Abstract
The impact of carbohydrates and milk on the bioavailability of catechin (C) and epicatechin (EC) from chocolate has been previously studied. However, little data exists regarding potential modulation of the phase-II metabolism by these chocolate matrix factors. The objectives of this study were to assess the impact of matrix composition on qualitative and quantitative profiles of circulating catechins and their metabolites following administration of commercially relevant chocolate confections. Sprague-Dawley rats were fed 1.5 g of a confection (reference dark, high sucrose, or milk chocolate) by intragastric gavage, and plasma samples were collected over 8 h. HPLC-MS analysis was performed to quantify C, EC and their metabolites. The predominant metabolites were O-glucuronides (2 metabolites), and O-Me-O-glucuronides (3 metabolites). Plasma concentrations of metabolites were generally the highest for high sucrose treatment and lowest for milk treatment, while reference dark treatment generally resulted in intermediate concentrations. The O-Me-(±)-C/EC-O-β-glucuronide (peak 4) was significantly higher for the high sucrose treatment (2325 nM*h) versus the milk treatment (1300 nM*h). Additionally, CMAX values for (±)-C/EC-O-β-glucuronide (peak 3), and two O-Me-(±)-C/EC-O-β-glucuronides (peaks 4 and 6) were significantly higher for high sucrose treatment (4012, 518, and 2518 nM, respectively) versus the milk treatment (2590, 240, and 1670 nM, respectively). Milk and sucrose appear to modulate both metabolism and plasma pharmacokinetics, and to a lesser extent, the overall bioavailability of catechins from chocolate confections.
Keywords: bioavailability, chocolate, flavan-3-ols, epicatechin, phase-II metabolism, Sprague-Dawley rat
INTRODUCTION
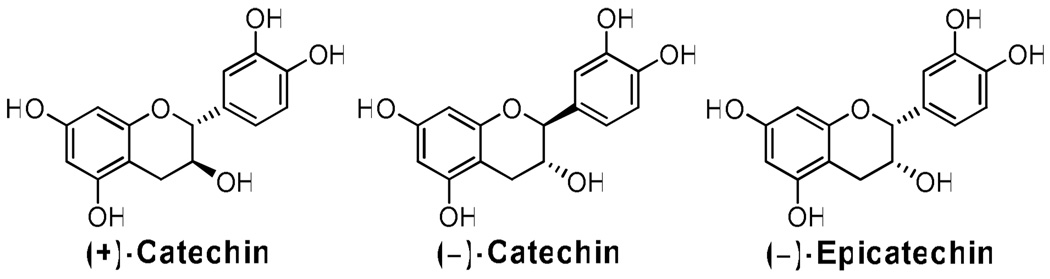
Chocolate from Theobroma cacao has been investigated as a functional food with potential health-protective and therapeutic activities against several chronic and degenerative diseases. Chocolate products are rich sources of a variety of monomeric, oligomeric, and polymeric flavan-3-ols (1–5). Although the profile of flavan-3-ol species present in chocolate can vary greatly, monomers are one of the major forms present (4, 6). The predominant flavan-3-ol monomers present in chocolate include (−)-epicatechin (EC), (+)-catechin and (−)-catechin (referred to as C, Figure 1) (1). Many of the proposed biological activities associated with chocolate have been linked to the flavan-3-ols, including C and EC. These activities include improved biomarkers of cardiovascular health and function (7–17), increased serum and/or plasma antioxidant capacity (17–18), and increased insulin sensitivity and decreased insulin resistance (11–13).
Figure 1.
Structures of (±)-catechin (C) and (−)-epicatechin (EC), the major monomeric flavan-3-ols present in chocolate.
Continued interest in the potential benefits of cocoa flavan-3-ols has underscored the need to better understand the bioavailability and metabolism of flavan-3-ol monomers in vivo. The poor absorption of flavan-3-ols (19–21) highlights the potential for optimization of bioavailability as an important strategy for maximizing the effects of chocolate flavan-3-ols in vivo. In order to accomplish this, a more detailed understanding of the effect of product matrix composition on absorption and metabolism of flavan-3-ols from commercial chocolate products is required.
Previous investigations of the overall bioavailability of C and EC from chocolate in human subjects have reported conflicting results regarding the influence of food matrix factors such as milk protein and carbohydrate (sucrose, starch, etc.). Serafini et al. (22) reported that the EC area under the plasma pharmacokinetic curve (AUC) was lower for a milk chocolate confection compared to a dark chocolate confection of equal EC content, while consumption of liquid milk with the dark chocolate confection resulted in an intermediate AUC value. However, Schramm et al. (23) reported that consumption of a cocoa beverage formulated with milk resulted in a slightly, but not significantly, higher AUC than consumption of a cocoa beverage formulated with water. Additionally, four separate studies found no difference in the overall bioavailability of EC between cocoa beverages formulated with liquid milk versus water (24–27). Interestingly, all studies of beverage formulations have shown no difference in overall bioavailability, while the study by Serafini is the only study performed with confections and also the only study to show a pronounced negative effect of milk on overall bioavailability.
Schramm et al. (23) also investigated the effect of carbohydrate, and found that co-consumption of cocoa beverages with sucrose or bread resulted in increased plasma AUC and maximal plasma concentrations (CMAX) of EC after consumption of cocoa with water. However, limited data exist regarding whether carbohydrate levels similarly affect EC bioavailability when chocolate is consumed in confection form. Recently, we investigated the influence of matrix composition and physical form (beverage vs. confection) of the bioavailability of EC from chocolate (28). We previously found that the overall bioavailability was generally similar between formulations, but that the physical form greatly modulated the serum pharmacokinetic behavior (CMAX and TMAX) of EC.
While the impact of matrix formulation on the bioavailability of flavan-3-ol monomers from chocolate has been extensively studied, there is little data regarding the potential modulation of phase-II metabolism and subsequent circulating profiles by milk and/or carbohydrate. Studies of the influence of matrix formulation on phase-II metabolite profiles have predominantly examined urine, with little data on plasma, and therefore do not completely reflect alterations of circulating profiles. Roura et al. (26) reported that consumption of a milk-based cocoa beverage by human subjects resulted in increased excretion of EC sulfates and decreased excretion of EC glucuronides in urine, with no effect on total bioavailability, compared to consumption of a water-based cocoa beverage. Mullen et al. reported that consumption of cocoa beverages resulted in the presence of C/EC sulfates and O-Me sulfates in plasma, and that the presence of milk increased the plasma elimination time and decreased the AUC of the sulfate metabolites relative to water-based beverages (29). Mullen et al. also reported that the total urinary excretion of C/EC metabolites (sulfates, O-Me sulfates, and O-glucuronides) was significantly lower from the milk-based beverages than from the water-based beverages from 0–2 h, 2–5 h, and for total (24 h) excretion. However, the studies by Roura et al. and Mullen et al. examined beverages, but not solid confections. Other studies in rats and humans have shown that C and EC are extensively metabolized in vivo, and are present in plasma as 3'-O-Me and 4'-O-Me derivatives, 5-O-β-glucuronides, 3'/4'-O-Me-5-O-β-glucuronides, and a variety of sulfated metabolites (26, 30–37). Although the reported quantitative profiles of these metabolites vary greatly, the 5-O-β-glucuronides appear to predominate, while the sulfated metabolites are typically present in low concentrations in the plasma as they are rapidly excreted into the urine.
The present study was designed to use a relevant animal model to further investigate the effects of matrix composition on the bioavailability of flavan-3-ols from cocoa which we previously observed in humans (28). The objectives of this study were to assess the impact of food matrix composition on the bioavailability and metabolism of C and EC monomers, and to characterize the qualitative and quantitative profiles of circulating native compounds and their predominant phase-II metabolites, following administration of commercially relevant chocolate confections. An understanding of how chocolate matrix factors potentially modulate systemic flavan-3-ol bioavailability and alter the profile of circulating species could potentially lead to the development of formulation and/or dietary strategies designed specifically to optimize the bioavailability and, by extension, the in vivo activities of monomeric cocoa flavan-3-ols.
METHODS AND MATERIALS
Reagents and Standards
(±)-C and (−)-EC standard material, formic acid, ascorbic acid, Na2EDTA, and NaCL were obtained from Sigma-Aldrich (St. Louis, MO). MeOH (LC-MS grade) was obtained from Mallinckrodt Baker (Phillipsburg, NJ). ACN (Optima grade) was obtained from Fisher Scientific (Pittsburg, PA). All H2O was distilled/deionized using a Barnstead MegaPure MP-1 system (Dubuque, IA).
Chocolate Confections
Chocolate matrices were prepared by Kraft Foods Research and Development (Munich, Germany). Three solid confection [reference dark chocolate (CDK), high sucrose (CHS), high milk protein (CMP)] providing commercially relevant levels of C and EC (approximately 1.37 mg C+EC/1.5 g serving for rats, equivalent to ~36.5 mg C+EC/40 g serving for humans, based on analysis of raw materials) from cocoa were formulated (Table 1). The flavan-3-ol content of the confections was determined as described previously (28). In order to balance serving sizes, the cocoa butter content was adjusted as needed. Therefore, the CDK treatment was higher in fat (0.75 g/1.5 g serving) than the CHS and CMP treatments, which had similar fat content (0.45 and 0.52 g/1.5 g serving, respectively).
Table 1.
Formulations of the Three Chocolate Confection Treatments.
| Product | |||
|---|---|---|---|
| CDK | CHS | CMP | |
| Trt | reference dark | high sucrose | milk protein |
| Ingredient | Solid components (mg/1.5 g serving) | ||
| cocoa butter | 750.00 | 450.00 | 525.00 |
| cocoa powder | 502.50 | 502.50 | 502.50 |
| sucrose | 245.55 | 545.55 | 245.55 |
| vanilla flavor | 1.20 | 1.20 | 1.20 |
| soya lecithin | 0.75 | 0.75 | 0.75 |
| milk protein* | 0.00 | 0.00 | 225.00 |
| total | 1500.00 | 1500.00 | 1500.00 |
| Flavan-3-ol | Composition (mg/1.5 g serving)† | ||
| C | 0.49 ± 0.03 | 0.51 ± 0.01 | 0.53 ± 0.04 |
| EC | 1.09 ± 0.03 | 1.13 ± 0.03 | 1.14 ± 0.01 |
| total | 1.58 ± 0.06 | 1.64 ± 0.04 | 1.67 ± 0.05 |
milk protein concentrate (80%)
flavan-3-ol composition was determined as described previously (28). Data represent mean of n=3 independent analyses formulated products, which were all formulated using equal amounts of the same cocoa powder. There were no significant differences between product compositions
Animal Pharmacokinetic Experiment
Animal care and experimental protocols were approved by Purdue University’s Animal Care and Use Committee (PACUC). Twenty four male Sprague-Dawley rats (~250 g) were obtained from Harlan (Indianapolis, IN) and maintained on a 12 h light/dark cycle in a climate-controlled facility. Rats were initially fed a 14% protein rodent maintenance diet (Teklad 2014, Harlan), followed by a nutritionally complete polyphenol-free purified maintenance diet (AIN-93M, Dyets, Inc., Bethlehem, PA) for 7 d prior to the study. Food and H2O were available ad libitum. After the 7 d diet adjustment period, rats were anaesthetized with isoflurane and implanted with jugular catheters exteriorized dorsally. Catheters were kept patent by flushing every 12 h post-surgery with heparinized saline (100 U/mL). Following a 48 h recovery period, food was removed for 6 h, and baseline blood samples (400 µL) were collected via the catheter. Rats then received 1.5 g of melted CDK, CHS, or CMP by intragastric gavage (n=8 per treatment, chocolate products were melted at 50 °C for 25 min prior to gavage). Following gavage, food was restored and blood samples were collected at 0.5, 1, 2, 4, 6, and 8 h. Blood samples were placed in Li-heparin tubes to prevent clotting, and then centrifuged (5000 rpm, 10 min, 4 °C). Following centrifugation, 100 µL of plasma was collected and stabilized by addition of 25 µL 1% aqueous ascorbic acid solution (w/v). Samples were then blanketed in N2 and stored at −80 °C prior to analysis.
Solid-phase Extraction
Plasma samples were thawed at room temperature and 1 mL of acidified saline [0.9% NaCl (w/v), 0.1% formic acid (v/v)] containing 20 mg/mL ascorbic acid and 1 mg/mL Na2EDTA was added to each sample. Sample were ultrasonicated for 15 s and centrifuged at 18000 rcf for 2 min to break the resulting foam. Solid phase extraction (SPE) was performed on Waters Oasis HLB (30 mg, 1 cc) SPE cartridges (Milford, MA). SPE cartridges were preconditioned with 1 mL methanol (MeOH) and 1 mL H2O, followed by loading of the plasma samples. Cartridges were washed with 2 mL 1.5 M aqueous formic acid (v/v) and 2 mL 5% aqueous MeOH (v/v). Compounds of interest were then eluted with 2 mL acidified MeOH (0.1% formic acid, v/v). Extracts were dried under vacuum for 20 minutes at 37 °C. Dried extracts were then solubilized in 150 µL 0.1% aqueous formic acid (v/v), vortexed for 10 s, and immediately analyzed by HPLC-MS (40 µL injection volume).
HPLC-MS Analysis
HPLC separations were performed on a Waters 2695 separations module equipped with a Waters XTerra RP-C18 column (2.1×100 mm, 3.5 µm particle size). Column temperature was 35 °C, and samples were maintained at 8 °C. The binary mobile phase system was comprised of 0.1% aqueous formic acid (v/v, Phase A) and 0.1% formic acid in acetonitrile (v/v, Phase B). System flow rate was 0.3 mL/min. Elution was performed based on the following linear gradient: 90% A at 0 min, 30% A at 5.5 min and held until 7 min, 0% A at 7.01 and held until 7.5 min, 90% A at 7.51 min and held until 12 min. The post-column effluent was split 1:1 prior to (−)-ESI-MS analysis on a Waters ZQ 2000 mass spectrometer. The ESI capillary voltage was −3.5 kV, and the source and desolvation temperatures were 150°C and 350 °C, respectively. The desolvation gas and cone gas were N2 at flow rates of 400 and 60 L/h, respectively. The cone, extractor and RF lens voltages were 30, 3 and 0.5 V, respectively. Selected Ion Response (SIR) detection was performed simultaneously for m/z 289, 303, 465, and 479 to detect native catechins, and their predominant phase-II metabolites including O-Me catechins (m/z 303), glucuronides (m/z 465) and O-Me glucuronides (m/z 479). SIR m/z spans were ±0.75 for each mass. Dwell times for individual SIRs were 0.3 s, with an inter-channel delay of 0.01 s and inter-scan delay of 0.05 s. SIR data was collected from 0–7 min.
Data Analysis
Native and metabolite species in plasma were quantified by MS peak area using a standard curve based on EC response. Statistical analyses were performed using SAS 9.1.3 software (SAS Institute Inc., Cary, NC). Plasma metabolite levels are reported as mean ± standard error of the mean (SEM). Areas under the plasma concentration vs. time curves (AUC) were calculated using the linear trapezoidal rule. The maximum plasma concentrations (CMAX), and the times at which the maximum plasma concentration (TMAX) were determined directly from individual plasma concentration vs. time curves and expressed as mean ± SEM. Differences in pharmacokinetic parameters of total as well as individual metabolites between treatments were determined by a paired Student's t-test (α = 0.05).
RESULTS
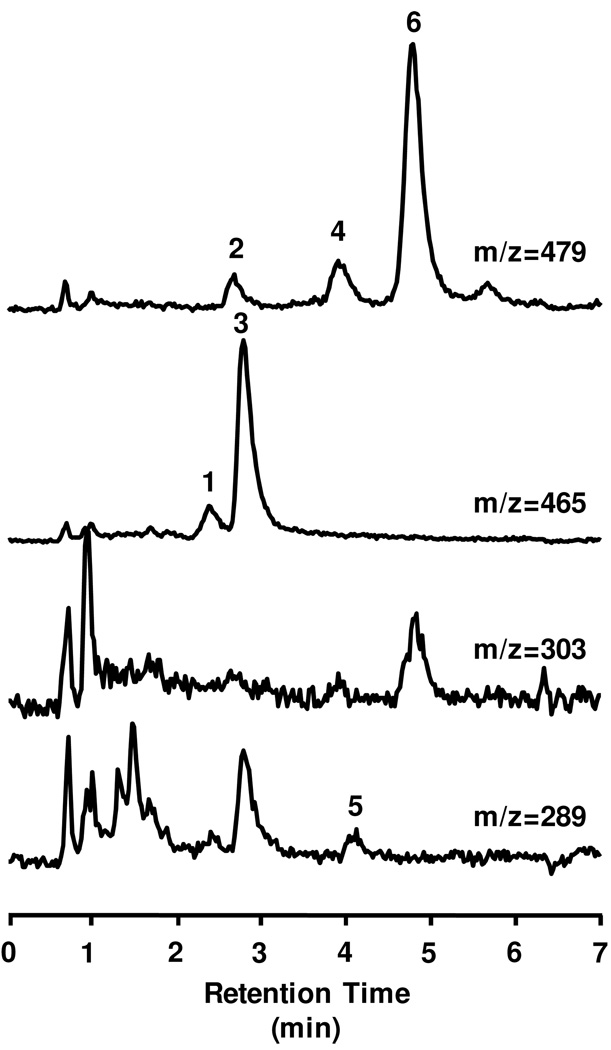
A representative SIR chromatogram from the HPLC-MS analysis of pooled plasma samples (0.5–8 h) collected from a rat following consumption of CMP and extracted by SPE as described above is shown in Figure 2. This separation demonstrates that 6 species arising from C and EC were detected in the plasma of rats fed the chocolate confections (none of the labeled peaks were detected in the 0 h baseline samples for any rat, data not shown). Peaks 2, 4, and 6 were assigned as O-Me glucuronides of C/EC due to the [M−H]− = 479 and the m/z 479 → m/z 303 fragmentation (loss of the glucuronyl residue to form O-Me-C/EC). Peaks 1 and 3 were assigned as glucuronides of C/EC due to the [M−H]− = 465 and the m/z 465 → m/z 289 fragmentation (loss of the glucuronyl residue to form C/EC). Peak 5 was assigned as (±)-EC based on the [M−H]− = 289 and identical retention time (approx. 4 min) to authentic EC standard material. Based on the results from previous studies of the phase-II metabolism of C and EC (30–35, 37), and elution order of each peak in relation to C and EC, the following tentative peak assignments were made: peak 1: (±)-C/EC-5-O-β-glucuronide, peak 2: O-Me-(±)-C/EC-5-O-β-glucuronide, peak 3: (±)-C/EC-5-O-β-glucuronide, peak 4: O-Me-(±)-C/EC-5-O-β-glucuronide, peak 5: (±)-EC, peak 6: O-Me-(±)-EC-5-O-β-glucuronide. HPLC-MS analysis conducted on these samples was not able to distinguish between 3’- and 4’- O-Me metabolites. As such, all methylated metabolites were identified as O-Me-C/EC derivatives. Additionally, the stereochemistry of these metabolites is unknown, as they may have arisen from (±)-C, which are both present in cocoa, or (±)-EC, due to isomerization post-consumption. Therefore each is designated as being a metabolites of (±)-C/EC. No intact (±)-C, (±)-C/EC sulfated metabolites or unconjugated O-Me-(±)-C/EC metabolites were detected in the plasma for any confection treatment. Based upon these data, O-glucuronides and O-Me glucuronides appeared to be the major phase-II metabolites present in plasma. Free EC was a minor component in blood consistent with previous studies on catechins in vivo in rats (32–33).
Figure 2.
Representative reverse-phase HPLC-MS separation of an extract of pooled rat plasma (0.5–8 h) obtained following consumption of 1.5 g CMP chocolate matrix. Selected Ion Response (SIR) chromatograms are shown for deprotonated pseudomolecular ions [M−H]− representing native monomers (m/z 289), and predominate phase-II metabolites: O-glucuronides (m/z 465) and O-methyl-O-glucuronides (479). Additionally, a SIR is shown for m/z 303 which is an MS fragment of the O-methyl-O-glucuronides.
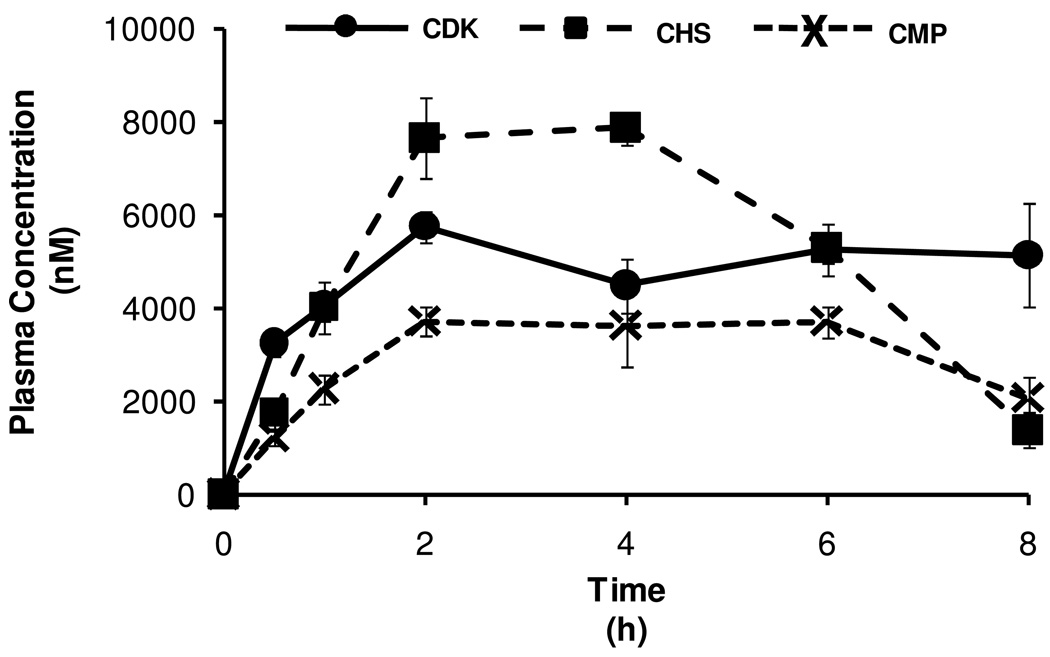
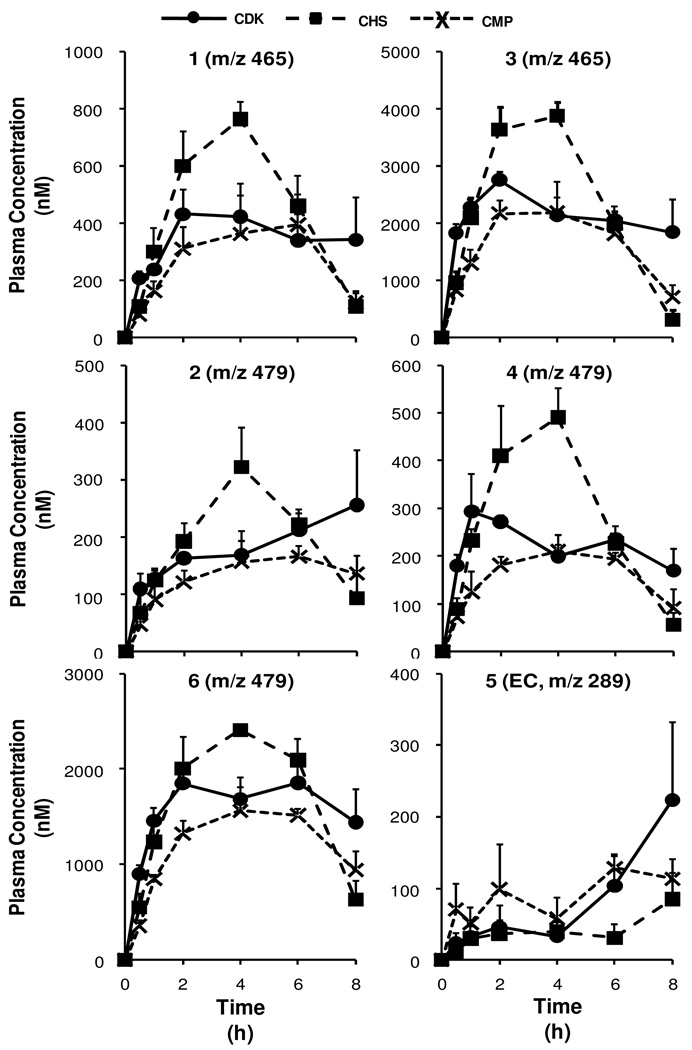
Following identification of native EC and the phase-II metabolites of C and EC in the plasma, pharmacokinetic curves of the mean plasma concentrations over 0–8 h were prepared for total metabolite sums (Figure 3) as well as for each compound (Figure 4). Examination of these curves shows that the plasma concentrations of the phase-II metabolites (total as well as individual metabolite species) were generally the highest for the CHS treatment and the lowest for the CMP treatment, while the CDK treatment generally resulted in intermediate concentrations, over the entire 8 h period. Additionally, plasma concentrations appeared to peak at approximately 4 h post-gavage.
Figure 3.
Plasma pharmacokinetic curves (0–8 h) of total monomeric flavan-3-ols (native and phase-II metabolites) from rats fed each of the chocolate confections. Curve values represent the mean plasma concentration from n=4 rats per time point for each treatment; error bars represent the SEM. Plasma concentrations are expressed as EC equivalents.
Figure 4.
Plasma pharmacokinetic curves (0–8 h) of native flavan-3-ols and predominant phase-II metabolites (glucuronides and O-Me glucuronides) from rats fed each of the chocolate confections. Peak 1: (±)-C/EC-O-glucuronide, peak 2: O-Me-(±)-C/EC-O-glucuronide, peak 3: (±)-C/EC-O-glucuronide, peak 4: O-Me-(±)-C/EC-O-glucuronide, peak 5: (±)-EC, peak 6: O-Me-(±)-C/EC-O-glucuronide. Curve values represent the mean plasma concentration from n=4 rats per time point for each treatment; error bars represent the SEM. Plasma concentrations are expressed as EC equivalents. Note the distinct scales for each set of curves.
Examination of the calculated pharmacokinetic parameters (Table 2) shows that the AUC was significantly lowered by CMP compared to CHS treatment for total circulating species as well as for the three EC metabolites detected: (±)-C/EC-O-glucuronide (peak 3), O-Me-(±)-C/EC-O-glucuronide (peak 4) and O-Me-(±)-C/EC-O-glucuronide (peak 6). Additionally, the AUC was significantly lowered by CMP compared to CDK treatment for O-Me-(±)-C/EC-O-glucuronide (peak 4). No significant differences in AUC values were observed between treatments for (±)-EC (which was present in low concentrations compared to the other metabolites), (±)-C/EC-O-glucuronide (peak 1) or O-Me-(±)-C/EC-O-glucuronide (peak 2).
Table 2.
Pharmacokinetic Parameters (0–8 h) of Native Flavan-3-ols and Predominant Phase-II Metabolites in Plasma of Rats.
| Pharmacokinetic Parameters* | |||||
|---|---|---|---|---|---|
| Compound | Peak | Trt | AUC (nM*h) |
CMAX (nM) |
TMAX (h) |
| CDK | 2796 ± 625a | 504 ± 125a | 4.0 ± 1.4a | ||
| (±)-C/EC-O-glucuronide | 1 | CHS | 3733 ± 584a | 763 ± 61a | 4.0 ± 0.0a |
| CMP | 2266 ± 516a | 458 ± 125a | 4.0 ± 0.8a | ||
| CDK | 16952 ± 1574ab | 2849 ± 214b | 3.5 ± 1.5a | ||
| (±)-C/EC-O-glucuronide | 3 | CHS | 19503 ± 1890a | 4012 ± 306a | 3.5 ± 0.5a |
| CMP | 13343 ± 1496b | 2590 ± 420b | 3.0 ± 0.6a | ||
| CDK | 1411 ± 188a | 292 ± 80a | 5.5 ± 1.5a | ||
| O-Me-(±)-C/EC-O-glucuronide | 2 | CHS | 1598 ± 259a | 322 ± 69a | 4.0 ± 0.0a |
| CMP | 1054 ± 230a | 190 ± 27a | 5.0 ± 0.6a | ||
| CDK | 1757 ± 122a | 341 ± 61ab | 3.8 ± 1.3a | ||
| O-Me-(±)-C/EC-O-glucuronide | 4 | CHS | 2325 ± 322a | 518 ± 72a | 3.5 ± 0.5a |
| CMP | 1300 ± 49b | 240 ± 18b | 5.0 ± 0.6a | ||
| CDK | 12843 ± 1325ab | 1988 ± 180ab | 5.0 ± 1.3ab | ||
| O-Me-(±)-C/EC-O-glucuronide | 6 | CHS | 13847 ± 1221a | 2518 ± 139a | 3.5 ± 0.5a |
| CMP | 9901 ± 830b | 1670 ± 203b | 5.5 ± 0.5b | ||
| CDK | 601 ± 164a | 229 ± 107a | 6.5 ± 1.5a | ||
| (±)-EC | 5 | CHS | 308 ± 86a | 104 ± 25a | 5.5 ± 1.5a |
| CMP | 708 ± 217a | 171 ± 38a | 3.6 ± 1.4a | ||
| CDK | 36360 ± 3856ab | 5916 ± 659b | 5.5 ± 1.5a | ||
| Total | 1–6 | CHS | 41314 ± 3876a | 8110 ± 530a | 3.5 ± 0.5a |
| CMP | 28573 ± 2926b | 5112 ± 757b | 4.5 ± 1.0a | ||
Values represent the mean ± SEM from n=4 rats per formulation. Pharmacokinetic parameters with different superscripts are significantly different for that compound or metabolite (P<0.05). The area under the pharmacokinetic curve (AUC), the time at which the maximum serum concentration was observed (TMAX), and the maximum serum concentrations observed (CMAX).
The CMAX values were significantly greater for CHS (CHS > CDK = CMP) for both total metabolites as well as (±)-C/EC-O-glucuronide (peak 3, the predominant metabolite observed). In addition, CMAX values were significantly lowered by CMP vs. CHS treatment for O-Me-(±)-C/EC-O-glucuronide (peak 4) and O-Me-(±)-C/EC-O-glucuronide (peak 6) (for this metabolite, the difference in CMAX approached significance between CDK and CHS, P = 0.058). Similar to AUC values, no significant differences in CMAX values were observed between treatments for (±)-EC, (±)-C/EC-O-glucuronide (peak 1) or O-Me-(±)-C/EC-O-glucuronide (peak 2).
The trends in the pharmacokinetic data consistently reflected AUC and CMAX values ordered CHS > CDK > CMP, with the exception of EC. The calculated pharmacokinetic parameters reflect the general shapes of the pharmacokinetic curves, which suggest that the CHS treatment resulted in significantly higher plasma concentrations of the predominant phase-II metabolites of C and EC than the CMP treatment, while CDK resulted in intermediate plasma concentrations.
TMAX values ranged from 3.0–6.5 h. Statistically significant differences between TMAX values were only observed for O-Me-(±)-C/EC-O-glucuronide (peak 6) between CHS (3.5 ± 0.5 h) and CMP (5.5 ± 0.5 h) treatments. No other significant differences were observed between TMAX values for different treatments. Generally, the CHS treatment resulted in the lowest TMAX values, with the exception of (±)-EC and (±)-C/EC-O-glucuronide (peak 3), for which the CMP treatment resulted in the lowest TMAX values.
DISCUSSION
Both absorption and metabolism of cocoa derived catechins appear to be influenced by chocolate matrix. Specific differences and trends observed for the AUC of C and EC metabolites [CHS had the highest AUC for all compounds except (±)-EC] generally agrees with the results of Schraam et al. (23), who reported that consumption of carbohydrates along with cocoa beverages increased the AUC of EC relative to control. Additionally, the significant differences and the general trend observed for the CMAX values [CHS had the highest CMAX for all compounds except (±)-EC] in the present study also agree with Schraam et al. (23), who demonstrated that carbohydrates increased the CMAX values of EC relative to control. The lower overall bioavailability of C relative to EC (38) may have minimized observed differences for any metabolites derived from (±)-C.
Several studies have been performed regarding the influence of milk protein on the bioavailability of EC from cocoa beverages and chocolate. Serafini et al. (22) reported that milk resulted in a reduced AUC for EC relative to control in chocolate confections, while Schroeter et al. (27), Schraam et al. (23), Roura et al. (25), and Keogh et al. (24) reported no statistical difference between the AUC of EC from cocoa beverages consumed with water or milk. The present results found a significantly lower AUC from the milk formulation (CHS > CMP) for total species as well as all three EC metabolites detected, as well as a trend was that the AUCs from CMP were the lowest from the milk formulation for all compounds except (±)-EC. It is critical to note that we examined confections, like Serafini et al. (22), while the studies demonstrating no difference between milk and control (23–25, 27) were performed using cocoa beverages.
Recently we performed a study in human subjects (28) using the same confections used in the present study, as well as beverages, and demonstrated that the AUC of EC from CMP was lower, though not significantly different, than CHS and CDK. Additionally, the CMAX of EC was lower from CMP than both CHS (statistically different) and CDK (not statistically different). However, the highest AUC and CMAX values in this study were observed from milk-containing beverage forms of these chocolate formulations. Taken together, these studies suggest that milk and sucrose appear to modulate the pharmacokinetics of EC and formation of predominant C/EC phase-II metabolites from confections. The presence of milk protein also appears to exert mild suppressive effect on the bioavailability of these compounds from confections. However, in most cases this affect is not large enough to be statistically significant. One explanation for this is that milk may drive the phase-II generation of sulfated metabolites (26), which are rapidly excreted into the urine, thus reducing the formation of glucuronides and O-Me glucuronides. While sulfated metabolites were not detected in rodent plasma in the current study, the extent to which food matrix factors influence the formation and clearance of these metabolites merits further investigation.
Milk does not appear to exert these effects to the same extent in beverages matrices when compared to confections, as our previous study demonstrated that milk-containing beverages produced generally higher serum AUC and CMAX values than confections formulated with or without milk (28). This may be due to the rapid emptying of beverages from the stomach, which facilitates more rapid appearance in the blood, as well as the relative ease of digestive release and solubilization from beverages compared to confections. This process would serve to facilitate subsequent catechin absorption. It is important to note that this increase in the formation of sulfated metabolites from cocoa was previously observed following consumption of milk beverages (26). A significant uncertainty therefore remains regarding the mechanisms by which matrix composition and physical form (beverage vs. confections) of chocolate products interact to modulate bioavailability and metabolism.
In conclusion, our data combined with that of previous investigations (28) (22) suggest that chocolate confections containing high levels of sucrose may enhance plasma levels of the predominant C and EC metabolites compared to milk chocolate confections, while confections containing moderate levels of sucrose and no milk deliver intermediate plasma levels of these compounds. However, the physical state of the product may significantly modulate this effect, as our prior study comparing confections and beverages (28) demonstrated that milk-containing beverages produced generally higher serum AUC and CMAX values than confections with or without milk, and numerous studies have shown no difference in the overall bioavailability of EC between cocoa beverages formulated with milk versus water (24–27). In the future, simultaneous investigation of the distribution of native species and phase-II metabolites into both plasma and urine will be required in order to quantitatively assess the influence of chocolate matrix composition and physical form on the bioavailability, metabolism and systemic distribution of C and EC from chocolate.
ACKNOWLEDGEMENT
The authors wish to thank Catrina Peters, Pamela Lachcik, and Angela Myracle (Department of Foods and Nutrition, Purdue University) for their assistance with the rat pharmacokinetic experiment. This research was supported by grants from Kraft Foods Global Brands, 801 Waukegan Rd., Glenview, IL 60025, and the Purdue-UAB Botanicals Center for Age Related Diseases NIH-NCCAM grant P50-AT00477.
ABBREVIATIONS USED
- EC
(−)-epicatechin
- C
(+)-catechin and (−)-catechin
- AUC
area under the plasma pharmacokinetic curve
- ESI
electrospray ionization
- CMP
high milk protein
- CHS
high sucrose
- HPLC-MS
high-performance liquid chromatography/mass spectrometry
- CMAX
maximal plasma concentrations
- SEM
mean ± standard error of the mean
- MeOH
methanol
- O-Me
O-methyl
- CDK
reference dark chocolate
- SIR
selected ion response
- SPE
solid phase extraction
- TMAX
time at which the maximum plasma concentration was observed
References
- 1.Cooper KA, Campos-Gimenez E, Alvarez DJ, Nagy K, Donovan JL, Williamson G. Rapid reversed phase ultra-performance liquid chromatography analysis of the major cocoa polyphenols and inter-relationships of their concentrations in chocolate. J. Agric. Food Chem. 2007;55:2841–2847. doi: 10.1021/jf063277c. [DOI] [PubMed] [Google Scholar]
- 2.Gu LW, Kelm M, Hammerstone JF, Beecher G, Cunningham D, Vannozzi S, Prior RL. Fractionation of polymeric procyanidins from lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC-MS fluorescent detection method. J. Agric. Food Chem. 2002;50:4852–4860. doi: 10.1021/jf020214v. [DOI] [PubMed] [Google Scholar]
- 3.Lee KW, Kim YJ, Lee HJ, Lee CY. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003;51:7292–7295. doi: 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- 4.Natsume M, Osakabe N, Yamagishi M, Takizawa T, Nakamura T, Miyatake H, Hatano T, Yoshida T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotech. Bioch. 2000;64:2581–2587. doi: 10.1271/bbb.64.2581. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Rabaneda F, Jauregui O, Casals I, Andres-Lacueva C, Izquierdo-Pulido M, Lamuela-Raventos RM. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao) J. Mass. Spectrom. 2003;38:35–42. doi: 10.1002/jms.395. [DOI] [PubMed] [Google Scholar]
- 6.Gu LW, House SE, Wu XL, Ou BX, Prior RL. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006;54:4057–4061. doi: 10.1021/jf060360r. [DOI] [PubMed] [Google Scholar]
- 7.Baba S, Osakabe N, Kato Y, Natsume M, Yasuda A, Kido T, Fukuda K, Muto Y, Kondo K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans'. Am. J. Clin. Nutr. 2007;85:709–717. doi: 10.1093/ajcn/85.3.709. [DOI] [PubMed] [Google Scholar]
- 8.Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, Kwak HK, Milbury P, Paul SM, Blumberg J, Mietus-Synder ML. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 9.Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am. J. Clin. Nutr. 2008;88:58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 10.Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, Serafini M, Luscher TF, Ruschitzka F, Noll G, Corti R. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–2382. doi: 10.1161/CIRCULATIONAHA.107.713867. [DOI] [PubMed] [Google Scholar]
- 11.Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 12.Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 13.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 14.Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. A. Coll. Cardiol. 2005;46:1276–1283. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 15.Mursu J, Voutilainen S, Nurmi T, Rissanen TH, Virtanen JK, Kaikkonen J, Nyyssoonen K, Salonen JT. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radical Bio. Med. 2004;37:1351–1359. doi: 10.1016/j.freeradbiomed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. P. Natl. Acad. Sci. USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Y, Vinson JA, Etherton TD, Proch J, Lazarus SA, Kris-Etherton P. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am. J. Clin. Nutr. 2001;74:596–602. doi: 10.1093/ajcn/74.5.596. [DOI] [PubMed] [Google Scholar]
- 18.Wang JF, Schramm DD, Holt RR, Ensunsa JL, Fraga CG, Schmitz HH, Keen CL. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J. Nutr. 2000;130:2115S–2119S. doi: 10.1093/jn/130.8.2115S. [DOI] [PubMed] [Google Scholar]
- 19.Auger C, Mullen W, Hara Y, Crozier A. Bioavailability of poluphenon E flavan-3-ols in humans with an ileostomy. J. Nutr. 2008;138:1535–1542. doi: 10.1093/jn/138.8.1535S. [DOI] [PubMed] [Google Scholar]
- 20.Donovan JL, Kasim-Karakas S, German JB, Waterhouse AL. Urinary excretion of catechin metabolites by human subjects after red wine consumption. Brit. J. Nutr. 2002;87:31–37. doi: 10.1079/bjn2001482. [DOI] [PubMed] [Google Scholar]
- 21.Henning SM, Niu YT, Lee NH, Thames GD, Minutti RR, Wang HJ, Go VLW, Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am. J. Clin. Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 22.Serafini M, Bugianesi R, Maiani G, Valtuena S, De Santis S, Crozier A. Plasma antioxidants from chocolate – Dark chocolate may offer its consumers health benefits the milk variety cannot match. Nature. 2003;424:1013–1013. doi: 10.1038/4241013a. [DOI] [PubMed] [Google Scholar]
- 23.Schramm DD, Karim M, Schrader HR, Holt RR, Kirkpatrick NJ, Polagruto JA, Ensunsa JL, Schmitz HH, Keen CL. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003;73:857–869. doi: 10.1016/s0024-3205(03)00373-4. [DOI] [PubMed] [Google Scholar]
- 24.Keogh JB, McInerney J, Clifton PM. The effect of milk protein on the bioavailability of cocoa polyphenols. J. Food Sci. 2007;72:S230–S233. doi: 10.1111/j.1750-3841.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 25.Roura E, Andres-Lacueva C, Estruch R, Mata-Bilbao ML, Izquierdo-Pulido M, Waterhouse AL, Lamuela-Raventos RM. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann. Nutr. Metab. 2007;51:493–498. doi: 10.1159/000111473. [DOI] [PubMed] [Google Scholar]
- 26.Roura E, Andres-Lacueva C, Estruch R, Bilbao MLM, Izquierdo-Pulido M, Lamuela-Raventos RM. The effects of milk as a food matrix for polyphenols on the excretion profile of cocoa (−)-epicatechin metabolites in healthy human subjects. Brit. J. Nutr. 2008;100:846–851. doi: 10.1017/S0007114508922534. [DOI] [PubMed] [Google Scholar]
- 27.Schroeter H, Holt RR, Orozoco TJ, Schmitz HH, Keen CL. Nutrition – Milk and absorption of dietary flavanols. Nature. 2003;426:787–788. doi: 10.1038/426787b. [DOI] [PubMed] [Google Scholar]
- 28.Neilson AP, George JC, Janle EM, Mattes RD, Rudolph R, Matusheski NV, Ferruzzi MG. Influence of Chocolate Matrix Composition on Cocoa Flavan-3-ol Bioaccessibility In Vitro and Bioavailability in Humans. J. Agric. Food Chem. 2009;57:9418–9426. doi: 10.1021/jf902919k. [DOI] [PubMed] [Google Scholar]
- 29.Mullen W, Borges G, Donovan JL, Edwards CA, Serafini M, Lean MEJ, Crozier A. Milk decreases urinary excretion but not plasma pharmacokinetics of cocoa flavan-3-ol metabolites in humans. Am. J. Clin. Nutr. 2009;89:1784–1791. doi: 10.3945/ajcn.2008.27339. [DOI] [PubMed] [Google Scholar]
- 30.Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radical Bio. Med. 2002;33:1693–1702. doi: 10.1016/s0891-5849(02)01137-1. [DOI] [PubMed] [Google Scholar]
- 31.Baba S, Osakabe N, Natsume M, Yasuda A, Takizawa T, Nakamura T, Terao J. Cocoa powder enhances the level of antioxidative activity in rat plasma. Brit. J. Nutr. 2000;84:673–680. [PubMed] [Google Scholar]
- 32.Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J. Absorption and urinary excretion of (−)-epicatechin after administration of different levels of cocoa powder or (−)-epicatechin in rats. J. Agric. Food Chem. 2001;49:6050–6056. doi: 10.1021/jf010965h. [DOI] [PubMed] [Google Scholar]
- 33.Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J. In vivo comparison of the bioavailability of (+)-catechin, (−)-epicatechin and their mixture in orally administered rats. J. Nutr. 2001;131:2885–2891. doi: 10.1093/jn/131.11.2885. [DOI] [PubMed] [Google Scholar]
- 34.Harada M, Kan Y, Naoki H, Fukui Y, Kageyama N, Nakai M, Miki W, Kiso Y. Identification of the major antioxidative metabolites in biological fluids of the rat with ingested (+)-catechin and (−)-epicatechin. Biosci. Biotech. Bioch. 1999;63:973–977. doi: 10.1271/bbb.63.973. [DOI] [PubMed] [Google Scholar]
- 35.Okushio K, Suzuki M, Matsumoto N, Nanjo F, Hara Y. Identification of (−)-epicatechin metabolites and their metabolic fate in the rat. Drug Metab. Dispos. 1999;27:309–316. [PubMed] [Google Scholar]
- 36.Roura E, Andres-Lacueva C, Jauregui O, Badia E, Estruch R, Izquierdo-Pulido M, Lamuela-Raventos RM. Rapid liquid chromatography tandem mass spectrometry assay to quantify plasma (−)-epicatechin metabolites after ingestion of a standard portion of cocoa beverage in humans. J. Agric. Food Chem. 2005;53:6190–6194. doi: 10.1021/jf050377u. [DOI] [PubMed] [Google Scholar]
- 37.Tsang C, Auger C, Mullen W, Bornet A, Rouanet JM, Crozier A, Teissedre PL. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Brit. J. Nutr. 2005;94:170–181. doi: 10.1079/bjn20051480. [DOI] [PubMed] [Google Scholar]
- 38.Donovan JL, Crespy V, Oliveria M, Cooper KA, Gibson BB, Williamson G. (+)-catechin is more bioavailable than (−)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radical Res. 2006;40:1029–1034. doi: 10.1080/10715760600868545. [DOI] [PubMed] [Google Scholar]