Abstract
Resveratrol, a polyphenol found in red wine, peanuts, soy beans, and pomegranates, possesses a wide range of biological effects. Since resveratrol’s properties seem ideal for treating neurodegenerative diseases, its ability to diminish amyloid plaques was tested. Mice were fed clinically feasible dosages of resveratrol for forty-five days. Neither resveratrol nor its conjugated metabolites were detectable in brain. Nevertheless, resveratrol diminished plaque formation in a region specific manner. The largest reductions in the percent area occupied by plaques were observed in medial cortex (−48%), striatum (−89%) and hypothalamus (−90%). The changes occurred without detectable activation of SIRT-1 or alterations in APP processing. However, brain glutathione declined 21% and brain cysteine increased 54%. The increased cysteine and decreased glutathione may be linked to the diminished plaque formation. This study supports the concept that onset of neurodegenerative disease may be delayed or mitigated with use of dietary chemo-preventive agents that protect against β-amyloid induced neuronal damage.
Keywords: Oxidative stress, mitochondria, Alzheimer’s disease, Aβ peptide, Stilbenes
Introduction
Mitochondrial dysfunction and oxidative stress have been implicated in multiple neurodegenerative diseases including Alzheimer’s disease (AD) (Balaban et al., 2005; Beal, 2005; Gibson et al., 2005), and the changes can be plausibly linked to plaque and tangle formation. Studies on postmortem tissues from AD patients reveal reductions in key thiamine-dependent enzymes of the pentose shunt (transketolase), the tricarboxylic acid cycle (TCA) (i.e., the α-ketoglutarate dehydrogenase complex; KGDHC) and the link of glycolysis and the TCA cycle (i.e., the pyruvate dehydrogenase complex; PDHC) in AD (Bubber et al., 2005; Gibson et al., 2000). Brains from AD patients also contain elevated levels of lipid peroxidation products, as well as protein and DNA oxidation (Nourooz-Zadeh et al., 1999; Pratico et al., 2000; Ramassamy et al., 2000; Smith et al., 1996). In a mouse model of plaque formation, partial knockout of manganese superoxide dismutase (MnSOD) elevates protein carbonyl levels, Aβ levels and increased plaque burden (Li et al., 2004). On the other hand, reducing iNOS diminishes the levels of protein tyrosine nitration products, lowers concentration of Aβ and diminishes amyloid plaque burden (Nathan et al., 2005). Furthermore, Aβ by itself is capable of producing free radicals and ROS (Butterfield et al., 1994; Hensley et al., 1994; Huang et al., 1999). Plausible mechanisms link elevated ROS to the AD-related changes in amyloid, tau and energy metabolism (Mark et al., 1997; Pappolla et al., 1998; Pratico, 2002; Smith et al., 1991).
The extensive data that supports an important role of oxidative stress in neurodegenerative disorders concomitantly support the beneficial effect of antioxidants as adjunctive or supportive therapy. Resveratrol (trans-3, 5, 4-trihydroxystilbene), possesses a wide range of biological effects that include anti-oxidative, anti-inflammatory and anti-carcinogenic properties. Numerous reports suggest that resveratrol acts as a potent antioxidant and induces endogenous antioxidants levels (Miller and Rice-Evans, 1995; Robb et al. 2008; Brunet et al., 2004). In vitro studies suggest that resveratrol may protect against β-amyloid induced oxidative cell damage in PC12 cell lines (Conte et al., 2003; Jang and Surh, 2003; Kim et al., 2007a; Kim et al., 2007b), and may promote Aβ clearance through promotion of intracellular proteosomal activity in cell lines expressing wild type or Swedish APP695 mutations (Marambaud et al., 2005).
Several clinical studies indicate that antioxidants may delay onset of neurodegenerative diseases (Engelhart et al., 2002; Morris et al., 2002). A double-blind, placebo-controlled, randomized, multicenter trial in AD patients with moderate severity demonstrated that α-tocopherol treatment slowed the progression of the disease (Sano et al., 1997). However, the effects were modest. Recent studies show that resveratrol increases longevity and delays the onset of aging in a manner similar to that of caloric restriction (Baur et al., 2006). In a model of AD and tauopathies, introduction of resveratrol directly into the brain ventricles reduces neurodegeneration in the hippocampus, prevents learning impairment, and decreases the acetylation of the known SIRT1 substrates PGC-1α and p53 (Kim et al., 2007a). The present studies tested whether administration of resveratrol to Tg19959 mice alters plaque pathology.
Materials and Methods
Animals
Tg19959 mice were produced by pronuclear microinjection of (FVB·129S6F1) embryos with a cosmid insert containing APP695 with two familial AD mutations (KM670/671NL and V717F) under the control of the hamster PrP promoter (Chishti et al., 2001). Male Tg19959 mice were backcrossed to (C57/B6SJL) F1 female breeders. Genotypes of the offspring were determined by PCR analysis of tail DNA. Animals were housed at constant temperature (22 ± 2°C), humidity (50 ± 5 %) and illumination (12 h light/dark cycles) with food and water provided ad libitum. The Institutional Animal Care and Use Committee of Weill Medical College of Cornell University approved all procedures with the animals.
Treatment Paradigm
A total of 19 Tg19959 mice at 45 days old were used. The control group included four males and five females, while the resveratrol group consisted of five males and five females. These animals were matched for age, sex and weight. The control group received a standard AIN-93G diet (Harlan Teklad, Madison, WI) ad libitum, and the resveratrol group received standard AIN-93G plus 0.2% resveratrol (Harlan Teklad, Madison, WI) ad libitum for 45 days. The calculated daily dosage in mice was 300 mg/kg (3 gm food per day for a 20 gm mouse). The human equivalent using a scaling factor of 0.08 (http://www.fda.gov/cber/gdlns/dose.htm) is 24 mg/kg or about 1.68 gms per day for a 70 kg individual. Resveratrol (> 98%) was purchased from Sigma Chemical Company (St Louis, MO) and mixed to homogeneity during manufacturing of the diets and stored at −80°C. Food was replaced fresh in all cages every four days. Stability of resveratrol in diet over time in cages and at room temperature was assessed using HPLC with electrochemical detection.
Tissue preparation
Mice were sacrificed by a lethal intraperitoneal dose of pentobarbitone sodium (200 mg/kg; i.p.; Abbott Laboratories, North Chicago, IL) and perfused with 100 ml of normal saline using a pump (Masterflex, Model 7518-00, Cole-Parmer Instrument Company, Barrington, IL) at 5ml/min. The brains were removed and bisected in the mid-sagittal plane. One-half was post-fixed in 4% paraformaldehyde in PBS for a minimum of 48 h and the other half was snap frozen in liquid nitrogen and stored at −80°C until analysis.
Analysis of resveratrol in diet and brain
The food was homogenized with 5% metaphosphoric acid (MPA) and methanol. The homogenate was transferred to 1.5 ml Eppendorf tube and centrifuged at 13,200 rpm for 2 min. The supernatant fraction was evaporated to dryness using a vacuum rotor vap and the residue was dissolved in 50% acetonitrile/water solution and centrifuged at 13,200 rpm for one min. After centrifugation, supernatant fractions were saved for HPLC determinations of resveratrol using electrochemical detection.
To analyze the resveratrol derivatives in the brain, about 40 mg of brain powder was incubated with 10μl of 10μg/ml estriol-3-(β-glucuronidase), 0.1 ml of 0.1 M ascorbic acid, 0.25 ml of 1.0 M ammonium acetate buffer, pH 5.0 and 1,000 units of β-glucuronidase from Helix pomatia. Glacial acetic acid (0.1 ml) was added and hydrolysates were washed with 5.0 ml of hexane and extracted with ethyl acetate and evaporated to dryness. The residue was dissolved in 50% methanol and centrifuged at 14,000 rpm for 10 min.
Concentrations of resveratrol and its derivatives were measured using a Perkin-Elmer Liquid Chromatograph equipped with an 8-channel coulometric array (CoulArray) detector (ESA, Inc., Chelmsford, MA). The supernatant fractions were injected onto a MD-150 column (3.0 × 150 mm; 3μm particle size; ESA, Inc., Chelmsford, MA) and eluted at ambient temperature with a mobile phase consisting of 26% acetonitrile (v/v) containing 75 mM citric acid and 25 mM ammonium acetate (pH 2.64) at a flow rate of 0.6 ml/min. A Rheodyne injection valve with a 5 μl sample loop was used to manually introduce samples. The 8-channel CoulArray detectors were set at 100, 200, 320, 380, 440, 500, 560 and 620 mV, respectively. To assure standardization between analyses, calibration standards were interspersed at intervals among sample runs. Peak areas were analyzed using ESA, Inc. software (Juan et al., 1999; Sale et al., 2005).
Analysis of Cysteine, Ascorbate, and GSH
For determination of selected redox active compounds, brain powders were homogenized in 10 volumes of deionized water (w/v) at 4 °C using a Potter-Elvehjem homogenizer fitted with a Teflon pestle attached to a motor. To precipitate proteins, a 100 μl aliquot of each homogenate was added to 400 μl of ice-cold 6.25% (w/v) MPA, and the sample was incubated for 10 min on ice. After centrifugation at 14,000 rpm for 5 min at 4 °C, aliquots of the 5% MPA supernatant fractions were saved for HPLC determination of redox active analytes using electrochemical detection. Concentrations of the redox-active ascorbate and the aminothiols, cysteine and GSH, were measured without prior derivatization using a Perkin-Elmer Liquid Chromatograph equipped with an 8-channel coulometric array (CoulArray) detector (ESA, Inc., Chelmsford, MA) (Lakritz et al., 1997; Pinto et al., 2005). The supernatant fractions from the 5% MPA homogenates were injected directly onto a Bio-Sil ODS-5S, 5 μm particle size, 4 × 250 mm C18 column (Bio-Rad) and eluted at ambient temperature with a mobile phase consisting of 50 mM NaH2PO4, 0.05 mM 1-octanesulfonic acid, 1% acetonitrile (v/v), and 0.5% N, N-dimethylformamide (v/v) (pH 2.70) at a flow rate of 1 ml/min. PEEK™ (polyetheretherketone) tubing was used throughout the HPLC system, and a 0.2 μm PEEK™ filters were placed pre- and post-column to protect both column and flow cells, respectively, from any particulate matter. A rheodyne injection valve with a 5 μl sample loop was used to manually introduce samples. The 8-channel CoulArray detectors were set at 250, 400, 450, 500, 550, 600, 650, and 700 mV, respectively. To assure standardization between analyses, calibration standards were interspersed at intervals among sample runs. Peak areas were analyzed using ESA, Inc. software. Concentrations of each redox active compound (cysteine, ascorbic acid, and GSH) were obtained from appropriate standard curves. Protein precipitates from 5% MPA preparations were dissolved in 500 ml of 0.1 N NaOH, and protein was quantitated by a spectrophotometric method using bicinchoninic acid reagent (Pierce Chemical Company, Rockford, IL).
Thioflavine S staining and quantitation of amyloid plaques
To demonstrate the fibrillar Aβ deposition thioflavine S staining was used. Briefly, sections were washed with distilled water for 5 min and stained in freshly prepared and filtered aqueous 0.5% thioflavine S solution for 5 min. These sections were treated with 70% ethanol, hydrated for 5 min and mounted with cytoseal. Sections were visualized and images were captured in Nikon Eclipse 80i microscope with a digital camera attached to a computer (Nikon, Melville, NY) and saved as Tagged image format files. Computer-aided quantification of Aβ deposits was performed using the MetaMorph 6.1 software (Universal Imaging Corp, Downington, PA). Three sections per mouse from lateral −0.94, −3.25, −3.72 were analyzed. Plaque counts and percentage occupied by the thioflavine S positive plaques were quantified in the cortex, hippocampus, neostriatum, hypothalamus, thalamus and cerebellum. The region of interest was drawn manually under 4 × magnification and threshold was kept constant throughout the analysis. The analysis was performed in a blinded fashion.
Immunohistochemistry
Serial sagittal sections (40 μm) thick were cut with a sliding microtome (Microm Lab GmbH, Walldorf, Germany) and processed as free floating sections. The staining protocol employed a modified ABC immunohistochemistry procedure (Karuppagounder et al., 2007; Robb et al.). Briefly, sections were washed with 0.1 M potassium phosphate buffered saline (PBS, pH 7.4) and incubated in 1% H2O2 for 30 min to quench the endogenous peroxidase. Then sections were treated with 0.1% Triton X-100 (Sigma Chemical Co., St. Louis, MO) for 15 min and blocked with 2% bovine serum albumin (BSA) in PBS for one hr. Sections were incubated with mouse monoclonal 6E10 against 1–16 residues of human Aβ, 1:1000 (Signet laboratories, Dedham, MA) in PBS containing 1% BSA (Sigma Chemical Co., St. Louis, MO), overnight at room temperature. After rinsing in PBS, sections were incubated with biotinylated anti-mouse 1:200 in PBS containing 0.25% BSA (Vector Laboratories Inc., Burlingame, CA) for one hr. Sections were then incubated in avidin-biotin-peroxidase complex for one hour 1:100 in PBS (Vector Laboratories Inc., Burlingame, CA;), rinsed in PBS and developed in 0.05% 3,3′-diaminobenzidine (DAB) (Vector Laboratories Inc., Burlingame, CA) and 0.003% H2O2 in PBS.
Immunoblot analysis
Hemi-brain powders were homogenized in cold lysate buffer (50 mM Tris-HCl pH 7.2, 0.4 % Triton X-100, 1mM DTT, 0.2 mM EGTA, 50 μM Leupeptin). Protein concentrations of the lysates were determined by a bicinchoninic acid (BCA) assay (Pierce Chemical Company, Rockford, IL). Forty μg of protein were electrophoresed through 10–20% Tris-Tricine gels, transferred onto nitrocellulose membranes (Pierce Chemical Company, Rockford, IL). Membrane blots were blocked with 50% Odyssey blocking buffer, 50% Tris-buffered saline (TBS) overnight at 4°C. After blocking, the membranes were incubated with primary antibodies, APP C-terminal specific polyclonal antibody G369 to epitopes 645–694 of APP695 (1:1000; Dr Sam Gandy), SIRT1 (1:1000; Santa Cruz Biotechnology, CA) or β-actin (1:10,000) (Sigma Chemical Company, St. Louis, MO) for 120 min at room temperature. Subsequently, blots were washed with TBS plus 0.1% Trition X-100, and incubated at room temperature for one hour with secondary Odyssey Goat anti-Rabbit IR Dye 680 antibody and Odyssey goat anti-Mouse IRDye 800 antibody (1:10,000; LICOR Biosciences, Lincoln, NB) and analyzed using the Odyssey infrared imaging program (LI-COR Biotechnology, Lincoln, NB).
Statistical Analysis
All values are reported as means ± SEM. P < 0.05 was considered significant. Statistical significance of group differences was tested by two-tailed Students t test or one way ANOVA analysis. SPSS (SPSS Co., Chicago, IL) software was used to perform statistical analysis.
Results
Resveratrol was stable in the diet and not detectable in brain
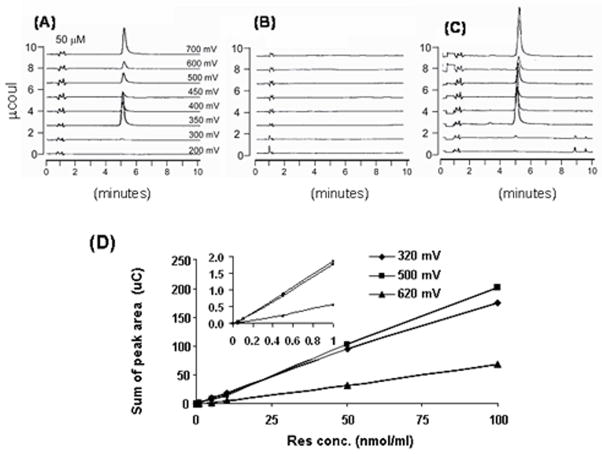
A method to extract resveratrol from diet or brain was developed. Linearity, characteristics plot, standard curve, and lower limits of detection were analyzed. The retention time of resveratrol was 5.3 min (Fig. 1A). At this time, the resveratrol standard curve was linear over a wide range (Fig. 1D). The method is very sensitive. The lowest point on the standard curves was 0.5 pmole/ml. Figure 1B represents no detectable levels of resveratrol in the control diet and Fig. 1C demonstrates the level of reseveratrol extracted from diet containing 0.2% resveratrol. The extraction efficiency from diet was determined by adding known quantities of resveratrol to both the control and resveratrol containing diets. The extraction efficiency was 67 ± 3.6%. HPLC chromatograms show Fig. 1A, a resveratrol standard (50 μM), the control diet (Fig. 1B) and the resveratrol diet (Fig. 1C).
Fig. 1. Standardization of the resveratrol assay to test the presence in brain and stability in diet.
Levels of resveratrol were determined by HPLC using electrochemical detection. Representative HPLC chromatograms from resveratrol standard show that the retention time of resveratrol peak was 5.3 min (A). Fig. B represents no detectable levels of resveratrol in the control diet and Fig. C demonstrates the level of reseveratrol extracted from diet containing 0.2% resveratrol. The standard curves for resveratrol measurements reveal a large linear range and a limit of detection of 0.5 picomole/mL (D). The inset is an expansion of the lower range of the curve.
Before delivering resveratrol in this manner, its stability in food was determined. Although several studies have reported on resveratrol stability in various diets, the results were inconclusive. A recent report suggested that resveratrol isolated from rhizoma polygoni cuspidati was unstable when exposed to light, heat and atmospheric oxygen (Chen et al., 2007). To test the stability of resveratrol in diet, the levels of resveratrol were determined by HPLC on diets that were kept for variable times at room temperature. The resveratrol content did not change significantly between the days analyzed. The percent resveratrol content was 0.2, 0.194 and 0.216 at 0, 4 and 7 days, respectively. In agreement with other studies (Bertelli et al., 1998; Prokop et al., 2006), resveratrol in the experimental diet was stable for a much longer duration than required to complete our studies.
The levels of free resveratrol or resveratrol conjugate in brain were analyzed in 90 day old Tg19959 mice that were fed diet containing 0.2% resveratrol for 45 days. The 0.2% resveratrol was well tolerated without any adverse effect on body weight gain or food intake. These results are consistent with those in rabbits fed a hypercholesterolemic diet supplemented with resveratrol (Wilson et al., 1996). Resveratrol is absorbed in the small intestine and undergoes glucuronidation before entering the blood (Kuhnle et al., 2000). The presence of glucuronide/sulfated conjugates of resveratrol was studied by pre-incubation of tissue samples with extracts from Helix Pomatia which contains sulfatase and β-glucuronidase. Interestingly, the brain levels of resveratrol or and its conjugated derivatives were below the detection limits (<0.5 pmol/ml/mg tissue).
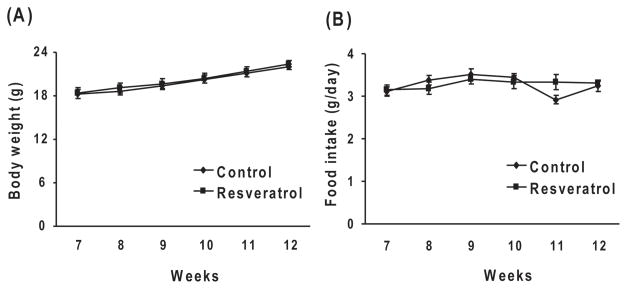
Resveratrol did not alter body weight or food intake in Tg19959 mice
The food intake of control diet or resveratrol fed animals in addition to body weights were monitored from 7 to 12 weeks. Before treatment (day 45), the control or resveratrol group mice weighed 18.2 ± 0.6 or 18.3 ± 0.7 g, respectively, and after treatment (90 days) animals weighed 22.0 ± 0.3 or 22.4 ± 0.43 g, respectively. Body weight did not vary between the control and resveratrol groups (Fig. 2A). The food intake in control and resveratrol fed groups were 3.11 ± 0.11 g and 3.15 ± 0.12 g, respectively at 45 days and 3.23 ± 0.13 g and 3.31 ± 0.06 g, respectively at 90 days. Food intake did not vary between groups (Fig. 2B).
Fig. 2. Resveratrol, body weight and food intake.
Forty five days old Tg19959 mice were fed AIN 93G diet with or without 0.2% resveratrol. The body weight (A) and the food intake (B) were determined in mice from 7 to 12weeks of age. Data represent means ± SEM of control (n=8) and 0.2% resveratrol (n=9) groups from 2–3 independent determinations.
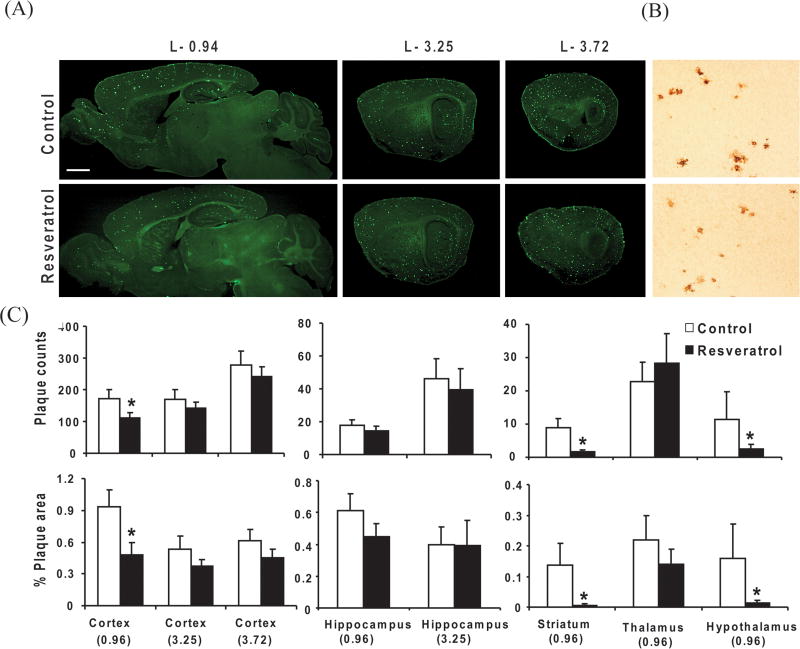
Resveratrol treatment reduced plaque pathology in Tg19959 mice
To test the effect of resveratrol on amyloid plaque pathology, 45 day old Tg19959 mice were fed a control AIN 93G diet with or without 0.2% resveratrol for 45 days. Resveratrol markedly reduced thioflavine S positive compact plaques and 6E10 positive diffuse plaques compared to mice on control diet (Fig. 3A,B). Quantification of thioflavine S positive plaques revealed significant reduction in plaque counts and plaque burden in medial cortex (P<0.05), striatum (P<0.05), hypothalamus (P<0.05) of resveratrol-fed mice compared to mice fed the control diet (Fig. 3C). Although, plaque counts and plaque burden declined in hippocampus, the change was statistically insignificant (Fig. 3C).
Fig. 3. Resveratrol and plaque pathology.
Forty five days old Tg19959 mice were fed AIN 93G diet with or without 0.2% resveratrol. Animals were sacrificed at 90 days old, and the brains were processed for thioflavine S staining. (A) Representative saggital brain sections from lateral levels − 0.94, − 3.25, − 3.72, respectively, showed that resveratrol decreased thioflavin S immunoreactivity (Scale bar, 500 μm). (B) Resveratrol also increased decreased 6E10 immunoreactivity stained with 6E10 antibody directed against 1–17 amino acids of Aβ in representative cortex sections (Scale bar, 200 μm). (C) Graphs shows the plaque counts and % area occupied by plaques quantified from the cortex, hippocampus, striatum, thalamus and hypothalamus region. Data represent means ± SEM of control (n=8) and 0.2% resveratrol (n=9) groups from 2–3 independent experiments. * Denotes control differs from resveratrol group (p<0.05).
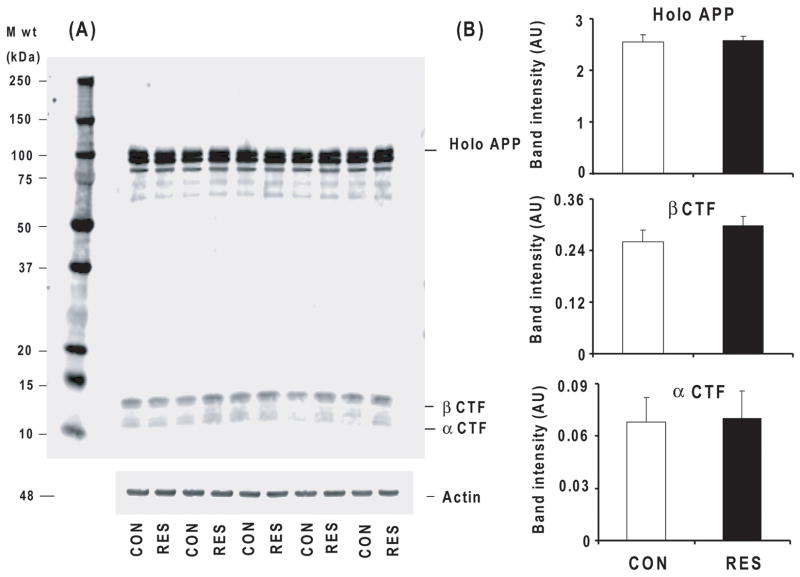
Resveratrol treatment did not alter amyloid precursor protein (APP) carboxyterminal fragments in Tg19959 mice
To investigate the mechanism responsible for the resveratrol-induced reduction in plaque counts, the APP cleavage products full length APP, β CTF (C99) and α CTF (C83) levels were determined by Western blot using APP G369 antibody (against the APP cytoplasmic tail). Resveratrol did not alter the levels of high molecular weight APP species (holo APP), β CTF (C99) or α CTF (C83) levels (Fig. 4A, B).
Fig. 4. Resveratrol and APP CTF levels.
Forty-five day old Tg19959 mice were fed AIN 93G diet with or without 0.2% resveratrol. Animals were sacrificed at 90 days. The whole hemi-brain extracts from control and resveratrol fed mice were analyzed by Western blot using G369 antibody. (A) Representative Western blots probed with G369 antibody, an antibody specific against both APP holo protein and C-terminal fragments for control and resveratrol groups are shown. (B) The densitometric analysis of APP holo protein and C-terminal fragments band intensity do not reveal any differences. Data are means ± SEM of control (n=5) and resveratrol (n=5) groups.
Resveratrol treatment increased the cysteine levels, but reduced the GSH levels in Tg19959 mice
To test the effect of resveratrol on redox status, cysteine, ascorbate and GSH levels were determined in control mice and in mice fed the resveratrol diet. Resveratrol feeding significantly increased the cysteine levels by 54% (Fig. 5A; P<0.05) compared to control. By contrast, resveratrol diminished GSH levels by 21% (Fig. 5B; P<0.05), and did not alter ascorbate levels (Fig. 5C).
Fig. 5. Resveratrol and brain redox status.
Forty-five day old Tg19959 mice were fed AIN 93G diet with or without 0.2% resveratrol for 45 days and sacrificed at 90 day. Values are expressed as nmol/mg protein. Data represent means ± SEM of control (n=8) and 0.2% Resveratrol (n=9) groups from 2–3 independent experiments. * Denotes control and resveratrol groups vary (p<0.05).
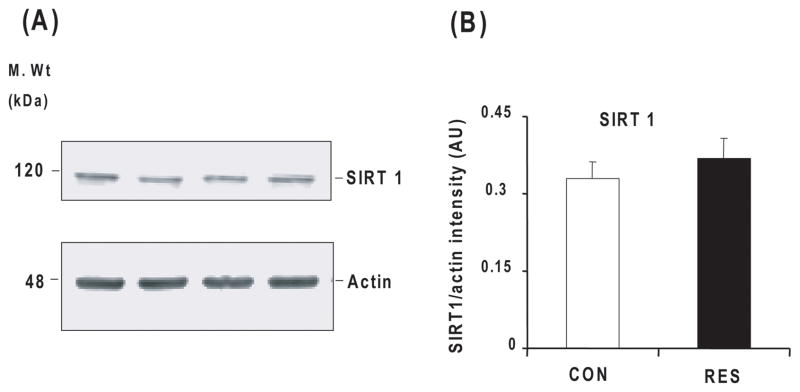
Resveratrol treatment did not alter SIRT 1 levels in Tg19959 mice
Since previous studies demonstrated that resveratrol activates the NAD-dependent protein deactylase SIRT1, a discovery that provided a molecular link to neurite stability and neuronal protection, we tested whether SIRT1 expression was upregulated in Tg19959 mice fed resveratrol. As demonstrated in Fig 6A and 6B, resveratrol treatment did not increase SIRT1 expression.
Fig. 6. Resveratrol and SIRT1 levels.
Forty-five day old Tg19959 mice were fed AIN 93G diet with or without 0.2% resveratrol and sacrificed at 90 days. Whole hemi-brain extracts were analyzed by Western blot using SIRT1 antibody (A) and the band was quantified by densitometry (B) Values represent mean ± SEM of control (n=5) and resveratrol (n=5) groups.
Discussion
Accumulating evidences from experimental and human studies suggest that oxidative stress and mitochondrial dysfunction are important causative factors in the development and progression of several neurodegenerative diseases including AD (Gibson et al., 2000; Ojaimi et al., 1999; Balaban et al., 2005. Induction of mild impairment of oxidative metabolism, oxidative stress and inflammation induced by thiamine deficiency alters BACE1 levels and metabolism of APP and/or Aβ and promotes accumulation of plaques in Tg 19959 mice (Karuppagounder et al., 2008). If oxidative stress is critical in the alteration of APP processing and the pathogenesis of AD, treatment with the appropriate antioxidants should be beneficial and diminish plaque formation.
The dosage of resveratrol that was used in the current studies (0.2% in diet) is a practical amount and was administered in a clinically relevant manner. Dietary resveratrol at the level administered in these studies had a striking effect on brain even though resveratrol was not measurable. Although resveratrol and its derivatives have been detected following gastric gavage, or intraperitoneal administration (Wang et al., 2002), they have not been detected following dietary administration (current results) (Asensi et al., 2002; Sale et al., 2005). This is true even though the daily dosage was higher in our experiments (300 mg/kg) than bolus injections in other experiments. This same dosage protected the vasculature of mice fed a high calorie diet (Baur et al., 2006). Thus, the data suggests that the striking biological effects of resveratrol in the diet result from trace levels entering the brain, an effect on brain vasculature or a peripheral change that affects brain.
The data demonstrate that resveratrol reduced plaque counts and plaque burden most effectively in medial cortex, striatum and hypothalamus. The causative factors or thresholds may differ in various brain regions. For example, thiamine deficiency (i.e., mild impairment of oxidative metabolism) exacerbates plaque formation in regions that will eventually get plaques and induces plaque formation in brain regions that do not normally contain plaques (Karuppagounder et al., 2008). The current results demonstrate that resveratrol can reduce plaques, but only in select regions. Thus, a more effective paradigm is required to reduce plaque burden throughout the brain. The relation of these restricted changes in plaque formation to learning and memory is critical. However, additional studies are required to optimize the response of the resveratrol or its derivatives before examining the interaction of learning and memory in multiple mouse models of plaque formation.
The exact mechanism(s) underlying reduction of plaque pathology by resveratrol is (are) unknown. The reduction in plaque pathology did not appear to be due to altered APP processing towards the non-amyloidgenic pathway. Resveratrol did not alter levels of α CTF or β CTF (C99) or high molecular weight APP species (holo APP). In vitro, resveratrol promotes Aβ clearance by increasing the intracellular proteosomal activity. To demonstrate the role of proteasomes in vitro, Marambaud et al., used a variety of selective proteasomal inhibitors and siRNA. These approaches are far more equivocal for in vivo studies. The effective concentrations about 40 μM (Marambaud et al., 2005) exceed those in brain in the current study (<0.5 nM) by orders of magnitude. Resveratrol has been postulated to have its beneficial effects on life span, neurodegeneration and prevents impaired memory by activation of SIRT1 (Kim et al., 2007a; Lagouge et al., 2006). However, we did not see activation of SIRT-1. Other reports suggest that resveratrol has other SIRT 1 independent actions (Dasgupta and Milbrandt, 2007).
In the current study, the surprising resveratrol-induced decline in glutathione and increase in cysteine is consistent with a modest oxidant effect in brain. The decline in GSH could reflect a decreased synthesis, increased degradation or altered redox status. GSH consists of glycine, cysteine, and glutamate. Thus, GSH serves as a storage form of cysteine. Addition of cysteinyl analogues to cells (e.g., N-acetylcysteine, 2-oxo-4-thiazolidine carboxylic acid) would normally increase glutathione. Thus, the decline in glutathione with increased cysteine suggests that brain glutathione is providing additional cysteine residues. Since the endogenous concentration of glutathione is much higher than that of cysteine in brain, a small decline in glutathione can lead to a large increase in cysteine. Further, an age-related decrease in plasma cysteine suggests that high cysteine may be important in diminishing plaque formation (Chen et al., 1989). Increasing intracellular cysteine protects against multiple oxidant injuries. In vitro and in vivo experiments have used N-acetylcysteine (NAC) to increase cellular cysteine. NAC protects SHSY-5Y neuroblastoma cells from oxidative stress and cell toxicity due to H2O2, UV light and amyloid-β1–42. Also, NAC promotes release of amyloid-β1–42 from cells and diminishes oxidant induced tau phosphorylation (Olivieri et al., 2001). NAC down regulates transcription of the amyloid precursor protein in human neuroblastoma cells (Studer et al., 2001). Thus, the resveratrol induced increase in cysteine may be protective and prevent plaque formation in the same way as NAC.
An alternative tantalizing speculation is that the resveratrol-induced reduction in plaques may be through cysteine or resveratrol chelation of copper or zinc. Cysteine chelates copper (Baker and Czarnecki-Maulden, 1987; Cakir et al., 2001; Hallman et al., 1971; Li and Manning, 1955) and zinc (Baker and Czarnecki-Maulden, 1987; Cakir et al., 2001; Chen and Liao, 2003; Hallman et al., 1971; Li and Manning, 1955). The chelation appears pharmacologically important. Resveratrol inhibits human low density lipoproteins (LDL) oxidation by chelating copper (Frankel et al., 1993; Belguendouz et al., 1997; Fremont et al., 1999). Cu and Zn are enriched in amyloid β deposits in AD, which are solubilized by Cu/Zn-selective chelators in vitro. A bioavailable Cu/Zn chelator decreases brain amyloid-β deposition by 49% (Cherny et al., 2001). Thus, the resveratrol induced increase in cysteine may help to reduce plaques by chelation of copper in a manner shown for studies of Cu/Zn chelators.
In conclusion, our results demonstrate that resveratrol treatment for 45 days reduced the plaque pathology in a region specific manner, decreased brain glutathione and increased its precursor product cysteine. Further studies need to be conducted to elucidate the precise mechanism(s) by which resveratrol reduces Aβ pathology and alters brain glutathione status. This study supports an important concept that onset of neurodegenerative disease may be delayed or mitigated with use of dietary chemo-preventive agents that protect against β-amyloid plaque formation and oxidative stress.
Acknowledgments
These studies were supported by Alzheimer’s Drug Discovery Foundation & Institute for the Study of Aging and NIH grants AG14600, and P01 AG14930.
References
- Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radical Biology & Medicine. 2002;33:387–398. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- Baker DH, Czarnecki-Maulden GL. Pharmacologic role of cysteine in ameliorating or exacerbating mineral toxicities. Journal of Nutrition. 1987;117:1003–1110. doi: 10.1093/jn/117.6.1003. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Annals of Neurology. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Belguendouz L, Fremont L, Linard A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochemical Pharmacology. 1997;53:1347–1355. doi: 10.1016/s0006-2952(96)00820-9. [DOI] [PubMed] [Google Scholar]
- Bertelli AA, Gozzini A, Stradi R, Stella S, Bertelli A. Stability of resveratrol over time and in the various stages of grape transformation. Drugs under Experimental and Clinical Research. 1998;24:207–211. [PubMed] [Google Scholar]
- Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Annals of Neurology. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Hensley K, Harris M, Mattson M, Carney J. Beta-Amyloid peptide free radical fragments initiate synaptosomal lipoperoxidation in a sequence-specific fashion: implications to Alzheimer’s disease. Biochemical and Biophysical Research Communications. 1994;200:710–715. doi: 10.1006/bbrc.1994.1508. [DOI] [PubMed] [Google Scholar]
- Cakir S, Bicer E, Eleman A. Synthesis, spectroscopic and voltammetric studies of mixed-ligand copper(II) complexes of amino acids. Transition Metal Chemistry. 2001;26:89–95. [Google Scholar]
- Chen CJ, Liao SL. Zinc toxicity on neonatal cortical neurons: involvement of glutathione chelation. Journal of Neurochemistry. 2003;85:443–453. doi: 10.1046/j.1471-4159.2003.01691.x. [DOI] [PubMed] [Google Scholar]
- Chen TS, Richie JP, Jr, Lang CA. The effect of aging on glutathione and cysteine levels in different regions of the mouse brain. Proceedings of the Society for Experimental Biology and Medicine. 1989;190:399–402. doi: 10.3181/00379727-190-42879. [DOI] [PubMed] [Google Scholar]
- Chen YB, Sun BX, Chen JX. Study on the stability of resveratrol in rhizoma polygoni cuspidati. Zhong Yao Cai. 2007;30:805–807. [PubMed] [Google Scholar]
- Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. Journal of Biological Chemistry. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Conte A, Pellegrini S, Tagliazucchi D. Synergistic protection of PC12 cells from beta-amyloid toxicity by resveratrol and catechin. Brain Research Bulletin. 2003;62:29–38. doi: 10.1016/j.brainresbull.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. Journal of the American Medical Association. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- Fremont L, Belguendouz L, Delpal S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Science. 1999;64:2511–2521. doi: 10.1016/s0024-3205(99)00209-x. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Haroutunian V, Zhang H, Park LC, Shi Q, Lesser M, Mohs RC, Sheu RK, Blass JP. Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Annals of Neurology. 2000;48:297–303. [PubMed] [Google Scholar]
- Gibson GE, Blass JP, Beal MF, Bunik V. The alpha-ketoglutarate-dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration. Molecular Neurobiology. 2005;31:43–63. doi: 10.1385/MN:31:1-3:043. [DOI] [PubMed] [Google Scholar]
- Hallman PS, Perrin DD, Watt AE. The computed distribution of copper(II) and zinc(II) ions among seventeen amino acids present in human blood plasma. Biochemical Journal. 1971;121:549–555. doi: 10.1042/bj1210549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Cuajungco MP, Atwood CS, Hartshorn MA, Tyndall JD, Hanson GR, Stokes KC, Leopold M, Multhaup G, Goldstein LE, Scarpa RC, Saunders AJ, Lim J, Moir RD, Glabe C, Bowden EF, Masters CL, Fairlie DP, Tanzi RE, Bush AI. Cu(II) potentiation of alzheimer abeta neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. Journal of Biological Chemistry. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radical Biology & Medicine. 2003;34:1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Juan ME, Lamuela-Raventos RM, de la Torre-Boronat MC, Planas JM. Determination of trans-resveratrol in plasma by HPLC. Analytical Chemistry. 1999;71:747–750. doi: 10.1021/ac9808831. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Shi Q, Xu H, Gibson GE. Changes in inflammatory processes associated with selective vulnerability following mild impairment of oxidative metabolism. Neurobiology of Disease. 2007;26:353–362. doi: 10.1016/j.nbd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder SS, Xu H, Shi Q, Chen LH, Pedrini S, Pechman DP, Baker HA, Beal MF, Gandy SE, Gibson GE. Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer’s mouse model. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. The EMBO Journal. 2007a;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee KW, Lee HJ. Protective effects of piceatannol against beta-amyloid-induced neuronal cell death. Annals of the New York Academy of Sciences. 2007b;1095:473–482. doi: 10.1196/annals.1397.051. [DOI] [PubMed] [Google Scholar]
- Kuhnle G, Spencer JP, Chowrimootoo G, Schroeter H, Debnam ES, Srai SK, Rice-Evans C, Hahn U. Resveratrol is absorbed in the small intestine as resveratrol glucuronide. Biochemical and Biophysical Research Communications. 2000;272:212–217. doi: 10.1006/bbrc.2000.2750. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lakritz J, Plopper CG, Buckpitt AR. Validated high-performance liquid chromatography-electrochemical method for determination of glutathione and glutathione disulfide in small tissue samples. Analytical Biochemistry. 1997;247:63–68. doi: 10.1006/abio.1997.2032. [DOI] [PubMed] [Google Scholar]
- Li NC, Manning RA. Some metal complexes of sulfur containing amino acids. Journal of American Chemical Society. 1955;77:5225–5228. [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. Journal of Neurochemistry. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Cao Z, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. Journal of Biological Chemistry. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. Journal of Neurochemsitry. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Rice-Evans CA. Antioxidant activity of resveratrol in red wine. Clinical Chemistry. 1995;41:1789. [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. Journal of the American Medical Association. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. Journal of Experimental Medicine. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourooz-Zadeh J, Liu EH, Yhlen B, Anggard EE, Halliwell B. F4-isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer’s disease. Journal of Neurochemsitry. 1999;72:734–740. doi: 10.1046/j.1471-4159.1999.0720734.x. [DOI] [PubMed] [Google Scholar]
- Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrne E. Mitochondrial respiratory chain activity in the human brain as a function of age. Mechanisms of Ageing and Development. 1999;111:39–47. doi: 10.1016/s0047-6374(99)00071-8. [DOI] [PubMed] [Google Scholar]
- Olivieri G, Baysang G, Meier F, Muller-Spahn F, Stahelin HB, Brockhaus M, Brack C. N-acetyl-L-cysteine protects SHSY5Y neuroblastoma cells from oxidative stress and cell cytotoxicity: effects on beta-amyloid secretion and tau phosphorylation. Journal of Neurochemsitry. 2001;76:224–233. doi: 10.1046/j.1471-4159.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- Pappolla MA, Chyan YJ, Omar RA, Hsiao K, Perry G, Smith MA, Bozner P. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer’s disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. American Journal of Pathology. 1998;152:871–877. [PMC free article] [PubMed] [Google Scholar]
- Pinto JT, Van Raamsdonk JM, Leavitt BR, Hayden MR, Jeitner TM, Thaler HT, Krasnikov BF, Cooper AJ. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: implications for the treatment of Huntington disease. Journal of Neurochemistry. 2005;94:1087–1101. doi: 10.1111/j.1471-4159.2005.03255.x. [DOI] [PubMed] [Google Scholar]
- Pratico D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, FitzGerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Annals of Neurology. 2000;48:809–812. [PubMed] [Google Scholar]
- Pratico D. Alzheimer’s disease and oxygen radicals: new insights. Biochemical Pharmacology. 2002;63:563–567. doi: 10.1016/s0006-2952(01)00919-4. [DOI] [PubMed] [Google Scholar]
- Prokop J, Abrman P, Seligson AL, Sovak M. Resveratrol and its glycon piceid are stable polyphenols. Journal of Medicinal Food. 2006;9:11–14. doi: 10.1089/jmf.2006.9.11. [DOI] [PubMed] [Google Scholar]
- Ramassamy C, Averill D, Beffert U, Theroux L, Lussier-Cacan S, Cohn JS, Christen Y, Schoofs A, Davignon J, Poirier J. Oxidative insults are associated with apolipoprotein E genotype in Alzheimer’s disease brain. Neurobiology of Disease. 2000;7:23–37. doi: 10.1006/nbdi.1999.0273. [DOI] [PubMed] [Google Scholar]
- Robb EL, Page MM, Wiens BE, Stuart JA. Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD. Biochemical and Biophysical Research Communications. 2008;367:406–412. doi: 10.1016/j.bbrc.2007.12.138. [DOI] [PubMed] [Google Scholar]
- Sale S, Tunstall RG, Ruparelia KC, Potter GA, Steward WP, Gescher AJ. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. International Journal of Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. The New England Journal of Medicine. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer’s. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Studer R, Baysang G, Brack C. N-Acetyl-L-Cystein downregulates beta-amyloid precursor protein gene transcription in human neuroblastoma cells. Biogerontology. 2001;2:55–60. doi: 10.1023/a:1010065103073. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Research. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- Wilson T, Knight TJ, Beitz DC, Lewis DS, Engen RL. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sciences. 1996;59:15–21. doi: 10.1016/0024-3205(96)00260-3. [DOI] [PubMed] [Google Scholar]
- Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB. Human, rat, and mouse metabolism of resveratrol. Pharmaceutical Research. 2002;19:1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]