Abstract
Conventional temperature measurements rely on material responses to heat, which can be detected visually. When Galileo developed an air expansion based device to detect temperature changes, Santorio, a contemporary physician, added a scale to create the first thermometer. With this instrument, patients’ temperatures could be measured, recorded and related to changing health conditions. Today, advances in materials science and bioengineering provide new ways to report temperature at the molecular level in real time. In this review the scientific foundations and history of thermometry underpin a discussion of the discoveries emerging from the field of molecular thermometry. Intracellular nanogels and heat sensing biomolecules have been shown to accurately report temperature changes at the nano-scale. Various systems will soon provide the ability to accurately measure temperature changes at the tissue, cellular, and even sub-cellular level, allowing for detection and monitoring of very small changes in local temperature. In the clinic this will lead to enhanced detection of tumors and localized infection, and accurate and precise monitoring of hyperthermia based therapies. Some nanomaterial systems have even demonstrated a theranostic capacity for heat-sensitive, local delivery of chemotherapeutics. Just as early thermometry moved into the clinic, so too will these molecular thermometers.
Introduction
Temperature is a master environmental variable that affects virtually all natural and engineered systems, from the molecular through the “systems” level. Conventional temperature measurements rely on repeatable physical manifestations of molecular effects, which report changes in material heat content on a macro-scale. The expansion of mercury, the ductility and conductivity changes of metal, and shifts in infrared reflections are calibrated to associate molecular level changes with easily interpretable scales, which we use—and take for granted—in everyday life. As more reliable and economically feasible methods have evolved for directly examining material changes at near molecular dimensions in real-time, it follows that one of the obvious outcomes is molecular thermometry. In this review, we define molecular thermometry as: a direct measurement of molecules or cohorts of molecules, which distinctly respond to heat transfer on a nanoscale — either alone or through the behavior of like-scale reporters which can be associated with physical/mathematical benchmarks that we (already) use to describe the heat content of a given substance. While these concepts are the traditional realm of thermodynamics, the biological context offers a fundamental path for such local temperature measurements at a cellular, and likely even subcellular, level.
In the clinic, molecular thermometry could be applied to images and possibly treat tumors and localized infections. The local increases in heat released from the sites of tumors and infections cause significant temperature gradients that have long been known (1–3), and molecular thermometry could aid detection in a manner similar to conventional contrast agents. With increased concern regarding emerging infections and multidrug resistant microorganisms, especially those of nosocomial origins, the potential benefit of imaging or opsonizing localized infections would yield obvious benefits (2). Further, as the field of theranostics evolves, sensing differential heat content with nano-scale biosensors that can respond with the local temperature-sensitive delivery of pharmaceuticals, including chemotherapeutics and antimicrobials, may be an indispensable facet of their application (4).
As the medical applications of bioelectromagnetics and the directed interaction of electromagnetic fields (EMF) with biological systems become mainstream, the field is rapidly yielding clinical devices and applications based on sound scientific understanding of molecular level effects. Many biological applications of EMF purposely deliver energy to rapidly change the temperature of local targets in tissues (lasers, cauterizations); but there are other therapeutic applications facilitated by directed EMFs with controlled heat transfer. Nanopulse EMF have been calculated to increase temperature at the subcellular level, heating the membrane without raising the intracellular temperature (De Vita A et al., Anomalous Heating in a Living Cell with Dispersive Membrane Capacitance Under Pulsed Electromagnetic Fields. Bioelectromagnetics Society Meetings, June 14–19, 2009 Davos, Switzerland. Abstract P-205). Other EMF-based therapies that have shown promise rely on an increase in cellular or tissue temperature; induced hyperthermia has been shown to enhance the effects of gamma irradiation (6, 7), and a recent study has shown hyperthermia can lead to tumor clearance, including metastases that were not directly treated, likely through an immune mediated mechanism (Ito A., Hyperthermia using functional magnetite nanoparticles. Bioelectromagnetics Society Meetings, June 14–19, 2009 Davos, Switzerland. Abstract Plenary 3-2). The reporting and control of local tissue temperatures will be critical for the success of these and other emerging therapeutic and surgical methods, and as such, in-situ systems engineered to accurately monitor temperature at a tissue and cellular level will become essential.
History of Thermometry
The earliest assessment of temperature was likely based on sensation or observation; hot versus cold weather; the vaporization of water as it boils; or from a physician’s view point, how ill patients’ bodies can often be notably warmer than that of their healthy counterparts. These early temperature associations lacked any basic understanding of material heat content, heat transfer processes, or empiric methods of their measurement. A physical understanding of temperature did not begin to arise until around 100BC when Hero of Alexandria observed that air volumes vary significantly with temperature; however, this observation had to wait over 1700 years to be utilized in a measurement device (9). In the late 1500’s, Galileo and a group of Venetian scientists and physicians developed the thermoscope and ultimately the first thermometer (10).
Galileo had read of the experiments of Hero, and repeated these same observations utilizing a tube containing air trapped above a column of water; when heated by his hands, the water column dropped as the air expanded, and as the column again cooled, the water rose (9). Galileo had invented the precursor of a modern thermometer, many of which also rely on expansion and contraction of liquid materials within a tube. This device, while absolute and repeatable in response, was lacking a critical standard: a scale would permit air volume changes to be related to heat content through simple mathematical means; that would, in turn, enable a standardized record, which could be compared over time.
The addition of a scale to the thermoscope, thus creating a calibrated thermometer, is commonly attributed to Santorio Santorio, a Venetian physician and contemporary of Galileo. Santorio devoted great efforts to measurements of the human body, his own and his patients. He utilized a pendulum (another of Galileo’s inventions) to accurately measure pulse, and regularly weighed himself, his food and beverage intake, and his wastes, and determined much of his intake was systematically lost through "insensible perspiration" (9, 11). It is of great significance to the birth of thermodynamics that Santorio was among the first to recognize that heat transfer occurs continuously, and progresses through both “sensible” and “insensible” mechanisms: that is, the heat content of materials changes in a sensible relationship to their temperature when they are of a single phase (liquid), as well as through an “insensible” relationship when materials change phase (evaporation). The latter entity, “insensible heat”, is the early thermodynamic concept of latent heat capacity – or the energy required for a substance to change phase, either from solid to liquid, or liquid to gas (12). When phase changes occur, they do so at constant temperature, while significant amounts of thermal energy are concomitantly transferred to the environment (perspiration and the welcome effect of evaporative cooling) (11). The phenomenon of latent heat transfer was termed “non-sensible” or “insensible” because no temperature change could be observed (seen) during this energy intensive process (11).
Santorio also identified that heat transfer processes could be quantified and scaled in a range that was germane to biological functions. It is thus no surprise that this fastidious “measurer” would adapt the thermoscope to systematically record human temperatures on a consistent scale. This adaptation was critical to the development of modern thermometry. Indeed, Santorio proved that by capturing the heat liberated from a living being in an adjacent but confined material, the heat transfered could manifest in a macro-scale reporting system (the density change of water and gases), such that he could record and compare cohorts of patients’ temperatures through changing health conditions (9, 10).
As such observations evolved to support the empirical science of thermodynamics, the understanding of temperature moved from the simple concept of sensible heat, to more advanced perspectives of specific heat content, latent heat capacity, and how thermal energy transiently moves in biological systems and the environment at large. It was later realized that the thermoscope and thermometers were leveraging intrinsic material properties that isolated heat content as a variable. Eventually, the invention of the sealed thermometer, a bulb and stem alcohol thermometer, by Ferdinando II de' Medici was able to measure temperature independently of barometric pressure, unlike Galileo and Santorio’s open thermoscope and thermometer (13). A similar device utilizing mercury, designed by Daniel Gabriel Fahrenheit, gave us the common glass tube thermometer calibrated to report on a Fahrenheit scale (°F) (13). Improved understanding of the nature of sensible heat and material heat content led to the continued development of temperature scales that were increasingly precise and molecularly relevant.
Thermodynamics and Thermometry
Classical thermodynamics hosts the convention for the temperature scales used in contemporary medicine, engineering, and bioengineering. The salient theme through which thermometry has evolved continues to be defined by thermodynamic thresholds associated with material phase transitions. These transitions are visible on the macroscale, where correlations of temperature and the behavior of ordinary substances —the boiling of water, or the expansion of mercury— are commonplace. They extend to wide-spread engineering applications where repeatable changes in material properties manifest from density changes. The ubiquitous thermocouple is calibrated to report temperatures based on a metal’s current capacity or physical conformation, as it expands in response to changing heat content – a microscale process, which is used for thermal control systems. We now leverage analytical tools that confidently report physical conformation on sub-cellular scales; where we can literally watch engineered and biological polymers undergo structural transitions that are as predictable as mercury expansion and the transition from ice to water. Phase transitions are the common denominator of thermometry, and the prospects for (bio)polymer-based temperature reporting is replete with power and in situ functional potential.
Throughout history, physicists, chemists, and more recently bioengineers have pursued thermometry on an absolute temperature scale, independent of the properties of individual substances, and thus report true heat content (12). Attempts at such manifest in the modern Rankine (R) scale in the English system, and the Kelvin (K) scale in the SI system (both these scales officially abandoned the degree designation in 1967 [e.g. ° K] to reflect their physical basis in absolute zero) (12). A scale that is thermodynamically identical to these absolute scales is based on the activity of ideal gases, where the temperature is defined as zero when the pressure they exert is zero (12). Using this basis, efforts toward improving the range and accuracy of direct temperature measurements have vastly improved over the last generation, and in large part rely on nanomaterial science to produce, contain, and control unique states of matter (e.g. plasma, Bose-Einstein condensates (14–16)) as well as a multitude of systems which deliver, detect, and record different forms of electromagnetic radiation (e.g. lasers and infrared lenses (17)). The international temperature scale of 1990 (ITS-90) reflects how recent advances in nanoscale science translate into improved applications (18). ITS-90 is based on the fusion and sublimation of water at its triple point – the thermodynamic condition (absolute pressure and temperature) where liquid, vapor, and solid ice exist in physical equilibrium.
While absolute scales have improved thermometry, absolute scales are typically used in science and engineering outside ranges that are germane to biological systems— except when supporting the development of localized destructive freezing practices for surgeries and long-term cryogenic storage (19, 20). The resolution and reporting of temperature is largely idiosyncratic to application, and often intrusive; nowhere is this more obvious than in medical settings. With notable exception to infrared reflectometry, which is local and surficial (2), temperature measurements within biological systems are notoriously difficult from a bioengineering perspective because of the range of their scale(s) and the fact that they are not in thermodynamic equilibrium.
Thermodynamics teaches us that fixed point calibration brings tremendous leverage to thermometry from an absolute basis, regardless of the reporting mechanism and how it is used in a thermometer: if water-ice and steam points are assigned respective values of 0 and 100, then the temperature scale will be identical to the Celsius scale. Any scale can thus be “embedded” in another, provided the fixed points defining the desired range are also based on a thermodynamic phase transition; thus, thermometry can be extended to any material(s) which undergoes structural transition, provided the time frame of the transition is germane to the system of concern, including, but not limited to, tissues, cells, and biopolymers they contain.
Nanomaterial Based Thermometry
Just as the first thermometer was utilized for medicine, as new thermometric technologies are developed, they too are adapted for medicine and biomedical research, though not always with the success Santorio enjoyed. An early example of a micro- or nano-scale approach to thermometry utilizing embedded scales and relevant to medical settings comes from a patent filed in the late1970s (21). This patent relies on the changes in the magnetic characteristics of ferromagnetic, paramagnetic, and diamagnetic materials corresponding to changes in temperature. Utilizing micron-sized particles, which were injected intravenously, the patent suggests that certain cells will absorb particles according to an equilibrium distribution, which is dependent on intracellular temperature. With calibration to known temperatures, a sensitive 3-dimensional scanner could then detect the changes in the magnetic properties of these intracellular particles. While the underlying physics is sound (22, 23), there are no published examples of this technology being utilized. This is likely due to problems achieving the broad cellular uptake, issues with biocompatability, and a lack (at that time) of viable 3D imaging technologies required to make implementation of such a method practical.
With the creation of nanomaterials, this same theory has been applied to develop a more practical cellular thermometry. The origins of this technology lie in the development of fluorescent quantum dots (QD), also known as semiconductor nanocrystals. These QDs have found many in vitro uses as fluorescent labels with size dependent emission wavelengths, bright fluorescence, and resistance to photobleaching (24). However, the main effect temperature has on QDs in a physiological range is to shorten their radiative-lifetimes with increasing temperature through 300K (26.8°C) (24–26). While QDs do respond to temperature changes in only a limited capacity, the fluorescence intensity they liberate is significantly affected by the makeup of their immediate surroundings (24). Thus, QDs can provide a simple, fluorescence-based detection system that is sensitive to environmental conditions.
Materials scientists have taken advantage of QD sensitivity to environment and developed nanogels that significantly change structure in response to temperature. These nanomaterial gels expand or contract with temperature changes, and this alteration in conformation at the molecular level will exclude ions and even water molecules (27). When QDs are placed in or conjugated to these nanogels, changes in temperature alter the local environment such that QDs significantly change the intensity of the fluorescence they liberate (27). These technologies have been successfully used to measure relevant temperatures in physiological buffers (27).
Recent advances in this nanogel technology no longer rely on QDs for reporting, but rather use chemical modifications that lend environmentally sensitive fluorescence to the nanogel itself. This type of engineered nanogel behaves similarly to QD containing nanogels; it changes conformation in response to temperature changes, altering the molecular environment and thus changing the emission of integrated fluorescent moieties (28). This fluorescent nanogel thermometer has been successfully microinjected into COS-7 cells and demonstrated temperature dependent fluorescence with a resolution of 0.31°C at ~28°C (28).
One of the largest concerns about the use of nanomaterials in the biological arena, and particularly in medicine, is biocompatability. Early studies of the interactions of nanomaterials in biological systems have shown that many nanomaterials are unpredictably reactive, and can produce reactive oxygen species, radicals, and a host of other by-products as a result of their highly ordered structures (29, 30). While there has been extensive testing of biocompatability with engineered nanomaterials that could potentially serve as in-situ thermometric systems (27, 28), due to the complex nature of biological systems, minor but significant effects on cellular systems may exist that have not yet been observed. Further, much of the biocompatibilty testing has been observed on short time scales, and longer-term interactions, as the biomolecules used for targeting are likely degraded, revealing the QD cores, must be studied; such systems have been examined in animal models with few effects, though long term effects have not been examined other than to note tissue specific accumulation (24). This lack of data poses significant problems for long term in vivo and ultimately clinical applications of this technology.
Biomolecular Based Molecular Thermometry
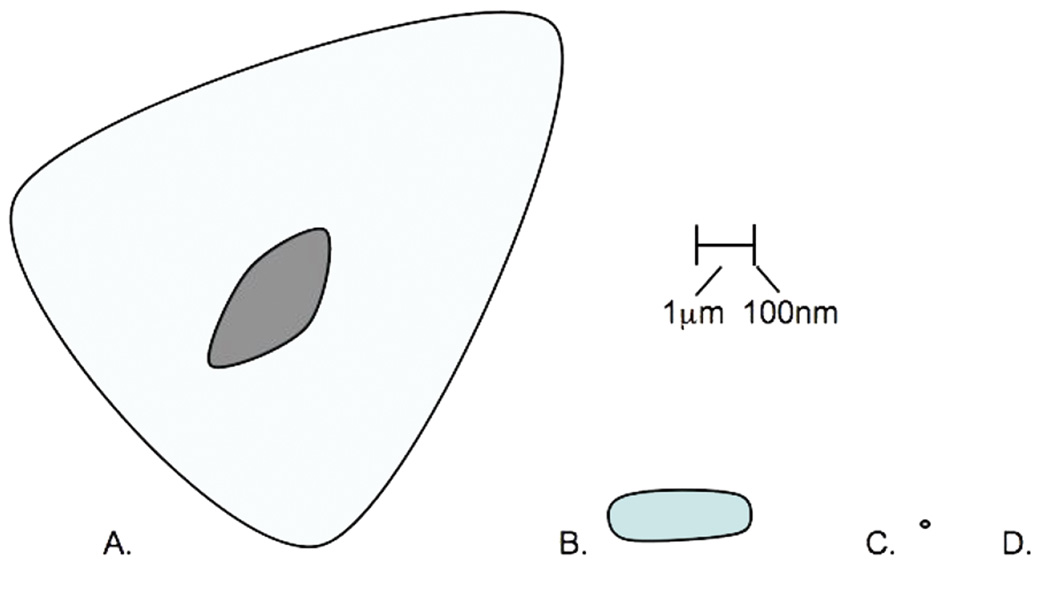
To circumvent the engineered nanomaterials-based biocompatibility question, other systems must be investigated. Perhaps the most biocompatible nanomaterials are biological macromolecules themselves. By their nature, biological molecules are naturally occurring nanomaterials; they are highly ordered molecular structures of the size range characteristic of engineered nanomaterials — less than 100 nanometer (nm) in any dimension (Figure 1). Further, biological macromolecules, by the nature of biological diversity, function at a wide range of temperatures. This eliminates the issue of a relevant scale seen with temperature sensing using QDs alone without nanogels. Further, engineering of specific biomolecules has become routine; thus, we can readily design and even evolve bionanomaterials to perform specific functions (e.g. aptamers (31)). The next step to designing biological molecules for intracellular thermometry is a relatively small one.
Figure 1.
Relative sizes of cells, proteins, and nanomaterials discussed in this review. A. Mammalian cell-10-110µm, B. Bacterium-1-5µm, C. EGFP-300nm diameter, D. QD-5-50nm (1/2 to 1/20 the size of the period)
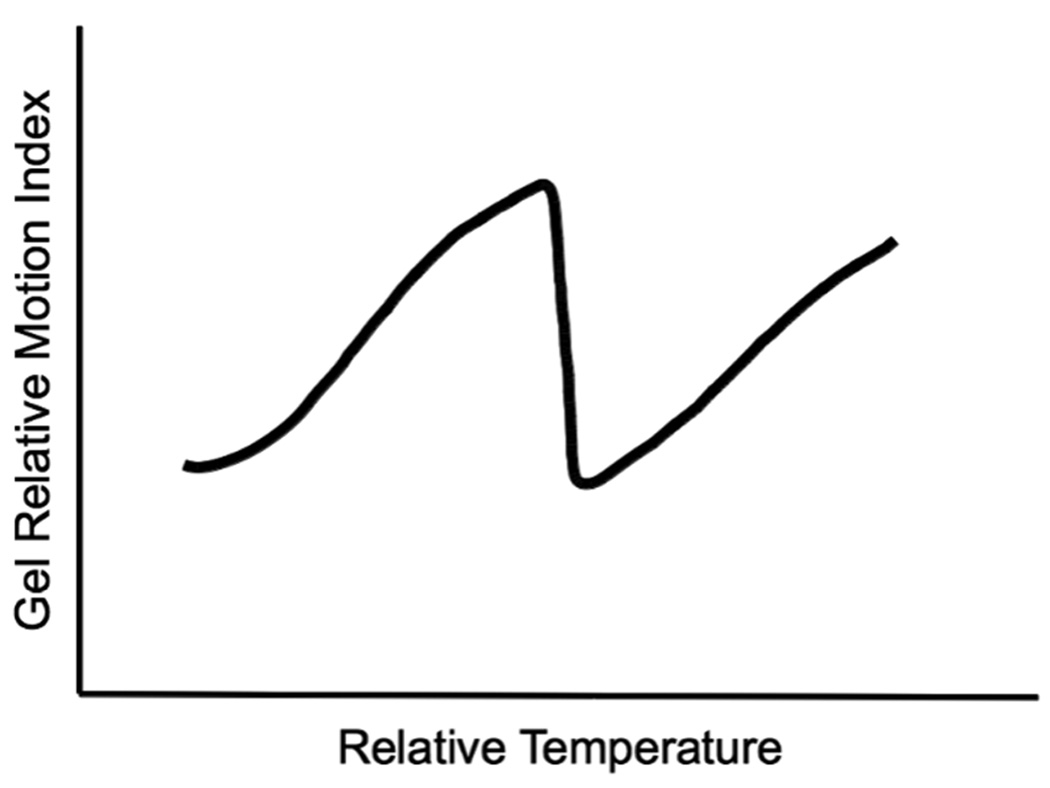
Some synthetic polymer gels share physical/chemical and thus structural features with the contents of living cells. Much like their synthetic counterparts, hydrogels that are constructed in whole or in part of natural biopolymers are gaining increased attention for thermometric applications. Such pseudo-natural gels, from a physical perspective, mimic nano-scale framework of actual intercellular matrices – such as cytoplasm, which is a unique and dynamic hydrogel created by short and long polymers biochemically associating with gelatinous qualities (32). Similar to the way water condenses to ice, the biopolymers, which constitute some hyrdogels, can experience significant physical transformations in response to temperature thresholds (33), and thus will be useful for thermometry. In this context, hydrogel transformations have been termed “phase transitions” by a growing cohort of biophysicists (34), which were first predicted through thermodynamic calculation by polymer scientists (35), and subsequently modeled by experiment (36, 37). These transitions, characterized by massive conformational changes, including protein denaturation or the melting of hybridized nucleotide complexes, are typically triggered at thresholds through small environmental shifts, much like the freezing and boiling of liquids. The denaturation of protein in a cooking egg provides an excellent example. Biopolymer interactions that maintain the character of hydrogels are pseudo-stable at a given heat (content), pH, ionic strength through a conglomerate of weak intermolecular interactions; by their nature these become unstable at physical/chemical thresholds. While the temperature-induced shift of selected biopolymer conformation (e.g. a specific protein) from one folded state to another is well recognized, the accompanying shift in the stability of gel-like plasma in which proteins reside, is less acknowledged. Yasunaga and colleagues attempted to model plasma-like gelling behavior with hydrogels of a common polymer (acronymed PNIPAM) (38, 39). Using nuclear magnetic resonance, they demonstrated that when the temperature of a PNIPAM-containing solution progressively increased, a surrogate metric for the motional freedom of associated water (T2/s) also increased to a marked threshold. Beyond a critical temperature, termed the “gellation temperature” water motion becomes remarkably restricted as the “system gels”. An analogous thermodynamic motif emerged which parallels the freezing of pure substances: where temperature thresholds predictably associate with profound structural changes can be leveraged for thermometry (Figure 2).
Figure 2.
Generalized structural motif representing how some engineered polymeric hydrogels respond to temperature shifts as judged by water exclusion. The fluidity of hydrogels is governed by weak electrostatic interactions between water and the polymers comprising the gel. When temperature reaches a “gel” threshold, water and polymer order undergoes formidable structural rearrangements. This behavior is parallel to the thresholds observed where pure substances experience conventional phase changes.
Some of the earliest studies of genetic mutation took advantage of these biopolymer transitions in response to changes in temperature (40, 41). The use of temperature sensitive (ts) mutants, or wild-type (wt) biomolecules for that matter, are typically considered only in the context of their biological function, however, they can also provide information about their environment. These mutants (both heat and cold sensitive) essentially function as biological thermometers; they have a detection system (i.e. the function of the biomolecule typically evidenced through a detectable phenotype) and a relevant scale can be readily defined (i.e. the biomolecule functions at 35°C, but not at 42°C). Though much of the literature surrounding ts mutants focuses on function as a measure of denaturation or structural change in an on/off manner, some studies have examined function through a range of temperatures.
Chao and coworkers developed a series of lacI-ts mutants to eliminate the need for biochemical analogs to activate lactose-triggered expression systems (42). Instead of using expensive reagents to induce the desired expression of a critical gene cassette (lacI), key regulatory proteins (LacI) were engineered such that they would thermally denature at predetermined temperature levels, and expose the transcription factor-binding site (lacO), which controlled the downstream production of a valued pharmaceutically active protein. In controlled steps between 1 and 3 °C, they examined the expression of the reporter protein LacZ through the range between 30–40°C, and demonstrated a significant response in LacZ activity corresponding to stepwise temperature increases through this physiologically relevant range (42). This group, in developing a temperature based expression system to reduce the cost of pharmaceutical production, has laid the groundwork for a ts mutant-based intracellular thermometer. Further work characterizing these vectors, perhaps with different reporter constructs or in different cell systems, will be required to confirm the clinical applicability of such a system as a biomolecular thermometer.
Even less complicated systems for a biomolecular thermometer can also be imagined. One report utilized loss of enhanced green fluorescent protein (EGFP) fluorescence as a marker for cellular heating, assuming heat denaturation led to this loss of fluorescence (Wood W, et al., The Use of Fluorescent Dyes and Other Markers to Measure Elevated Temperature in Cells During RF Exposure. Bioelectromagnetics Society Meetings, June 14–19, 2009 Davos, Switzerland. Abstract P-63). However, this group observed a sharp reduction in fluorescence through a narrow temperature threshold, limiting the sensitivity and applicable range of this system using EGFP. This approach can likely be applied to other reporter proteins, wt or engineered. As a result of their individual structural features and stabilities, other proteins would likely behave differently, showing different temperatures at which the reporter denatures or perhaps broader temperature ranges of detection. Several reporters could be used in concert to achieve a broad range sensitive thermometric system in a cell utilizing native wt proteins.
Another approach to an EGFP based thermometric system would be to examine, not the loss of fluorescence, but rather changes in the emission spectrum of a fluorescing protein. The various EGFP derivatives with different excitation and emission spectra represent minor structural changes to the molecule. Much in the way ts mutants alter the sensitivity of a protein to temperature, these subtle changes in excitation and emission could be created by changes in temperature. A recent biophysics paper has in fact demonstrated changes in the emission wavelengths of wt-green fluorescent protein with increasing temperatures (44). The temperatures tested range from 85K–294K (−188.5-+20.85°C) (44). While just shy of a clinically relevant range, this trend would likely continue and could be seen in EGFP and other protein based fluorophores. Perhaps most importantly, changes in emission are quite easy to detect with high levels of sensitivity.
There are a variety of molecular mechanisms by which temperature is sensed in the cell aside from proteins; heat-shock induced expression changes provide an RNA-based example (45). In one system, the temperature dependent control of heat-shock uses the ROSE RNA element (46). This ROSE sequence is found in the mRNA of a heat-shock transcript and regulates translation of the mRNA. At 30°C a ribosomal binding sequence is occluded in the ROSE stem-loop structure, however, as the cell heats to 42°C the hydrogen bonds forming the stem-loop become unstable or "melt", exposing the ribosomal binding sequence, leading to translation (46). Thus this paper demonstrated the ability of 3D structure of a biomolecule to act as a thermometer in vivo.
The melting curves of the ROSE and a mutant were measured at various ion concentrations, from 0–100°C. The melting curves manifest into distinct distributions, suggesting that the melting of the stem loop structure did not occur in all molecules simultaneously (46). This population distribution of melted and intact stem-loop structures at any given temperature indicates that a standard could be determined which would relate temperature to different families of reporter systems. Incorporation of the ROSE element into a marker transcript, EGFP, LacZ, etc., would provide such a detection system and lead to RNA-based thermometry within a cell.
Just as the melting of the stem loop structure of the ROSE element manifests into a distribution through a given temperature range, small DNA hairpins would likely behave in a similar manner. Hairpin probes designed for quantitative polymerase chain reaction (qPCR) have fluorophores at both ends of the strand that quench when in a hairpin, but fluoresce when melted and bound to the target amplicon. Much in the way heat “melts” a ROSE element, exposing a ribosomal binding sequence, a DNA hairpin, tagged with a quenched fluorophore, would also fluoresce beyond a denaturing temperature threshold. The base order, AT / GC content, as well as hairpin length of a family of such tagged oligonucleotides, could be engineered for desired melting temperatures, and thus provide control through relevant temperature ranges which could be assessed by individual hairpins. The relative fluorescence could then be calibrated against known temperatures and thus provide relevant scales.
Summary
In this review, we have presented hallmarks circumscribing the development and application of modern thermometry in biological applications, as well as some of the historical path through which thermometry has emerged and progressed into contemporary thermodynamics and physics. We close with an acknowledgement and perspective of the unfortunate overlapping usage of the term “scales” in this context: one with respect to the scale of the systems we desire to measure (organism, tissue, or cell); another with respect to the scale of the reporting system of local heat content (a thermocouple, a contained thermometer, or intracellular biopolymer); as well as the various mathematical scales benchmarked to unique material behaviors (the freezing of water or the denaturing of proteins) used by thermometric systems to report heat content. This multiple usage reflects a parallel in the overlap of many fields – thermodynamics, molecular biology, materials science, and physics— that have precipitated the potential for molecular thermometry to serve important functions in medical applications.
We find the art and science of thermometry experiencing an unprecedented acceleration over the past decade and, fortunately, following technological trends of miniaturization into the micro- and even nano-scale. At the intracellular scale, a variety of molecular thermometers have emerged with markedly different physical bases: some are based on magnetic behaviors, others in cutting-edge nanomaterials, and yet others based on biopolymer behaviors, both native and engineered. All of these thermometric systems share two things with conventional thermometry: a method of detecting temperature changes (i.e. the loss or gain of energy through heat transfer), and a relevant scale such that temperature readings can be normalized and compared. These systems cover a tremendous range of relative complexity from individual molecules or particles, to hydrogels, to transcription control systems. Advancements in nanomaterials and bioengineering will no doubt lead to thermometry improvements which we cannot yet envision.
It is important to note that many of the molecular thermometers presented herein are hypothetical and have yet to mature into reliable temperature reporting systems, and this is particularly true of those based on biomolecular functions. In this realm, the only systems actually serving a functional thermometer beyond the context of immediate biological function is that incorporating EGFP denaturation. While it may seem that the use of biomolecules as thermometers may be lost in the complex nature of cells, far more complicated biological systems have been used as thermometers for over one hundred years. It had long been recognized that the activity of common crickets varies with temperature in temperate climates, but in 1898, Dolbear conducted an extensive study that demonstrated that the rate of audible cricket chirps closely correlates with temperature. His observations showed temperature in °F equals fifty plus the number of chirps let in a minute, minus forty, divided by four; thus providing the relevant scale to a macro level observation turning a cricket into a thermometer (47). Indeed, the further evolution of thermometry will require classical thermodynamics to embrace other disciplines, and it is an exciting time for those collaborating in this unique cusp.
Acknowledgments
Grant Support:
The State of Colorado and NIH Grant #1RO1 EB 009115-01 A1
Abbreviations
- EMF
Electromagnetic Fields
- QD
Quantum Dot
- ts
Temperature Sensitive
- EGFP
Enhanced Green Fluorescent Protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donovan JA, Jochimsen PR, Folk GE, Jr, Loh PM. Temperature over tumors in hairless mice. Cryobiology. 1985;22:499–502. doi: 10.1016/0011-2240(85)90163-4. [DOI] [PubMed] [Google Scholar]
- 2.Saxena AK, Willital GH. Infrared thermography: Experience from a decade of pediatric imaging. Eur J Pediatr. 2008;167:757–764. doi: 10.1007/s00431-007-0583-z. [DOI] [PubMed] [Google Scholar]
- 3.Sterns EE, Zee B. Thermography as a predictor of prognosis in cancer of the breast. Cancer. 1991;67:1678–1680. doi: 10.1002/1097-0142(19910315)67:6<1678::aid-cncr2820670633>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Kettering M, Zorn H, Bremer-Streck S, Oehring H, Zeisberger M, Bergemann C, Hergt R, Halbhuber K-J, Kaiser WA, Hilger I. Characterization of iron oxide nanoparticles adsorbed with cisplatin for biomedical applications. Phys Med Biol. 2009;54:5109–5121. doi: 10.1088/0031-9155/54/17/003. [DOI] [PubMed] [Google Scholar]
- 5.Latorre M, Rinaldi C. Applications of magnetic nanoparticles in medicine: magnetic fluid hyperthermia. P R Health Sci J. 2009;28:227–238. [PubMed] [Google Scholar]
- 6.Tong L, Wei Q, Wei A, Cheng JX. Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects. Photochem Photobiol. 2009;85:21–32. doi: 10.1111/j.1751-1097.2008.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulcahy R. Medical Technology: Inventing the Instruments. Minneapolis: Oliver Press; 1996. pp. 17–24. [Google Scholar]
- 8.Bolton HC. Evolution of the Thermometer, 1592–1743. Whitefish: Kessinger Publishing, LLC; 2007. pp. 7–27. [Google Scholar]
- 9.Krzysztof Cena JA, Politechnika Wroctawska. Bioengineering, thermal physiology and comfort. New York: Elsevier Science; 1981. pp. 82–85. [Google Scholar]
- 10.Çengel YA, Turner RH, Cimbala JM. Fundamentals of Thermal-Fluid Sciences. New York: McGraw-Hill; 2008. [Google Scholar]
- 11.Spink WW. Infectious Diseases: Prevention and Treatment in the Nineteenth and Twentieth Centuries. Minneapolis: University of Minnesota Press; 1978. [Google Scholar]
- 12.Lewanndowski HJ, Harber DM, Whitaker DL, Cornell EA. Simplified system for creating a bose-einstein condensate. J Low Temp Phys. 2003;132:309–367. [Google Scholar]
- 13.Donley EA, Claussen NR, Cornish SL, Roberts JL, Cornell EA, Wieman CE. Dynamics of collapsing and exploding Bose-Einstein condensates. Nature. 2001;412:295–299. doi: 10.1038/35085500. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JL, Claussen NR, Cornish SL, Donley EA, Cornell EA, Wieman CE. Controlled Collapse of a Bose-Einstein Condensate. Phys Rev Lett. 2001;86:4211–4214. doi: 10.1103/PhysRevLett.86.4211. [DOI] [PubMed] [Google Scholar]
- 15.Childs PR, Greenwood JR, Long CA. Review of temperature measurement. Rev Sci Instrum. 2000;71:2959–2978. [Google Scholar]
- 16.Preston-Thomas H. The international temperature scale of 1990 (ITS-90) Metrologia. 1990;27:3–10. [Google Scholar]
- 17.Lustgarten DL, Keane D, Ruskin J. Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Progr Cardiovasc Dis. 1999;41:481–498. doi: 10.1016/s0033-0620(99)70024-1. [DOI] [PubMed] [Google Scholar]
- 18.Spurr EE, Wiggins NE, Marsden KA, Lowenthal RM, Ragg SJ. Cryopreserved human haematopoietic stem cells retain engraftment potential after extended (5–14 years) cryostorage. Cryobiology. 2002;44:210–217. doi: 10.1016/s0011-2240(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 19.Gordon RT. Intracellular Temperature Measurement. [Accessed 1/28/2010];US Patent No. 4,136,683. 1979 http://patft.uspto.gov.
- 20.Gilbert W. De magnete. Mineola: Dover Publications; 1991. [Google Scholar]
- 21.Uyeda C, Tanaka K, Takashima R, Sakakibara M. Characteristics of paramagnetic and diamagnetic anisotropy which induce magnetic alignment of micron-sized non-ferromagnetic particles. Mater Trans JIM. 2003;44:2594–2598. [Google Scholar]
- 22.Alivisatos AP, Gu W, Larabell C. Quantum dots as cellular probes. Annu Rev Biomed Eng. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 23.Borri P, Langbein W, Schneider S, Woggon U, Sellin RL, Ouyang D, Bimberg D. Ultralong Dephasing Time in InGaAs Quantum Dots. Phys Rev Lett. 2001;87:157401. doi: 10.1103/PhysRevLett.87.157401. [DOI] [PubMed] [Google Scholar]
- 24.XB Zhang RD. Thermal effects on the luminescent properties of quantum dots. In: Schwartz JA, Contescu CI, Putyera K, editors. Dekker Encyclopedia of Nanoscience and Nanotechnology. New York: Marcel Dekker, Inc.; 2004. pp. 3873–3881. [Google Scholar]
- 25.Hardison D, Deepthike HU, Senevirathna W, Pathirathne T, Wells M. Temperature-sensitive microcapsules with variable optical signatures based on incorporation of quantum dots into a highly biocompatible hydrogel. J Mater Chem. 2008;18:536–5375. [Google Scholar]
- 26.Gota C, Okabe K, Funatsu T, Harada Y, Uchiyama S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J Am Chem Sci. 2009;131:2766–2767. doi: 10.1021/ja807714j. [DOI] [PubMed] [Google Scholar]
- 27.Park EJ, Yi J, Chung KH, Ryu DY, Choi J, Park K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett. 2008;180:222–229. doi: 10.1016/j.toxlet.2008.06.869. [DOI] [PubMed] [Google Scholar]
- 28.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 29.Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005;56:555–583. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- 30.Porter KR, Beckerle M, McNivan M. The cytoplasmic matrix. Mod Cell Biol. 1983;2:259–302. [Google Scholar]
- 31.Hoffman A. Conventional and environmentally sensitive hydrogels for medical and industrial uses: a review paper. Polymer Gels. 1991;268:82–87. [Google Scholar]
- 32.Pollack GH. Cells, Gels and the Engines of Life. Seattle: Ebner and Sons Publishing; 2001. [Google Scholar]
- 33.Dusek K, Patterson D. A transition in swollen polymer networks induced by intramolecular condensation. J Polymer Sci Polymer Chem. 1968;6:1209–1216. [Google Scholar]
- 34.Tanaka T. Gels. Sci Am. 1981;244:124–136. doi: 10.1038/scientificamerican0181-124. 138. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka T, Annaka M, Ilmain F, Ishii K, Kokufuta E, Suzuki A, Tokita M. Phase transitions of gels. In: Karalis TK, editor. Mechanics of Swelling: From Clays to Living Cells and Tissues. Berlin: Springer-Verlag, Inc.; 1992. pp. 683–703. [Google Scholar]
- 36.Yasunaga H, Ando I. Effect of cross-linking on the molecular motion of water in polymer gel as studied by pulse 1H NMR and PGSE 1H NMR Polymer Gels and Networks. 1993;1:267–274. [Google Scholar]
- 37.Yasunaga H, Kobayashi M, Matsukawa S, Kurosu H, Ando I. Structure and dynamics of polymer gel systems viewed from NMR spectroscopy. Annu Rep NMR Spectros. 1997;34:39–104. [Google Scholar]
- 38.Chakshusmathi G, Mondal K, Lakshmi GS, Singh G, Roy A, Ch RB, Madhusudhanan S, Varadarajan R. Design of temperature-sensitive mutants solely from amino acid sequence. Proc Natl Acad Sci USA. 2004;101:7925–7930. doi: 10.1073/pnas.0402222101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fried M. Cell-Transforming Ability of a Temperature-Sensitive Mutant of Polyoma Virus. Proc Natl Acad Sci USA. 1965;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chao YP, Chern JT, Wen CS, Fu H. Construction and characterization of thermo-inducible vectors derived from heat-sensitive lacI genes in combination with the T7 A1 promoter. Biotechnol Bioeng. 2002;79:1–8. doi: 10.1002/bit.10304. [DOI] [PubMed] [Google Scholar]
- 41.Leiderman P, Huppert D, Agmon N. Transition in the temperature-dependence of GFP fluorescence: from proton wires to proton exit. Biophys J. 2006;90:1009–1018. doi: 10.1529/biophysj.105.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narberhaus F, Waldminghaus T, Chowdhury S. RNA thermometers. FEMS Microbiol Rev. 2006;30:3–16. doi: 10.1111/j.1574-6976.2005.004.x. [DOI] [PubMed] [Google Scholar]
- 43.Chowdhury S, Maris C, Allain FH, Narberhaus F. Molecular basis for temperature sensing by an RNA thermometer. EMBO J. 2006;25:2487–2497. doi: 10.1038/sj.emboj.7601128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eric R, Eaton KK. Kaufman Field Guide to Insects of North America. Geneva: Houghton Mifflin Harcourt; 2007. p. 82. [Google Scholar]