell et al., 1988) and animal (Brown & Hasser, 1996b) limb muscle studies. There is evidence, however, from human(Lexell, 1995) and animal (Brown & Hasser, 1996b; Gutmann, Hanzlikova, & Vyskocil, 1971; Holloszy, Chen, Cartee, & Young, 1991; Prakash & Sieck, 1998) work that aging does not affect all muscles or muscle fiber types equally and that differences in degree of age-related change may be due to the function and composition of the muscle. For example, studies in animals have shown that muscle atrophy and altered neuromuscular junction configuration may be greater for postural, weight-bearing muscles versus nonweight-bearing muscles (Brown & Hasser, 1996a; Gutmann et al., 1971; Holloszy et al., 1991). Greater age-related atrophy of fast-twitch (Type II) fibers relative to slow-twitch (Type I) fibers has also been reported in humans (Lexell, 1995) and rats (Prakash & Sieck, 1998). Therefore, it is reasonable to expect that differential aging effects may be apparent across limb and tongue muscle systems, given the differences in function. However, there have not been previous in vivo physiological studies in animals in which limb and tongue contractile properties in the same experimental subjects have been investigated to discover potential differences in the manifestations of aging.
Because invasive procedures, such as hypoglossal nerve stimulation and the recording of in vivo tongue muscle contractile properties, are difficult to perform comfortably with human participants, the use of an animal model is required. A rat model was chosen for the present study on the basis of a number of scientific considerations. These considerations included the following: (a) the relatively short median lifespan of the rat (approximately 33 months; Turturro et al., 1999) that allows physiological and morphological changes associated with aging to be realized in a relatively short period of time; (b) the ease of handling rats, which permits rigorous experimental control, measurement of multiple parameters, and examination of relationships among variables; (c) the large knowledge base of prior work in aging rat muscle and nervous systems, including some studies within the cranial sensorimotor system (Fuller, Mateika, & Fregosi, 1998; Fuller, Williams, Janssen, & Fregosi, 1999;Hodges etal., 2004; Inagi, Connor, Ford, et al., 1998; Inagi, Connor, Schultz, et al., 1998; Nagai et al., in press; Ota et al., 2005; Shiotani & Flint, 1998); and (d) the relative magnitude of age-related muscle loss in rodents, which corresponds to that typically reported for humans (cf. Cartee, 1995). In gerontological research, the rat has been the most frequently used species for examining the neuromuscular sequelae of aging (Cartee, 1995; Gill, 1985). Because a large body of research exists, our results can be placed in context with data found abundantly within the literature that were derived via study of other muscles.
In the current study, we examined age-related changes in muscle contractile properties in the rat tongue and in a muscle of the hindlimb within young and old rats. The extensor digitorum longus (EDL) muscle was chosen as the muscle of interest in the hindlimb because, like the tongue (DePaul & Abbs, 1996; Stal et al., 2003), the EDL is composed of fast-twitch muscle fibers in the rat (Eddinger, Cassens, & Moss, 1986). This well-studied muscle also consistently demonstrates age-related changes. In aged rats, the following morphological and physiological sequelae have been reported in aged EDL relative to young adult EDL: a reduced muscle cross-sectional area (Eddinger, Moss, & Cassens, 1985) and the presence of increased axonal spouting, increased end plate area, decreased number of nerve terminal branches per end plate (Rosenheimer, 1990), reduced contractile force (Brown & Hasser, 1996b; Fisher & Brown, 1998), longer contraction times, longer half-relaxation times, and prolonged rate of peak twitch tension development (Gutmann et al., 1971).
The purpose of this study was to determine whether muscle contraction properties of the rat hindlimb (EDL muscle) and tongue are differentially affected by aging in a common set of animals. Our hypothesis was that differential age-related changes would be found in tongue and hindlimb muscle contractile properties.
Method
This study was performed in accordance with the Public Health Service policy on care and use of laboratory animals, the National Institutes of Health guide for care and use of laboratory animals, and the Animal Welfare Act. The animal use protocol was approved by the Institutional Animal Care and Use Committee of the University of Wisconsin.
Thirteen male Fischer 344-Brown Norway rats were studied. This strain has a median lifespan of approximately 33 months for male rats (Turturro et al., 1999). Six of the rats were young adults, 8–10 months of age, and the remaining 7 rats were old, 30–32 months of age. All rats were anesthetized with an intraperitoneal injection of a mixture of ketamine (90 mg/kg) and 1% xylazine (9 mg/kg).
The EDL is located deep in the hindlimb and extends from the knee to the ankle and digits; and contraction extends the digits (Netter, 2003). There are four muscle bellies in the EDL, each with an associated tendon, that insert into Digits 2–4 (Netter, 2003). The EDL was studied first in all animals, and recordings took 20–30 min, followed by tongue contraction studies, which also took 20–30 min to complete. The EDL recordings were completed first because they did not involve manipulation of the airway and thus could be completed more safely to ensure complete acquisition of data. Following the EDL recordings, additional anesthesia, to effect, was provided via intraperitoneal injection if needed. Degradation of the tongue muscles prior to the in vivo recordings was not expected with our experimental design because EDL recordings were not lengthy, surgery on the neck was not initiated until after the EDL recordings were completed, animal temperature and health were constantly monitored, and consistent anesthesia levels were maintained as assessed via behavioral methods. Force and temporal properties were recorded for all muscle contractions.
Muscle contractile properties were measured using standard physiological testing methods that are routinely employed in muscle physiology laboratories to characterize contraction forces (i.e., twitch and tetanic force) and temporal properties of muscle contraction (contraction time and half decay time). The following measurements were made: (a) Twitch contraction time was measured as the interval (in milliseconds) between the onset of stimulation and the point of 50% maximal force; (b) half decay time was the interval (in milliseconds) between the onset of stimulation and the point of 50% decay from peak force; (c) maximum twitch force was the peak force (in grams) generated by the muscle following a single electrical stimulus; (d) tetanic force was the maximal force of each stimulated fused wave; and (e) fatigue index was determined from repetitive stimulation of the muscles. That is, muscles were stimulated repeatedly at 100 Hz for 2 min. The fatigue index was calculated by constructing a ratio of the average tetanic force at the end of 2 min of simulation relative to the initial force and by multiplying by 100 to express the value as the percentage of initial force. As such, a high fatigue index indicates a resistance to fatigue (van Lunteren, Vafaie, & Salomone, 1995).
Peak twitch force (in grams) was a measure of maximum tension generated by a muscle, or a group of muscles, from a single supramaximal stimulation of themotor nerve supplying that muscle. Tetanic force (i.e., fused tetanus) was a measure of the amount of force generated by repeated stimulations, and it represented a fused force signal. The fatigue ratio was based on the change in tetanic force levels over time and was a measure of the reduction in force found as a muscle is continuously driven to contract. This measure has been used previously in other studies that examined fatiguing characteristics of the rat tongue (Fuller & Fregosi, 2000). Force (twitch and tetanic force) and fatigue measures are physiologically significant because they reflect the strength capabilities of the muscles being stimulated and the resistance to fatigue of these muscles.
The temporal variables of contraction time and half decay time were measured from the twitch force signal and were associated with how rapidly muscles contract—or were able to recover from a contraction, respectively—following stimulation. Accordingly, the physiological entity of muscle contraction speed was associated with the twitch contraction time and half decay time. Contraction times may be altered with aging, and thus it was important to measure temporal factors in studies of muscle contraction changes with aging (Degens & Alway, 2003). Accordingly, our goal was to examine these temporal elements for the tongue and EDL. These temporal measures are physiologically significant because they are associated with the speed, or velocity, of muscle contraction.
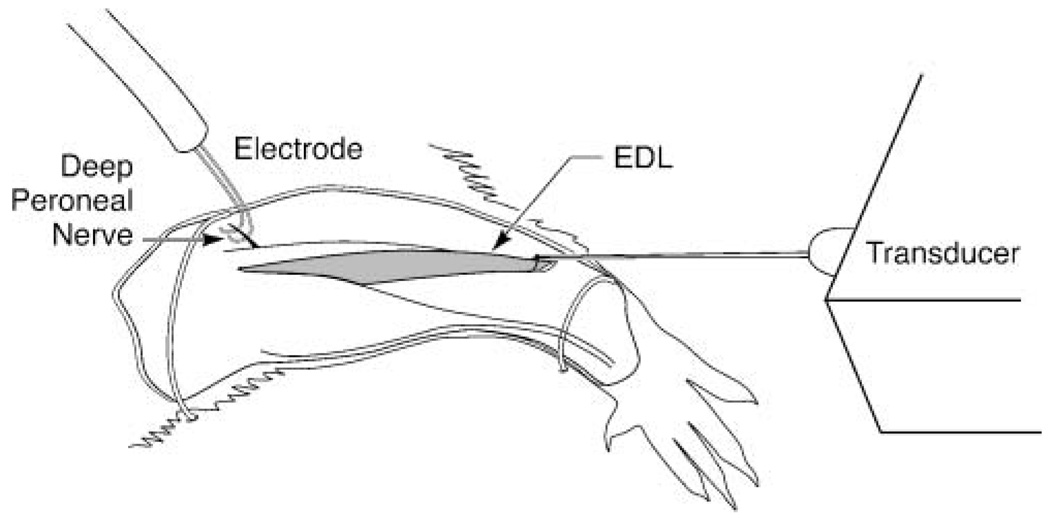
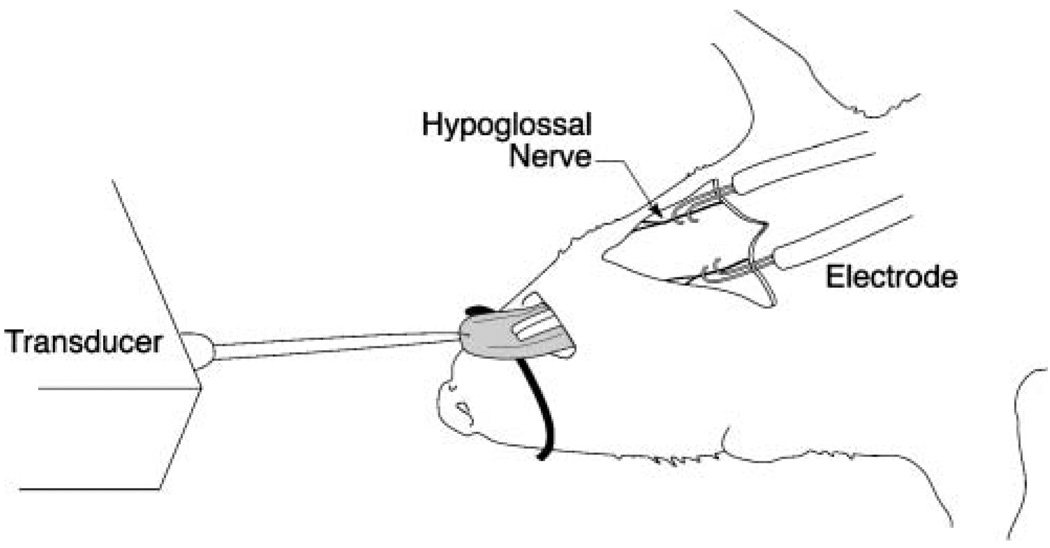
As shown in Figures 1 and 2, the rat was secured in supine position, and the hypoglossal nerves (bilateral) and either the left or right deep peroneal nerves were exposed (randomly determined). Bipolar silver–silver chloride hook electrodes were placed around the whole nerve and were covered with an insulated sheet to prevent current spread. A constant body temperature of 37 °C was maintained throughout the experiment.
Figure 1.
Schematic of extensor digitorum longus (EDL) apparatus. EDL contractile properties were recorded in the same anesthetized animals as the tongue recordings, following stimulation of the deep peroneal nerve on either the left or right leg (determined randomly).
Figure 2.
Schematic of tongue force experiment apparatus. Tongue muscle contractile data were obtained in anesthetized animals following bilateral supramaximal stimulation of the hypoglossal nerves.
For the EDL muscle recordings, the distal tendon of the muscle was dissected free and attached, via a silk suture, to a load cell force transducer. Applied stretch (preload) of the EDL and tongue were between 3 and 5 g. For the tongue recordings, the tongue was manually protruded from the mouth prior to stimulation, which does not adversely affect tongue contraction times (Hellstrand, 1981), and a 3.0 silk suture was placed through the tip of the tongue and then attached to a force load transducer (Kent Scientific, Torrington, CT).Optimal line tension was determined for each animal to yield maximum muscle twitch forces during supramaximal stimulation (1.5 times maximum), using the method of Fuller et al. (1998).
For both EDL and tongue, twitch force and temporal characteristics were measured in response to stimulation by 0.1-ms rectangular pulses at supramaximal levels (1.5 times maximum stimulation level; A-M Systems Differential AC Amplifier, Model 177; A-M Systems Isolated Pulse Stimulator, Model 2100; A-M Systems Analog Stimulus Amplification Unit, Model 2200, Carlsborg, WA). For the deep peroneal nerve, stimulation required between 500 and 900 µA, whereas hypoglossal nerve stimulation was generally delivered between 300 and 500 µA. The stimulator maintained a constant current level. Supra-maximal stimulation levels controlled for small differences in stimulation electrode placement and contact. These stimulation parameters have been reported previously (Gilliam & Goldberg, 1995). Thirty 1-s trials, with a 1-min rest period between trials, were recorded per muscle. The nerves were stimulated independently, and recordings were made of force generation and temporal properties of muscle contraction of the EDL and tongue muscles. The force signal and stimulation signal were acquired digitally at 2000 Hz on a personal computer, equipped with an A/D transformer, using data acquisition software developed for this purpose (Acquire 2.0, Madison, WI).
Data Analysis
All measurements were made with available computer software (Acquire 2.0, Madison, WI) on a personal computer. The old rats weighed significantly more than the young rats (old rats: mean weight = 534.3 g, SD = 72.1; young rats: mean weight = 450 g, SD = 30.3), F(1, 11) = 7.06, p = .02. Because this difference in body weight between groups may have affected force output (Jaric, 2002), data from the old and young groups were compared using analysis of covariance (ANCOVA) with weight as a covariate. That is, 10 separate ANCOVAs were used for measures of muscle contraction when comparisons were made between the young and old groups. When comparisons were made within the young and the old groups, such as EDL versus tongue comparisons within the same animals, a weight covariate was not necessary, and statistical comparisons were made using paired t tests. It is the practice of our laboratory to use body weight as a covariate for force comparisons because this method of normalization is generally accepted (Jaric, 2002) and is not dependent on accuracy of tongue muscle dissections in the manner of muscle weight normalizations. SAS statistical software was used for all analyses (SAS Institute, Cary, NC). The critical value for obtaining statistical significance was set at α = .05 level. We did not make any adjustments for multiple statistical tests, such as use of the Bonferroni correction, because such corrections might have limited our ability to detect true differences in the population.
Results
Stimulation of the hypoglossal nerve bilaterally resulted in retrusion of the tongue (Gilliam & Goldberg, 1995), and all tongue forces were measured during this retrusive tongue contraction. Group means, standard deviations, F values, and degrees of freedom for ANCOVAs are found in Tables 1 and 2. Statistically significant differences are also highlighted in Tables 1 and 2.
Table 1.
Weight-adjusted means and standard deviations of muscle contractile properties within each group during elicited retrusive actions of the tongue.
| Maximum twitch force (g) |
Twitch contraction time (ms) |
Half decay time (ms) |
Tetanic force (g) | Fatigue index (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | M | SD | M | SD | M | SD |
| Young | 24.55 | 6.8 | 11.75a | 0.7a | 46.63 | 5.5 | 64.27 | 17.3 | 73.84 | 8.2 |
| Old | 27.24 | 4.7 | 13.68 | 1.1 | 49.68 | 5.0 | 71.5 | 18.1 | 74.50 | 10.3 |
| F (dfs) | 0.39 (1, 10) | 10.53 (1, 10) | 0.69 (1, 10) | 0.30 (1, 10) | 0.01 (1, 10) | |||||
Note. Group means, standard deviations, F values (and degrees of freedom [dfs]) for analyses of covariance are shown.
Significantly different (p < .05).
Table 2.
Weight-adjusted means and standard deviations of muscle contractile properties within each group during elicited contractions of the extensor digitorum longus.
| Maximum twitch force (g) |
Twitch contraction time (ms) |
Half decay time (ms) |
Tetanic force (g) | Fatigue index (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | M | SD | M | SD | M | SD |
| Young | 48.84 | 14.1 | 21.04 | 2.9 | 67.24 | 15.0 | 162.17a | 38.9a | 29.32 | 9.0 |
| Old | 33.28 | 8.3 | 20.06 | 1.8 | 65.37 | 11.6 | 97.77 | 33.4 | 34.56 | 9.0 |
| F (dfs) | 3.38 (1, 10) | 0.32 (1, 10) | 0.04 (1, 10) | 5.78 (1, 10) | 0.61 (1, 10) | |||||
Note. Group means, standard deviations, F values (and degrees of freedom [dfs]) for analyses of covariance are shown.
Significantly different (p < .05).
Maximum Twitch Force
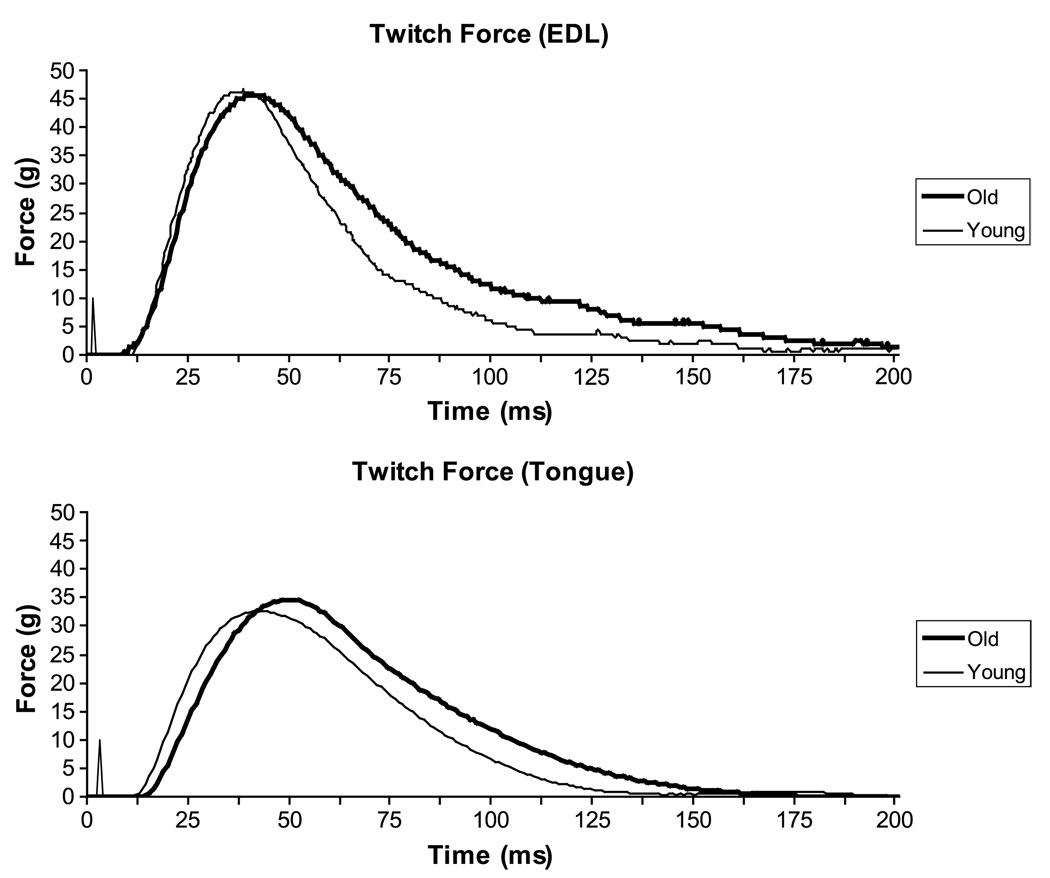
Representative twitch force signals are shown for EDL and tongue from one young and one old rat in Figure 3. No significant differences were found with ANCOVA in maximum twitch force between old and young rats for the EDL (p = .10) or the tongue (p = .54).
Figure 3.
Representative twitch force signals for the EDL (top) and the tongue (bottom). No statistically significant differences (p < .05) in twitch force were found between young and old groups. For the EDL, no statistically significant differences were observed between groups in temporal measures of muscle contraction (contraction time, half decay time). For the tongue, twitch contraction time was significantly longer in the old group relative to the young group.
Maximum twitch forces for the EDL muscle were significantly greater than those for the tongue on paired t tests, t(12) = 3.9, p = .002, in both young and old animals. However, the difference in maximum twitch forces between the EDL and tongue was significantly larger in young animals when compared with old animals using ANCOVA, F(1, 10) = 5.79, p = .04.
Tetanic Force
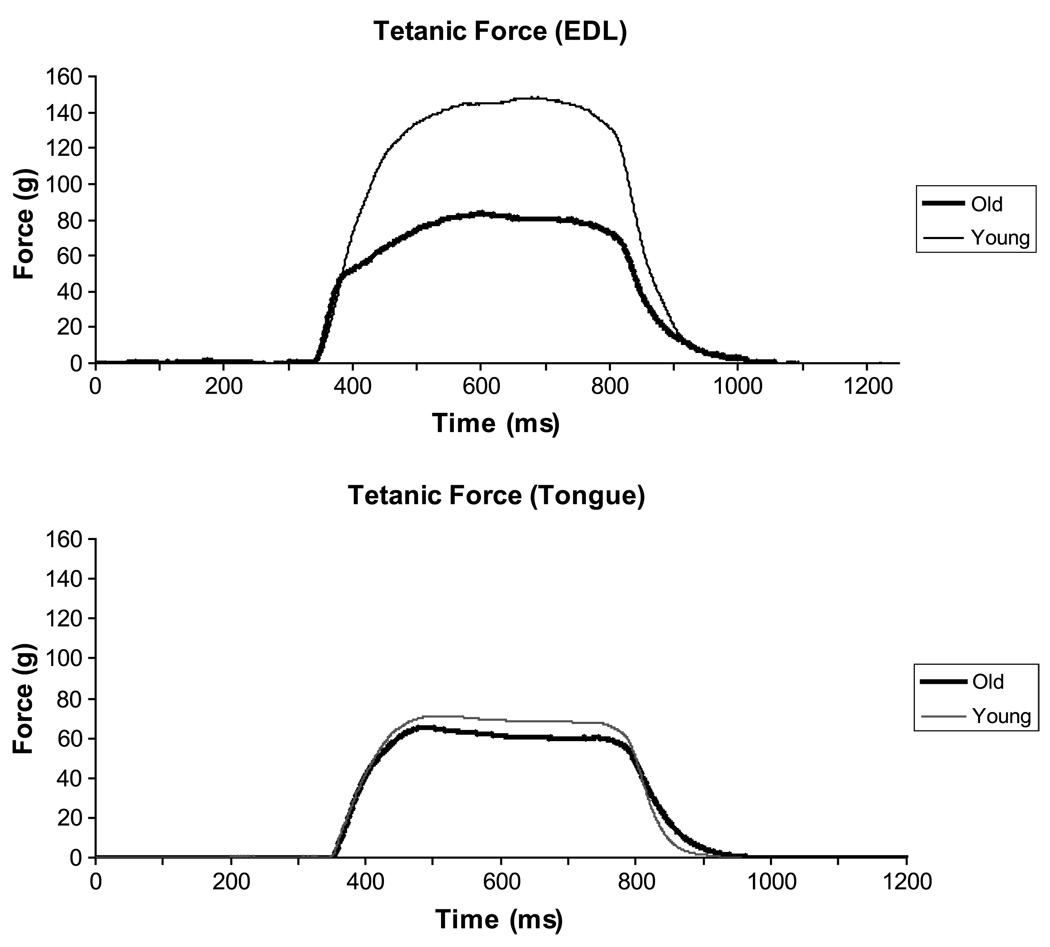
Representative tetanic force signals from one old and one young rat are shown in Figure 4 for EDL and tongue. Old animals evidenced significantly smaller tetanic EDL forces in comparison with young animals with ANCOVA (p = .04), whereas tetanic tongue forces were not significantly different between groups (p = .60).
Figure 4.
Representative tetanic force signals for the EDL (top) and the tongue (bottom). For the EDL, tetanic forces were significantly reduced (p < .05) in the old group relative to young group, whereas no statistically significant differences were observed between groups for the tongue.
Tetanic forces for the EDL muscle were significantly greater than those for the tongue on paired t tests, t(12) = 4.44, p = .0008, in both young and old animals. As with twitch forces, the difference in tetanic forces between the EDL and tongue was significantly larger in young animals when compared with old animals using ANCOVA, F(1, 10) = 7.98, p = .02.
Fatigue Index
A fatigue index value closer to 100% reflects a resistance to fatigue (van Lunteren et al., 1995). As evidenced by mean values contained in Tables 1 and 2, the old and young groups did not differ significantly in fatigue index for tongue (ANCOVA; p = .93) or EDL (ANCOVA; p = .45) muscle contractions.
Resistance to fatigue (i.e., fatigue indices) for the tongue was significantly greater than for the EDL on paired t tests, t(12) = 13.5, p < .0001, for both young and old animals. There was not a statistically significant difference between young and old animals on fatigue index difference values for the tongue and EDL on ANCOVA, F(1, 10) = 0.32, p = .58.
Temporal Measures
As suggested by the force signals found in Figure 3 and the mean data in Tables 1 and 2, old animals evidenced significantly longer contraction times for retrusive tongue twitch than young animals (p = .009). However, contraction time was not significantly different for EDL twitch contractions (p = .58). Half decay time was not found to differ significantly for tongue (p = .43) or EDL (p = .85) in young versus old rats on ANCOVA.
Contraction time and half decay time values for the EDL muscle were significantly greater than those for the tongue on paired t tests in both young and old animals, t(12) = 9.28, p < .0001, and t(12) = 4.14, p = .001, respectively. On ANCOVA, the difference in contraction time, F(1, 10) = 1.83, p = .21, and half decay time values, F(1, 10) = 0.16, p = .70, between the EDL and tongue was not significantly different as a function of age.
Discussion
The purpose of this study was to determine whether muscle contraction properties of the rat hindlimb and tongue are differentially affected by aging in a common set of animals. On the basis of previous research showing differential age-related changes across muscles, our hypothesis was that aging would affect muscles of the tongue and hindlimb differently in a common set of animals. Our results support this hypothesis. In old rats, the EDL muscle evidenced significantly reduced tetanic forces when compared with young rats, but contraction times were unaffected by aging. In contrast, contraction times for evoked, retrusive tongue muscle contractions in old animals were significantly slower, but twitch and tetanic forces in the tongue were not different as a function of aging. Moreover, the EDL had increased force properties, longer contraction times, and increased fatigability relative to the tongue. These findings reinforce that aging is not manifested equally across muscle systems of the body in the rat and that temporal components of muscle contraction are particularly impaired for retrusive tongue actions (Ota et al., 2005).
Muscles in the cranial motor system with a role in speech and swallowing may have different morphological and physiological characteristics than other skeletal muscles found in the body. In a recent review, “The Uniqueness of Speech Among Motor Systems,” Kent (2004) presented evidence in the form of histologic, morphologic, and physiologic data to argue that cranial and limb muscle systems differ at multiple levels of investigation and across the lifespan. Our results reinforce Kent’s hypothesis that lingual muscles in the rat may have unique properties relative to hindlimb muscles, perhaps because of the inherent complexity of critical head and neck functions. Specifically, in our study, contraction times were affected by aging in lingual muscles, whereas forces were reduced in the limbs. The hindlimb manifested increased fatigability across age groups. These findings are consistent with the idea of the tongue specialization for the production of rapid articulatory movements rather than the generation of large forces (Kent, 2004), as for limb musculature.
Temporal aspects of lingual actions are particularly relevant for swallowing in humans. Older individuals swallow more slowly (Nicosia et al., 2000; Robbins, Connor, & Barczi, 2006; Robbins et al., 1992). If increases in tongue muscle contraction times are found in elderly people, these temporal delays may influence the timing of tongue muscle actions and, moreover, may contribute to the temporal abnormalities of the oral phase of the swallow reported in previous human swallow research (Ekberg & Feinberg, 1991; Robbins et al., 1992; Shaw et al., 1995). Accordingly, temporal properties of tongue muscle contractions that contribute to the oral swallow may be particularly affected by aging, and they may represent a putative mechanism for the age-related degradation in swallowing function observed in some elderly individuals.
Muscles in the tongue and hindlimb were found to differ significantly in terms of contraction forces, temporal features, and fatigability. Specifically, the EDL muscle in the hindlimb generated greater forces, evidenced greater fatigability, and had increased contraction times relative to the tongue. These findings can be explained by the substantial differences in size, innervation ratio, muscle fiber type, and biomechanics for muscles of the tongue and hindlimb (Delp & Duan, 1996; Sutlive, McClung, & Goldberg, 1999). For example, the tongue has no bony or cartilaginous skeleton, in contrast to limb motor structures, and therefore muscle actions and morphological properties must be uniquely managed to produce movement in a manner distinct from limb biomechanics (Kier, 1985; Kier & Smith, 1985).
The differences in biomechanics of the two muscle systems examined must be considered when interpreting our data. That is, the muscle systems studied may not be equivalent, and it may not be possible to create equivalence for in vivo study. Because the present study measured forces produced by the entire tongue, both retrusive and protrusive extrinsic muscles as well as intrinsic muscles were activated by the bilateral, whole-nerve stimulation of the hypoglossal nerves. The net result of stimulation was an evoked retrusive tongue action (Gilliam & Goldberg, 1995). In contrast, the EDL muscle recordings were isolated to a single hindlimb muscle. However, comparisons of EDL with tongue were not the findings of greatest interest presented in this work because of known differences in biomechanics and muscle size. As such, it might be expected that forces in EDL would be greater than those of the tongue. Other comparisons presented between old and young tongue and old and young EDL were much more interesting and resulted in a differential pattern of impairment in the old animal within the tongue and hindlimb. Contraction time differences in the aged rat tongue may be important, if also characteristic of humans, when placed in the larger context of physiological requirements for speech and swallowing.
Our use of an animal model must be considered when interpreting our data and in attempting to generalize our results to humans. Although cross-species studies of tongue structure and function have not been forthcoming in the literature, it appears that there are more similarities than differences between the rat and human tongue. For instance, both species have been found to contain primarily fast-contracting (Type II) muscle fiber types (Stal et al., 2003; Volz et al., 2007); both humans and rats have been found to evidence coactivation of protruser and retruser muscles via stimulation of the whole hypoglossal nerve, resulting in visible retrusion, as also noted in this study (Bailey & Fregosi, 2003; Fuller et al., 1998, 1999; Gilliam & Goldberg, 1995; Schwartz et al., 2001). Therefore, differences in anatomical size and shape of the tongue across rats and humans still allow for very similar neural control of the tongue musculature (R. Fregosi, personal communication, October 19, 2004). Accordingly, the rat is a useful and valid model for exploration of the basic principles of tongue muscle physiology reported here. As stated in a review on the topic, “… research with animal models has greatly advanced the understanding of the biological basis for age-related changes in human skeletal muscle” (Cartee, 1995, p. 140). However, any model, in the best of circumstances, can only serve as an approximation to the human clinical situation that it is designed to reflect. As such, our findings should be interpreted with the appropriate caution that should always be applied to animal experimentation in the medical and social sciences.
Differences in muscle contractile properties across cranial and spinal motor systems in a common set of animals are important because they suggest that research findings concerning isolated target muscles in the limbs may not be directly applicable to cranial muscles. The findings reported here, and the structural and functional differences among limb and cranial muscles, underscore the need to directly examine the cranial system. Thus, direct examination of cranial muscles is critical to a complete understanding of age-related muscular changes and to interventions targeted at particular cranial functions.
Acknowledgment
This study was partially supported by Grants R01DC005935 and R01DC008149 from the National Institute on Deafness and Other Communication Disorders. We gratefully acknowledge the assistance of Jack Jiang, who provided us with a computer program for making our force measurements.
Contributor Information
Nadine P. Connor, University of Wisconsin–Madison
Fumikazu Ota, Jikei University, Tokyo, Japan.
Hiromi Nagai, Kitasato University, Kanagawa, Japan.
John A. Russell, University of Wisconsin–Madison
Glen Leverson, University of Wisconsin–Madison.
References
- Bailey EF, Fregosi RF. Pressure–volume behavior of the rat upper airway: Effects of tongue muscle activation. Journal of Physiology. 2003;548:563–568. doi: 10.1113/jphysiol.2002.029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Hasser EM. Complexity of age-related change in skeletal muscle. Journal of Gerontology. 1996a;51A:B117–B123. doi: 10.1093/gerona/51a.2.b117. [DOI] [PubMed] [Google Scholar]
- Brown M, Hasser EM. Differential effects of reduced muscle use (hindlimb unweighting) on skeletal muscle with aging. Aging: Clinical and Experimental Research. 1996b;8:99–105. doi: 10.1007/BF03339562. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Factors influencing the repair and adaptation of muscles in aged individuals: Satellite cells and innervation. Journal of Gerontology. 1995;50:96–100. doi: 10.1093/gerona/50a.special_issue.96. [DOI] [PubMed] [Google Scholar]
- Cartee GD. What insights into age-related changes in skeletal muscle are provided by animal models? Journal of Gerontology. 1995;50A:137–141. doi: 10.1093/gerona/50a.special_issue.137. [DOI] [PubMed] [Google Scholar]
- Clark HM, Henson PA, Barber WD, Stierwalt JAG, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. American Journal of Speech-Language Pathology. 2003;12:40–50. doi: 10.1044/1058-0360(2003/051). [DOI] [PubMed] [Google Scholar]
- Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle and Nerve. 2003;27:339–347. doi: 10.1002/mus.10314. [DOI] [PubMed] [Google Scholar]
- Delp M, Duan C. Composition and size of Type I, IIA, IID/ X, and IIB fibers and citrate synthase activity of rat muscle. Journal of Applied Physiology. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- DePaul R, Abbs JH. Quantitative morphology and histochemistry of intrinsic lingual muscle fibers in Macaca fascicularis. Acta Anatomica. 1996;155:29–40. doi: 10.1159/000147787. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. Aging and sarcopenia. Journal of Applied Physiology. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Eddinger TJ, Cassens RG, Moss RL. Mechanical and histochemical characterization of skeletal muscles from senescent rats. American Journal of Physiology. 1986;251:C421–C430. doi: 10.1152/ajpcell.1986.251.3.C421. [DOI] [PubMed] [Google Scholar]
- Eddinger TJ, Moss RL, Cassens RG. Fiber number and type composition in extensor digitorum longus, soleus, and diaphragm muscles with aging in Fischer 344 rats. Journal of Histochemistry and Cytochemistry. 1985;33:1033–1041. doi: 10.1177/33.10.2931475. [DOI] [PubMed] [Google Scholar]
- Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: Radiologic findings in 56 cases. American Journal of Roentgenology. 1991;156:1181–1184. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Brown M. Immobilization effects on contractile properties of aging rat skeletal muscle. Aging: Clinical and Experimental Research. 1998;10:59–66. doi: 10.1007/BF03339635. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Fregosi RF. Fatiguing contractions of tongue protrudor and retractor muscles: Influence of systemic hypoxia. Journal of Applied Physiology. 2000;88:2123–2130. doi: 10.1152/jappl.2000.88.6.2123. [DOI] [PubMed] [Google Scholar]
- Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. Journal of Physiology. 1998;507:265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. Journal of Physiology. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ, Napadow VJ. Three-dimensional muscular architecture of the human tongue determined in vivo with diffusion tensor magnetic resonance imaging. Dysphagia. 2005;20:1–7. doi: 10.1007/s00455-003-0505-9. [DOI] [PubMed] [Google Scholar]
- Gill TJ. The rat in biomedical research. Physiologist. 1985;28:9–17. [PubMed] [Google Scholar]
- Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: Effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. Journal of Neurophysiology. 1995;74:547–555. doi: 10.1152/jn.1995.74.2.547. [DOI] [PubMed] [Google Scholar]
- Gutmann E, Hanzlikova V, Vyskocil F. Age changes in cross striated muscle of the rat. Journal of Physiology. 1971;219:331–343. doi: 10.1113/jphysiol.1971.sp009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand E. Contraction times of the cat's tongue muscles measured by light reflection: Innervation of individual tongue muscles. Acta Physiologica Scandinavica. 1981;111:417–423. doi: 10.1111/j.1748-1716.1981.tb06757.x. [DOI] [PubMed] [Google Scholar]
- Hodges SH, Anderson A, Connor NP. Neuromuscular junction changes in aged rat genioglossus. Annals of Otology, Rhinology and Laryngology. 2004;113:175–179. doi: 10.1177/000348940411300301. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: Differential changes in the three fiber types. Mechanisms of Aging and Development. 1991;60:199–213. doi: 10.1016/0047-6374(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Inagi K, Connor NP, Ford CN, Schultz E, Rodriquez AA, Bless DM, et al. Physiologic assessment of botulinum toxin effects in the rat larynx. Laryngoscope. 1998;108:1048–1054. doi: 10.1097/00005537-199807000-00018. [DOI] [PubMed] [Google Scholar]
- Inagi K, Connor NP, Schultz E, Ford CN, Cook CH, Bless DM, Heisey DM. Increased acute and chronic mitotic activity in rat laryngeal muscles after botulinum toxin injection. Laryngoscope. 1998;108:1055–1061. doi: 10.1097/00005537-199807000-00019. [DOI] [PubMed] [Google Scholar]
- Jaric S. Muscle strength testing: Use of normalisation for body size. Sports Medicine. 2002;32:615–631. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed] [Google Scholar]
- Kent RD. The uniqueness of speech among motor systems. Clinical Linguistics & Phonetics. 2004;18:495–505. doi: 10.1080/02699200410001703600. [DOI] [PubMed] [Google Scholar]
- Kier WM. The musculature of squid arms and tentacles: Ultrastructural evidence for function differences. Journal of Morphology. 1985;185:223–239. doi: 10.1002/jmor.1051850208. [DOI] [PubMed] [Google Scholar]
- Kier WM, Smith KK. Tongues, tentacles and trunkcs: The biomechanics of movement in muscular-hydrostats. Zoological Journal of the Linnean Society. 1985;83:307–324. [Google Scholar]
- Lazarus CL, Logemann JA, Pauloski BR, Rademaker AW, Larson CR, Mittal BB, Pierce M. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. Journal of Speech, Language, and Hearing Research. 2000;43:1011–1023. doi: 10.1044/jslhr.4304.1011. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. Journal of Gerontology. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of ageing atrophy? Total number, size, and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the Neurological Sciences. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- McHenry MA, Minton JT, Hartley LL, Calhoun K, Barlow SS. Age-related changes in orofacial force generation in women. Laryngoscope. 1999;109:827–830. doi: 10.1097/00005537-199905000-00027. [DOI] [PubMed] [Google Scholar]
- Miller JL, Watkin KL, Chen MF. Muscle, adipose, and connective tissue variations in intrinsic musculature of the adult human tongue. Journal of Speech, Language, and Hearing Research. 2002;45:51–65. doi: 10.1044/1092-4388(2002/004). [DOI] [PubMed] [Google Scholar]
- Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: Relationship to apnoea/hypopnoea index and age. Journal of Sleep Research. 2000;9:389–393. doi: 10.1046/j.1365-2869.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- Mu L, Sanders I. Neuromuscular organization of the canine tongue. Anatomical Record. 1999;256:412–424. doi: 10.1002/(SICI)1097-0185(19991201)256:4<412::AID-AR8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Nagai H, Russell JA, Jackson MA, Connor NP. Effect of aging on tongue protrusion forces in rats. Dysphagia. doi: 10.1007/s00455-007-9103-6. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M. Histological study on aging changes in the human tongue. Nippon Jibiinkoka Gakkai Kaiho. 1991;94:541–555. doi: 10.3950/jibiinkoka.94.541. [DOI] [PubMed] [Google Scholar]
- Netter FH. Atlas of human anatomy. 3rd ed. Teterboro, NJ: Icon Learning Systems; 2003. [Google Scholar]
- Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55:M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- Oliven A, Carmi N, Coleman R, Majed O, Silbermann M. Age-related changes in upper airway muscles morphological and oxidative properties. Experimental Gerontology. 2001;36:1673–1686. doi: 10.1016/s0531-5565(01)00127-9. [DOI] [PubMed] [Google Scholar]
- Ota F, Connor NP, Konopacki RA. Alterations in contractile properties of tongue muscles in old rats. Annals of Otology, Rhinology and Laryngology. 2005;114:799–803. doi: 10.1177/000348940511401010. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle and Nerve. 1998;21:887–895. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Robbins JA, Connor NP, Barczi S. Effects of aging on swallowing. In: Calhoun K, Eibling D, Wax M, Kost K, editors. Geriatric otolaryngology. New York: Taylor & Francis; 2006. pp. 277–291. [Google Scholar]
- Robbins JA, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind J. The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society. 2005;53:1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- Robbins JA, Hamilton JW, Lot GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- Rosenheimer JL. Ultraterminal sprouting in innervated and partially denervated adult and aged rat muscle. Neuroscience. 1990;38:763–770. doi: 10.1016/0306-4522(90)90069-g. [DOI] [PubMed] [Google Scholar]
- Saigusa H, Niimi S, Yamashita K, Gotoh T, Kumada M. Morphological and histochemical studies of the genioglossus muscle. Annals of Otology, Rhinology and Laryngology. 2001;110:779–784. doi: 10.1177/000348940111000815. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Bennett JL, Smith PL, De Backer W, Hedner J, Boooudewyns A, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Archives of Otolaryngology–Head and Neck Surgery. 2001;127:1216–1223. doi: 10.1001/archotol.127.10.1216. [DOI] [PubMed] [Google Scholar]
- Shaw DW, Cook IJ, Gabb M, Holloway RH, Simula ME, Panagopoulos V, Dent J. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. American Journal of Physiology. 1995;268:G389–G396. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- Shiotani A, Flint PW. Myosin heavy chain composition in rat laryngeal muscles after denervation. Laryngoscope. 1998;108:1225–1229. doi: 10.1097/00005537-199808000-00023. [DOI] [PubMed] [Google Scholar]
- Stal P, Marklund S, Thornell L-E, DePaul R, Eriksson P-O. Fiber composition of human intrinsic tongue muscles. Cells Tissues Organs. 2003;173:147–161. doi: 10.1159/000069470. [DOI] [PubMed] [Google Scholar]
- Sutlive TG, McClung R, Goldberg SJ. Whole-muscle and motor-unit contractile properties of the styloglossus muscle in rat. Journal of Neurophysiology. 1999;82:584–592. doi: 10.1152/jn.1999.82.2.584. [DOI] [PubMed] [Google Scholar]
- Sutlive TG, Shall MS, McClung JR, Goldberg SJ. Contractile properties of the tongue’ s genioglossus muscle and motor units in the rat. Muscle and Nerve. 2000;23:416–425. doi: 10.1002/(sici)1097-4598(200003)23:3<416::aid-mus14>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1999;54A:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Vafaie H, Salomone RJ. Comparative effects of aging on pharyngeal and diaphragm muscles. Respiration Physiology. 1995;99:113–125. doi: 10.1016/0034-5687(94)00077-d. [DOI] [PubMed] [Google Scholar]
- Volz LM, Mann LB, Russell JA, Jackson MA, Leverson GE, Connor NP. Biochemistry of anterior, medial, and posterior genioglossus muscle in the rat. Dysphagia. 2007;22:210–214. doi: 10.1007/s00455-006-9075-y. [DOI] [PubMed] [Google Scholar]