Abstract
We reviewed cardiac T2* assessments from 77 thalassemia major patients between the ages of 2.5 and 18 years to study optimal timing of cardiac iron screening by magnetic resonance imaging. No patient under 9.5 years of age showed detectable cardiac iron in contrast to 36% of patients between the ages of 15–18 years old, corresponding to an odds-ratio of 1.28 (28%) per year. All patients with cardiac iron had received at least 35 grams of transfusional iron. Liver iron and ferritin failed to predict cardiac iron loading. Initiation of cardiac magnetic resonance imaging assessment should be determined according to age and transfusional burden rather than indices of iron overload. When appropriate chelation therapy has been administered since birth, cardiac magnetic resonance imaging can be postponed until 8 years of age when anesthesia is not required. Patients with suboptimal chelation, increased transfusional requirements, or who have initiated transfusions later in life should be tested sooner.
Keywords: thalassemia major, heart, MRI, iron overload, children
Introduction
Patients with thalassemia major develop life threatening cardiac complications from iron overload in their teens and twenties. Pathological data1 and previous observational trials2 suggest that patients have to reach a critical transfusional exposure before cardiac symptoms are presented. Cardiac MRI identifies pre-clinical cardiac iron deposition, but it is not known at what age T2* screening should be initiated. In a pilot study of 19 thalassemia patients between the ages of 7 and 26, we found cardiac iron in 8/10 patients older than 13 years of age and 0/9 patients under 13 years of age.3 The present study involved two institutions and extends our previous work in 77 pediatric patients with thalassemia major.
Design and Methods
The study was performed at the Ospedale Regionale Microcitemie in Cagliari and the Childrens’ Hospital Los Angeles (CHLA). Retrospective review of medical records, waiver of informed consent, and coded exchange of clinical data was authorized by the Institutional Review Boards at CHLA and Cagliari. Consent was not obtained for MRI examinations since these were performed for clinical indications. All pediatric patients undergoing cardiac MRI before July of 2007 were included. Children under 8 years of age were generally studied freely breathing under propofol sedation. Cardiac T2* and cardiac function of subjects at both institutions were assessed using validated techniques on a 1.5 T General Electric CVi scanner.4 Cardiac T2* was assessed by a multiecho, gradient echo sequence with 8 echo times spaced from 2 to 18 ms.4,5 Individual pixels in the image were fitted to a monoexponential and the average was calculated from a full thickness region of interest drawn in the ventricular septum.6 Patients at CHLA also underwent validated MRI-based liver iron measurements as previously described.7 All MRI acquisitions were collected using a single signal average for patients who could breath-hold and three signal averages if they could not. Left ventricular ejection fraction (LVEF) was measured by MRI at CHLA and by echocardiography at Cagliari using standard techniques. All patients were on regular transfusion therapy, consisting of 10–15 cc/kg transfusions administered at intervals to maintain a pre-transfusion hemoglobin of 9–10 g/dL. Hematocrit-corrected transfusional iron burden was measured in the Cagliari patients and was expressed in grams of iron. All patients had mainly used deferoxamine chelation, beginning 1.1±0.9 years after first transfusion. At the first MRI examination, 37 patients remained on deferoxamine monotherapy, 29 patients were using deferasirox (18.2±10.5 months) and 5 patients were using deferiprone alone or in combination with deferoxamine (36.6±23.3 months).
Serial MRI data was recorded in 31 patients, but associations between cardiac T2* and age were determined using only the results of the first patient MRI scan. Linear and logistic regressions were performed using JMP5.1 (SAS, Cary, North Carolina). Receiver Operator Characteristic curves were also used to compare the ability of ferritin, hepatic iron concentration (HIC), and age to predict the presence of detectable cardiac iron (T2* <20 ms).
Results and Discussion
Patient age ranged from 8.0 to 18.0 years of age at Cagliari (n=36) and 2.5 to 17.9 years of age at CHLA (n=41), reflecting the use of MRI at CHLA to monitor liver iron in patients <8 years of age. Iron burden was moderate to severe with HIC of 12.7±9.8 mg/g dry weight (CHLA) and ferritin values of 2,308±1,569 ng/mL (all patients). At CHLA, ferritin was a reasonable surrogate for HIC, having an r-value of 0.85 (p<0.0001). In the patients from Cagliari, transfusion burden was 47.7±24.5g [15.6–90.6], corresponding to 11.9±3.8 [4–17.4] years of chronic transfusion therapy. Transfusional burden was closely correlated with age (r=0.95) and with years of transfusion therapy (r=0.93). In both populations, median cardiac T2* was 30.2 ms and ranged from 3.4 to 72.8 ms. There was no significant difference with respect to chelator type.
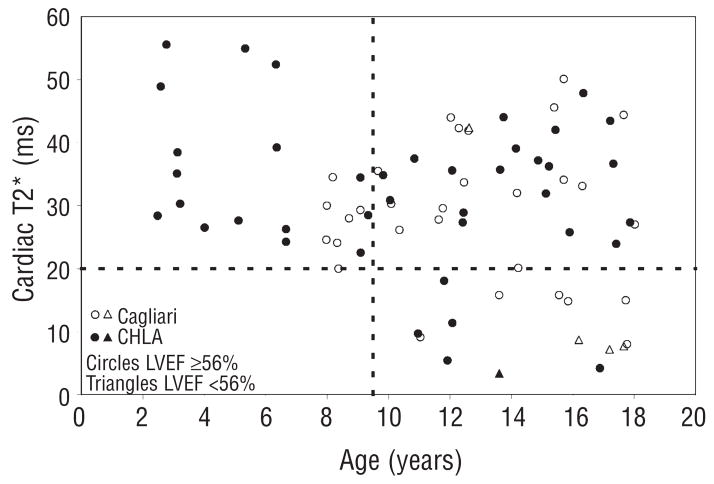
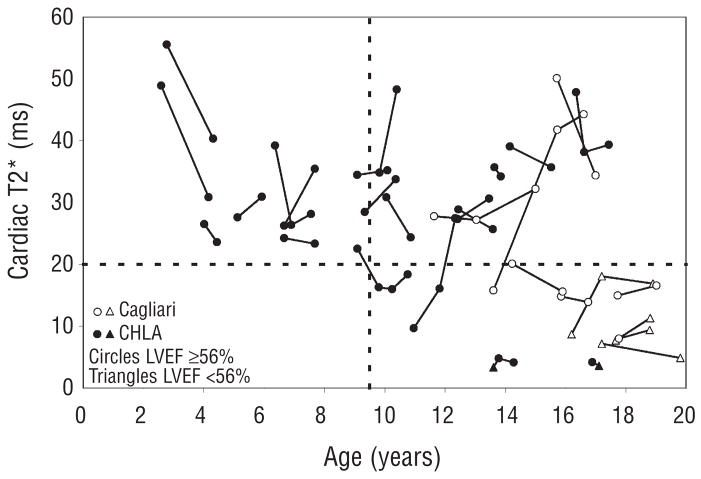
The decrease in cardiac T2* with increasing chronological age are shown in Figure 1. Although linear regression was weakly positive (r=0.25, p=0.03), the relationship between T2* and age was fundamentally non-linear. No patient under 9.5 years of age demonstrated an abnormal cardiac T2* (<20 ms) while 24% of patients aged 9.5–15 and 36% of patients aged of 15–18 years had detectable cardiac iron. There was no difference in ferritin or liver iron concentration between patients with and without detectable cardiac iron. Five patients had an ejection fraction less than 56% (open and filled triangle symbols). Four of these patients had a cardiac T2* <10 ms. Serial data from 31 patients are shown in Figure 2. Although the global trend is downward with age, half of the patients improve their T2* over time. In particular, 7/11 patients with detectable cardiac iron improved their cardiac T2*, most likely representing physician and patient response to the abnormal test value. There was insufficient clinical data to determine whether improvements reflected improved changes in the chelator dose, type or compliance, but these data do reinforce the importance of regular cardiac T2* screening.
Figure 1.
Plot of first cardiac T2* as a function of age in 77 patients from Cagliari (open circles) and Los Angeles (closed symbols). Circles indicate patients with normal cardiac function (LVEF >=56%) and triangles indicate left ventricular dysfunction.
Figure 2.
Serial T2* values in 31 patients from Cagliari (open circles) and Los Angeles (closed symbols). Circles indicate patients with normal cardiac function (LVEF >=56%) and triangles indicate left ventricular dysfunction.
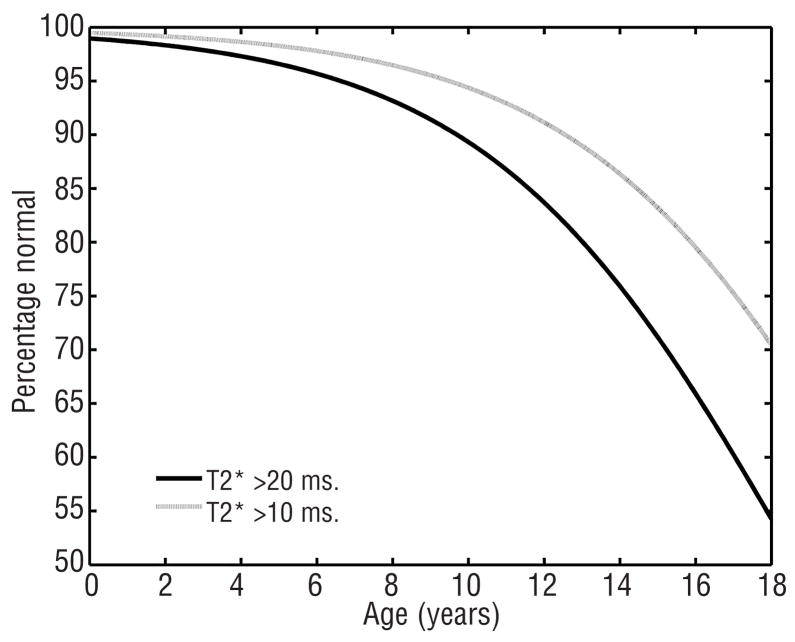
The logistic regression curves modeling the prevalence of detectable (T2* <20) and severe (T2* <10) cardiac iron as a function of age (r= 0.35 and r=0.33, p<0.005) are shown in Figure 3. This emphasizes the sharp increase in cardiac iron prevalence throughout the early teen years. From these data, the odds-ratio for developing cardiac iron is 1.28 (28%) per year and for developing severe cardiac iron 1.1 (10%) per year. It is not possible to extrapolate this curve beyond 18 years of age but cardiac iron is observed in nearly 2/3 of adult thalassemia patients.8–11 We did not include older patients in the logistical regression because survival and referral bias are introduced as patients are transferred from pediatric to adult health care centers.
Figure 3.
Probability of having undetectable cardiac iron (T2* >20, black line) as a function of age. The prevalence of cardiac iron loading increases dramatically in the second decade. Since the risk of cardiac dysfunction increases steeply for T2* <10 ms, the probability of having T2* >10 ms is shown for comparison (grey line).
The T2* cut-off of 20 ms is generally used as the lower limit of normal although good data levels of age and ethnicity matched controls.11 In fact, our laboratory previously reported a lower limit of normal of 25 ms in adults.3 As a result, we usually report T2* values of 20–25 ms as borderline rather than negative. If this cutoff were applied to the current data, the logistic regression curve would shift leftward but retain its fundamental shape. There are 5/12 patients between the ages of 6.5 and 9.5 years of age with cardiac T2* in this borderline zone. Therefore, the choice of threshold represents a trade-off between sensitivity and false-positive diagnosis.
In many locations characterization of the onset of cardiac iron deposition has important practical as well as theoretical implications. MRI resources are limited. In addition, MRI scans in children under 8 years of age generally require deep sedation or general anesthesia, increasing the risk and cost of the procedure. We obtain acceptable image quality in freely breathing patients, but some centers prefer to intubate and ventilate children for cardiac MRI studies. These data suggest that cardiac MRI can safely be deferred in well-chelated patients until they are able to undergo the study without anesthesia. However, Figure 3 only applies to patients who had received regular transfusions and early, consistent iron chelation therapy (beginning ~ one year after first transfusion). Patients who have increased transfusion requirements, or prolonged intervals from iron chelation may accumulate cardiac iron more aggressively. In developing nations, where access to chelation is more difficult, patients may develop cardiac iron at an earlier age. Similarly, patients who start transfusions later in life or have other chronic anemias may exhibit different relative cardiac risk profiles.3 For example, Jensen et al. demonstrated detectable cardiac iron in unchelated, adult myelodysplasia patients after less than four years of chronic transfusion therapy.12
From a theoretical perspective, cardiac iron loading appears to have a significant latency compared with liver iron loading. In the present study, all patients with abnormal cardiac T2* had been transfused with at least 35 grams of iron. Appropriately, this level is slightly lower than the 200 units (~40 g) and 14 mmol/kg (44–51 g) thresholds established using cardiac death as an endpoint.1,2 On the other end of the spectrum, Jensen found MRI-detectable cardiac iron at a significantly lower transfusion threshold of ~15 g (77 units). However, his patients were older and were naïve to iron chelation therapy.12 In our population, age, transfusional burden, and years of chelation therapy were so tightly correlated, it was impossible to determine whether age, transfusional burden, noncompliance with chelation therapy in adolescence, or any combination of these, represents the relevant risk factor. We also cannot exclude the potential role for maturational, hormonal, or nutritional changes with age and genetic factors in facilitating cardiac iron uptake.
These data also confirm the observation that total body iron stores have little immediate predictive value with respect to the presence or absence of cardiac iron, even in pediatric patients. The area under the Receiver Operator Characteristic Curve (AUROC) was 0.5 for ferritin and 0.49 for hepatic iron concentration. This is pratically identical to values predicted by random chance. This is surprising since liver iron is an excellent metric of total body iron stores as well as indirectly reflecting patient compliance with iron chelation.2,13 For example, when patients were divided according to their serum ferritin (<1,500 ng/mL vs. >1,500 ng/mL), the prevalence of detectable cardiac iron was statistically identical (23% vs. 18%). We had anticipated greater predictive power for somatic iron indices in this relatively young and homogenous patient cohort. By contrast, patient age, years of chelation therapy, and transfusional burden had AUROC values of 0.73, 0.81 and 0.79. This implies that cardiac risk is either conveyed by positive iron balance over a prolonged period of time or by positive iron balance after a critical age/transfusional burden has been reached. Nonetheless, serial ferritin and liver iron measurements still remain the best metrics of overall chelation compliance and may have more direct predictive value in other organ toxicity, such as the liver and endocrine glands.14,15
Acknowledgments
this work was supported by the NHLBI (1 RO1 HL075592-01A1), the General Clinical Research Center at Childrens’ Hospital Los Angeles (RR000043-43), Center for Disease Control (Thalassemia Center Grant U27/CCU922106) and Novartis Pharma.
Footnotes
Authorship and Disclosures
JW: designed the study, data acquisition and analysis, wrote the paper; RO, AA and GM: data acquisition and analysis, reviewed the paper; TC: data analysis, reviewed the paper; RG: designed the study, data acquisition and analysis, reviewed the manuscript; JC Wood has a consulting agreement with Novartis regarding the assess cardiac iron and chelation efficacy.
The authors reported no potential conficts of interest.
References
- 1.Buja LM, Roberts WC. Iron in the heart. Etiology and clinical significance. Am J Med. 1971;51:209–21. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 2.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 3.Wood JC, Tyszka JM, Ghugre N, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle-cell disease. Blood. 2004;103:1934–6. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 4.Wood JC, Enriquez C, Powell A, Ghugre N, Nelson MD, Polzin J, et al. Single-echo versus multiple echo cardiac T2* estimates for cardiac iron estimation in iron overload. J Cardiovasc Magn Reson. 2005;7:97. [Google Scholar]
- 5.Ghugre NR, Enriquez CM, Gonzalez I, Nelson MD, Jr, Coates TD, Wood JC. MRI detects myocardial iron in the human heart. Magn Reson Med. 2006;56:681–6. doi: 10.1002/mrm.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghugre NR, Enriquez CM, Coates TD, Nelson MD, Jr, Wood JC. Improved R2* measurements in myocardial iron overload. J Magn Reson Imaging. 2006;23:9–16. doi: 10.1002/jmri.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–5. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aessopos A, Fragodimitri C, Karabatsos F, Hatziliami A, Yousef J, Giakoumis A, et al. Cardiac magnetic resonance imaging R2* assessments and analysis of historical parameters in patients with transfusion-dependent thalassemia. Haematologica. 2007;92:131–2. doi: 10.3324/haematol.10455. [DOI] [PubMed] [Google Scholar]
- 9.Tanner MA, Galanello R, Dessi C, Westwood MA, Smith GC, Nair SV, et al. Myocardial iron loading in patients with thalassemia major on deferoxamine chelation. J Cardiovasc Magn Reson. 2006;8:543–7. doi: 10.1080/10976640600698155. [DOI] [PubMed] [Google Scholar]
- 10.Tanner MA, Westwood M, Galanello R, Pennell DJ. Baseline findings of a CMR driven randomized controlled trial of iron chelation therapy in thalassemia major. JCMR. 2005;7:31–2. [Google Scholar]
- 11.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 12.Jensen PD, Jensen FT, Christensen T, Eiskjaer H, Baandrup U, Nielsen JL. Evaluation of myocardial iron by magnetic resonance imaging during iron chelation therapy with deferrioxamine: indication of close relation between myocardial iron content and chelatable iron pool. Blood. 2003;101:4632–9. doi: 10.1182/blood-2002-09-2754. [DOI] [PubMed] [Google Scholar]
- 13.Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327–31. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 14.Telfer PT, Prestcott E, Holden S, Walker M, Hoffbrand AV, Wonke B. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110:971–7. doi: 10.1046/j.1365-2141.2000.02298.x. [DOI] [PubMed] [Google Scholar]
- 15.Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–93. [PubMed] [Google Scholar]