Abstract
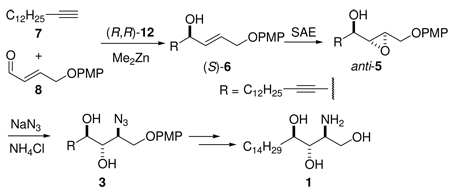
An asymmetric synthesis of d-ribo-phytosphingosine (1) was achieved by utilizing the ProPhenol-catalyzed alkynylation of aldehyde 8 to afford allylic propargylic alcohol (S)-6 followed by asymmetric epoxidation and opening of propargylic epoxy alcohol anti-5 with NaN3/NH4Cl. Deprotection and reduction of the resulting acyclic azide 3 then gave 1. Alkyne-azide 3 was subjected to an intramolecular click reaction, generating a bicyclic triazole, which was found to have unexpected vicinal coupling constants. Application of the advanced Mosher method verified the configurations of the three contiguous stereogenic centers of 1. An alkynyl-azide analogue of 1, which may be useful as a glycosyl acceptor in the synthesis of α-galactosylceramide derivatives, was also readily prepared by this route.
Introduction
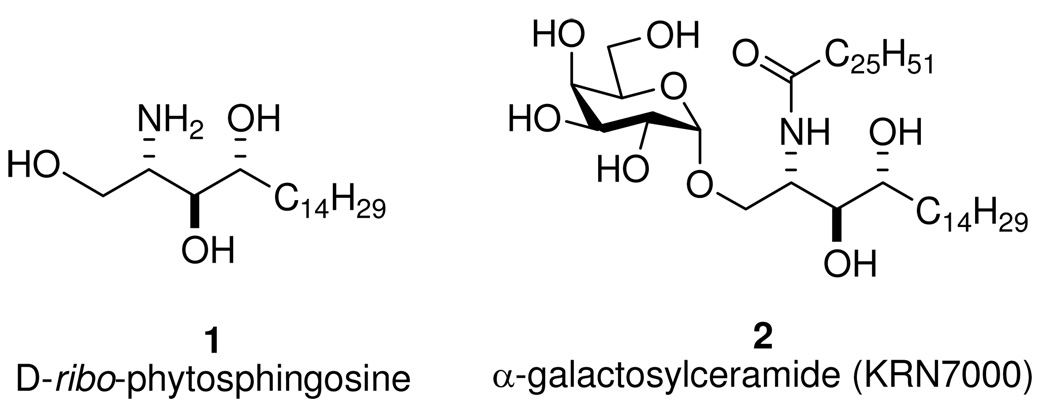
2S,3S,4R-(d-ribo)-Phytosphingosine (4d-hydroxysphinganine, PHS, 1) is distributed ubiquitously, including in membranes of fungi, plants, bacteria, marine organisms, and mammalian tissues.1 In addition to its structural role in membranes, 1 regulates cellular growth2 and mediates the heat stress response of yeast.3 Moreover, 1 serves as a metabolic precursor of important lipid mediators such as PHS 1-phosphate,3b,4 inositol phosphorylceramide,5 and KRN7000 (2, the α-anomer of galactosylceramide, an immunostimulant of invariant natural killer T (iNKT) cells).6
Because of the biological importance of PHS, there has been considerable interest in the synthesis of 1 and its stereoisomers.7 The construction of the three contiguous stereogenic centers poses an interesting and demanding challenge. Historically, natural chiral pools have played a significant role in their syntheses, but asymmetric reactions have emerged as a more favorable strategy for reasons of chirality economy and efficiency. Recently, the catalytic asymmetric alkynylation reaction developed by Trost et al. has been used as a key step in the syntheses of natural products.8 As an extension of our previous studies on the synthesis of phytosphingolipids7c,7e,9 and of glycolipid-based iNKT cell agonists,10 we describe here a stereocontrolled synthesis of 1 via a sequence of catalytic alkynylation and Sharpless asymmetric epoxidation (SAE)11 reactions to generate the intermediate chiral propargylic epoxy alcohol 5 which was converted to 3 by ring-opening attack by azide ion. Although the ring opening was expected to result in an inversion of configuration at C-2, the stereochemical outcome required verification because of a previous report of unexpected retention of configuration during a Ti(O-i-Pr)2(N3)2-mediated epoxide ring-opening reaction.12 To determine the relative configurations in azido diol 3, we used an intramolecular click reaction13 to form rigid bicyclic triazole 15, which, however, proved to have unusual vicinal coupling constants. The configurations of the three contiguous stereogenic centers of 1 were instead determined by application of the advanced Mosher method.14
Interestingly, 2-azido alcohols have been found to be more favorable glycosyl acceptors than the corresponding 2-amido alcohols (ceramides).15,16 Azido-alkynyl alcohol 4 was readily obtained by the synthetic procedure described here. Thus the route to 1 described herein may be used to prepare a glycosyl acceptor for the preparation of 2 and other galactosylceramide derivatives.6,17
Results and Discussion
Outline of the Synthetic Plan
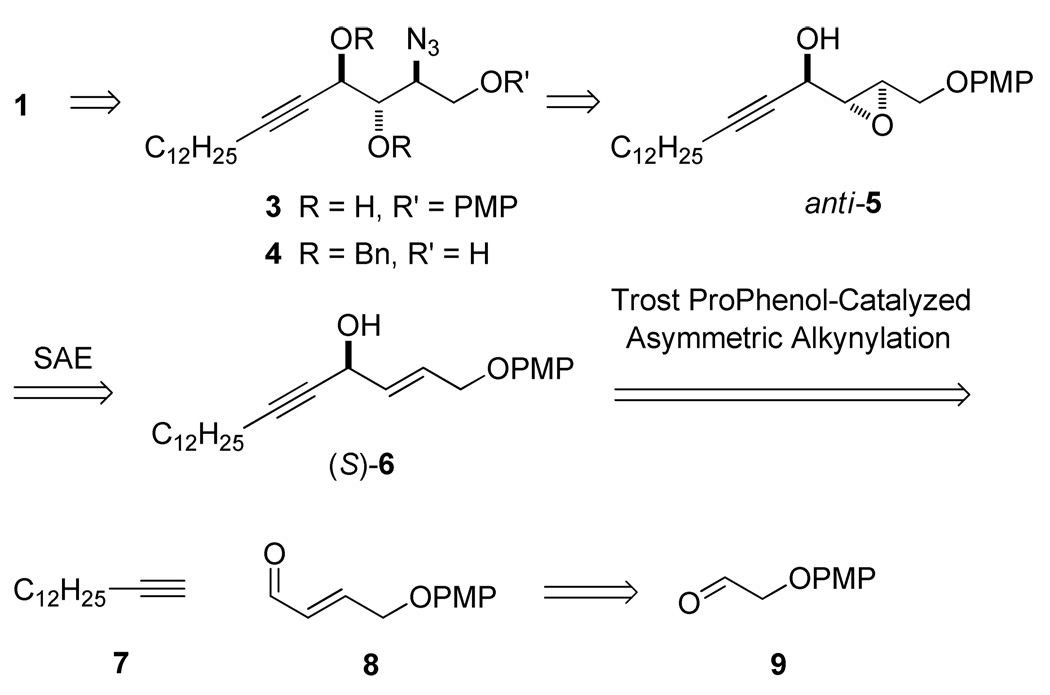
As illustrated in Scheme 1, we envisaged 1 and 4 to be accessible from azido diol 3. The 2S,3S configuration in 3 can be generated by SAE followed by opening of the resulting epoxy alcohol anti-5 with NaN3/NH4Cl. SAE of (S)-6 under kinetic resolution conditions can improve the diastereomeric excess of anti-5. Although the enantiomeric excess of (S)-6 will not affect the following stereoselectivity, it plays a key role in maximizing the yield of SAE. The enantiomeric excess of (S)-6 can be enhanced via catalytic alkynylation of enal 8 with alkyne 7. Asymmetric alkynylation of α,β-unsaturated aldehydes has been used in the synthesis of many complicated molecules with high efficiency,18 but often requires stoichiometric or catalytic titanium in addition to zinc. Trost and co-workers have recently simplified and expanded the scope of this reaction.8a We have employed the Trost protocol to make 6 starting with enal 8, which was prepared from commercially available aldehyde 9 using our recently developed two-step HWE/AlH3 reduction protocol.19 This synthetic route may permit access to other stereoisomers of 1 because the catalytic alkynylation and SAE reactions can be used to generate the opposite configurations by use of their counterpart ligands.
Scheme 1.
Retrosynthetic Plan
Asymmetric Synthesis of Enynol (S)-6
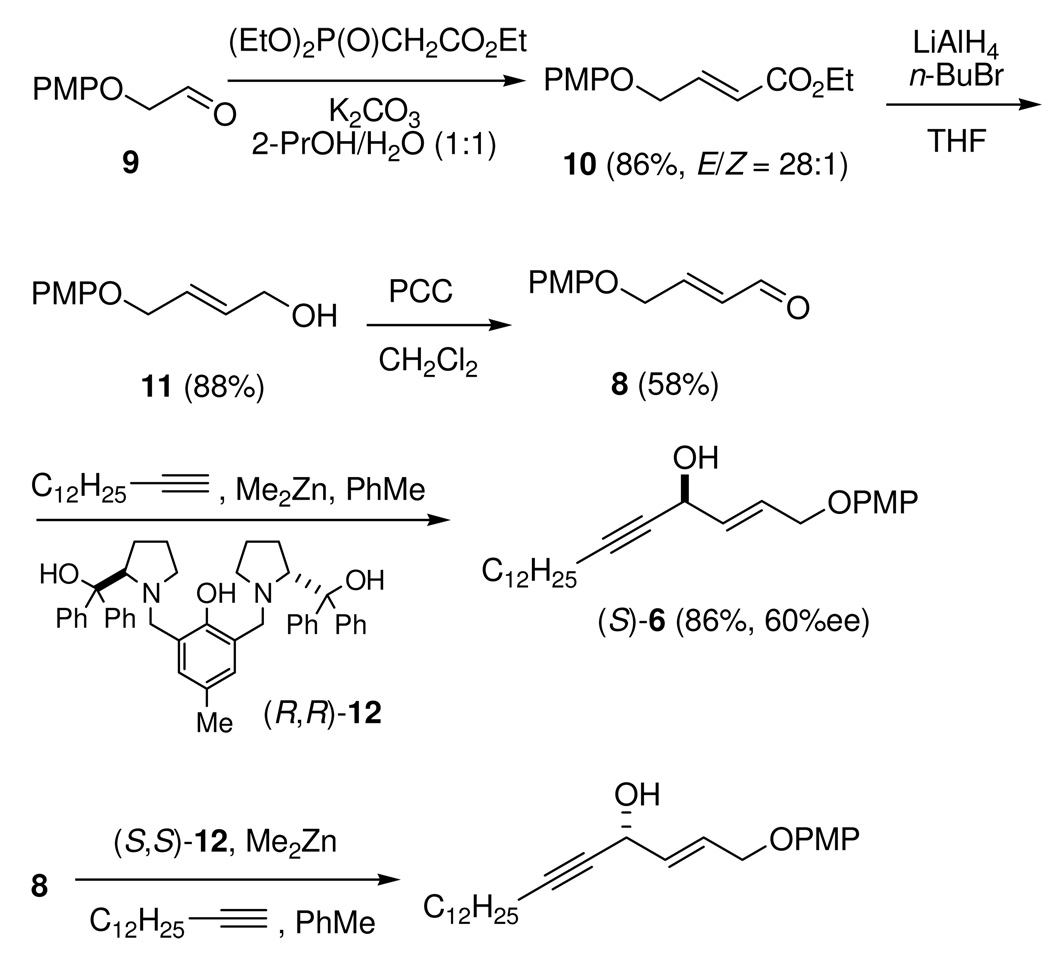
On the basis of the retrosynthetic analysis depicted in Scheme 1, the first target, the conjugated (E)-enynol (S)-6, can be prepared by coupling of enal 8 with 7 by catalytic alkynylation. As shown in Scheme 2, allylic alcohol 11 was prepared from (4-methoxyphenoxy)-acetaldehyde (9)20 via a two-step HWE/reduction protocol.19 Previously, because it is difficult to achieve high E-selectivity by the HWE olefination reaction, (E)-α,β-unsaturated ester 10 was obtained by nucleophilic substitution of alkyl 4-bromocrotonate with 4-methoxyphenol.21 HWE reaction of aldehyde 9 with triethyl phosphonoacetate in the presence of K2CO3 in H2O/2-PrOH (1:1) produced ester 10 in 86% yield and high E-selectivity (E/Z = 28:1). Reduction of ester 10 by AlH3 (generated from LAH and n-BuBr in THF and used in situ)19 provided allylic alcohol 11 in 88% yield. Oxidation of allyl alcohol 11 with PCC gave (E)-α,β-unsaturated enal 8 in 58% yield.
Scheme 2.
Synthesis of Allylic Propargylic Alcohols (S)-6 and (R)-6
Chiral allylic propargylic alcohols have been accessible with high ee either by reduction of the corresponding ketone with a stoichiometric amount of pinanyl-borane22 or by lipase-catalyzed resolution of racemic allylic propargylic alcohols.23 In our hands, alkynylation of enal 8 with 1-tetradecyne catalyzed by ProPhenol ligand (R,R)-1224 reproducibly provided a high yield of the desired (E)-enynol (S)-6 (86%), but the enantiomeric excess was only moderate at best (60% ee). Under degassed reaction conditions, the chemical yield was improved, but the enantioselectivity was not. However, to our surprise, the alkynylation reaction catalyzed by ProPhenol ligand (S,S)-12 provided E-enynol (R)-6 with a high enantioselectivity (85% ee).25 The enantiomeric excess and absolute configuration of E-enynols (S)-6 and (R)-6 were determined by preparing the (R)- and (S)-MTPA esters and analyzing their 1H NMR spectra by the subtraction protocol of the advanced Mosher method (see Supporting Information, pp. S12, S13, S16, S17).14
Epoxidation of E-Enynol 6
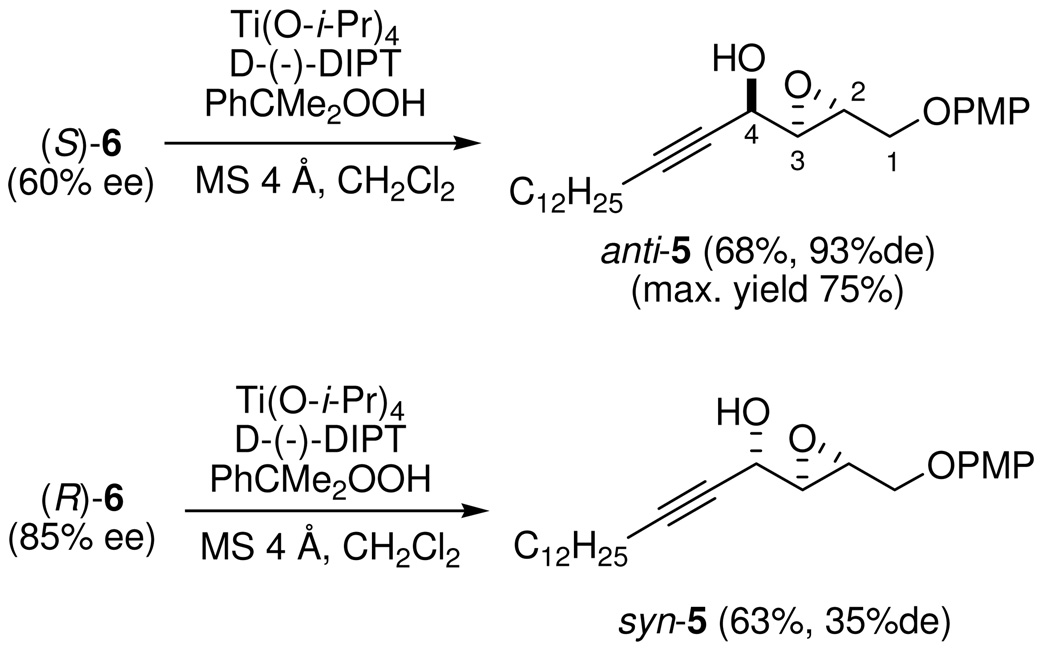
As shown in Schemes 1 and 3, we intended to use SAE as one of the key steps to build the other two stereogenic centers from enynols (S)- and (R)-6. Under Sharpless kinetic resolution conditions, epoxidation of (S)-6 (60% ee) with cumene hydroperoxide (CHP) in the presence of substoichiometric amounts of catalysts (d-(−)-DIPT, Ti(OPr-i)4) and 4Å molecular sieves gave epoxy alcohol anti-5 in good yield (68%; the maximum yield was 75%). After the unreacted substrate was removed by chromatography, the desired propargylic epoxy alcohol anti-5 was obtained in high diastereomeric excess (93% de). In contrast, epoxidation of (R)-6 (85% ee) under the same conditions led to syn-5 in a much lower diastereomeric excess (35% de), indicating that the R configuration eroded the diastereomeric induction dominated by the catalyst. This mismatched effect brought about by the acetylenic moiety may be more pronounced than that of the alkyl group.
Scheme 3.
Synthesis of Epoxy Alcohols anti-5 and syn-5
Opening of Epoxy Alcohol anti-5
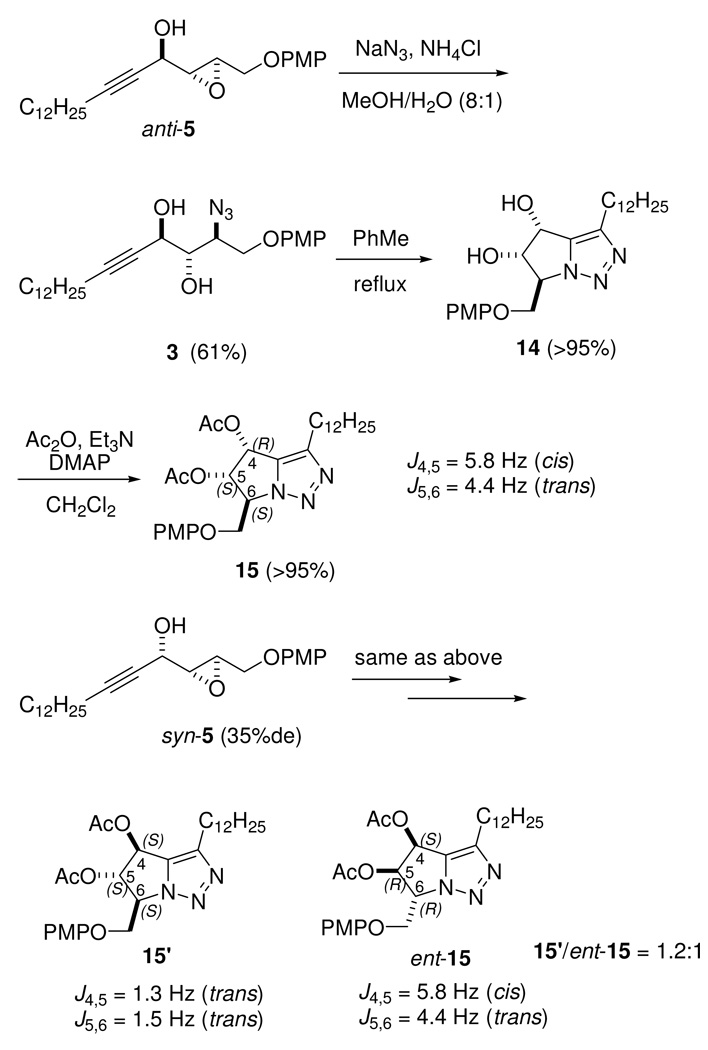
As shown in Scheme 4, opening of epoxy alcohol anti-5 with NaN3/NH4Cl26 provided azido diol 3 in 61% yield, together with other unidentified compounds. Although this reaction was not very clean, alternative methods, such as Ti(O-i-Pr)2(N3)2,27 were not attempted because in our prior experience this reagent gave rise to many unidentified compounds,10,28 including the Ti-catalyzed semi-pinacol rearrangement product of α-hydroxy epoxides.29
Scheme 4.
Opening of anti-5 and Unexpected Coupling Constants in Bicyclic Triazole Derivative 15
Investigation of the Stereochemistry of the Azido Diols from syn- and anti-5 Based on J Values of Bicyclic Derivatives
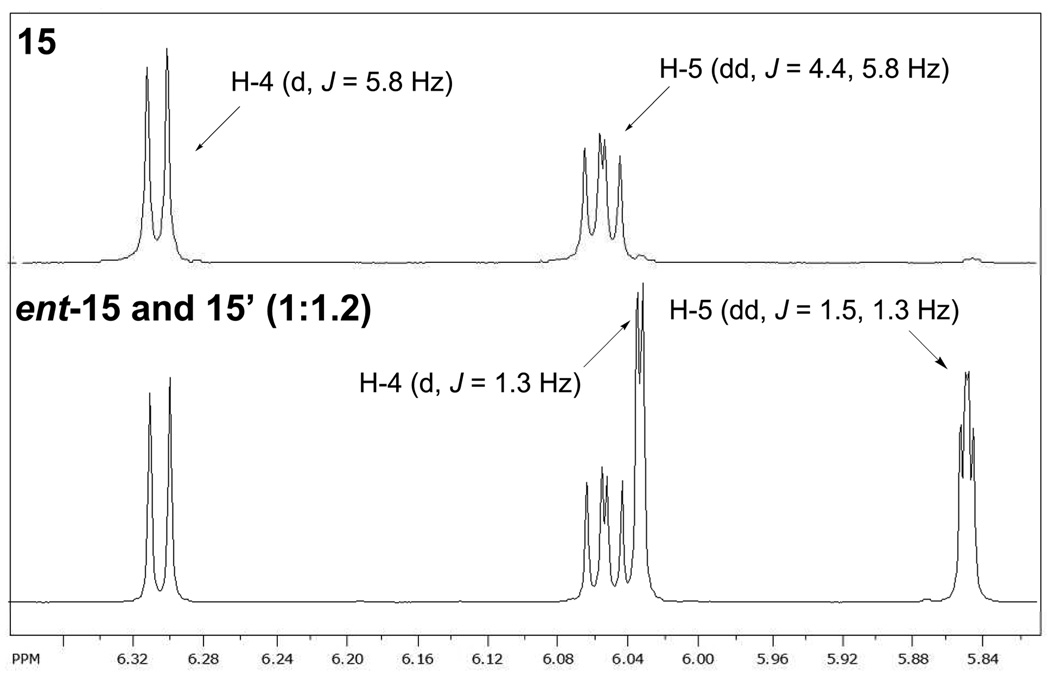
To verify the stereochemistry of azido diols 3, propargylic epoxy alcohol anti-5 was converted to bicyclic dihydroxy triazole 14 via a copper-free intramolecular click reaction in refluxing toluene for 48 h (Scheme 4).13 Diol 14 was then converted to diacetate 15. However, when syn-5 (which has a low de) was subjected to the same reaction sequence, we obtained a mixture of diastereoisomer 15’ [4S,5S,6S] and ent-15 (the enantiomer of 15, [4S,5R,6R]) in a ratio of 1.2:1 (Scheme 4). The partial 1H-NMR spectra of triazoles 15 and 15’ are reported in Figure 1.
Figure 1.
Partial 1H-NMR spectra of 15 (top) and ent-15/15' (ratio 1:1.2, bottom).
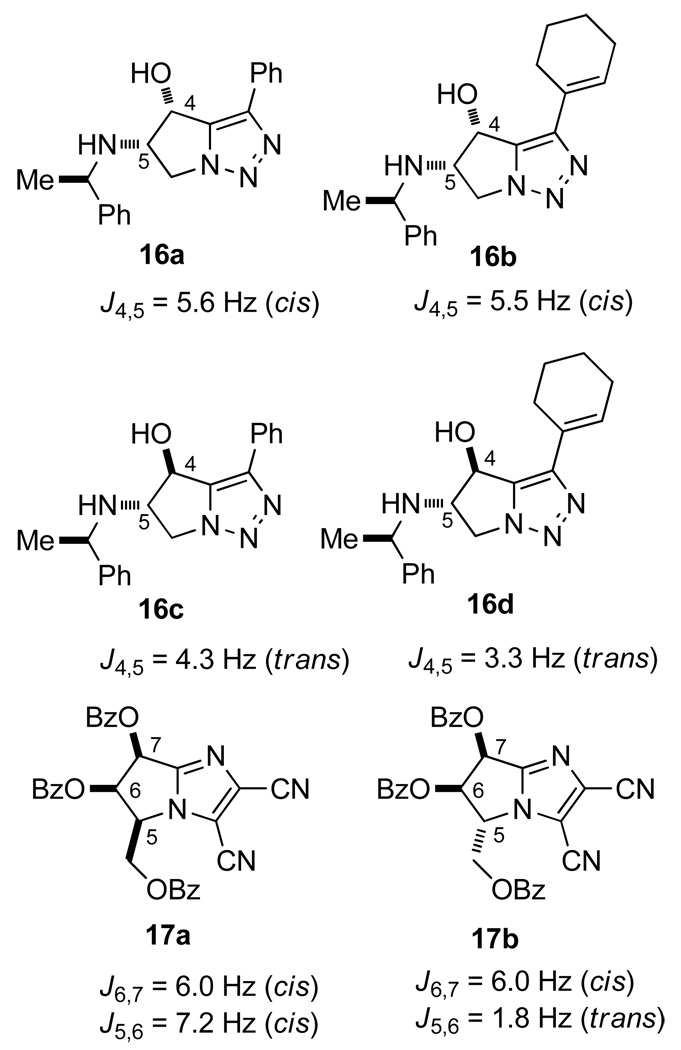
In 2005, Kim et al. prepared bicyclic triazoles 16a–16d from chiral aziridines (Chart 2).30a The coupling constant in the cis relationship is 5.5–5.6 Hz, whereas that in the trans is 3.3–4.3 Hz. Although H-4 in diol 14 gave a doublet signal (J = 5.5 Hz) and H-6 gave a quartet signal (J = 3.4 Hz), H-5 demonstrated an abnormal doublet of doublets (4.3 Hz and 5.0 Hz), probably because of intramolecular hydrogen bonding between the hydroxy and amino groups. The coupling constants of 14 match those of 16a–16d; therefore, these data may tentatively confirm the relative configuration of the three contiguous stereogenic centers. However, it must be pointed out that the intramolecular hydrogen bonds in 14 and 16a–16d are different, which leads to uncertainty regarding the values of the coupling constants. Furthermore, because of the absence of the substituent at the 6 position, 16a–16d may adopt different conformations compared with 14. In 1988, based on coupling constants,30b Ferris and Devades reported a conformational analysis in the pyrrolo-imidazole ring system 17a and 17b, which is very similar to our bicyclic triazole 15 (Chart 2). Since 17a and 17b were fully protected, the impact of intramolecular hydrogen bonds is avoided. Their investigation revealed that the value of the trans coupling constant (J5,6 in 17) was 1.8 Hz, whereas the cis coupling constants (J6,7 in 16 and 17, J5,6 in 16) were 6.0 and 7.2 Hz, respectively.30b
Chart 2.
Reported coupling constants in similar bicyclic systems.30a,b
The coupling constants of cis J4,5 and trans J5,6 in bicyclic triazole 15 (Figure 1) were expected to differ largely. However, to our surprise, we found that they have two very close J values (J4,5 = 5.8 Hz and J5,6 = 4.4 Hz). Furthermore, the two J5,6 values of the trans coupling constants in 15 and 15’ are markedly different (4.4 Hz in 15, 1.5 Hz in 15'). Based on this analysis, it is possible that one of the known stereocontrolled steps did not proceed in the normal way. Therefore, we decided to verify the course of the construction of the three contiguous stereogenic centers by examining: (1) the configuration at C-4 in anti-5 to check which enynol reacted [(S)-6 or (R)-6] in SAE, (2) the configurations at the C-2 and C-3 positions in anti-5, and (3) the opening of anti-5 to verify the configuration at C-2 of 3.
Verification of the Sharpless Kinetic Resolution
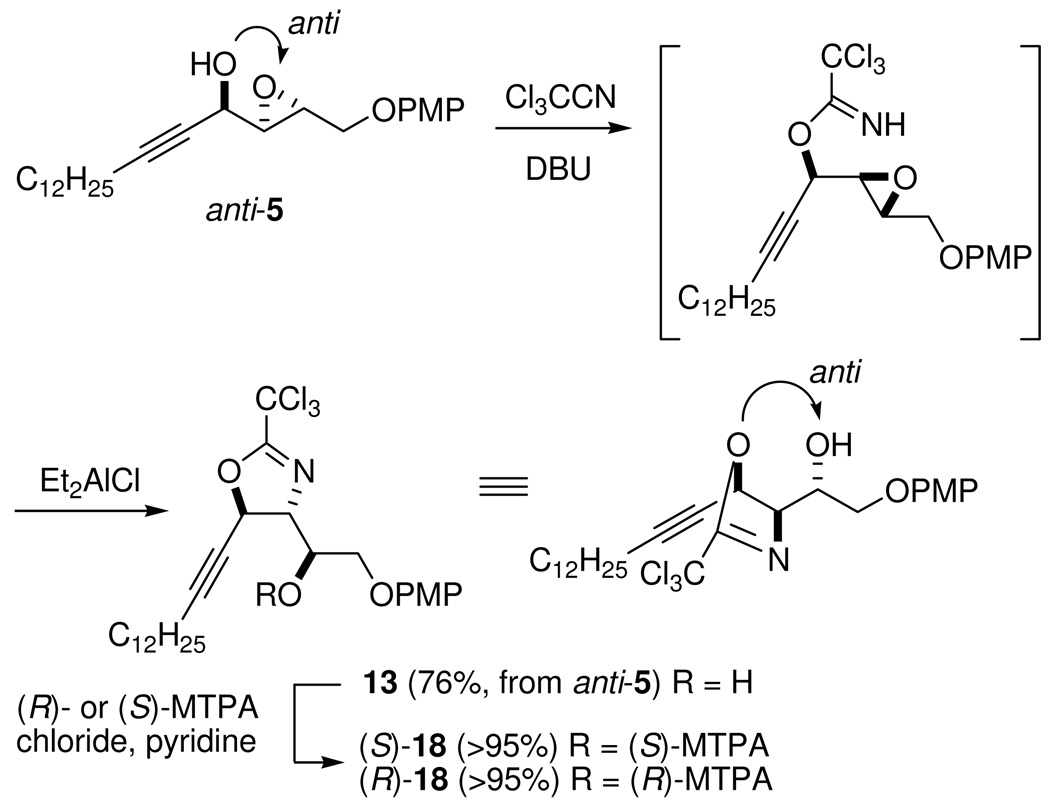
According to the empirical rule established by Sharpless and co-workers, the Sharpless kinetic resolution of secondary allylic alcohols favors the reaction in which the R enantiomer of the racemic mixture forms the epoxide, while the S enantiomer is recovered in an optically enriched form when d-(−)-DIPT is used.31 For allylic propargylic alcohols, the S enantiomer should react faster with d-(−)DIPT because the acetylenic moiety has a higher priority than the olefinic group. Allylic propargylic alcohols have already been proved to uphold the empirical rule,32 although the acetylenic moiety would cause less steric crowding in the transition states for SAE in comparison with alkyl groups. However, the verification is limited to hydrocarbon substrates.32 In order to confirm the Sharpless kinetic resolution in our case, the (R)- and (S)-MTPA esters of anti-5 were prepared to verify the configuration at C-4 (see Supporting Information, p. S20). Analysis of the Δδ values of the protons indicates that the reacted allylic propargylic alcohol was in fact (S)-6, confirming the prediction made by the empirical rule.
Epoxide Configuration in anti-5
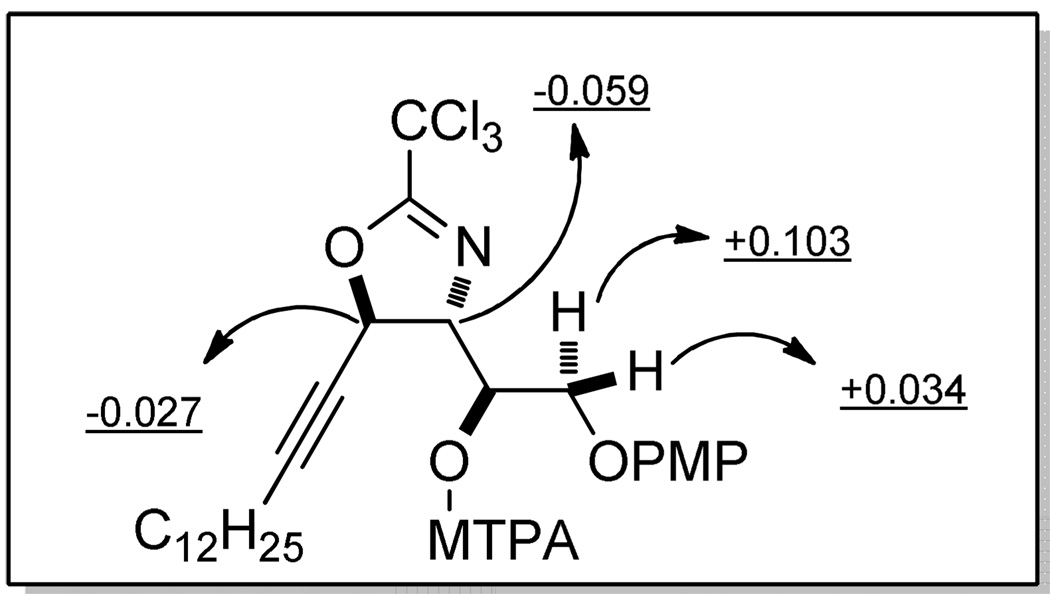
The absolute stereochemistry of an epoxide in chiral epoxy alcohols is generally assigned by the advanced Mosher method using (R)- and (S)-MTPA esters of the corresponding ring-opened diol or by established empirical mnemonics developed for different asymmetric epoxidation strategies.33 Parker and Katsoulis32c determined the absolute configuration of the epoxide in propargylic epoxy alcohols by converting the epoxide to a 1,3-diol and analyzing the corresponding acetonide by the commonly used [13C]-acetonide method developed by Rychnovsky et al.34 This method needs a two-step derivatization. We selected the Et2AlCl-catalyzed cyclization of epoxytrichloroacetimidates35 to transfer the chiral information of the epoxide to the newly formed secondary hydroxy group in an oxazoline or dihydrooxazine (Scheme 5). Generally, cyclization takes place preferentially at the more polarized center of the epoxide with complete inversion of stereochemistry.35 As a result, after this transformation, the newly formed hydroxy group and C-4 oxygen will retain the same relative configuration as that in the epoxy alcohol between the epoxide and C-4 hydroxy group. By determining the configuration of the newly formed hydroxy group, we can assign the chirality of the epoxide in anti-5. Reaction of anti-5 with trichloroacetonitrile in the presence of Et2AlCl and DBU gave the corresponding 2,3-epoxy-1-trichloroacetimidate, which delivered oxazoline 13 in a two-step yield of 76% (Scheme 5). The regiochemistry of cyclization was judged by analysis of the 1H-1H COSY spectrum of 13 (see Supporting Information, p. S28). Analysis of the (R)- and (S)-MTPA esters of 13 ((R)-and (S)-18) revealed the anti relationship between the C-2 hydroxy group and C-4 oxygen (Figure 2), indicating that SAE of enynol (S)-6 followed the normal prediction made by Sharpless et al. for allylic alcohols bearing saturated alkyl groups.
Scheme 5.
Conversion of anti-5 to (S)- and (R)-18
Figure 2.
Absolute stereochemistry determination of 13 via the advanced Mosher method.
Caption to Fig. 2: Δδ values for the MTPA derivatives (S)-18 and (R)-18 (Δδ = δS − δR ppm, 500 MHz).
Configuration of C-2
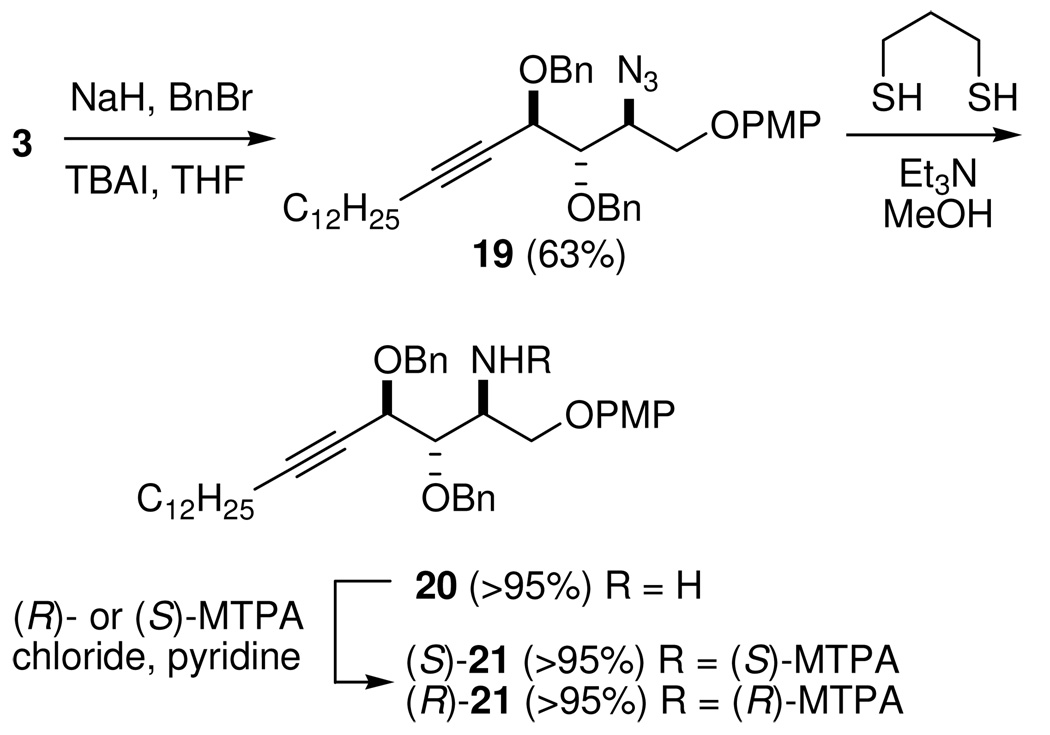
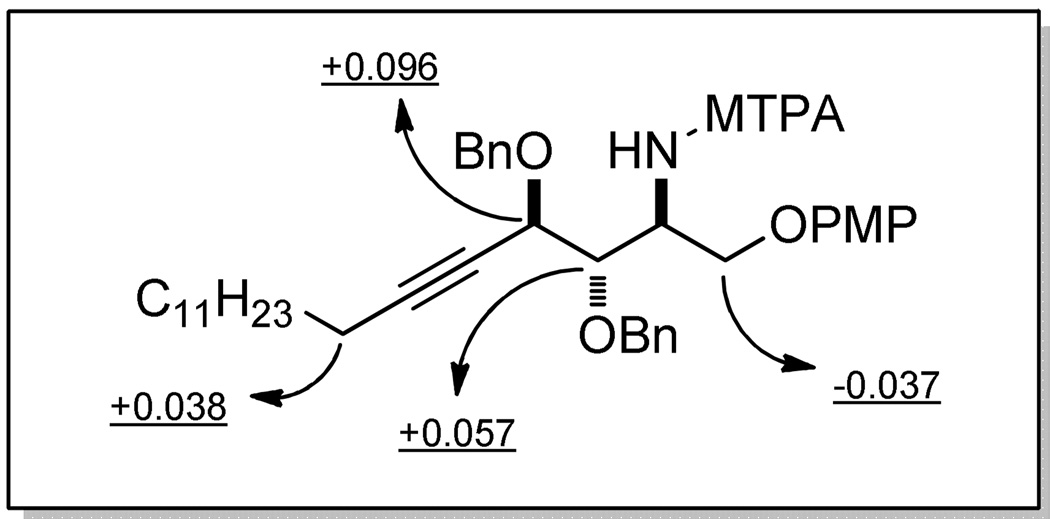
At this stage, the stereochemistry of the opening of epoxy alcohol anti-5 (the first step of Scheme 4) requires verification. For this purpose, we planned to determine the configuration at C-2 of 3 by preparing the (R)- and (S)-MTPA amides (Scheme 6). Reaction of diol 3 with BnBr and NaH in the presence of a catalytic amount of TBAI provided azide 19 in 63% yield. Several methods were explored for the reduction of azide 19. We found that azide 19 was smoothly converted to the corresponding amine 20 by using 1,3-dithiopropane as the reducing agent.36 In situ reaction of 20 with (R)- and (S)-MTPA chlorides gave the (S)- and (R)-MTPA amides ((S)- and (R)-21), respectively. Analysis of the two MTPA amides demonstrated the syn relationship between the azide at C-2 and oxygen at C-4, indicating that opening of epoxy alcohol anti-5 took place by a simple SN2 inversion (Figure 3). Therefore, the evidence showed that the construction of the three contiguous stereogenic centers was correct. The unexpected coupling constants in 15 and 15' may result from the two different conformations they adopted. This result indicates the need for caution when coupling constants are used to judge the relative configuration in bicyclic triazole and related similar systems.
Scheme 6.
Conversion of Azido Diol 3 to (S)- and (R)-MTPA Amide 21
Figure 3.
Absolute stereochemistry determination of 20 by the advanced Mosher method.
Caption to Fig. 2: Δδ values for the MTPA amides (S)-21 and (R)-21 (Δδ= δS − δR ppm, 500 MHz).
Completion of the Preparation of 1 and 4
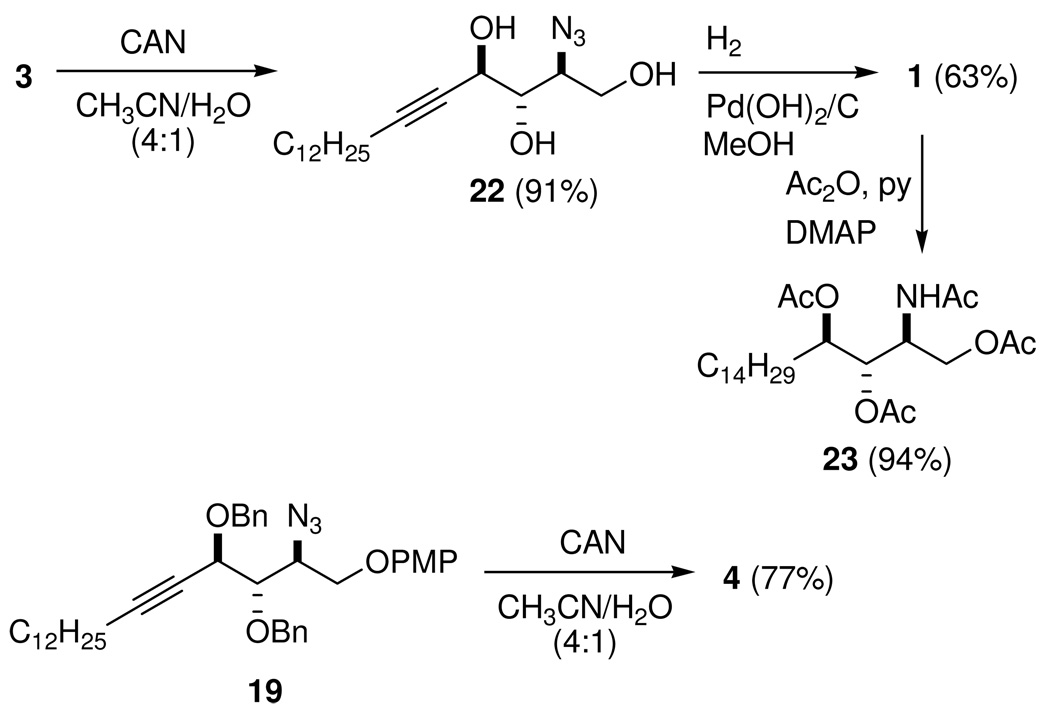
As shown in Scheme 7, deprotection of the PMP group by CAN in 3 followed by hydrogenation of the resulting triol 22 using Pearlman's catalyst (Pd(OH)2/C) in MeOH afforded 1. Its 1H and 13C NMR spectra and specific rotation matched the previously reported data. The structure of 1 was further confirmed by conversion to its tetraacetyl derivative 23.
Scheme 7.
Completion of the Syntheses of 1 and 4 from 3 and 19, Respectively
In the synthesis of glycosylceramides, the choice of the glycosyl acceptor is a critical consideration (together, of course, with the selection of the glycosyl donor). A free amino group at the C-2 position of the sphingolipid is not a viable choice in the acceptor; moreover, the amide functionality of ceramide is not suitable because it deactivates the primary hydroxy group of the acceptor through unfavorable hydrogen bonding interactions.15 Since an azide is apparently devoid of hydrogen-bonding interactions with the adjacent hydroxy group and can be readily converted to an amide in two steps after the glycosidation reaction,16 we decided to prepare 4. The saturated analogue of azido PHS 4 has been prepared from 1 by a tedious protecting group manipulation involving the conversion of an amino group to an azide by a diazo-transfer reaction.37 In contrast, 2-azido carbinol 4, which is accessible from anti-5 via deprotection of 19, is an attractive alternative glycosyl acceptor because the azido group is introduced into the phytosphingosine backbone at an early stage of the synthesis. Since modification of the lipid chain length in α-galactosylceramide analogues influences an array of cytokines release from activated iNKT cells and demonstrates a profound relationship between structure and activity,38 the synthetic route described here allows modification of the chain length with ease.
Conclusion
A stereocontrolled synthetic route to 1 from aldehyde 9 and 1-tetradecyne (7) has been developed. HWE reaction of aldehyde 919 and AlH3 reduction provided allylic alcohol 11, and PCC oxidation afforded α,β-unsaturated aldehyde 8. Catalytic alkynylation of 8 with 7 and SAE of (S)-6 followed by regioselective NaN3/NH4Cl opening of the resulting propargylic epoxy alcohol anti-5 delivered (2S,3S,4R) azido diol 3 with the desired three contiguous stereogenic centers in good yield and high chiral purities. Deprotection of 3 with CAN and catalytic hydrogenation gave 1. A key intermediate, alkynyl-azido 3, can be readily converted to glycosyl acceptor 4 via O,O-dibenzylation and CAN deprotection. An advanced Mosher method clarified the unexpected values of the coupling constants in bicyclic triazoles 15 and 15' generated by a copper-free intramolecular click reaction of 3 and confirmed the configurations of the stereogenic centers.
Experimental Section
(E)-4-(4'-Methoxyphenoxy)-2-butenal (8)
To a cooled, rapidly stirred suspension of PCC (15.0 g, 69.6 mmol) and Celite (16 g) in 250 mL of CH2Cl2 was added alcohol 11 (8.44 g, 43.5 mmol) in one portion. After the mixture had stirred for 3 h at rt, the resulting dark mixture was diluted with 150 mL of Et2O. Filtration through a pad of Florisil left a dark solid residue that was washed with Et2O. The combined filtrates were concentrated, and the residue was purified by flash chromatography (a gradient of hexane/EtOAc 6:1 to 3:1) to afford 8 (5.5 g, 66%) as a slightly yellow solid. 1H NMR (400 MHz, CDCl3) δ 3.77 (s, 3H), 4.76 (dd, J = 1.9, 4.0 Hz, 2H), 6.46 (ddt, J = 15.8, 7.8, 1.9 Hz, 1H), 6.82–6.87 (m, 4H), 6.94 (dt, J = 15.8, 4.0 Hz, 1H), 9.62 (d, J = 7.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 55.7, 67.1, 114.7, 115.6, 132.2, 151.3, 151.9, 154.3, 193.0.
(4S,2E)-1-(4'-Methoxyphenoxy)-2-octadecen-5-yn-4-ol ((S)-6)
A flame-dried round-bottom flask was charged with commercially available Trost’s ProPhenol ligand (R,R)-12 (1.0 g, 1.57 mmol), alkyne 7 (9.2 g, 47.1 mmol), and 300 mL of toluene. A solution of Me2Zn (39.3 mL, 1.2 M in toluene, 47.1 mmol) was added rapidly via syringe. The reaction mixture was stirred for 90 min at rt, and gas slowly evolved. A solution of α,β-unsaturated aldehyde 8 (3.0 g, 15.6 mmol) in a minimal amount of toluene was added via syringe over ~10 s. The reaction mixture was sealed and cooled to 4 °C for 4 days without stirring. Then the reaction mixture was slowly quenched with aqueous saturated NH4Cl solution, and the layers were separated. The aqueous layer was extracted with EtOAc (3 × 200 mL), and the combined organic layers were dried (Na2SO4), filtered, and concentrated. Purification of the residue by flash chromatography (a gradient of hexane/EtOAc 6:1 to 7:2) provided (S)-6 (5.2 g, 86%, 60% ee); 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H), 1.19–1.40 (m, 18H), 1.46–1.54 (m, 2H), 2.21 (dt, J = 2.0, 7.2 Hz, 2H), 2.45 (d, J = 4.3 Hz, 1H), 3.74 (s, 3H), 4.46–4.49 (m, 2H), 4.88–4.92 (m, 1H), 5.95 (ddt, J = 5.3, 15.4, 1.4 Hz, 1H), 6.08 (ddt, J = 1.2, 15.4, 5.3 Hz, 1H), 6.78–6.85 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 14.0, 18.6, 22.6, 28.5, 28.8, 29.0, 29.3, 29.4, 29.5, 29.6, 31.8, 55.5, 62.3, 68.0, 78.8, 87.2, 114.5, 115.5, 127.1, 132.4, 152.5, 153.7; ESI-HRMS [M + Na]+ calcd for m/z C25H38NaO3 + 409.2713, found 409.2717.
(4R,2E)-1-(4'-Methoxyphenoxy)-2-octadecen-5-yn-4-ol ((R)-6)
(R)-6 was prepared in 82% yield and 85% ee according to the procedure used to prepare (S)-6. The 1H and 13C NMR spectra were identical to those of (S)-6; ESI-HRMS [M + Na]+ calcd for C25H38NaO3 + 409.2713, found 409.2715.
General Preparation and Analysis of MTPA Esters or Amide
The reactions were generally run on a 0.02-mmol scale. A mixture of pyridine (4.0 equiv) and substrate (1.0 equiv) in CH2Cl2 (0.6 mL) was treated with neat (R)- or (S)-MTPA chloride (3.0 equiv). The solution was stored in a desiccator until no starting material was observed by TLC. It is important to monitor the reaction by TLC to ensure complete reaction, because kinetic resolution of an incomplete reaction may significantly alter the ee or de measurements. The reaction mixture was passed through a short plug of silica gel to remove polar impurities, and the plug was washed with EtOAc/hexane (the ratio made the Rf = 0.5). After the filtrate was concentrated, the residue was dried under high vacuum (0.2 Torr, 1 h) and dissolved in CDCl3. The (S)- and (R)-MTPA esters and amides were prepared by using (R)- and (S)-MTPA chlorides, respectively.
(R)-1-{(2'R,3'R)-3'-[(4"-Methoxyphenoxy)methyl]oxiran-2'-yl}pentadec-2-yn-1-ol (anti-5)
4Å Molecular sieves (the amount is not critical if the allyl propargyl alcohol, CH2Cl2, and cumene hydroperoxide are pre-dried) were added to a solution of d-(−)-DIPT (363 mg, 1.55 mmol) in 50 mL of dry CH2Cl2. The mixture was stirred at rt for 30 min before it was cooled to −40 °C. Ti(OPr-i)4 (367 mg, 1.29 mmol) was added to the reaction mixture, which was stirred for 30 min. After cumene hydroperoxide (590 mg, 80% technical grade, 3.10 mmol) was added, the reaction mixture was stirred for 30 min. A solution of (S)-6 (1.33 g, 3.45 mmol, 60% ee) in a minimal amount of dry CH2Cl2 was added, and the reaction mixture was sealed and stored at −20 °C without stirring for 3 days. An aqueous pre-cooled (0 °C) solution of tartaric acid (10 mL, 10% w/v) was added dropwise, and the mixture was allowed to warm to rt over 1 h, after which time the solution became transparent. The organic layer was separated, washed with brine, and concentrated. The residue was dissolved in Et2O at 0 °C, and the solution was treated with a solution (4 mL) of 30% w/v NaOH in saturated brine. The two-phase mixture was stirred vigorously for 1 h at 0 °C. The phases were separated, the aqueous layer was extracted with Et2O, and the combined organic layers were dried over Na2SO4. The solvent was evaporated, and the residue was purified by flash chromatography (hexane/EtOAc 5:1 to 4:1) to afford anti-5 (949 mg, 68% (maximum yield, 75%), 93% de); 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H), 1.20–1.41 (m, 18H), 1.47–1.55 (m, 2H), 2.03 (br s, 1H), 2.22 (dt, J = 7.2, 1.9 Hz, 2H), 3.30 (t, J = 2.5 Hz, 1H), 3.50–3.53 (m, 1H), 3.77 (s, 3H), 3.99 (dd, J = 11.5, 5.3 Hz, 1H), 4.26 (dd, J = 11.5, 2.7 Hz, 1H), 4.65–4.68 (m, 1H), 6.80–6.88 (m, 4H); 13C NMR (125 MHz, CDCl3) δ 14.1, 18.7, 22.7, 28.4, 28.9, 29.1, 29.3, 29.5, 29.63, 29.65, 31.9, 53.8, 55.7, 57.4, 60.8, 68.0, 76.0, 88.1, 114.6, 115.8, 152.5, 154.2; ESI-HRMS [M + NH4 +] calcd for C25H42NO4 + 420.3108, found 420.3114.
(S)-1-{(2'R,3'R)-3'-[(4"-Methoxyphenoxy)methyl]oxiran-2'-yl}pentadec-2-yn-1-ol (syn-5)
Compound syn-5 was prepared in 63% yield and 35% de from (R)-6 (85% ee) according to the procedure used to prepare anti-5. 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H), 1.22–1.40 (m, 18H), 1.47–1.55 (m, 2H), 2.22 (dt, J = 7.2, 1.9 Hz, 2H), 3.27 (dd, J = 2.2, 4.1 Hz, 1H), 3.40–3.43 (m, 1H), 3.77 (s, 3H), 3.98 (dd, J = 11.5, 5.3 Hz, 1H), 4.23 (dd, J = 11.5, 2.9 Hz, 1H), 4.38–4.42 (m, 1H), 6.81–6.88 (m, 4H); 13C NMR (125 MHz, CDCl3) δ 14.1, 18.7, 22.7, 28.4, 28.9, 29.1, 29.3, 29.5, 29.62, 29.64, 31.9, 54.2, 55.7, 58.2, 60.4, 61.8, 76.9, 87.6, 114.6, 115.7, 152.5, 154.2.
(1S)-2-(4'-Methoxyphenoxy)-1-[(4'R,5'R)-2'-(trichloromethyl)-4',5'-dihydro-5'-(tetradec-1"-ynyl)oxazol-4'-yl]ethanol (13)
To an ice-cold solution of anti-5 (30 mg, 74.5 µmol) in 1 mL of CH2Cl2 were added DBU (1.1 µL, 7.5 µmol) and trichloroacetonitrile (15 µL, 149 µmol). After being stirred at 0 °C until no starting material was observed on TLC, the reaction mixture was diluted with CH2Cl2 (10 mL), quenched with saturated NH4Cl solution (5 mL), and extracted with CH2Cl2. The combined organic extracts were washed with saturated aqueous NH4Cl solution, dried (Na2SO4), and concentrated. The residue was dissolved in Et2O and passed through a short column packed with anhydrous Na2SO4 and silica gel. Evaporation of Et2O gave a residue that was dried under high vacuum (0.2 Torr, overnight) and used directly in the subsequent cyclization reaction without further purification.
To an ice-cold solution of epoxy trichloroacetimidates in CH2Cl2 (1 mL) was added Et2AlCl (37.3 µL, 1.0 M solution in hexane, 37.3 µmol). After being stirred at rt until no starting material was observed on TLC, the reaction mixture was quenched with saturated aqueous NaHCO3 solution. The reaction mixture was diluted with Et2O, washed with water, dried, and evaporated. The residue was purified by flash chromatography (hexane/EtOAc 5:1) to afford oxazoline 13 (31 mg, 76%); 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H), 1.21–1.36 (m, 18H), 1.43–1.50 (m, 2H), 2.21 (dt, J = 1.9, 7.1 Hz, 2H), 2.48 (d, J = 4.8 Hz, 1H), 3.77 (s, 3H), 4.05 (dd, J = 6.0, 9.5 Hz, 1H), 4.12 (dd, J = 4.2, 9.5 Hz, 1H), 4.15–4.20 (m, 1H), 4.46 (dd, J = 5.8, 7.0 Hz, 1H), 5.61 (dt, J = 1.9, 7.0 Hz, 1H), 6.81–6.90 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 14.1, 18.8, 22.7, 28.2, 28.8, 29.1, 29.3, 29.5, 29.62, 29.63, 29.66, 31.9, 55.7, 69.5, 70.5, 74.9, 75.8, 76.1, 91.2, 114.7, 115.6, 152.3, 154.3, 162.7; ESI-HRMS [M + H]+ calcd for C27H38Cl3NO4 + 546.1939, found 546.1944.
(2S,3S,4R)-1-(4'-Methoxyphenoxy)-2-azidooctadec-5-yne-3,4-diol (3)
To epoxy alcohol anti-5 (95 mg, 0.246 mmol) in 4.5 mL of MeOH/H2O (8:1) were added NH4Cl (66 mg, 1.23 mmol) and NaN3 (160 mg, 2.46 mmol). The reaction mixture was heated at reflux for 48 h. The reaction mixture was allowed to cool to rt, and the solvents were evaporated. The residue was extracted with CH2Cl2 (3 × 10 mL). The combined extracts were dried (Na2SO4) and concentrated. Purification of the residue by flash chromatography (elution with EtOAc/hexane, 5:1 to 3:1 to 5:2) afforded 3 (68 mg, 62%); [α]25D +22.8 (c 1.2, MeOH); 1H NMR (CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H), 1.19–1.33 (m, 16H), 1.33–1.41 (m, 2H), 1.48–1.56 (m, 2H), 2.25 (dt, J = 2.0, 7.1 Hz, 2H), 2.53 (br s, 1H), 2.60 (br s, 1H), 3.76–3.80 (m, 4H), 3.85–3.90 (m, 1H), 4.18 (dd, J = 7.1, 10.0 Hz, 1H), 4.42 (dd, J = 3.3, 10.0 Hz, 1H), 4.67–4.71 (m, 1H), 6.82–6.91 (m, 4H); 13C NMR (CDCl3) δ 14.1, 18.7, 22.7, 28.5, 28.9, 29.1, 29.3, 29.5, 29.6, 29.7, 31.9, 55.7, 62.2, 64.4, 69.1, 72.7, 76.1, 89.3, 114.7, 115.7, 152.3, 154.3; ESI-HRMS [M + Na]+ calcd for C25H39N3NaO4 + 468.2833, found 468.2834.
(4R,5S,6S)-6-[(4’-Methoxyphenoxy)methyl]-3-dodecyl-5,6-dihydro-4H-pyrrolo[1,2-c][1,2,3]triazole-4,5-diol (14)
A solution of 20 mg (45 µmol) of diol 3 in 2 mL of toluene was stirred at 90 °C for 48 h. The solvent was evaporated and the residue was purified by column chromatography (elution with hexane/EtOAc 1:1 to 2:3) to provide 14 (19 mg, 95%); [α]25D −20.8 (c 0.48, MeOH); 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.0 Hz, 3H), 1.19–1.39 (m, 16H), 1.53–1.65 (m, 4H), 1.66–1.75 (m, 2H), 2.71 (t, J = 7.7 Hz, 2H), 3.75 (s, 3H), 4.42–4.46 (m, 1H), 4.48–4.53 (m, 1H), 4.73 (q, J = 3.4 Hz, 1H), 4.96–4.99 (m, 1H), 5.21 (d, J = 5.5 Hz, 1H), 6.72–6.80 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 14.1, 22.7, 25.4, 29.1, 29.4, 29.62, 29.65, 29.68, 31.9, 55.7, 64.5, 64.8, 66.9, 77.9, 114.7, 115.9, 137.8, 143.3, 152.0, 154.6; ESI-HRMS [M + H]+ calcd for C25H40N3O4 + 446.3013, found 446.3006.
(4R,5S,6S)-6-[(4’-Methoxyphenoxy)methyl]-3-dodecyl-5,6-dihydro-4H-pyrrolo[1,2-c][1,2,3]triazol-4,5-di-yl Acetate (15)
To a solution of 10 mg (22 µmol) of 14 in 1 mL of CH2Cl2 was added 100 µL (717 µmol) of Et3N and 50 µL (530 µmol) of Ac2O. The solution was stirred overnight at rt. After the solvent was removed, the residue was further dried under high vacuum (0.7 Torr) for 1 h, dissolved in a minimal amount of CH2Cl2, and filtered through a pad of silica gel in a buret. The pad was rinsed with 10 mL of hexane/EtOAc 4:1. Concentration gave diacetate 15 (11 mg, 98%) as a colorless syrup. 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.2 Hz, 3H), 1.20–1.37 (m, 18H), 1.61–1.70 (m, 2H), 2.13 (s, 3H), 2.14 (s, 3H), 2.73 (t, J = 7.7 Hz, 2H), 3.75 (s, 3H), 4.48 (dd, J = 2.7, 10.4 Hz, 1H), 4.59 (dd, J = 3.0, 10.4 Hz, 1H), 4.87 (dt, J = 4.3, 2.9 Hz, 1H), 6.05 (dd, J = 4.3, 5.7 Hz, 1H), 6.31 (d, J = 5.8 Hz, 1H), 6.73–6.81 (m, 4H); 13C NMR (125 MHz, CDCl3) δ 14.1, 20.4, 20.5, 22.7, 25.3, 29.2, 29.3, 29.4, 29.57, 29.62, 29.64, 29.7, 31.9, 55.6, 62.2, 64.6, 66.4, 76.8, 114.6, 116.1, 134.6, 143.7, 151.7, 154.7, 169.5, 169.6.
(4S,5S,6S)-6-[(4’-Methoxyphenoxy)methyl]-3-dodecyl-5,6-dihydro-4H-pyrrolo[1,2-c][1,2,3]triazol-4,5-di-yl Acetate (15’)
Compound 15' was prepared from syn-5 (35% de) according to the same sequence used to convert anti-5 to 15 and was contaminated with 15 (ratio of 15':ent-15 = 1.2:1). 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.2 Hz, 3H), 1.20–1.37 (m, 18H), 1.61–1.70 (m, 2H), 2.14 (s, 3H), 2.15 (s, 3H), 2.69–2.72 (m, 2H), 3.76 (s, 3H), 4.51–4.53 (m, 2H), 4.72–4.75 (m, 1H), 5.85 (dd, J = 1.5, 1.9 Hz, 1H), 6.03 (d, J = 1.3 Hz, 1H), 6.73–6.81 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 14.1, 20.4, 20.5, 22.7, 25.3, 29.2, 29.3, 29.4, 29.57, 29.62, 29.64, 29.7, 31.8, 55.6, 64.0, 67.0, 69.4, 84.3, 114.6, 115.8, 134.9, 143.6, 151.9, 154.5, 169.7, 169.8.
(2S,3S,4R)-1-(4’-Methoxyphenyl)-2-azido-3,4-benzyloxy-5-octadecyn-1,3,4-triol (19)
To a mixture of 222 mg (5.56 mmol) of NaH (60%) and 620 mg (1.39 mol) of diol 3 in 10 mL of freshly distilled THF were added 343 µL (6.95 mmol) of benzyl bromide and 3 mg (8 µmol) of TBAI at rt. The mixture was stirred at rt overnight and then was quenched with 5 mL of MeOH. The reaction mixture was poured into a mixture of ice and EtOAc. The organic layer was separated, washed with aqueous 1 M HCl, water, saturated aqueous NaHCO3 solution, and brine, and then dried (Na2SO4). The solvent was evaporated, and the residue was purified by flash chromatography (hexane/EtOAc 40:1) to afford 19 (546 mg, 63%) as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H), 1.20–1.30 (m, 12H), 1.37–1.44 (m, 2H), 1.51–1.60 (m, 2H), 2.28 (dt, J = 1.6, 7.0 Hz, 2H), 3.77 (s, 3H), 3.82 (t, J = 4.9 Hz, 1H), 4.00–4.06 (m, 2H), 4.18–4.23 (m, 1H), 4.40–4.42 (m, 1H), 4.51 (d, J = 11.6 Hz, 1H), 4.65 (d, J = 11.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 14.1, 18.9, 22.7, 28.6, 29.0, 29.2, 29.4, 29.55, 29.64, 29.66, 31.9, 55.7, 61.5, 68.3, 70.0, 70.8, 74.3, 75.6, 80.1, 89.1, 114.6, 115.6, 127.8, 128.0, 128.2, 128.3, 128.4, 137.6, 137.8, 152.5, 154.1; ESI-HRMS [M + Na]+ calcd for C39H51N3O4Na+ 648.3772, found 648.3776.
(2S,3S,4R)-1-(4’-Methoxyphenoxy)-3,4-bis(benzyloxy)octadec-5-yn-2-amine (20)
To a solution of 19 (23 mg, 37 µmol) in MeOH (1 mL) were added Et3N (102 µL, 0.73 mmol) and 1,3-dithiopropane (73 µL, 0.73 mmol). The reaction mixture was stirred overnight at 50 °C. The white precipitate was removed by filtration and washed twice with MeOH. After the solvent was evaporated, the residue was dried under high vacuum (0.2 Torr, overnight) and used directly in the subsequent MTPA ester analysis without further purification.
(2S,3S,4R)-2-Azidooctadec-5-yne-1,3,4-triol (22)
Diol 3 (75 mg, 0.17 mmol) was dissolved in 2.5 mL of CH3CN/H2O (4:1) at rt, and CAN (461 mg, 0.84 mmol) was added. The mixture was stirred at rt until completion as monitored by TLC (about 1 h) and diluted with CHCl3. The resulting solution was washed with H2O and brine. The organic layer was dried (Na2SO4) and concentrated. The residue was purified by flash chromatography (hexane/EtOAc 3:2) to afford triol 22 (52 mg, 91%); [α]25D +43.2 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 0.90 (t, J = 7.1 Hz, 3H), 1.23–1.35 (m, 16H), 1.39–1.46 (m, 2H), 1.49–1.56 (m, 2H), 2.25 (dt, J = 2.0, 7.0 Hz, 2H), 3.52–3.57 (m, 2H), 3.69 (dd, J = 7.2, 11.5 Hz, 1H), 3.96–4.00 (m, 1H), 4.45–4.47 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 14.1, 18.7, 22.7, 28.5, 28.9, 29.1, 29.3, 29.5, 29.62, 29.65, 31.9, 62.7, 63.9, 64.2, 73.7, 76.2, 89.3; ESI-HRMS [M + Na]+ calcd for C18H33N3O3Na+ 362.2414, found 362.2412.
d-ribo-Phytosphingosine (1)
To a solution of 34 mg (0.10 mmol) of triol 22 in 5 mL of MeOH was added 11 mg (0.020 mmol) of 20% Pd(OH)2/C. The resulting suspension was degassed three times and was stirred with a balloon filled with H2 overnight. The crude reaction mixture was filtered through a short pad of Celite, which was washed with 30 mL of MeOH. The combined filtrates were concentrated and purified by flash chromatography (CHCl3/MeOH/concd NH4OH 130:25:4) to afford 1 (20 mg, 63%) as a white solid. The product was dissolved in a minimum volume of CHCl3 and passed through a 0.45-µm filter to remove the suspended silica gel; mp 99–101 °C [lit.9 mp 98.5–101.5 °C]; [α]25D +8.0 (c 0.8, C5H5N) [lit. 9 [α]25D +7.3 (c 0.9, C5H5N)]; 1H NMR (500 MHz, CD3OD) δ 0.89 (t, J = 7.1 Hz, 3H), 1.22–1.41 (m, 24H), 1.49–1.60 (m, 1H), 1.68–1.77 (m, 1H), 2.94–2.97 (m, 1H), 3.33 (dd, J = 5.4, 7.8 Hz, 1H), 3.47–3.52 (m, 1H), 3.55 (dd, J = 6.8, 10.9 Hz, 1H), 3.75 (dd, J = 4.1, 10.9 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ 14.4, 23.8, 26.6, 30.5, 30.79, 30.82, 31.0, 33.1, 34.8, 55.8, 64.0, 74.4, 76.4; ESI-HRMS [M + Na]+ calcd for C18H39NNaO3 + 340.2822, found 340.2823.
d-ribo-Phytosphingosine Tetraacetate (23)
Compound 23 was prepared from 1 according to ref. 9. [α]25D +22.6 (c 0.7, CHCl3) [lit. 39 [α]20 d +21.9 (c 1.1, CHCl3)]; 1H NMR (500 MHz, CHCl3) δ 0.88 (t, J = 7.0 Hz, 3H), 1.16–1.39 (m, 24H), 1.57–1.72 (m, 2H), 2.03 (s, 1H), 2.05 (s, 6H), 2.09 (s, 3H), 4.00 (dd, J = 2.8, 11.7 Hz, 1H), 4.29 (dd, J = 4.7, 11.7 Hz, 1H), 4.44–4.51 (m, 1H), 4.93 (t, J = 9.9, 2.8 Hz, 1H), 5.11 (dd, J = 2.8, 8.4 Hz, 1H), 6.06 (d, J = 9.4 Hz, 1H); 13C NMR (125 MHz, CHCl3) δ 14.1, 20.75, 20.78, 21.1, 22.7, 23.3, 25.5, 28.0, 29.28, 29.34, 29.5, 29.57, 29.61, 29.64, 29.66, 31.9, 47.5, 62.8, 71.8, 73.0, 169.8, 170.1, 170.9, 171.2.
(2S,3S,4R)-2-Azido-3,4-bis(benzyloxy)octadec-5-yn-1-ol (4)
Compound 19 (546 mg, 0.872 mmol) was dissolved in 25 mL of CH3CN/H2O (4:1) at rt, and CAN (2.39 g, 4.36 mmol) was added. The mixture was stirred at rt until completion, as monitored by TLC (about 1 h), and was then diluted with CHCl3. The resulting solution was washed with H2O and brine. The organic layer was dried (Na2SO4) and concentrated. The residue was purified by flash chromatography (elution with hexane/EtOAc 10:1 to 6:1) to afford 349 mg (77%) of alcohol 4 as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H), 1.20–1.33 (m, 16H), 1.36–1.45 (m, 2H), 1.50–1.58 (m, 2H), 2.28 (dt, J = 1.9, 7.1 Hz, 2H), 2.32 (br s, 1H), 3.72–3.81 (m, 3H), 3.81–3.87 (m, 1H), 4.37–4.40 (m, 1H), 4.50 (d, J = 11.8 Hz, 1H), 4.63 (d, J = 11.4 Hz, 1H), 4.81 (d, J = 11.4 Hz, 1H), 4.86 (d, J = 11.8 Hz, 1H), 7.26–7.38 (m, 10H); 13C NMR (125 MHz, CDCl3) δ 14.1, 18.8, 22.7, 28.6, 28.9, 29.1, 29.3, 29.5, 29.60, 29.63, 31.9, 62.1, 63.0, 69.6, 70.7, 73.9, 75.3, 80.8, 89.3, 127.85, 127.89, 128.0, 128.1, 128.38, 128.39, 137.3, 137.5; ESI-HRMS [M + Na]+ calcd for C32H45N3NaO3 + 542.3353, found 542.3356.
Supplementary Material
Chart 1.
Structures of d-ribo-phytosphingosine and α-galactosylceramide (KRN7000).
Acknowledgments
This work was supported in part by National Institutes of Health Grant HL-083187. Z. L. thanks a Doctoral Student Research Grant from the CUNY Graduate Center. We thank Dr. William F. Berkowitz for helpful comments in the preparation of the manuscript.
Footnotes
Supporting Information Available: Copies of 1H NMR and 13C NMR spectra of all new compounds are available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.(a) Karlsson K-A, Martensson E. Biochim. Biophys. Acta. 1968;152:230–233. doi: 10.1016/0005-2760(68)90029-5. [DOI] [PubMed] [Google Scholar]; (b) Carter HE, Hirschberg CB. Biochemistry. 1968;7:2296–2300. doi: 10.1021/bi00846a036. [DOI] [PubMed] [Google Scholar]
- 2.Veerman ECI, Valentijn-Benz M, van't Hof W, Nazmi K, van Marle J, Amerongen AVN. Biol. Chem. 2010;391:65–71. doi: 10.1515/BC.2010.001. [DOI] [PubMed] [Google Scholar]
- 3.(a) Jenkins GM, Richards A, Wahl T, Mao C, Obeid L, Hannun Y. J. Biol. Chem. 1997;272:32566–32572. doi: 10.1074/jbc.272.51.32566. [DOI] [PubMed] [Google Scholar]; (b) Skrzypek MS, Nagiec MM, Lester RL, Dickson RC. J. Bacteriol. 1999;181:1134–1140. doi: 10.1128/jb.181.4.1134-1140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Meier KD, Deloche O, Kajiwara K, Funato K, Riezman H. Mol. Biol. Cell. 2006;17:1164–1175. doi: 10.1091/mbc.E05-11-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Kim MK, Park KS, Lee H, Kim YD, Yun T, Bae YS. Exp. Mol. Med. 2007;39:185–194. doi: 10.1038/emm.2007.21. [DOI] [PubMed] [Google Scholar]; (b) Cowart LA, Shotwell M, Worley ML, Richards AJ, Montefusco DJ, Hannun YA, Lu X. Mol. Syst. Biol. 2010;6 doi: 10.1038/msb.2010.3. article number 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Smith SW, Lester RC. J. Biol. Chem. 1974;249:3395–3405. [PubMed] [Google Scholar]; (b) Rhome R, Del Poeta M. Annu. Rev. Microbiol. 2009;63:119–131. doi: 10.1146/annurev.micro.091208.073431. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Björkbom A, Ohvo-Rekilae H, Kankaanpaeae P, Nyholm TKM, Westerlund B, Slotte JP. Biochim. Biophys. Acta. 2010;1798:453–460. doi: 10.1016/j.bbamem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 6.For reviews of KRN7000 and its analogues, see: Franck RW, Tsuji M. Acc. Chem. Res. 2006;39:692–701. doi: 10.1021/ar050006z. Savage PB, Teyton L, Bendelac A. Chem. Soc. Rev. 2006;35:771–779. doi: 10.1039/b510638a. Zheng L, Ye X-S. J. Chin. Pharmaceut. Sci. 2008;17:263–271.
- 7.For reviews of PHS syntheses prior to 2004, see: Howell AR, Ndakla AJ. Curr. Org. Chem. 2002;6:365–391. Liao J, Tao J, Lin G, Liu D. Tetrahedron. 2005;61:4715–4733. For additional references on subsequent syntheses of 1, see: Lu X, Byun H-S, Bittman R. J. Org. Chem. 2004;69:5433–5438. doi: 10.1021/jo0493065. Singh OV, Kampf DJ, Han H. Tetrahedron Lett. 2004;45:7239–7242. Lu X, Bittman R. Tetrahedron Lett. 2005;46:3165–3168. Lombardo M, Capdevila MG, Pasi F, Trombini C. Org. Lett. 2006;8:3303–3305. doi: 10.1021/ol0612096. Jeon J, Shin M, Yoo JW, Oh JS, Bae JG, Jung SH, Kim YG. Tetrahedron Lett. 2007;48:1105–1108. Chang C-W, Chen Y-N, Adak AK, Lin K-H, Tzou D-LM, Lin C-C. Tetrahedron. 2007;63:4310–4318. Abraham E, Candela-Lena JI, Davies SG, Georgiou M, Nicholson RL, Roberts PM, Russell AJ, Sanchez-Fernandez EM, Smith AD, Thomson JE. Tetrahedron: Asymmetry. 2007;18:2510–2513. Kim S, Lee N, Lee S, Lee T, Lee YM. J. Org. Chem. 2008;73:1379–1385. doi: 10.1021/jo702147y. Abraham E, Brock EA, Candela-Lena JI, Davies SG, Georgiou M, Nicholson RL, Perkins JH, Roberts PM, Russell AJ, Sánchez-Fernández EM, Scott PM, Smith AD, Thomson JE. Org. Biomol. Chem. 2008;6:1665–1673. doi: 10.1039/b801671b. Llaveria J, Diaz Y, Matheu MI, Castillon S. Org. Lett. 2009;11:205–208. doi: 10.1021/ol802379b.
- 8.(a) Trost BM, Weiss AH, Jacobi von Wangelin A. J. Am. Chem. Soc. 2006;128:8–9. doi: 10.1021/ja054871q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Trost BM, Weiss AH. Org. Lett. 2006;8:4461–4464. doi: 10.1021/ol0615836. [DOI] [PubMed] [Google Scholar]; (c) Trost BM, Weiss AH. Angew. Chem., Int. Ed. 2007;46:7664–7666. doi: 10.1002/anie.200702637. [DOI] [PubMed] [Google Scholar]; (d) Trost BM, Sieber JD, Qian W, Dhawan R, Ball ZT. Angew. Chem., Int. Ed. 2009;48:5478–5481. doi: 10.1002/anie.200901907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He L, Byun H-S, Bittman R. J. Org. Chem. 2000;65:7618–7626. doi: 10.1021/jo001225v. [DOI] [PubMed] [Google Scholar]
- 10.Lu X, Song L, Metelitsa LS, Bittman R. ChemBioChem. 2006;7:1750–1756. doi: 10.1002/cbic.200600197. [DOI] [PubMed] [Google Scholar]
- 11.For a review of SAE, see: Katsuki T, Martin VS. Org. React. 1996;48:1–299.
- 12.By comparing the synthetic final product with authentic samples of other diastereoisomers, Pu and Franck found that retention took place during the Ti(O-i-Pr)2(N3)2-mediated opening of a primary epoxy alcohol. The reason for retention is unclear. Pu J, Franck RW. Tetrahedron. 2008;64:8618–8629. doi: 10.1016/j.tet.2008.06.007. Pu J. Ph.D. Dissertation. The City University of New York; 2006.
- 13.Pearson WH, Bergmeier SC, Chytra JA. Synthesis. 1990:156–159. [Google Scholar]
- 14.For the advanced Mosher method for determining the absolute configuration by 1H-NMR spectroscopy, see: Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J. Am. Chem. Soc. 1991;113:4092–4096.
- 15.(a) Schmidt RR, Zimmermann P. Angew. Chem. Int. Ed. Engl. 1986;25:725–726. [Google Scholar]; (b) Polt R, Szabo L, Treiberg J, Li Y, Hruby VJ. J. Am. Chem. Soc. 1992;114:10249–10258. [Google Scholar]
- 16.For the use of azido sphingosine analogues for the synthesis of β-glycosylsphingolipids, see: Bittman R. Chem. Phys. Lipids. 2004;129:111–131. doi: 10.1016/j.chemphyslip.2004.01.004. Lankalapalli RS, Baksa A, Liliom K, Bittman R. ChemMedChem. 2010;5:682–686. doi: 10.1002/cmdc.201000018.
- 17.(a) Veerapen N, Brigl M, Garg S, Cerundolo V, Cox LR, Brenner MB, Besra GS. Bioorg. Med. Chem. Lett. 2009;19:4288–4291. doi: 10.1016/j.bmcl.2009.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Leung L, Tomassi C, Van Beneden K, Decruy T, Trappeniers M, Elewaut D, Gao Y, Elliott T, Al-Shamkhani A, Ottensmeier C, Werner JM, Williams A, Van Calenbergh S, Linclau B. ChemMedChem. 2009;4:329–334. doi: 10.1002/cmdc.200800348. [DOI] [PubMed] [Google Scholar]; (c) Chen W, Xia C, Wang J, Thapa P, Li Y, Nadas J, Zhang W, Zhou D, Wang PG. J. Org. Chem. 2007;72:9914–9923. doi: 10.1021/jo701539k. [DOI] [PubMed] [Google Scholar]; (d) Ding N, Li C, Liu Y, Zhang Z, Li Y. Carbohydr. Res. 2007;342:2003–2013. doi: 10.1016/j.carres.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 18.For reviews of catalytic asymmetric organozinc additions to carbonyl compounds, see: Pu L, Yu HB. Chem. Rev. 2001;101:757–824. doi: 10.1021/cr000411y. Pu L. Tetrahedron. 2003;59:9873–9886.
- 19.Liu Z, Gong Y, Byun H-S, Bittman R. New J. Chem. 2010;34:470–475. [Google Scholar]
- 20.Piggott MJ, Wege D. Aust. J. Chem. 2003;56:691–702. [Google Scholar]
- 21.He L, Byun H-S, Smit J, Wilschut J, Bittman R. J. Am. Chem. Soc. 1999;121:3897–3903. [Google Scholar]
- 22.Midland MM, McDowell DC, Hatch RL, Tramontano A. J. Am. Chem. Soc. 1980;102:867–869. [Google Scholar]
- 23.Burgess K, Jennings LD. J. Am. Chem. Soc. 1991;113:6129–6139. [Google Scholar]
- 24.Trost BM, Ito H. J. Am. Chem. Soc. 2000;122:12003–12004. [Google Scholar]
- 25.The reason for the different ee values is not clear, but the chiral purities of the ligands appear to differ based on our determination of their specific rotations: (S,S)-12: [α]25D +38.7 (c 0.63, CHCl3), [lit.24 [α]25D +49.8 (c 3.0, CHCl3), Sigma-Aldrich catalog: [α]25D +50 (c 1.0, CHCl3)]; (R,R)-12: [α]25D −40.9 (c 0.65, CHCl3), [Sigma-Aldrich catalog: [α]25D −50 (c 1.0, CHCl3)].
- 26.Behrens CH, Ko SY, Sharpless KB, Walker FJ. J. Org. Chem. 1985;50:5687–5696. [Google Scholar]
- 27.Caron M, Carlier PR, Sharpless KB. J. Org. Chem. 1988;53:5185–5187. [Google Scholar]
- 28.Liu Z, Byun H-S, Bittman R. unpublished results. [Google Scholar]
- 29.Snape TJ. Chem. Soc. Rev. 2007;36:1823–1842. doi: 10.1039/b709634h. [DOI] [PubMed] [Google Scholar]
- 30.(a) Kim MS, Yoon HJ, Lee BK, Kwon JH, Lee WK, Kim Y, Ha H-J. Synlett. 2005:2187–2190. [Google Scholar]; (b) Ferris JP, Devadas B. J. Org. Chem. 1987;52:2355–2361. [Google Scholar]
- 31.Martin VS, Woodard SS, Katsuki T, Yamada Y, Ikeda M, Sharpless KB. J. Am. Chem. Soc. 1981;103:6237–6240. [Google Scholar]
- 32.(a) Rao AVRama, Khrimian AP, Krishna PRadha, Yadagiri P, Yadav JS. Synth. Commun. 1988;18:2325–2330. [Google Scholar]; (b) Yadav JS, Radhakrishna P. Tetrahedron. 1990;46:5825–5832. [Google Scholar]; (c) Parker KA, Katsoulis IA. Org. Lett. 2004;6:1413–1416. doi: 10.1021/ol049735p. [DOI] [PubMed] [Google Scholar]
- 33.(a) Gao Y, Hanson RM, Klunder JM, Ko SY, Masamune H, Sharpless KB. J. Am. Chem. Soc. 1987;109:5765–5780. [Google Scholar]; (b) Jacobsen EN. Asymmetric Catalytic Epoxidation of Unfunctionalized Olefins. In: Ojima I, editor. Catalytic Asymmetric Synthesis. New York: Wiley-VCH; 1993. pp. 159–202. [Google Scholar]; (c) Johnson RA, Sharpless KB. Catalytic Asymmetric Epoxidation of Allylic Alcohols. In: Ojima I, editor. Catalytic Asymmetric Synthesis. New York: Wiley-VCH; 1993. pp. 103–158. [Google Scholar]; (d) Wang ZX, Tu Y, Frohn M, Zhang JR, Shi Y. J. Am. Chem. Soc. 1997;119:11224–11235. [Google Scholar]; (e) Zhang W, Basak A, Kosugi Y, Hoshino Y, Yamamoto H. Angew. Chem., Int. Ed. 2005;44:4389–4391. doi: 10.1002/anie.200500938. [DOI] [PubMed] [Google Scholar]
- 34.(a) Rychnovsky SD, Rogers BN, Richardson TI. Acc. Chem. Res. 1998;31:9–17. [Google Scholar]; (b) Evans DA, Rieger DL, Gage JR. Tetrahedron Lett. 1990;31:7099–7100. [Google Scholar]; (c) Rychnovsky SD, Skalitzky DJ. Tetrahedron Lett. 1990;31:945–948. [Google Scholar]
- 35.(a) Hatakeyama S, Matsumoto H, Fukuyama H, Mukugi Y, Irie H. J. Org. Chem. 1997;62:2275–2279. doi: 10.1021/jo9618278. [DOI] [PubMed] [Google Scholar]; (b) Liu Z, Byun H-S, Bittman R. Org. Lett. 2010;12 doi: 10.1021/ol1009976. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.(a) Bayley H, Standring DN, Knowles JR. Tetrahedron Lett. 1978;39:3633–3634. [Google Scholar]; (b) Sun C, Bittman R. J. Org. Chem. 2004;69:7694–7699. doi: 10.1021/jo0487404. [DOI] [PubMed] [Google Scholar]
- 37.Xia C, Schümann J, Emmanuel R, Zhang Y, Chen W, Zhang W, De Libero G, Wang PG. J. Med. Chem. 2007;50:3489–3496. doi: 10.1021/jm0701066. [DOI] [PubMed] [Google Scholar]
- 38.(a) Miyamoto K, Miyake S, Yamamura T. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]; (b) Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, Teyton L, Bendelac A, Savage PB. J. Am. Chem. Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 39.Shirota O, Nakanishi K, Berova N. Tetrahedron. 1999;55:13643–13658. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.