Abstract
Iron cardiomyopathy remains the leading cause of death in patients with thalassemia major. Magnetic resonance imaging (MRI) is ideally suited for monitoring thalassemia patients because it can detect cardiac and liver iron burdens as well as accurately measure left ventricular dimensions and function. However, patients with thalassemia have unique physiology that alters their normative data. In this article, we review the physiology and pathophysiology of thalassemic heart disease as well as the use of MRI to monitor it. Despite regular transfusions, thalassemia major patients have larger ventricular volumes, higher cardiac outputs, and lower total vascular resistances than published data for healthy control subjects; these hemodynamic findings are consistent with chronic anemia. Cardiac iron overload increases the relative risk of further dilation, arrhythmias, and decreased systolic function. However, many patients are asymptomatic despite heavy cardiac burdens. We explore possible mechanisms behind cardiac iron-function relationships and relate these mechanisms to clinical observations.
Keywords: iron, heart, MRI, ejection fraction, cardiac function, T2*
INTRODUCTION
Thalassemia, although relatively uncommon in the United States, is the most common genetic disease worldwide.1 With increasing East Asian immigration to the Pacific States in the last two decades, thalassemia major is becoming an important domestic as well as international health challenge. Regular transfusion therapy, while improving patient quality of life, creates a state of iron overload, a second devastating disease. Once reticuloendothelial stores saturate, iron deposition increases in parenchymal tissues such as endocrine glands, hepatocytes, and myocardium. Typically silent for many years, cardiac iron deposition produces arrhythmias, systolic and diastolic dysfunction, and congestive heart failure in the second or third life decade.
The introduction of an effective iron chelation agent, deferoxamine, transformed thalassemia management in the 1980s. Administered as a continuous subcutaneous infusion, 8–12 hours per day, 5–7 days per week, deferoxamine therapy remains an onerous lifeline for thalassemia patients. Although chelation does prolong length and quality of life for thalassemia patients, cardiac toxicity remains the leading cause of death, generally striking patients in their third or fourth decade.2 Chelation noncompliance contributes to these deaths; however, some patients die despite apparently adequate liver iron chelation.3 Conventional cardiac surveillance, consisting of annual ECG, Holter, and echocardiogram, has proved remarkably ineffective in detecting preclinical cardiac iron overload. Electrocardiogram changes reflect primarily left ventricular hypertrophy and nonspecific ST–T wave changes from volume overload. Conduction abnormalities, consisting primarily of atrioventricular and bundle-branch block, typically present after symptomatic disease. Patients may complain of palpitations as the earliest clinical symptom; hence Holter monitoring has merit to document atrial and ventricular irritability. Iron toxicity arrhythmias are labile and often automatic rather than reentrant in nature, typically presenting with polymorphic atrial and ventricular arrhythmias.4 Although iron deposition and scarring does occur in the cardiac conduction system, deposits are not correlated with clinical presentation.
Abnormalities of ventricular systolic function on echocardiogram are nearly universal but are often not detectable until patients are in overt congestive heart failure. Echocardiographic assessment of myocardial function may be confounded by segmental wall motion abnormalities. As a result, measurements of resting ejection fraction by radionucleide angiography and magnetic resonance imaging (MRI) are more robust than echocardiography and are better at recognizing preclinical systolic dysfunction.5 While systolic dysfunction carries a grave prognosis, patients can be “rescued” by continuous deferoxamine administration, provided they are willing to comply with several years of this therapy.5,6
Although many left ventricular filling abnormalities have been described previously in thalassemia,7 most reports have failed to acknowledge the critical role of chronic anemia.8 Thalassemia patients have elevated cardiac output and stroke volumes, leading to elevated mitral inflow velocities and shorter “deceleration” times, regardless of cardiac iron status.8 Restrictive physiology may be observed in advanced disease but is often accompanied by systolic dysfunction or severe pulmonary hypertension.8 Impaired myocardial relaxation, common in hypertensive and idiopathic hypertrophic cardiomyopathy, has not been well documented in thalassemic cardiomyopathy.7 While diastolic dysfunction measures have the potential for earlier diagnosis of cardiac iron toxicity, standard techniques are confounded by sensitivity to the volume overload state (discussed in next section).
NORMAL CARDIAC PHYSIOLOGY IN THALASSEMIA MAJOR
Some of the limitations of conventional cardiac monitoring can be put in perspective by considering the normal cardiac physiology of thalassemia patients. Transfusion therapy was initiated in thalassemia major to stop the destructive effects of ineffective erythropoiesis and marrow expansion. Typically, this can be achieved by keeping pretransfusion hemoglobin levels between 9 and 10 g/dL, leaving patients with a mild chronic anemia.
Because hemoglobin is responsible for oxygen transport, the body compensates for chronic anemia in three important ways.9 Since oxygen delivery represents the product of cardiac output, hemoglobin, and hemoglobin saturation (usually >95% regardless of anemia), the body can compensate for low hemoglobin levels by increasing cardiac output. This measure can be easily documented through MRI. Table 1 demonstrates cardiac index (cardiac output indexed to body surface area) in 19 patients with thalassemia major compared with historical control subjects.10,11 Cardiac index was increased nearly 60% in the thalassemia patients compared with control subjects, comparable to their degree of anemia, resulting in relatively normal oxygen delivery.
TABLE 1.
Hemodynamic variables in thalassemia major
| Parameter | Thalassemia major patients | Reference range |
|---|---|---|
| Cardiac index (L/min/m2) | 4.6 ± 0.8 | 2.9 ± 0.6 |
| Pretransfusion hemoglobin (mg/dL) | 9.2 ± 0.6 | 14.5 ± 1 male 14.0 ± 2 female |
| Heart rate (bpm) | 84.1 ± 12.6 | 85 ± 15 |
| Left ventricular end-diastolic volume index (mL/m2) | 85.9 ± 15.9 | 58 ± 11.2 |
| Left ventricular end-systolic volume index (mL/m2) | 29.1 ± 11.6 | 18 ± 6.6 |
| Left ventricular ejection fraction (%) | 67 ± 6.9 | 69 ± 4.1 |
| Systolic blood pressure (mmHg) | 105 ± 8 | 104 ± 11 |
| Diastolic blood pressure (mmHg) | 60 ± 4 | 71 ± 10 |
Increased cardiac index can be achieved either by increased ventricular stroke volume index or by increased heart rate. We compared heart rate, cardiac index, cardiac volumes, and ejection fraction measured by MRI with vital signs and hemoglobin levels measured during routine blood transfusion visits (Table 1). Average heart rate was comparable to that of age-matched control subjects. Ejection fraction was within reference limits, but end-diastolic, end-systolic, and stroke-volume indices were elevated compared with those of controls.
Therefore, thalassemia major represents a chronic, high-output state, produced by volume-loaded ventricles rather than increased heart rate. To maintain a normal mean systemic blood pressure in the presence of high cardiac output, the body would have to lower the systemic vascular resistance.9 This response, similar to the physiologic compensation observed during exercise, occurs through peripheral arteriolar vasodilation and leads to wide pulse pressures and low diastolic pressures. Systolic blood pressures were comparable to those of age-matched control subjects; however, diastolic pressures were significantly decreased in our thalassemia patients (Table 1).
To summarize, the “normal” heart in thalassemia pumps at larger volumes (pre-load) and against lower peripheral resistance (afterload) than a normal heart. As a result, the expected cardiac parameters for non–iron-overload thalassemia major patients remains poorly characterized. Should the ejection fraction be higher, lower, or the same as that in an age- and sex-matched healthy volunteer? For now, the question remains unanswered. Understanding the normal physiologic baseline is critical to interpreting cardiac tests in these patients and understanding their response to pathologic stimuli.
EARLY DETECTION OF IRON CARDIOMYOPATHY
Since cardiac function remains normal until late in the spectrum of iron cardiomyopathy, other tools are necessary to anticipate and prevent iron cardiomyopathy. Liver iron provides a good index of total body iron stores, and high levels may convey future cardiac risk.12 Although traditionally estimated by biopsy or SQUID, liver iron level can now be accurately estimated using MRI. Iron shortens the MRI relation parameters T2 and T2* (and lengthens R2 and R2*) in a predictable and reproducible manner. These MRI techniques can be used to assess iron levels in the heart as well. Both myocardial T2 and T2* shorten in thalassemia patients.10,13-15 Patients with a normal T2* have normal function, but the relative prevalence of myocardial dysfunction and arrhythmias increases with lower T2* (high iron).10,14-16 Ventricular function is impaired in approximately 10% of patients having a T2* of 10 ms but nearly 70% for patients having a T2* of 4 ms.16 Like many other biomarkers, such as serum cholesterol level, abnormal T2* conveys only a relative risk; many patients with relatively high iron burdens are asymptomatic at the time of study. The predictive value of abnormal T2* is strongly implied but has not yet been demonstrated.
Although liver iron level has been used as a surrogate for cardiac iron for many years,12,17 the link between cardiac iron and liver iron is quite complicated. Some patients develop ventricular dysfunction despite low liver iron concentrations. In fact, there is little or no correlation between cardiac T2* (or cardiac function) and liver iron level in cross-sectional analyses.10,14,15 This observation raised concerns that cardiac T2* did not reflect cardiac iron level. However, recent work in animals indicates that cardiac T2 and cardiac T2* are determined primarily by cardiac iron concentration.18,19 The apparent paradox between cross-sectional and longitudinal studies of liver iron can be understood in the context of organ-specific iron transport and elimination.
PATHOPHYSIOLOGY OF IRON CARDIOMYOPATHY
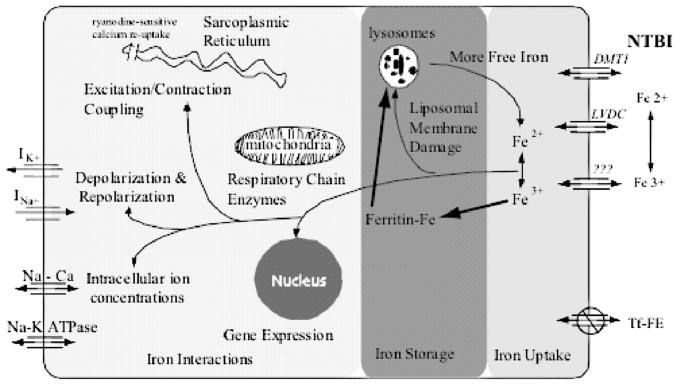
Figure 1 is a schematic illustration of the pathophysiology of iron cardiomyopathy, dividing the disease process into iron uptake, iron storage, and iron interactions. Characterization of iron transport mechanisms is important because it may represent an independent therapeutic target to complement chelation therapy. Iron uptake occurs primarily through uptake of non–transferrin-bound iron (NTBI).20-23 Both ferric and ferrous ions can be absorbed in tissue culture, and there are membrane-bound enzymes that facilitate conversion from one species to the other.20 Dimethyl transferase 1 (DMT1) levels have been implicated in intestinal iron transport but have not been definitively linked to cardiac iron transport.21 L-Type voltage-dependent channels (LVDCs) appear to mediate murine cardiac iron transport, accounting for at least half of cardiac iron uptake.23 Interestingly, neonatal rat myocytes do not use LVDCs, but it is unclear whether this represents species or maturational specificity.20
FIGURE 1.
Uptake, storage, and toxicity of cardiac iron in a myocyte. Fe2+ and Fe3+ enter the cell and are rapidly buffered and stored (bold arrows). Only stored iron is MRI active. Toxicity occurs when the iron buffering system is overwhelmed.
In cell culture, NTBI uptake can be quite rapid.22 Preexposure of myocytes to iron increases their uptake rate dramatically, suggesting a positive-feedback regulatory mechanism.22,24 Clinically, this finding indicates that relatively brief periods of very poor chelator compliance might lead to significant cardiac iron deposition in vulnerable individuals. Abnormal cardiac T2* is rarely found before the age of 10 years, even in patients with high liver iron concentrations.10 However, the prevalence of abnormal T2* jumps to more than 50% in late adolescence and early adulthood, suggesting relatively “precipitous” iron loading. This transition corresponds with the most difficult years for chelation compliance, but one cannot exclude contributions from developmental factors such as puberty.
Both the level and duration of NTBI exposure are probably important components of cardiac iron uptake. The level of NTBI appears to be a function of transferrin saturation and the liver's ability to buffer and store iron. Jensen and colleagues demonstrated that liver iron levels in excess of 19.5 mg/g led to dramatic increases in NTBI or “chelatable” iron in unchelated patients with myelodysplasia,25 suggesting a “saturation” threshold for the liver. In turn, excess chelatable iron was strongly correlated with cardiac iron loading by MRI (signal intensity ratio technique). Similar cardiac-risk “thresholds” for liver iron in this range have been suggested by the work of Brittenham12 and Mariotti.44 Liver diseases, such as cirrhosis, that modify the liver's ability to buffer and store iron could also increase vulnerability to extrahepatic iron deposition.26
Although high liver iron levels probably increase cardiac risk, low levels do not guarantee cardiac safety. Chronic exposure to lower levels of NTBI may be sufficient for cardiac iron overload. Labile iron species are suppressed during deferoxamine therapy but rebound within a few hours of stopping infusion.27 As a result, the hours per day of deferoxamine therapy may be as important to the heart as the grams per day of the drug.
Once NTBI enters the myocyte, it is rapidly buffered by ferritin, limiting its potential for redox damage or other harmful interactions in the cell.28-30 Within hours, the ferritin–iron complexes begin to appear in intracellular siderosomes for long-term storage. Some studies have suggested a “last-in, first-out” pattern of iron accessibility.28 Although this supposition is intuitive, these studies are limited to relatively short-term observations in tissue culture.
From a magnetic perspective, the “free-iron” species have little effect on MRI T2 or T2* values at physiologic concentrations. Once bound to ferritin, iron produces greater inhomogeneities in the magnetic field, leading to detectable changes in T2 and T2*. However, clusters of ferritin molecules or their breakdown products, such as are found in siderosomes, produce much larger changes (up to sixfold) in T2 or T2* for the same amount of iron than freely diffusing ferritin molecules.31 Hence, MRI is measuring predominantly long-term storage depots of iron rather than the functionally active iron. This observation explains why some individuals can have massive cardiac iron deposition without cardiac symptoms.
Nonetheless, all buffering systems have limited capacity or can be disrupted by other factors. Once this occurs, free iron levels rise within the cell, wreaking havoc through redox reactions, gene modulation, and direct interaction with ion channels.30,32-36 Through the Haber–Weiss reaction, iron catalyzes production of free radicals, leading to oxidative membrane damage throughout the cell. One membrane target is the siderosomes, increasing their fragility and competency.32,37 This, in turn, could potentially lead to release of additional redox-active iron species, setting up the potential for a positive-feedback system. Such a phenomenon may explain the catastrophic hemodynamic collapse seen in some patients.
Other membranes involved include the mitochondrial membranes. Iron is avidly taken up by mitochondria.30,38 Oxidative phosphorylation is impaired, although the mechanisms of this disruption are not completely understood. Chronic impairment of mitochondrial energy production causes dilated cardiomyopathy in many diseases and may represent a mechanism for the asymptomatic functional abnormalities observed in early iron cardiomyopathy.39
Elevated myocyte iron levels also lead to alterations in gene expression.33 Whether these changes represent controlled interactions through iron response elements or nonspecific effects from redox damage is unclear. Myocytes appear to tonically suppress fibroblast proliferation, but this paracrine effect is reduced by myocyte iron loading.40 This observation represents one potential mechanism for iron-induced cardiac fibrosis in the thalassemic heart.
Ferrous iron has similar size and charge to that of calcium irons, the major mediator of excitation–contraction coupling and a major determinant of the cardiac action potential. Hence it is not surprising that iron overload results in arrhythmias and poor cardiac function.4,35,36,41 Ferrous iron can directly interact with the ryanodine-sensitive calcium channel in the sarcoplasmic reticulum.34 This channel is responsible for activation of contraction and also modulates calcium reuptake in the sarcoplasmic reticulum. Ryanodine channel dysfunction is the common denominator for a wide variety of congenital and acquired arrhythmogenic cardiomyopathies.42
Intracellular iron also impairs function of membrane-bound fast-sodium channels as well as delayed-rectifier potassium currents.36 The former channels are responsible for the rapid upstroke of the cardiac action potential. Channel blockage or other interference will slow cardiac conduction, broadening the QRS of the EKG36,41 and delayed-action potential spread across the myocardium.41 Both calcium and potassium channel modification may be responsible for repolarization abnormalities such as early or delayed afterdepolarizations and QTc prolongation. These changes are associated with both triggered ventricular arrhythmias and reentrant mechanisms such as torsade-de-pointes.42
Once arrhythmias or cardiac dysfunction develops, aggressive chelation must be initiated, regardless of the total iron burden. The current standard of care remains continuous deferoxamine therapy because it provides a continuous “sink” for free iron species.5,6 Continuous administration also may overcome unfavorable transport kinetics of deferoxamine across the myocyte membrane. Cardiac symptoms typically stabilize in a period of weeks to months once the “free” iron levels are consistently suppressed. Sustained recovery, however, often necessitates continued therapy for several years, suggesting that cardiac iron stores deplete more slowly.5,6 In fact, it has been demonstrated by MRI that the rate of iron elimination in the heart is nearly sixfold slower than that in the liver.15,43 The rate-limiting step in cardiac iron excretion is not known but may reflect ferritin turnover.
The asymmetry of cardiac iron loading and elimination compared with that in the liver effectively weakens or destroys any cross-sectional correlation between liver and cardiac iron levels. Thus, an elevated liver iron level has no predictive value for whether the heart is currently iron loaded, but it may convey prospective risk for subsequent cardiac iron loading. MRI offers a unique tool to prospectively study the interplay between hepatic and extrahepatic iron stores.
CONCLUSION
Despite transfusion therapy, thalassemia major represents a chronically anemic condition resulting in volume-loaded ventricles and increased peripheral vasodilation.9 As a result, there is a paucity of appropriate normative data for thalassemia patients. The mechanisms and kinetics of iron entry and clearance differ markedly in the heart and liver, leading to a complicated relationship between the two parameters.20,22,43 Increased levels of stored iron can be detected using MRI and are associated with increased relative risk of poor ventricular function.10,15
ACKNOWLEDGMENTS
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (1 R01 HL75592-01A1), the General Clinical Research Center at Childrens Hospital Los Angeles (RR0043-43), Department of Pediatrics at Childrens Hospital Los Angeles, and Novartis Pharma AG, Basel, Switzerland.
REFERENCES
- 1.Weatherall DJ. Thalassemia in the next millennium. Keynote address. Ann. N.Y. Acad. Sci. 1998;850:1–9. doi: 10.1111/j.1749-6632.1998.tb10456.x. [DOI] [PubMed] [Google Scholar]
- 2.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 3.Aldouri MA, Wonke B, Hoffbrand AV, et al. High incidence of cardiomyopathy in beta-thalassaemia patients receiving regular transfusion and iron chelation: reversal by intensified chelation. Acta Haematol. 1990;84:113–117. doi: 10.1159/000205046. [DOI] [PubMed] [Google Scholar]
- 4.Vecchio C, Derchi G. Management of cardiac complications in patients with thalassemia major. Semin. Hematol. 1995;32:288–296. [PubMed] [Google Scholar]
- 5.Davis BA, O'Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–269. doi: 10.1182/blood-2003-08-2841. [DOI] [PubMed] [Google Scholar]
- 6.Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000;95:1229–1236. [PubMed] [Google Scholar]
- 7.Gharzuddine WS, Kazma HK, Nuwayhid IA, et al. Doppler characterization of left ventricular diastolic function in beta-thalassaemia major. Evidence for an early stage of impaired relaxation. Eur. J. Echocardiogr. 2002;3:47–51. doi: 10.1053/euje.2001.0114. [DOI] [PubMed] [Google Scholar]
- 8.Kremastinos DT, Tsiapras DP, Tsetsos GA, et al. Left ventricular diastolic Doppler characteristics in beta-thalassemia major. Circulation. 1993;88:1127–1135. doi: 10.1161/01.cir.88.3.1127. [DOI] [PubMed] [Google Scholar]
- 9.Aessopos A, Farmakis D, Hatziliami A, et al. Cardiac status in well-treated patients with thalassemia major. Eur. J. Haematol. 2004;73:359–366. doi: 10.1111/j.1600-0609.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 10.Wood JC, Tyszka TM, Ghugre N, et al. Myocardial iron loading in transfusion-dependent thalassemia and sickle-cell disease. Blood. 2004;103:1934–1936. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz CH, Walker ES, Morgan VL, et al. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 1999;1:7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- 12.Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N. Engl. J. Med. 1994;331:567–573. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 13.Voskaridou E, Douskou M, Terpos E, et al. Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassaemia and sickle cell disease. Br. J. Haematol. 2004;126:736–742. doi: 10.1111/j.1365-2141.2004.05104.x. [DOI] [PubMed] [Google Scholar]
- 14.Wood JC, Ghugre N, Carson S, et al. Predictors of abnormal myocardial function and T2* in children and young adults with thalassemia major. Blood. 2003;102:952a. [Google Scholar]
- 15.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 16.Westwood MA, Anderson LJ, Tanner MA, Pennell DJ. The relationship between myocardial iron deposition and left ventricular dysfunction in thalassemia using cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2005;7:46–47. [Google Scholar]
- 17.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761. [PubMed] [Google Scholar]
- 18.Wood JC, Otto-Duessel M, Aguilar M, et al. Cardiac MRI (T2, T2*) predicts cardiac iron in the gerbil model of iron cardiomyopathy. Circulation. 2005;112:535–543. doi: 10.1161/CIRCULATIONAHA.104.504415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZJ, Lian L, Chen Q, et al. 1/T2 and magnetic susceptibility measurements in a gerbil cardiac iron overload model. Radiology. 2005;234:749–755. doi: 10.1148/radiol.2343031084. [DOI] [PubMed] [Google Scholar]
- 20.Parkes JG, Olivieri NF, Templeton DM. Characterization of Fe2+ and Fe3+ transport by iron-loaded cardiac myocytes. Toxicology. 1997;117:141–151. doi: 10.1016/s0300-483x(96)03566-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Parkes JG, Templeton DM. Differential accumulation of non-transferrin-bound iron by cardiac myocytes and fibroblasts. J. Mol. Cell. Cardiol. 2003;35:505–514. doi: 10.1016/s0022-2828(03)00072-5. [DOI] [PubMed] [Google Scholar]
- 22.Parkes JG, Hussain RA, Olivieri NF, Templeton DM. Effects of iron loading on uptake, speciation, and chelation of iron in cultured myocardial cells. J. Lab. Clin. Med. 1993;122:36–47. [PubMed] [Google Scholar]
- 23.Oudit GY, Sun H, Trivieri MG, et al. L-Type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003;9:1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 24.Randell EW, Parkes JG, Olivieri NF, Templeton DM. Uptake of non-transferrin-bound iron by both reductive and nonreductive processes is modulated by intracellular iron. J. Biol. Chem. 1994;269:16046–16053. [PubMed] [Google Scholar]
- 25.Jensen PD, Jensen FT, Christensen T, et al. Evaluation of myocardial iron by magnetic resonance imaging during iron chelation therapy with deferrioxamine: indication of close relation between myocardial iron content and chelatable iron pool. Blood. 2003;101:4632–4639. doi: 10.1182/blood-2002-09-2754. [DOI] [PubMed] [Google Scholar]
- 26.Buja LM, Roberts WC. Iron in the heart. Etiology and clinical significance. Am. J. Med. 1971;51:209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 27.Esposito BP, Breuer W, Sirankapracha P, et al. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102:2670–2677. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 28.Shiloh H, Iancu TC, Bauminger ER, et al. Deferoxamine-induced iron mobilization and redistribution of myocardial iron in cultured rat heart cells: studies of the chelatable iron pool by electron microscopy and Mossbauer spectroscopy. J. Lab. Clin. Med. 1992;119:428–436. [PubMed] [Google Scholar]
- 29.Stuhne-Sekalec L, Xu SX, Parkes JG, et al. Speciation of tissue and cellular iron with on-line detection by inductively coupled plasma-mass spectrometry. Anal. Biochem. 1992;205:278–284. doi: 10.1016/0003-2697(92)90435-a. [DOI] [PubMed] [Google Scholar]
- 30.Iancu TC, Shiloh H, Link G, et al. Ultrastructural pathology of iron-loaded rat myocardial cells in culture. Br. J. Exp. Pathol. 1987;68:53–65. [PMC free article] [PubMed] [Google Scholar]
- 31.Wood JC, Fassler J, Meade T. Mimicking liver iron overload using liposomal ferritin preparations. Magn. Reson. Med. 2004;51:607–611. doi: 10.1002/mrm.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Link G, Pinson A, Hershko C. Iron loading of cultured cardiac myocytes modifies sarcolemmal structure and increases lysosomal fragility. J. Lab. Clin. Med. 1993;121:127–134. [PubMed] [Google Scholar]
- 33.Parkes JG, Liu Y, Sirna JB, Templeton DM. Changes in gene expression with iron loading and chelation in cardiac myocytes and non-myocytic fibro-blasts. J. Mol. Cell. Cardiol. 2000;32:233–246. doi: 10.1006/jmcc.1999.1068. [DOI] [PubMed] [Google Scholar]
- 34.Kim E, Giri SN, Pessah IN. Iron(II) is a modulator of ryanodine-sensitive calcium channels of cardiac muscle sarcoplasmic reticulum. Toxicol. Appl. Pharmacol. 1995;130:57–66. doi: 10.1006/taap.1995.1008. [DOI] [PubMed] [Google Scholar]
- 35.Link G, Athias P, Grynberg A, et al. Effect of iron loading on transmembrane potential, contraction, and automaticity of rat ventricular muscle cells in culture. J. Lab. Clin. Med. 1989;113:103–111. [PubMed] [Google Scholar]
- 36.Kuryshev YA, Brittenham GM, Fujioka H, et al. Decreased sodium and increased transient outward potassium currents in iron-loaded cardiac myocytes. Implications for the arrhythmogenesis of human siderotic heart disease. Circulation. 1999;100:675–683. doi: 10.1161/01.cir.100.6.675. [DOI] [PubMed] [Google Scholar]
- 37.Selden C, Owen M, Hopkins JM, Peters TJ. Studies on the concentration and intracellular localization of iron proteins in liver biopsy specimens from patients with iron overload with special reference to their role in lysosomal disruption. Br. J. Haematol. 1980;44:593–603. doi: 10.1111/j.1365-2141.1980.tb08714.x. [DOI] [PubMed] [Google Scholar]
- 38.Link G, Saada A, Pinson A, et al. Mitochondrial respiratory enzymes are a major target of iron toxicity in rat heart cells. J. Lab. Clin. Med. 1998;131:466–474. doi: 10.1016/s0022-2143(98)90148-2. [DOI] [PubMed] [Google Scholar]
- 39.Russell LK, Finck BN, Kelly DP. Mouse models of mitochondrial dysfunction and heart failure. J. Mol. Cell. Cardiol. 2005;38:81–91. doi: 10.1016/j.yjmcc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Templeton DM. The effects of cardiac myocytes on interstitial fibroblasts in toxic iron overload. Cardiovasc. Toxicol. 2001;1:299–308. doi: 10.1385/ct:1:4:299. [DOI] [PubMed] [Google Scholar]
- 41.Laurita KR, Chuck ET, Yang T, et al. Optical mapping reveals conduction slowing and impulse block in iron-overload cardiomyopathy. J. Lab. Clin. Med. 2003;142:83–89. doi: 10.1016/S0022-2143(03)00060-X. [DOI] [PubMed] [Google Scholar]
- 42.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc. Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br. J. Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 44.Mariotti E, Angelucci E, Agostini A, et al. Evaluation of cardiac status in iron loaded thalassemia patients following bone marrow transplantation during reduction in body iron burden. Br. J. Haematol. 1998;103:916–921. doi: 10.1046/j.1365-2141.1998.01099.x. [DOI] [PubMed] [Google Scholar]