Abstract
It is well documented that disability accumulation in multiple sclerosis is correlated with axonal injury and that the extent of axonal injury is correlated with the degree of inflammation. However, the interdependence between focal inflammation, diffuse inflammation and neurodegeneration, and their relative contribution to clinical deficits, remains ambiguous. A hypothesis might be that early focal inflammation could be the pivotal event from which all else follows, suggesting the consideration of multiple sclerosis as a two-stage disease. This prompted us to define two phases in the disease course of multiple sclerosis by using two scores on the Kurtzke Disability Status Scale as benchmarks of disability accumulation: an early phase, ‘Phase 1’, from multiple sclerosis clinical onset to irreversible Disability Status Scale 3 and a late phase, ‘Phase 2’, from irreversible Disability Status Scale 3 to irreversible Disability Status Scale 6. Outcome was assessed through five parameters: Phase 1 duration, age at Disability Status Scale 3, time to Disability Status Scale 6 from multiple sclerosis onset, Phase 2 duration and age at Disability Status Scale 6. The first three were calculated among all patients, while the last two were computed only among patients who had reached Disability Status Scale 3. The possible influence of early clinical markers on these outcomes was studied using Kaplan–Meier estimates and Cox models. The analysis was performed in the Rennes multiple sclerosis database (2054 patients, accounting for 26 273 patient-years) as a whole, and according to phenotype at onset (1609 relapsing/445 progressive onset). Our results indicated that the disability progression during Phase 2 was independent of that during Phase 1. Indeed, the median Phase 2 duration was nearly identical (from 6 to 9 years) irrespective of Phase 1 duration (<3, 3 to <6, 6 to <10, 10 to <15, ≥15 years) in the whole population, and in both phenotypes. In relapsing onset multiple sclerosis, gender, age at onset, residual deficit after the first relapse and relapses during the first 2 years of multiple sclerosis were found to be independent predictive factors of disability progression, but only during Phase 1. Our findings demonstrate that multiple sclerosis disability progression follows a two-stage process, with a first stage probably dependant on focal inflammation and a second stage probably independent of current focal inflammation. This concept has obvious implications for the future therapeutic strategy in multiple sclerosis.
Keywords: multiple sclerosis, disability progression, prognosis, course, age
Introduction
Several clinical courses are usually distinguished in multiple sclerosis (Jekyll Island Meeting of MS Society 1995, reported in Lublin, 1996), but it remains uncertain whether they reflect different neuropathological mechanisms. It is well established that axonal injury is a feature of multiple sclerosis (Charcot, 1880), that the extent of axonal injury is correlated with the degree of inflammation (Trapp et al., 1998) at least in relapsing multiple sclerosis, and that a close association between inflammation and neurodegeneration might exist in all disease stages of multiple sclerosis (Kutzelnigg et al., 2005; Frischer et al., 2009). However, the interdependence between focal inflammation, diffuse inflammation and neurodegeneration, and their relative contribution to clinical deficits remain ambiguous. Nevertheless, this point is central for understanding the mechanism of tissue injury in multiple sclerosis, which may have an effect on treatment.
It has been demonstrated that relapses produce a measurable and sustained effect on disability progression in patients with relapsing-remitting multiple sclerosis (Lublin et al., 2003), but others did not find a consistent effect of on-study relapses on the subsequent development of sustained disability increase, during a typical clinical study observation period (Young et al., 2006). Moreover, a number of trials have suggested that β-interferon has an impact on both relapses and disability progression at an early stage in patients with relapsing multiple sclerosis and in patients with a first clinical event suggestive of multiple sclerosis, at least over a short-term period (Jacobs et al., 1996; The PRISMS study group, 1998; Kappos et al., 2007). In contrast, several observational studies about multiple sclerosis natural history (Confavreux et al., 2003, 2006a; Kremenchutzky et al., 2006) showed that relapses did not influence disability progression in patients who subsequently develop a secondary progressive course, suggesting that focal inflammation may have only a small influence on disability accumulation. Those studies also showed that the median age at the onset of the progressive phase was similar in secondary progressive cases and in primary progressive cases, suggesting that multiple sclerosis may correspond to a chronic neurodegenerative age-related disease that is not affected by the initial course (Confavreux, 2006b). Moreover, several well-designed trials in secondary progressive multiple sclerosis (Rice et al., 2000; SPECTRIMS Study Group, 2001; Cohen et al., 2002; Panitch et al., 2004) showed at this late stage an apparent dissociation between the impact of therapeutics on focal inflammatory markers (frequency of relapses and MRI activity) and their impact on delaying disability progression in the same time; suggesting that agents with a short term effect on relapses may not necessarily delay the development of disability in the long term.
A unifying hypothesis might be that early focal inflammation could be the pivotal event from which all else follows (Compston, 2006). Such a hypothesis implies that multiple sclerosis is a two-stage disease, with a first stage mainly dependent on focal inflammation and a second stage dependent on diffuse inflammation and neurodegeneration, but independent of current focal inflammation. This prompted us to define two phases in the multiple sclerosis disease course, an early phase and a late phase. In this article we have reviewed the Rennes Multiple Sclerosis database and attempted to contrast disability progression in those two phases, as well as to identify potential predictive factors of their duration.
Patients and methods
Patients and data collection
Patients were identified through the Rennes Multiple Sclerosis Clinic, which is a regional referral centre for multiple sclerosis in West France (Leray et al., 2007). Patients referred to Rennes Multiple Sclerosis Clinic mainly live in Brittany (60%), Pays de Loire (20%) or bordering regions (14%), the remainder coming from other French or European regions (6%). Since January, 1976, any new case of multiple sclerosis referred to our centre was systematically registered, whatever the date of first multiple sclerosis symptoms (from 1947 to 2004 in our series). Historical data (date of clinical onset, symptoms at onset, relapses and disability) were obtained at the first visit, and follow-up data were prospectively collected and entered into the medical records. In 1996, all these data were computerized using the standardized European Database for Multiple Sclerosis (EDMUS) software (Confavreux et al., 1992). From this time onwards, the Rennes EDMUS database was regularly expanded with newly referred patients and prospectively updated with data recorded at each follow-up visit. Individual reports include the following information: identification and demographic data, medical history, multiple sclerosis course (first event, relapses, irreversible disability and progression) and treatments. The database received approval from the French ‘Commission Nationale Informatique et Libertés’.
Definition of cases
By October, 2004, our database was locked with a total of 2290 cases. For all of them, diagnosis of multiple sclerosis was established according to Poser’s classification (Poser et al., 1983). The date for the likely first symptom of the disease was considered to mark the clinical onset of multiple sclerosis. A relapse of multiple sclerosis was defined as the occurrence, the recurrence or the worsening of symptoms of neurological dysfunction lasting over 24 h and usually ending up in partial or complete remission (Confavreux et al., 1992; Lublin, 1996), and a progressive disease was defined by at least 1 year of continuous deterioration, regardless of the rate of worsening that was not attributable to relapses (Kremenchutzky et al., 2006). Disease phenotype at onset was considered as either relapsing or progressive, and overall multiple sclerosis course was categorized according to the standardized classification of multiple sclerosis (Lublin, 1996). However, we decided to incorporate progressive-relapsing patients into the primary progressive category, leading to three clinical multiple sclerosis courses: relapsing-remitting, secondary progressive and primary progressive.
Clinical assessment
Disability was graded using the Kurtzke Expanded Disability Status Scale (EDSS) (Kurtzke, 1983). However, for the data analysis, each half unit was lumped with the corresponding whole unit, leading to a reduction of the original 20-step EDSS to a 10-step scale, very similar if not exactly similar to the original Disability Status Scale (DSS) (Kurtzke, 1961). Residual deficit from the first relapse was defined as the persistence of neurological signs, corresponding to an irreversible EDSS score of at least 2. For the data analysis on disability accumulation, we have further reduced the scale by focusing on the irreversible score 3 (defined as moderate disability) and on the irreversible score 6 (defined as unilateral assistance required to walk 100 m). Those two scores can be quite easily identified in our database, even when medical records are scored retrospectively. A score was qualified as irreversible when it persisted for at least 6 months, and up to the last visit.
Disability milestones
The two benchmarks of disability progression were the assignment of an irreversible score of DSS 3 and DSS 6. Hypothesizing that DSS 3 corresponded to a key step in the disease process, we defined two phases in multiple sclerosis course: an early phase, ‘Phase 1’, from clinical onset to irreversible DSS 3 and a later phase, ‘Phase 2’, from irreversible DSS 3 to irreversible DSS 6. Outcome measurements consisted in five parameters estimated in two different populations: time to reach DSS 3 from multiple sclerosis clinical onset (‘Phase 1’ duration); age at DSS 3; time to reach DSS 6 from clinical onset of multiple sclerosis; time to reach DSS 6 from DSS 3 (’Phase 2’ duration); and age at DSS 6. The first three were estimated among all patients with multiple sclerosis, while the last two were used only in those patients who had reached DSS 3.
Moreover, to explore the relationship between disability progression during ‘Phase 1’ and that during ‘Phase 2’, patients with multiple sclerosis who had reached irreversible DSS 3 were classified into five subgroups defined according to ‘Phase 1’ duration (0 to <3, 3 to <6, 6 to <10, 10 to <15 and ≥15 years), the thresholds being selected to give groups of comparable size, and to allow statistical comparisons.
Prognostic factors
Initial characteristics (gender, age, residual deficit of the first relapse and number of relapses during the first 2 years of multiple sclerosis) were assessed as potential prognostic factors of ‘Phase 1’ and ‘Phase 2’ durations, in relapsing onset and progressive onset separately. Duration of ‘Phase 1’ (considered as a categorical variable) and the occurrence of relapses after DSS 3 were added as potential predictors of ‘Phase 2’ duration.
Statistical analysis
Quantitative and qualitative variables were presented as number of patients (%) and mean ± standard deviation (SD), respectively, and were compared using appropriate statistical tests.
Outcome measurements were analysed as survival data (event defined as either the attainment of DSS 3 or 6). Thus, whenever the milestone had not been reached, data were censored at the date of the last visit. The total number of patients, the number of patients who reached the milestone (number of events) and the number of censored patients are presented in the tables. Median times were estimated using the Kaplan–Meier method (Kaplan, 1958), and survival curves were compared using the log-rank test. Influence of the putative risk factors was then investigated using multivariate Cox models (Cox, 1972).
P-values of less than 0.05 on two-tailed tests were considered as statistically significant. Statistical analysis was performed with Statistical Package for the Social Sciences 15.0 for Windows (SPSS, 2002).
Results
Characteristics of the population
Of the 2290 patients registered in the database, 236 patients did not meet the Poser criteria for clinically definite multiple sclerosis and were excluded from the study. The demographic and clinical characteristics according to initial multiple sclerosis course of the 2054 remaining patients are presented in Tables 1 and 2. Overall, the sex ratio female:male was 2.30, the mean age at multiple sclerosis clinical onset was 31.4 ± 9.8 years, and the mean duration of follow-up from multiple sclerosis clinical onset to the last visit was 12.8 ± 9.4 years (accounting for 26 273 patient-years). During the follow-up period, 1415 patients (68.9%) reached irreversible DSS 3, of whom 718 patients (35%; 51.7% of those who had reached DSS 3) reached irreversible DSS 6. Considering disease phenotype at onset, there were 445 patients (21.7%) with a progressive onset disease and 1609 patients (78.3%) with a relapsing onset disease. In the latter group, 237 patients (14.9%) had residual deficit after the first relapse, and 853 patients (53.0%) had two relapses or more within the first 2 years after clinical onset (mean number of relapses during the first 2 years of multiple sclerosis: 1.8 ± 1.2). During the follow-up, 618 patients with relapsing onset (38.4%) had converted to secondary progressive phase, after a median time from multiple sclerosis clinical onset of 16.0 years (14.7–17.3) and at a median age of 40.4 years (39.3–41.4).
Table 1.
Demographic characteristics of patients in the whole multiple sclerosis population and according to the disease phenotype at onset of multiple sclerosis
| All multiple sclerosis patients | Disease phenotype at onset of multiple sclerosis |
P-valuea | ||
|---|---|---|---|---|
| Relapsing onset | Progressive onset | |||
| Number of patients | 2054 | 1609 | 445 | – |
| Female : male sex ratio | 2.30 (1431:623) | 2.67 (1171:438) | 1.41 (260:185) | <0.001 |
| Mean age at onset of multiple sclerosis (years) ± SD | 31.4 ± 9.8 | 29.5 ± 8.8 | 38.5 ± 10.1 | <0.001 |
| Age group at onset of multiple sclerosis | 0.0001 | |||
| <20 years | 236 (11.5%) | 223 (13.9%) | 13 (2.9%) | |
| 20 to <30 years | 771 (37.5%) | 688 (42.8%) | 83 (18.7%) | |
| 30 to <40 years | 630 (30.7%) | 488 (30.3%) | 142 (31.9%) | |
| 40 to <50 years | 337 (16.4%) | 183 (11.4%) | 154 (34.6%) | |
| ≥50 years | 80 (3.9%) | 27 (1.7%) | 53 (11.9%) | |
| Initial symptoms of multiple sclerosis (n=1876) | <0.001 | |||
| Isolated long tracts | 962 (51.3%) | 686 (45.3%) | 276 (76.0%) | |
| Isolated brainstem | 218 (11.6%) | 216 (14.3%) | 2 (0.6%) | |
| Isolated optic neuritis | 404 (21.5%) | 367 (26.2%) | 7 (1.9%) | |
| Combined symptoms | 292 (15.6%) | 214 (14.1%) | 78 (21.5%) | |
| Mean follow-up duration from onset (years) ± SD | 12.8 ± 9.4 | 13.1 ± 9.8 | 11.4 ± 7.9 | <0.001 |
a Comparison relapsing onset versus progressive onset.
Table 2.
Disability characteristics of patients in the whole multiple sclerosis population and according to the disease phenotype at onset of multiple sclerosis
| All multiple sclerosis patients | Disease phenotype at onset of multiple sclerosis |
P-valuea | ||
|---|---|---|---|---|
| Relapsing onset | Progressive onset | |||
| All patients | 2054 | 1609 | 445 | – |
| Patients with a progressive course | – | 618 (38.4%) | 445 (100.0%) | – |
| Patients who had reached DSS 3 during follow-up | 1415 (68.9%) | 995 (61.8%) | 420 (94.4%) | <0.001 |
| Patients who had reached DSS 6 during follow-up | 718 (35.0%) | 467 (29.0%) | 251 (56.4%) | <0.001 |
| Median time to progression onset from multiple sclerosis clinical onset (years)b | – | 16.0 (14.7 – 17.3) | 0 | – |
| Median time to DSS 3 from multiple sclerosis clinical onset (years)b | 7.4 (6.9–7.9) | 10.0 (9.4–10.6) | 2.0 (1.8–2.2) | <0.0001 |
| Median time to DSS 6 from multiple sclerosis clinical onset (years)b | 18.0 (16.8–19.2) | 21.7 (20.6–22.9) | 10.0 (9.1–10.9) | <0.0001 |
| Median age at the assignment of DSS 3 (years)b | 42.3 (41.742.8) | 42.4 (41.6–43.1) | 41.9 (40.8–42.9) | 0.236 |
| Patients who had reached DSS 3 | 1415 | 995 | 420 | – |
| Median time to DSS 6 from EDSS 3 (years)b | 7.0 (6.4–7.6) | 7.4 (6.8–8.0) | 6.6 (6.2–7.0) | 0.003 |
| Median age at the assignment of DSS 6 (years)b | 51.4 (50.1–52.6) | 51.1 (49.7–52.6) | 52.1 (49.7–54.6) | 0.915 |
a Comparison relapsing onset versus progressive onset.
b Kaplan–Meier estimated median with 95% confidence interval; comparison of survival curves using LogRank test.
A total of 1154 patients (56.2%) received disease-modifying drugs for at least 6 months, accounting for 4515 patient-years spent with disease modifying drugs (17.2% of the total number of patient-years; 16.9% in the relapsing onset and 18.1% in the progressive onset, non-significant difference). Treatment was started 7.7 ± 7.1 years after clinical onset of multiple sclerosis on average, and consisted of β-interferon (28.5%), mitoxantrone (26.7%), azathioprine (21.4%), methotrexate (13.0%), cyclophosphamide (6.7%) and glatiramer acetate (2.2%).
Among the 2054 patients, 540 patients (26.3%) had their last follow-up visit within the year preceding the closing of the database, 1042 patients (50.7%) within the previous 2 years and 1337 patients (65.1%) within the previous 3 years. Only 14.2% of patients did not have updated information in the 5 years preceding the closing of the database for analysis. Disease characteristics and disability progression did not differ according to the delay between the closing of the database and the last visit at the Multiple Sclerosis Centre (within the last 2 years versus before the last 2 years, data not shown).
Disability progression during Phase 1 and Phase 2
To evaluate the potential relationship between disability progression during Phase 1 and Phase 2, we focused on the 1415 patients with multiple sclerosis (995 patients with relapsing onset and 420 patients with progressive onset) who reached DSS 3, and classified them into five subgroups according to Phase 1 duration: subgroup 1 (0 to <3 years, 523 patients), subgroup 2 (3 to <6 years, 290 patients), subgroup 3 (6 to <10 years, 254 patients), subgroup 4 (10 to <15 years, 172 patients) and subgroup 5 (≥15 years, 176 patients). For progressive onset multiple sclerosis, due to the low number of patients, the subgroups over 6 years were collapsed into one, leading to three subgroups (0 to <3 years, 270 patients; 3 to <6 years, 90 patients; ≥6 years, 60 patients). Two categories of patients were described in the analysis: those who had reached DSS 6, and those who had not. Among the 718 patients who had reached DSS 6, the mean time to reach DSS 6 from DSS 3 was 5.47 years, with variations from 3.8 to 6.4 years when considering each year of duration of Phase 1 (data not shown). Almost identical mean times were observed in the five subgroups of Phase 1 duration, either in the whole multiple sclerosis population (between 4.88 and 5.74 years, P = 0.764), in relapsing onset multiple sclerosis (between 4.93 and 6.31 years, P = 0.394), or in progressive onset multiple sclerosis (between 4.74 and 6.10 years, P = 0.444) (Table 3).
Table 3.
Mean time to reach DSS 3 from clinical onset (Phase 1 duration), mean time and Kaplan-Meier estimated median time to reach DSS 6 from DSS 3 (Phase 2 duration) in all the patients who had reached DSS 3, and according to the disease phenotype at onset of multiple sclerosis
| Disease phenotype at onset of multiple sclerosis | Time to EDSS 3 from multiple sclerosis clinical onset (years) | Number of patients who had reached DSS 3 | DSS 3 and DSS 6 reached |
EDSS 3 reached but not DSS 6 |
Kaplan-Meier estimated median time to DSS 6 from DSS 3 (years) in patients who had reached DSS 3c | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients who had reached DSS 6 (%)a | Mean time to DSS 3 from multiple sclerosis clinical onset (years) | Mean time to DSS 6 from DSS 3 (years) | Number of patients who did not reach DSS 6 (%)b | Mean time to DSS 3 from multiple sclerosis clinical onset (years) | Mean time to last visit from DSS 3 (years) | ||||
| All patients | 0 to <3 | 523 | 277 (53.0) | 1.26 | 5.54 | 246 (47.0) | 1.13 | 5.34 | 6.8 (6.2–7.5) |
| 3 to <6 | 290 | 142 (49.0) | 4.42 | 5.74 | 148 (51.0) | 4.52 | 4.92 | 7.2 (5.6–8.7) | |
| 6 to <10 | 254 | 125 (49.2) | 7.84 | 5.34 | 129 (50.8) | 7.76 | 4.79 | 7.0 (5.9–8.1) | |
| 10 to <15 | 172 | 94 (54.7) | 12.20 | 4.88 | 78 (45.3) | 12.17 | 5.34 | 7.0 (5.7–8.3) | |
| ≥15 | 176 | 80 (45.5) | 21.25 | 5.66 | 96 (54.5) | 20.36 | 4.82 | 9.2 (6.6–11.7) | |
| Total | 1415 | 718 (50.7) | 6.69 | 5.47 | 697 (49.3) | 6.96 | 5.08 | 7.0 (6.4–7.6) | |
| Relapsing onset | 0 to <3 | 253 | 111 (43.9) | 1.43 | 5.78 | 142 (56.1) | 1.19 | 5.42 | 7.4 (6.2–8.7) |
| 3 to <6 | 200 | 90 (45.0) | 4.49 | 6.31 | 110 (55.0) | 4.62 | 5.24 | 8.2 (6.6–9.7) | |
| 6 to <10 | 208 | 96 (46.2) | 8.00 | 5.06 | 112 (53.8) | 7.78 | 4.57 | 7.0 (5.8–8.2) | |
| 10 to <15 | 161 | 91 (56.5) | 12.22 | 4.93 | 70 (43.5) | 12.23 | 5.21 | 6.5 (5.3–7.7) | |
| ≥15 | 173 | 79 (45.7) | 21.20 | 5.60 | 94 (54.3) | 20.37 | 4.66 | 9.2 (6.3–12.0) | |
| Total | 995 | 467 (46.9) | 8.82 | 5.54 | 528 (53.1) | 8.18 | 5.04 | 7.4 (6.8–8.0) | |
| Progressive onset | 0 to <3 | 270 | 166 (61.5) | 1.16 | 5.37 | 104 (38.5) | 1.04 | 5.22 | 6.5 (5.6–7.4) |
| 3 to <6 | 90 | 52 (57.8) | 4.31 | 4.74 | 38 (42.2) | 4.24 | 4.02 | 6.0 (5.0–7.0) | |
| ≥6 | 60 | 33 (55.0) | 8.25 | 6.10 | 27 (45.0) | 9.70 | 6.79 | 7.0 (6.8–7.2) | |
| Total | 420 | 251 (59.8) | 2.74 | 5.33 | 169 (40.2) | 3.14 | 5.20 | 6.6 (6.2–7.0) | |
a In Kaplan-Meier analysis (last column) the number of patients who had reached both DSS 3 and DSS 6 corresponds to the number of events.
b In Kaplan–Meier analysis (last column) the number of patients who did not reach DSS 6 corresponds to the number of censored patients.
c Kaplan–Meier estimated median time with 95% confidence interval.
In addition, the proportion of patients who had reached DSS 6 did not differ significantly in the five different subgroups of the whole multiple sclerosis population (45.5–54.7%), of relapsing onset multiple sclerosis (43.9–56.5%) and of progressive onset multiple sclerosis (55.0–61.5%). To exclude any bias potentially linked to a shorter follow-up duration of patients who had not reached DSS 6, we also compared the mean time to reach DSS 6 from DSS 3 in patients who had reached EDSS 6 with the mean time of follow-up from DSS 3 to the last visit in patients who had not reach DSS 6. No significant difference was found when considering overall data (718 versus 697 patients, respectively), and each subgroup of Phase 1 duration.
Moreover, the Kaplan–Meier method allowed us to calculate the median time to reach DSS 6 from DSS 3 by taking into account censored data (the time from DSS 3 to the last visit for patients who had not reached DSS 6). This confirmed that the duration of Phase 2 was nearly identical irrespective of the duration of Phase 1 in the whole multiple sclerosis population (from 6.8 to 9.2 years, P = 0.651), in relapsing onset multiple sclerosis (from 6.5 to 9.2 years, P = 0.073) and in progressive onset multiple sclerosis (from 6.0 to 7.0 years, P = 0.118).
As a large part of our population received disease-modifying treatments, and the impact of those treatments is not well known on the long-term disability progression, we performed the same analysis on the untreated population (n = 900). In this population, we confirmed that almost identical mean durations of Phase 2 were observed in the five subgroups of Phase 1 duration, either in the whole multiple sclerosis population, in relapsing onset multiple sclerosis or in progressive onset multiple sclerosis (data not shown).
Finally, of the 995 patients with relapsing onset who had reached DSS 3, 416 patients (41.8%) had converted into secondary progressive multiple sclerosis during Phase 1, which indicated that conversion into secondary progressive multiple sclerosis was not concomitant with the assignment of DSS 3.
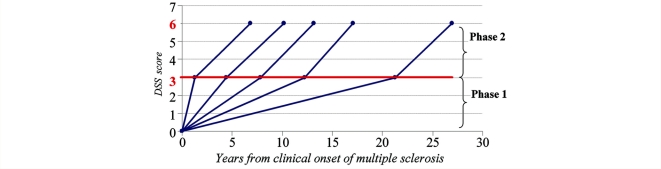
In summary, these results indicated that the disability progression during Phase 2 is independent of the disability progression during Phase 1, as clearly illustrated in Fig. 1.
Figure 1.
Disability progression during Phase 2 (mean time from DSS 3 to DSS 6) in five subgroups defined according to the duration of Phase 1 (mean time from multiple sclerosis clinical onset to DSS 3) in the 718 multiple sclerosis patients who had reached both DSS 3 and DSS 6.
Age and disease duration to disability milestones DSS 3 and DSS 6 according to disease phenotype at multiple sclerosis onset (relapsing or progressive onset)
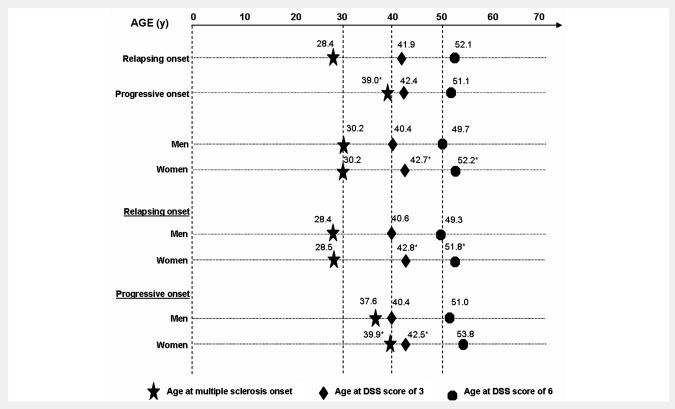
To assess the relationship between disease progression and the phenotype at multiple sclerosis clinical onset, we separately examined relapsing onset and progressive onset multiple sclerosis with respect to the time to reach the two disability milestones (DSS 3 and DSS 6) from multiple sclerosis clinical onset (i.e. disease duration) or from birth (i.e. age). The proportion of patients who had reached both irreversible DSS 3 and 6 were significantly higher in progressive onset multiple sclerosis than in relapsing onset multiple sclerosis (DSS 3: 94.4 versus 61.0%, respectively, P < 0.001; DSS 6: 56.4 versus 29.0%, P < 0.001). This would indicate that multiple sclerosis with progressive onset had a more rapid disability progression than multiple sclerosis with relapsing onset during a given follow-up period, as will be demonstrated below. As expected, patients with progressive onset multiple sclerosis were older at onset than those with relapsing onset (median 39.0 versus 28.4 years, P < 0.0001; Fig. 2). However, their age at the assignment of both DSS 3 and 6 did not differ significantly from that of multiple sclerosis patients with relapsing onset (median age at DSS 3: 41.9 versus 42.4 years, respectively; P = 0.236; median age at DSS 6: 52.1 versus 51.1 years, respectively, P = 0.915). On the contrary, the median durations from multiple sclerosis clinical onset to both DSS 3 and 6 were significantly shorter in progressive onset multiple sclerosis than in relapsing onset multiple sclerosis (DSS 3: 2.0 versus 10.0 years, respectively, P < 0.0001; DSS 6: 10.0 versus 21.7 years respectively, P < 0.0001). The median duration of Phase 2 (time to reach DSS 6 from DSS 3) was also shorter in progressive compared to patients with relapsing onset multiple sclerosis but the difference was less significant (6.6 versus 7.4 years, respectively, P < 0.003) (Table 4).
Figure 2.
Kaplan–Meier estimated median age at multiple sclerosis clinical onset and at DSS score of 3 in the 2054 patients with multiple sclerosis, and Kaplan-Meier estimated median age at DSS score of 6 in the 1415 patients who had reached DSS 3, according to (i) disease phenotype at onset, (ii) gender and (iii) gender by disease phenotype at onset. Asterisk denotes the significant comparison (P < 0.05, progressive versus relapsing onset, and females versus males, respectively).
Table 4.
Potential risk factors affecting the median times from clinical onset of multiple sclerosis to the assignment of irreversible DSS score of 3 (Phase 1), and to the assignment of irreversible DSS score of 6 among the 2054 patients with multiple sclerosis, and the median time from DSS 3 to the assignment of an irreversible DSS score of 6 (Phase 2) among the 1415 patients with multiple sclerosis who had reached DSS 3
| Variables | Time from multiple sclerosis clinical onset to DSS 3 (years) (n=2054) |
Time from multiple sclerosis clinical onset to DSS 6 (years) (n=2054) |
Time from DSS 3 to DSS 6 (years) (n=1415) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of events | Number of censored | Kaplan–Meier estimated mediana | Number of events | Number of censored | Kaplan–Meier estimated mediana | Number of events | Number of censored | Kaplan–Meier estimated mediana | ||
| All | 1415 | 639 (31.1%) | 7.4 (6.9–7.9) | 718 | 1336 (65.0%) | 18.0 (16.8–19.2) | 718 | 697 (49.3%) | 7.0 (6.4–7.6) | |
| Phenotype | ||||||||||
| Relapsing onset | 995 | 614 (38.2%) | 10.0 (9.4–10.6) | 467 | 1142 (71.0%) | 21.7 (20.6–22.9) | 467 | 528 (53.1%) | 7.4 (6.8–8.0) | |
| Progressive onset | 420 | 25 (5.6%) | 2.0 (1.8–2.2) | 251 | 194 (43.6%) | 10.0 (9.1–10.9) | 251 | 169 (40.2%) | 6.6 (6.2–7.0) | |
| P < 0.0001 | P < 0.0001 | P < 0.003 | ||||||||
| Gender | ||||||||||
| Male | 477 | 146 (12.4%) | 6.0 (5.3–6.7) | 265 | 358 (57.5%) | 16.0 (15.0–17.0) | 265 | 212 (44.4%) | 7.0 (6.3–7.7) | |
| Female | 938 | 493 (34.5%) | 8.0 (7.5–8.5) | 453 | 978 (68.3%) | 20.0 (18.4–21.6) | 453 | 485 (51.7%) | 7.0 (6.4–7.6) | |
| P < 0.0001 | P < 0.0001 | P = 0.404 | ||||||||
| Gender by phenotype Relapsing onset | ||||||||||
| Male | 302 | 136 (31.1%) | 9.0 (7.6–10.4) | 158 | 280 (63.9%) | 18.0 (15.8–20.2) | 158 | 144 (47.7%) | 7.0 (5.7–8.3) | |
| Female | 693 | 478 (40.8%) | 10.0 (9.1–10.9) | 309 | 862 (73.6%) | 22.7 (21.3–24.1) | 309 | 384 (55.4%) | 7.7 (7.0–8.5) | |
| Progressive onset | P < 0.005 | P < 0.006 | P = 0.371 | |||||||
| Male | 175 | 10 (5.4%) | 2.0 (1.6–2.4) | 107 | 78 (42.2%) | 10.1 (9.1–11.1) | 107 | 68 (38.9%) | 6.8 (6.3–7.3) | |
| Female | 245 | 15 (5.8%) | 2.0 (1.8–2.2) | 144 | 116 (44.6%) | 9.5 (8.4–10.6) | 144 | 101 (41.2%) | 6.0 (5.0–7.0) | |
| P = 0.871 | P = 0.847 | P = 0.832 | ||||||||
| Age at multiple | ||||||||||
| sclerosis onset (years) | 142 | 94 (39.8%) | 14.0 (12.1–15.9) | 76 | 160 (67.8%) | 29.0 (23.1–34.9) | 76 | 66 (46.5%) | 7.3 (6.1–8.5) | |
| <20 | 496 | 275 (35.7%) | 10.2 (9.2–11.2) | 258 | 513 (66.5%) | 21.0 (19.6–22.4) | 258 | 238 (48.0%) | 7.5 (7.0–8.0) | |
| 20 to <30 | 445 | 185 (29.4%) | 6.3 (5.7–7.0) | 230 | 400 (63.5%) | 16.8 (15.4–18.3) | 230 | 215 (48.3%) | 6.7 (6.2–7.2) | |
| 30 to <40 | 263 | 74 (22.0%) | 3.5 (3.0–4.0) | 117 | 220 (65.3%) | 12.7 (11.1–14.3) | 117 | 146 (55.5%) | 6.5 (5.8–7.3) | |
| 40 to <50 | 69 | 11 (13.8%) | 2.2 (1.8–2.7) | 37 | 43 (53.8%) | 9.0 (7.6–10.4) | 37 | 32 (46.4%) | 6.0 (4.6–7.4) | |
| ≥50 | P < 0.0001 | P < 0.0001 | P = 0.122 | |||||||
a Kaplan–Meier estimated median time with 95% confidence interval; comparison of survival curves using LogRank test.
In summary, the disease phenotype at multiple sclerosis onset was found to be correlated not only with age at onset, but also with the disability progression. While patients with relapsing and progressive multiple sclerosis onset reached DSS 3 and DSS 6 at the same age, our data showed that the progressive onset phenotype significantly shortened the duration of Phase 1, and to a lesser extent the duration of Phase 2, compared with the relapsing onset phenotype.
Age and disease duration to disability milestones DSS 3 and DSS 6 according to gender
Although males and females had the same age at multiple sclerosis clinical onset (median 30.2 years, P =0.784), males were significantly younger than females when they reached the two disability milestones (DSS 3: 40.4 versus 42.7 years, P <0.0001; DSS 6: 49.7 versus 52.2 years, P <0.010; Fig. 2). Consistently, the time to reach DSS 3 from clinical onset of multiple sclerosis was shorter in males than in females (median 6.0 versus 8.0 years, P < 0.0001). The time to reach DSS 6 from clinical onset of multiple sclerosis was also shorter in males than in females (median 16.0 versus 20.0 years, respectively, P < 0.0001), but not the time to reach DSS 6 from DSS 3 (median 7.0 years in both genders, P = 0.404; Table 4). In the same line, although the follow-up duration from multiple sclerosis clinical onset was similar in males and females (13.3 ± 8.8 years and 12.6 ± 9.7 years, respectively, P = 0.119), males had more frequently reached both disability milestones than females (DSS 3: 76.6 versus 66.5%, respectively, P < 0.0001; DSS 6: 42.5 versus 31.7%, respectively, P < 0.0001). This might be due to the fact that the ‘progressive onset:relapsing onset’ ratio was higher in males. We thus examined gender effect according to the disease phenotype at onset. In the group of patients with progressive onset multiple sclerosis, males had a younger age than women, the difference being significant both at multiple sclerosis onset (median 37.6 versus 39.9 years, P < 0.004) and at DSS 3 (median 40.4 versus 42.5 years, P < 0.004), and almost significant at DSS 6 (median 51.0 versus 53.8 years, P = 0.09). In addition, males and females with progressive onset had similar durations of both Phase 1 (median 2.0 years, P = 0.871) and Phase 2 (6.8 versus 6.0 years, P = 0.832). On the contrary, in the group of patients with relapsing onset multiple sclerosis, males and females had similar age at onset (median 28.4 versus 28.5 years, respectively, P = 0.881), but men reached DSS 3 at a younger age than women (median 40.6 versus 42.8 years, P < 0.005), and DSS 6 as well (median 49.3 versus 51.8 years, P < 0.048). Consistently, in relapsing onset multiple sclerosis, the duration of Phase 1 was shorter in males than in females (9.0 versus 10.0 years, P < 0.005), while the duration of Phase 2 was similar in both genders (7.0 versus 7.7 years, P = 0.371).
Interestingly, males with progressive or relapsing onset multiple sclerosis reached DSS 3 at the same age (median 40.4 versus 40.6 years, P = 0.363) as did females (median 42.5 versus 42.8 years, P = 0.710), supporting the idea that gender rather than phenotype at onset influenced the age at the assignment of DSS 3.
In summary, when males were compared with females, they were characterized by a younger age at clinical onset in progressive onset multiple sclerosis only, a shorter Phase 1 duration in relapsing onset multiple sclerosis only and a younger age at DSS 3 in both phenotypes. On the contrary, gender did not influence the duration of Phase 2, whatever the disease phenotype at onset.
Age and disease duration to disability milestones DSS 3 and DSS 6 according to age at clinical onset of multiple sclerosis
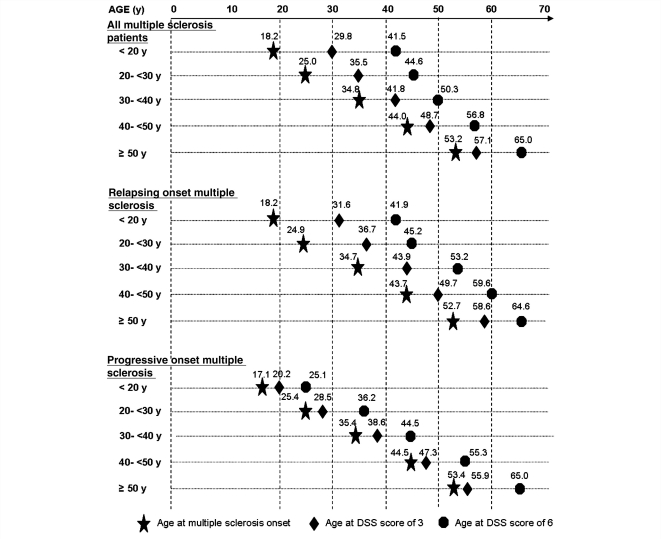
Considering age at onset as a categorical variable, we looked for a correlation between age at onset and disability progression. First, the younger the age at onset, the younger were the median ages at both EDSS 3 and 6. The age at DSS 3 significantly increased from the youngest to the oldest age group at onset (median from 29.8 to 57.1 years, P < 0.0001). The same was true for the age at DSS 6 (median from 41.5 to 65.0 years, P < 0.0001). Those correlations were observed in both relapsing onset and progressive onset multiple sclerosis (Fig. 3). Second, the younger the age at onset, the longer were the durations from clinical onset of multiple sclerosis to irreversible DSS 3 and 6. Indeed, the median times from multiple sclerosis clinical onset to DSS 3 significantly decreased as age of multiple sclerosis onset increased. Only the group of relapsing onset multiple sclerosis accounted for this correlation (median from 14.4 years to 3.3 years, P < 0.0001), which was not observed in the group of patients with progressive onset multiple sclerosis (median 2.0 years for all age groups, P = 0.295). The median times from clinical onset of multiple sclerosis to DSS 6 also decreased with increasing age at onset (median from 29.0 to 9.0 years, P < 0.0001). On the contrary, the time from DSS 3 to DSS 6 remained stable irrespective of the age group at onset (between 6.0 and 7.5 years, P = 0.122; Table 4).
Figure 3.
Kaplan–Meier estimated median age at clinical onset of multiple sclerosis and at DSS score of 3 in the 2054 patients with multiple sclerosis, and age at DSS score of 6 in the 1415 patients, who had reached DSS 3, according to age at onset, in the total multiple sclerosis population and in patients with multiple sclerosis classified by disease phenotype at multiple sclerosis onset.
In summary, a strong correlation was found between age at onset and disability progression in the whole multiple sclerosis population, and especially in relapsing onset multiple sclerosis. While patients younger at onset were also younger when reaching DSS 3 and 6, they exhibited a slower disability progression during Phase 1. On the contrary, age at onset did not influence the duration of Phase 2.
Age and disease duration to disability milestones DSS 3 and DSS 6 according to relapse history in relapsing onset multiple sclerosis
Results on relapse history are presented in Table 5. First, the presence of residual deficit (EDSS ≥ 2) after the first relapse was strongly correlated with subsequent disability progression. Patients with early residual deficit reached DSS 3 more rapidly than others (median 5.0 versus 11.0 years, respectively, P < 0.0001). They also reached DSS 6 more rapidly (median 18.0 versus 22.0 years, respectively, P < 0.0001), despite a slightly longer Phase 2 than patients without residual deficit after the first relapse (median 8.2 versus 7.0 years, respectively, P < 0.011).
Table 5.
Influence of relapses history on the median time from multiple sclerosis clinical onset to the assignment of irreversible DSS score of 3 (Phase 1), and to the assignment of irreversible DSS score of 6 among the 1609 patients with relapsing onset multiple sclerosis, and on the median time from DSS 3 to the assignment of an irreversible DSS score of 6 (Phase 2) among the 995 patients with relapsing onset multiple sclerosis who had reached DSS 3
| Variables | Time from multiple sclerosis clinical onset to DSS 3 (years) (n=1609) |
Time from multiple sclerosis clinical onset to DSS 6 (years) (n=1609) |
Time from EDSS 3 to DSS 6 (years) (n=995) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of events | Number of censored | Kaplan–Meier estimated mediana | Number of events | Number of censored | Kaplan–Meier estimated mediana | Number of events | Number of censored | Kaplan–Meier estimated mediana | |
| Residual deficit from the first relapse | |||||||||
| No | 813 | 559 (40.7%) | 11.0 (10.4–11.6) | 385 | 987 (71.9%) | 22.0 (20.6–23.4) | 385 | 428 (52.6%) | 7.0 (6.4–7.6) |
| Yes | 182 | 55 (23.2%) | 5.0 (4.2–5.8) | 82 | 155 (65.4%) | 18.0 (15.5–20.5) | 82 | 100 (54.9%) | 8.2 (5.8–10.6) |
| P < 0.0001 | P < 0.0001 | P < 0.011 | |||||||
| Number of relapses during the first 2 years of multiple sclerosis | 513 | 243 (32.1%) | 12.2 (11.2–13.2) | 271 | 485 (64.2%) | 22.3 (20.8–23.8) | 271 | 242 (47.2%) | 7.0 (6.1–7.9) |
| 1 | 482 | 371 (43.5%) | 7.7 (6.8–8.5) | 196 | 657 (77.0%) | 20.3 (18.4–22.2) | 196 | 286 (59.3%) | 7.7 (6.7–8.7) |
| ≥2 | P < 0.0001 | P < 0.001 | P = 0.200 | ||||||
| Occurrence of relapse after DSS 3 | |||||||||
| No | 235 | 252 (51.7%) | 6.0 (5.2–6.8) | ||||||
| Yes | 232 | 276 (54.3%) | 8.9 (8.1–9.7) | ||||||
| P < 0.0001 | |||||||||
| Occurrence of relapse after DSS 3, excluding the 416 patients with secondary progressive multiple sclerosis at DSS 3 (n=579) | |||||||||
| No | 39 | 135 (77.6%) | 12.0 (6.9–17.1) | ||||||
| Yes | 184 | 221 (54.6%) | 9.0 (7.8–10.2) | ||||||
| P = 0.677 | |||||||||
| Conversion to secondary progression at DSS 3 or before (n=995) | |||||||||
| No (relapsing–remitting multiple sclerosis patients with or without relapses after DSS 3) | 223 | 356 (61.5%) | 9.1 (7.9–10.3) | ||||||
| Yes | 244 | 172 (41. 3%) | 6.0 (5.2–6.8) | ||||||
| P < 0.0001 | |||||||||
| Conversion to secondary progression at DSS 6 or before (n=995) | |||||||||
| No (relapsing–remitting multiple sclerosis patients with or without relapses after DSS 3) | 104 | 314 (75.1%) | 12.0 (7.0–17.0) | ||||||
| Yes | 363 | 214 (37.1%) | 6.5 (5.9–7.2) | ||||||
| P < 0.0001 | |||||||||
a: Kaplan–Meier estimated median time with 95% confidence interval; comparison of survival curves using LogRank test.
Second, the number of relapses during the first 2 years in patients with relapsing multiple sclerosis onset was strongly correlated with a shorter duration from multiple sclerosis clinical onset to DSS 3 (median 7.7 years if two relapses or more versus 12.2 years if only one relapse, P < 0.0001), and to DSS 6 (median 20.3 versus 22.3 years, P < 0.001), but did not modify the duration from DSS 3 to DSS 6 (7.7 versus 7.0 years, respectively, P = 0.200).
Third, we focused on patients with relapsing onset multiple sclerosis who had reached DSS 3 and assessed, in this subgroup, the influence of relapses occurring after DSS 3. At first, the occurrence of relapses after DSS 3 would appear negatively correlated with the time to reach DSS 6 from DSS 3, since patients without relapses after DSS 3 had a shorter Phase 2 than patients who experienced relapses (median 6.0 versus 8.9 years, respectively, P < 0.0001). However, up to 64.3% of them had converted into secondary progressive multiple sclerosis during Phase 1, and only 20.3% of those who had relapses during Phase 2 (P < 0.0001). Thus, to evaluate the real impact on disability progression of relapses occurring during Phase 2, we excluded the 416 patients who had converted to secondary progressive multiple sclerosis at DSS 3 or before. Among the 579 patients whose disease was still relapsing at DSS 3, Phase 2 was longer among patients without relapses after DSS 3 than among patients who experienced relapses, but the difference was not significant (median 12.0 versus 9.0 years, P = 0.677), which allowed us to collapse these two groups into one. Finally, the 579 patients still relapsing at DSS 3 (with or without relapses) were found to have a longer Phase 2 than the 416 patients with secondary progressive multiple sclerosis (median 9.1 versus 6.0 years, P < 0.0001).
In summary, in relapsing onset multiple sclerosis, the presence of a residual deficit after the first relapse and the occurrence of relapses during the first two years of multiple sclerosis significantly shortened the duration of Phase 1 but did not influence disability progression during Phase 2. During Phase 2, disability progression was more influenced by a previous conversion to secondary progressive than by occurrence of relapses.
Independent predictive factors of disability progression in Phases 1 and 2 using multivariate analysis according to disease phenotype at onset
In relapsing onset multiple sclerosis, factors detected by Kaplan–Meier analyses as influencing disability progression during Phase 1 (i.e. gender, age at multiple sclerosis onset, residual deficit after the first relapse and relapses during the first 2 years of multiple sclerosis) were used as covariates in the Cox multivariate model. All were identified as independent predictive factors of disability progression during Phase 1. Likewise, the multivariate Cox model confirmed that only two factors (residual deficit after the first relapse, and early conversion into secondary progression) were predictive of the duration of Phase 2. In particular, the duration of Phase 2 was not influenced by the duration of Phase 1 (defined as a categorical variable).
As for progressive onset multiple sclerosis, no factor was found to be associated with disability progression during Phase 1 (neither gender, nor age at multiple sclerosis clinical onset) and Phase 2 (neither gender, nor age at multiple sclerosis clinical onset, nor duration of Phase 1; data not shown) (Table 6).
Table 6.
Results from Cox models about potential risk factors affecting the time from multiple sclerosis clinical onset to the assignment of an irreversible DSS score of 3 (Phase 1) among the 1609 patients with relapsing onset multiple sclerosis, and the time from DSS 3 to the assignment of an irreversible DSS score of 6 (Phase 2) among the 995 patients with relapsing onset multiple sclerosis who had reached DSS 3
| Variable | Time from multiple sclerosis clinical onset to DSS 3 (n=1609) |
Time from DSS 3 to DSS 6 (n=995) |
||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | P-value | Hazard ratio (95% confidence interval) | P-value | |
| Gender | 0.004 | 0.729 | ||
| Male | 1 | 1 | ||
| Female | 1.22 (1.06–1.40) | 1.04 (0.85–1.27) | ||
| Age at multiple sclerosis clinical onset (years) | 0.0001 | 0.223 | ||
| <20 | 1 | 1 | ||
| 20 to <30 | 1.32 (1.08–1.60) | 1.02 (0.77–1.35) | ||
| 30 to <40 | 1.64 (1.34–2.02) | 1.06 (0.78–1.44) | ||
| 40 to <50 | 2.94 (2.28–3.79) | 1.13 (0.76–1.70) | ||
| ≥50 | 4.28 |2.56–7.14) | 1.61 |0.75–3.44) | ||
| Number of relapses during the first 2 years of multiple sclerosis | 0.0001 | 0.536 | ||
| 1 | 1 | 1 | ||
| ≥2 | 1.77 (1.56–2.02) | 0.94 (0.77–1.15) | ||
| Residual deficit from the first relapse | 0.0001 | 0.021 | ||
| No | 1 | 1 | ||
| Yes | 2.44 (2.07–2.88) | 0.72 (0.55–0.95) | ||
| Time from multiple sclerosis clinical onset to DSS 3 (years) | – | – | 0.240 | |
| 0 to <3 | – | 1.10 (0.78–1.55) | ||
| 3 to <6 | – | 0.95 (0.69–1.31) | ||
| 6 to <10 | – | 1.16 (0.85–1.59) | ||
| 10 to <15 | – | 1.32 (0.97–1.79) | ||
| ≥15 | 1 | |||
| Conversion to secondary progression at DSS 3 or before | – | – | 0.0001 | |
| No | – | 1 | ||
| Yes | 1.62 (1.33–1.98) | |||
Discussion
In our population of 2054 patients referred to the Rennes Multiple Sclerosis Clinic in France, the age and sex distributions were similar to those previously reported (Weinshenker et al., 1989b; Phadke, 1990; Runmarker, 1993; Confavreux et al., 2000; Tremlett et al., 2006). In most series, including ours, the follow-up duration from onset of multiple sclerosis was 10–12 years (Phadke, 1990; Runmarker, 1993; Confavreux et al., 2000, 2003), except for the Canadian cohorts where it was 20 years (Tremlett et al., 2006) and 25 years (Kremenchutzky et al., 2006). In our series, there were 445 patients (21.7%) with a progressive onset disease. The proportion of primary progressive patients in the published series varies from 12.4% (352/2837) in Vancouver (Tremlett et al., 2006), 15.3% (282/1844) in Lyon (Confavreux et al., 2000), 19.8% (216/1089) in London Ontario (Cottrell et al., 1999), to 33.7% (367/1089) in the initial paper coming from London Ontario (Weinshenker et al., 1989a). There is at present no good explanation for these differences (except for the last one, as some misclassifications were identified in a second assessment). Unlike the other multiple sclerosis populations, a large proportion of our patients received disease-modifying treatments, and time spent on disease modifying drugs was 17.2% of the total follow-up. Nevertheless, to date, there is no proven efficacy of those treatments on reducing the long-term progression of irreversible disability in multiple sclerosis. Moreover, our database was closed in 2004, i.e. before the new therapeutic strategies in favour of a treatment administered earlier in multiple sclerosis history. Indeed, none of our patients received a treatment after the first episode (mean multiple sclerosis duration before the first treatment: 7.7 years), and when restricting the analysis to the untreated population our results on the two phases remained similar. The time from multiple sclerosis clinical onset to reach DSS 6 in our population was in line with those found in the main multiple sclerosis series (about 18–20 years in relapsing onset in both Lyon cohort (Confavreux et al., 2000) and London Ontario cohort (Weinshenker et al., 1989a; Kremenchutzky et al., 2006) whatever the mean follow-up duration (11 years in Lyon cohort, 25 years in London Ontario cohort), but was lower than in British Columbia (27.9 years; Tremlett et al., 2006). A methodological reason may account for this difference as pinpointed by Confavreux (2008): Tremlett et al. are the only authors who excluded the patients who had already reached the selected outcome before the first visit at the Multiple Sclerosis Clinic (left-censored patients), resulting in a potential over-estimation of the times to disability milestones.
In this observational study we clearly demonstrate that disability progression in the first phase of multiple sclerosis (defined by the period until irreversible DSS 3) does not influence disability progression during the second phase (defined by the period from irreversible DSS 3 to irreversible DSS 6). Contrasting with the high variability of Phase 1 duration, the Phase 2 duration remained remarkably constant, as previously shown in the British Columbia multiple sclerosis population (Tremlett et al., 2006). In this article, the median time from EDSS 3 to EDSS 6 was estimated between 4.4 and 6.8 years in the four subgroups defined according to the time to EDSS 3 from onset (<5, 5 to <10, 10 to <15, ≥15 years). While some authors (Confavreux et al., 2000, 2003; Confavreux, 2006a, b; Debouverie et al., 2008) used a score of 4 as early benchmark of disability accumulation, we chose to use the score of 3. Indeed, during the data collection we paid a special attention to this hallmark, as did others (Weinshenker et al., 1989; Cottrell et al., 1999; Kremenchutzky et al., 2006; Tremlett et al., 2006). In our opinion, more than the choice of score on the disability scale, the important fact is that both define an early and reliable threshold of irreversible disability.
We also confirmed previous results showing the role of phenotype on disability progression. In relapsing onset multiple sclerosis, the disability progression during Phase 1 was much slower, and that during Phase 2 was somehow slower, than in progressive onset multiple sclerosis. Phenotype also influenced the age at onset, but not the age at DSS 3 and DSS 6. This was in line with some reports (Confavreux, 2006a; Kremenchutzky et al., 2006; Koch et al., 2007) showing that the age at the onset of progression was not significantly different between secondary progressive and primary progressive multiple sclerosis.
Our most important and original result was the finding that the factors influencing disability progression during Phase 1 were restricted to relapsing onset multiple sclerosis. These comprised gender, age at multiple sclerosis onset and relapse history (relapses within the first 2 years, and residual deficit after the first relapse).
In relapsing onset multiple sclerosis, gender influenced disability progression during Phase 1 only. Females had a slower progression, and thus were older at DSS 3 and DSS 6 than males. In progressive onset multiple sclerosis, although males were younger at onset of DSS 3 and DSS 6 than females, gender did not influence the disability progression during Phase 1 and Phase 2. Other studies on the whole multiple sclerosis population have showed a slower disability progression in females than in males (Weinshenker et al., 1989b; Runmarker, 1993; Confavreux et al., 2003). One of these studies (Confavreux et al., 2003) also showed that in the whole multiple sclerosis population, gender influenced the time from multiple sclerosis clinical onset to DSS 4, but not the time from DSS 4 to DSS 6 or 7. Our data demonstrated that only the relapsing onset phenotype accounted for this observation.
In relapsing onset multiple sclerosis, age at multiple sclerosis clinical onset deserves special consideration as it dramatically influenced the disability progression during Phase 1 (but not during Phase 2); the younger the age at onset, the slower the disability progression during Phase 1. In contrast, in progressive onset multiple sclerosis, age at onset did not influence disability progression during Phase 1 and Phase 2. Age at onset also influenced age at DSS 3 and DSS 6 in both phenotypes: the younger the age at onset, the younger the age at DSS 3 and DSS 6. While the influence of age at onset has already been described in the whole multiple sclerosis population (Phadke, 1990; Runmarker, 1993; Confavreux, 2006b; Tremlett et al., 2006; Stankoff et al., 2007), our data demonstrated that only the relapsing onset phenotype accounted for this observation.
In relapsing onset multiple sclerosis, the occurrence of at least two relapses during the first 2 years of multiple sclerosis and the presence of residual deficit from the first relapse made the disability progression faster during Phase 1. The correlation between early relapses and early disability progression has also been shown in previous studies (Weinshenker et al., 1989b; Confavreux et al., 2000; Lublin et al., 2003; Tremlett et al., 2009). In contrast, relapses after DSS 3 did not have a similar influence on disability progression in the large majority of patients with multiple sclerosis. However, when secondary progressive multiple sclerosis was excluded, the relapses after DSS 3 might still have some influence on disability, although the comparison did not reach statistical significance due to the small numbers of patients. More than the occurrence of relapses, the conversion to secondary progressive multiple sclerosis influenced disability progression in Phase 2. The dissociation between later relapses and later disability progression has already been observed (Confavreux et al., 2000, 2003; Kremenchutzky et al., 2006; Young et al., 2006; Tremlett et al., 2009). Our data pointed out the influence of early focal inflammatory clinical markers on disability progression restricted to Phase 1.
Finally, the Cox multivariate analysis confirmed that all the above factors were independently predictive of disability progression during Phase 1 in relapsing onset multiple sclerosis. While these factors have already been identified in the whole multiple sclerosis population (Weinshenker et al., 1989b; Phadke, 1990; Runmarker, 1993, Kantarci et al., 1998; Confavreux et al., 2003; Ebers, 2005), we demonstrated that their influence was restricted to the duration of Phase 1 and to the relapsing onset phenotype. Indeed, except for the conversion to secondary progressive multiple sclerosis and the presence of a residual deficit after the first relapse, we did not find a clear predictive factor of disability progression after irreversible DSS 3 in relapsing onset multiple sclerosis, or during Phases 1 and 2 in progressive onset multiple sclerosis. This was consistent with other observational studies (Confavreux et al., 2003; Debouverie et al., 2008) yielding the conclusion that once a clinical threshold of irreversible disability is reached, the progression of disability is amnesic of the prior clinical history of the disease.
The understanding about the dissociation between early and later disability progression and about the role of prognostic factors is central to the debate on the putative mechanisms of disability progression in multiple sclerosis. Some studies (Confavreux, 2006b; Kremenchutzky et al., 2006, Stankoff et al., 2007) have suggested that age is a key player (if not the only one) in the natural history of multiple sclerosis, leading to the concept of multiple sclerosis as a single-stage disorder with a chronic age-related neurodegeneration since the onset of the disease (Confavreux, 2006a). However, our data gave evidence for a two-stage disability progression in multiple sclerosis. In relapsing onset phenotype, the independency between Phase 1 and Phase 2 suggested a two-stage disease, with a first stage during which focal inflammatory lesions influence disability progression and a second stage during which disability progression is independent of focal inflammatory markers. In contrast, in progressive onset phenotype, focal inflammatory lesions are clinically asymptomatic for a long period of time and only detectable on MRI, restricting the clear identification of the first stage of the disease. This concept of multiple sclerosis as a two-stage disease is not contradictory with the influence of age in disability progression. Age might influence the multiple sclerosis course in different ways: age-related decrease in central nervous system remyelination (Stankoff et al., 2007), age-related plasticity of brain injury (Compston, 2008) and age-related change in immune factors (Weiner, 2009).
The concept of multiple sclerosis as a two-stage disease is also supported by some MRI data, especially the plateauing relationship between T2 burden of disease and disability for EDSS value above 4.5 (Li et al., 2006), and the strong correlation between T2 lesion load change within the first 5 years of multiple sclerosis and disability status at 20 years of disease duration (Fisnicu et al., 2008). It is also supported by therapeutic experience. With early therapeutic intervention, it is now easier to demonstrate a relationship between effects on the focal inflammatory lesions (relapses or new MRI lesions) and delaying confirmed disability progression in the short-term (The CAMMS223 Trial Investigators, 2008). However, at a later stage of relapsing-remitting or secondary progressive multiple sclerosis, the impact of these same therapies on disability progression remains uncertain (Panitch et al., 2004, Coles et al., 2006). Finally, this concept of multiple sclerosis as a two-stage disease has obvious implications for the future therapeutic strategies in multiple sclerosis, reinforcing the concept of a therapeutic window of opportunity, as suggested by Coles et al. (2006).
Funding
Association pour la Recherche sur la Sclérose en Plaques (ARSEP) and academic funding for clinical research.
Acknowledgements
The authors are indebted to the patients for their participation in the Rennes Multiple Sclerosis Database; Drs De Burghgraeve, De Marco, Hinzelin, Lallement, Laplaud, Merienne, Taurin and Wiertlewski for their contribution to the development of the database; Prof Chaperon for his scientific expertise on data analysis; Profs Confavreux, Debouverie, Ebers, Gonsette, Hommes, Kremenchutzky and Narayana, for fruitful discussions and comments on the manuscript; Prof Confavreux and the European Database for Multiple Sclerosis (EDMUS) Coordinating Center for their essential contribution to the development and update of the EDMUS system.
Glossary
Abbreviations
- DSS
Disability Status Scale
- EDMUS
European Database for Multiple Sclerosis
- EDSS
Expanded Disability Status Scale
References
- CAMMS223 Trial Investigators. Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, et al. Alemtuzumab vs Interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- Charcot JM. Paris: A Delahaye; 1880. Leçons sur les maladies du système nerveux faites à la Salpétrière. [Google Scholar]
- Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR, Kooijmans MF, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59:679–87. doi: 10.1212/wnl.59.5.679. [DOI] [PubMed] [Google Scholar]
- Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- Compston A. Making progress on the natural history of multiple sclerosis. Brain. 2006;129:561–3. doi: 10.1093/brain/awl034. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1520–7. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006a;129:606–16. doi: 10.1093/brain/awl007. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006b;129:595–605. doi: 10.1093/brain/awh714. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability: an amnesic process. Brain. 2003;126:770–82. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343:1430–8. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Compston DA, Hommes OR, McDonald WI, Thompson AJ. EDMUS a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry. 1992;55:671–6. doi: 10.1136/jnnp.55.8.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confavreux C, Ritleng C, Debouverie M, Durand-Dubief F, Marignier R, Androdias G, et al. Defining the natural history of MS: the need for complete data and rigorous definitions. Answer to Dr Tremlett et al. Mult Scler. 2008;14:1144–7. doi: 10.1177/1352458508088625. [DOI] [PubMed] [Google Scholar]
- Cottrell DA, Kremenchutzky M, Rice GPA, Koopman WJ, Hader W, Baskerville J, et al. The natural history of multiple sclerosis: a geaographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain. 1999;122:625–39. doi: 10.1093/brain/122.4.625. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- Debouverie M, Pittion-Vouyovitch S, Louis S, Guillemin F. Natural history of multiple sclerosis in a population-based cohort. Eur J Neurol. 2008;15:916–21. doi: 10.1111/j.1468-1331.2008.02241.x. [DOI] [PubMed] [Google Scholar]
- Ebers GC. Prognostic factors for multiple sclerosis: the importance of natural history studies. J Neurol. 2005;252(Suppl 3):iii15–iii20. doi: 10.1007/s00415-005-2012-4. [DOI] [PubMed] [Google Scholar]
- Fisnicu LK, Brex PA, Altmann TR, Riskiel KA, Benton CE, Lanyon R, et al. Disability and T2 MRI lesions: a 20 year follow-up of patients with relapse onset of MS. Brain. 2008;131:808–17. doi: 10.1093/brain/awm329. [DOI] [PubMed] [Google Scholar]
- Frischer J, Brasmow S, Lucchinetti C, Raushka H, Schimdbauer M, Laursen H, et al. The relationship between inflammation and neurodegeneration in multiple sclerosis. Brain. 2009;132:1175–89. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular Interferon Beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996;39:285–94. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- Kantarci O, Siva A, Eraksoy M, Karabudak R, Sütlas N, Agaoglu J, et al. Survival and predictors of disability in Turkish MS patients. Neurology. 1998;51:765–72. doi: 10.1212/wnl.51.3.765. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- Kappos L, Freedman M, Polman C, Edan G, Hartung P, Miller DH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet. 2007;370:389–97. doi: 10.1016/S0140-6736(07)61194-5. [DOI] [PubMed] [Google Scholar]
- Koch M, Mostert J, Heersema D, De Keyser J. Progression in multiple sclerosis: further evidence of an age dependant process. J Neurol Sci. 2007;255:35–41. doi: 10.1016/j.jns.2007.01.067. [DOI] [PubMed] [Google Scholar]
- Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically bases study. 9: Observations on the progressive phase of the disease. Brain. 2006;129:584–94. doi: 10.1093/brain/awh721. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. On the evaluation of disability in multiple sclerosis. Neurology. 1961;11:686–94. doi: 10.1212/wnl.11.8.686. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–12. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Leray E, Morrissey S, Yaouanq J, Coustans M, Le Page E, Chaperon J, et al. Long term survival of patients with multiple sclerosis in West France. Mult Scler. 2007;13:865–74. doi: 10.1177/1352458507077410. [DOI] [PubMed] [Google Scholar]
- Li DK, Held U, Petkau J, Daumer M, Barkhof F, Fazekas F, et al. MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability. Neurology. 2006;66:1384–89. doi: 10.1212/01.wnl.0000210506.00078.5c. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Baier M, Cutter G. Effect of relapse on development of residual deficit in multiple sclerosis. Neurology. 2003;61:1528–32. doi: 10.1212/01.wnl.0000096175.39831.21. [DOI] [PubMed] [Google Scholar]
- Panitch H, Miller A, Paty D, Weinshenker B. North American Study Group on interferon beta 1b in secondary progressive MS. Neurology. 2004;63:1788–95. doi: 10.1212/01.wnl.0000146958.77317.3e. [DOI] [PubMed] [Google Scholar]
- Phadke JG. Clinical aspects of multiple sclerosis in north-east Scotland with particular references to its course and prognosis. Brain. 1990;113:1597–628. doi: 10.1093/brain/113.6.1597. [DOI] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, Mc Donald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- PRISMS Study Group (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Randomised double-blind placebo controlled study of interferon beta-1a in relapsing-remitting multiple sclerosis. Lancet. 1998;352:1498–504. [PubMed] [Google Scholar]
- Rice GP, Filippi M, Comi G. Cladribine and progressive multiple sclerosis: clinical and MRI outcomes of a multicenter controlled trial. Cladribine MRI Study Group. Neurology. 2000;54:1145–55. doi: 10.1212/wnl.54.5.1145. [DOI] [PubMed] [Google Scholar]
- Runmarker B, Andersen O. Pronostic factors in a multiple sclerosis incidence cohort with twenty five years of follow-up. Brain. 1993;116:117–34. doi: 10.1093/brain/116.1.117. [DOI] [PubMed] [Google Scholar]
- SPECTRIMS Study Group (Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon beta-1a in MS) Randomized controlled trial of interferon-beta-1a in secondary progressive MS: Clinical results. Neurology. 2001;56:1496–504. doi: 10.1212/wnl.56.11.1496. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Mrejen S, Tourbah A, Fontaine B, Lyon-Caen O, Lubetzki C, et al. Age at onset determines the occurrence of the progressive phase of multiple sclerosis. Neurology. 2007;68:779–81. doi: 10.1212/01.wnl.0000256732.36565.4a. [DOI] [PubMed] [Google Scholar]
- Chicago: SPSS; 2002. Statistical Package for Social Science [program] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transaction in the lesion of multiple sclerosis. N Engl J Med. 1998;338:278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology. 2006;66:172–77. doi: 10.1212/01.wnl.0000194259.90286.fe. [DOI] [PubMed] [Google Scholar]
- Tremlett H, Zhao Y, Devonshire V. Natural history comparisons of primary and secondary progressive multiple sclerosis reveals differences and similarities. J Neurol. 2009;256:374–81. doi: 10.1007/s00415-009-0039-7. [DOI] [PubMed] [Google Scholar]
- Tremlett H, Yousefi M, Devonshire V, Rieckmann P, Zhao Y. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology. 2009;73:1616–23. doi: 10.1212/WNL.0b013e3181c1e44f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol. 2009;65:239–48. doi: 10.1002/ana.21640. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Bass B, Rice GP, Noseworthy JH, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989a;112:133–46. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Bass B, Rice GP, Noseworthy JH, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain. 1989b;112:1419–28. doi: 10.1093/brain/112.6.1419. [DOI] [PubMed] [Google Scholar]
- Young PY, Lederer C, Eder K, Daumer M, Neiss A, Polman C, et al. Relapses and subsequent worsening of disability in relapsing-remitting multiple sclerosis. Neurology. 2006;67:804–8. doi: 10.1212/01.wnl.0000234064.17156.03. [DOI] [PubMed] [Google Scholar]