Abstract
The relationship of relapses to long-term disability in multiple sclerosis is uncertain. Relapse reduction is a common therapeutic target but clinical trials have shown dissociation between relapse suppression and disability accumulation. We investigated relationships between relapses and disability progression for outcomes of requiring assistance to walk, being bedridden and dying from multiple sclerosis [Disability Status Scale 6, 8, 10] by analysing 28 000 patient-years of evolution in 806-bout onset patients from the London Ontario natural history cohort. Having previously shown no effect of relapse frequency among progressive multiple sclerosis subtypes, here we examined these measures in the pre-progressive or relapsing–remitting phase. Survival was compared among groups stratified by (i) early relapses—number of attacks during the first 2 years of multiple sclerosis; (ii) length of first inter-attack interval; (iii) interval between onset and Disability Status Scale 3 (moderate disability); (iv) number of attacks from the third year of disease up to onset of progression; and (v) during the entire relapsing–remitting phase. Early clinical features can predict hard disability outcomes. Frequent relapses in the first 2 years and shorter first inter-attack intervals predicted shorter times to reach hard disability endpoints. Attack frequencies, in the first 2 years, of 1 versus ≥3, gave differences of 7.6, 12.8 and 20.3 years in times from disease onset to Disability Status Scale 6, 8 and 10, respectively. Time to Disability Status Scale 3 highly and independently predicted time to Disability Status Scale 6, 8 and 10. In contrast, neither total number of relapsing–remitting phase attacks nor of relapses experienced during the relapsing–remitting phase after the second year up to onset of progression showed a deleterious effect on times from disease onset, from progression onset and from Disability Status Scale 3 to these hard endpoints. The failure of a regulatory mechanism tied to neurodegeneration is suggested. Relapse frequency beyond Year 2 does not appear to predict the key outcome of secondary progression or times to Disability Status Scale 6, 8 or 10, highlighting two distinct disease phases related to late outcome. These appear to be separated by a watershed within the relapsing–remitting phase, just a few years after clinical onset. Higher early relapse frequencies and shorter first inter-attack intervals herald more rapid deterioration via interaction with the neurodegeneration characterizing secondary progression. They increase the probability of its occurrence, its latency and influence—to a lesser degree—its slope. The prevention or delay of the progressive phase of the disease is implicated as a key therapeutic target in relapsing–remitting patients.
Keywords: multiple sclerosis, relapses, long-term disability, natural history
Introduction
Relapses are the most florid clinical feature of multiple sclerosis. Typical relapses in appropriate anatomical locations are sufficient for experienced clinicians to diagnose multiple sclerosis with high reliability. Although relapses are diagnostically useful and can produce temporary or even permanent loss of function, progressive unremitting disability is at the heart of the medical, social and economic impact of multiple sclerosis. The vast majority of patients experience an initial relapsing–remitting phase, followed in more than 80% by secondary progression of disability with or without superimposed relapses (Weinshenker et al., 1989a; Lublin and Reingold, 1996).
Relationships among cumulative relapses, onset of secondary progression and increasing disability are highly relevant to clinical practice. Suppression of relapses and their surrogates, MRI T2 or gadolinium enhancing lesions, have been ubiquitous clinical endpoints for evaluating treatment efficacy. Belief that disability in multiple sclerosis results from serial exacerbations, each adding to cumulative disability, is widespread. Clearly a ‘staircase’ worsening characterizes neuromyelitis optica and some with early multiple sclerosis (Wingerchuk et al., 1999) but it is not as well defined in long-term multiple sclerosis outcomes.
Biological mechanisms leading to acute attacks probably differ from those responsible for unremitting disability (Bjartmar et al., 2001; DeLuca et al., 2006; Trapp and Nave, 2008). Dissociation between therapeutic effects on relapse frequency versus effects on progression of disability first became clear in the interferon studies (IFNB Multiple Sclerosis Study Group, 1993, 1995; Jacobs et al., 1996; European Study Group, 1998; PRISMS Study Group, 1998), was extended with cladribine (Beutler et al., 1996; Rice et al., 2000) and is no more clearly evident than with alemtuzumab (Coles et al., 1999). Therapeutic reduction of early relapse rates holds promise for the reduction of subsequent disability progression but remains unvalidated in terms of hard outcomes.
The predictive value of early relapse number and first inter-attack interval for long-term disability outcomes has been addressed (Confavreux et al., 1980, 2000, 2003; Weinshenker et al., 1989b; Kantarci et al., 1998). The prognosis was shown to be worse in patients who had more frequent relapses within 2–5 years (Weinshenker et al., 1989b; Kantarci et al., 1998; Confavreux et al., 2003) from onset and who had a shorter first inter-attack interval (Weinshenker et al., 1989b; Phadke, 1990; Confavreux et al., 2003), seemingly driven by the first 2 years’ events. The independent impact of relapses after the second year of disease has not been previously analysed.
There is no clear indication whether the association of early relapses with disability is causal or associative. Relapses could be related to long-term outcome indirectly or represent associated concomitants of predetermined rapidity of clinical course. The predictive effect of early relapse rate on disease progression disappears once the progressive course supervenes (Confavreux et al., 2003). However, the probability of progression and time to progression are relatively unexplored measures with profound clinical relevance (Kremenchutzky et al., 2006). There are little or no data on the relationships between later relapses and hard outcomes, from time to cane requirement through to death from multiple sclerosis.
In primary-progressive and progressive-relapsing multiple sclerosis, superimposed relapses do not affect long-term outcomes (Kremenchutzky et al., 1999) and once progression has begun in the secondary progressive phase, its rate has been reported to be both independent of preceding factors (Confavreux et al., 2000, 2003) and homogeneous among primary progressive and grouped secondary progressive multiple sclerosis subtypes stratified by relapse frequencies (Kremenchutzky et al., 2006).
In this context, we sought to clarify further the role of early relapses in long-term outcomes (Weinshenker et al., 1989b). In a population-based series of 806 patients with relapsing–remitting onset multiple sclerosis from the London Ontario database and with an extended 28 years follow-up, we revaluated the predictive value of relapses. Distinctively, the focus was on the period prior to the onset of the progressive course and on the attainment of high disability levels [Disability Status Scale (DSS) 6–8–10].
Methods
The London Multiple Sclerosis Clinic (London Health Sciences Centre, Canada), established in 1972, provides long-term care for patients with multiple sclerosis from its referral area of south-western Ontario. Clinic and database characteristics have been extensively outlined (Weinshenker et al., 1989a; Cottrell et al., 1999; Kremenchutzky et al., 2006). Patients were evaluated annually or semi-annually regardless of clinical course. Disability was assessed using the DSS (Kurtzke, 1955). Data collection was performed through separate research charts containing data forms completed at patient visits, with the observation period ending in 2000. The shortest follow-up was 16 years. Within the total population, two subpopulations were identified: (i) the subgroup from Middlesex County, encompassing 90% of patients in Middlesex County with multiple sclerosis (Hader et al., 1988) and (ii) subgroup of patients seen from disease onset, the vast majority within 12 months from the diagnosis. The database was recently (2009) subjected to a rigorous data quality process.
Population and definitions
Among 806 relapsing–remitting onset patients (Lublin and Reingold, 1996) from the original population-based natural history cohort, exacerbations were defined as acute development of new symptoms or worsening of existing symptoms, lasting >24 h (Poser et al., 1983; Lublin and Reingold, 1996). Clinical onset was the date (year) of the first symptom. Occurrences of attacks and disability scores were obtained from attack-related visits and yearly follow-ups (Kremenchutzky et al., 2006). Neurological systems involved at onset were grouped into motor (pyramidal), sensory, cerebellar, brainstem, visual (optic nerve) and bowel/bladder; for patients not seen at onset, DSS scores (Kurtzke, 1955) were determined retrospectively from outside records. Progressive disease was defined by at least 1 year of continuous deterioration, regardless of the rate of worsening. Transitory plateaus and trivial temporary improvements in the relentlessly progressive course were allowed in the long term, although steady progression was the rule. Evaluations at yearly intervals had the distinct advantage of longer retrospect than for treatment trials.
Documentation collected for the hard endpoints of requiring aids for walking (DSS 6), for restriction to bed with effective arm use (DSS 8) and death from multiple sclerosis (DSS10) left little ambiguity. If DSS scores were unrecorded, they were derived from the description of the neurological findings only when unambiguous, otherwise the database was left blank for that specific visit.
The basic underlying hypotheses being tested were posed in Nat Hist 9 (Kremenchutzky et al., 2006), namely that relapse frequency and initial location determine late disability outcomes. Here the focus was on the relapsing–remitting phase.
Statistical methods
We investigated relationships between disability outcome and the following variables: (i) number and type of neurological systems involved at clinical onset; (ii) number of relapses in Year 1, in Year 2 and combined (Y1–Y2); (iii) time between the first and the second attack; (iv) time from disease onset to attainment of DSS 3 (moderate disability); (v) number of relapses from Year 3 up to the onset of progression; and (vi) total number of relapses before onset of the progressive phase. When investigating the predictive effect of time to reach DSS 3, time to attain later endpoints was adjusted to the interval from DSS 3 in order to make parameters independent from each other.
Patients were grouped according to (i) number of neurological systems involved at clinical onset; (ii) number of attacks (low, intermediate and high); (iii) length of interval between first and second attack; and (iv) between disease onset and attainment of DSS 3 (short, intermediate and long), as defined in Table 2. Grouping aimed for similar numbers of patients in each category; additional stratifications provided internal controls to confirm results.
Table 2.
Stratification of patients by features of the early clinical course
| No. of patients | ||
|---|---|---|
| No. of neurological systems involved at clinical onset | ||
| Low | 1 | 535 |
| Intermediate | 2 | 187 |
| High | ≥3 | 75 |
| No. of relapses in the first 2 years | ||
| Low | 1 | 389 |
| Intermediate | 2 | 183 |
| High | ≥3 | 158 |
| First inter-attack interval (years) | ||
| Short | 0–2 | 388 |
| Intermediate | 3–5 | 141 |
| Long | ≥6 | 155 |
| Time to reach DSS 3 (years) | ||
| Short | 0–2 | 123 |
| Intermediate | 3–7 | 192 |
| Long | ≥8 | 463 |
| No. of relapses from the third year to onset of progressive phasea | ||
| Low | 0 | 107 |
| Intermediate | 1–2 | 164 |
| High | ≥3 | 165 |
| Total no. of relapses before the onset of progressive phasea | ||
| Low | 1–2 | 158 |
| Intermediate | 3–4 | 138 |
| High | ≥5 | 163 |
a Patients with secondary progressive multiple sclerosis only.
Kaplan–Meier technique estimated times for conversion to secondary progressive, times to reach DSS 6–8–10 from both disease onset and from onset of progressive phase, and times from DSS 3 to DSS 6–8–10. Log rank tests investigated differences observed; survival was compared against groups with more relapses or with longer first inter-attack interval or with longer interval between disease onset and DSS 3. Using Cox proportional hazard analysis (Cox and Oakes, 1984) relapses, first inter-attack intervals and times to DSS 3 were also analysed as continuous variables to estimate the risk of attaining endpoints according to numbers of attacks experienced, increasing times between the first two attacks and increasing times between disease onset and accumulation of moderate disability (DSS 3).
Hazard ratios (HR) were obtained through comparison versus the hypothetical scenario where patients experienced 0 relapses or 0 years interval between the first two attacks and between disease onset and the attainment of DSS 3. Information on time to every DSS level was not always available, resulting in slightly different numbers of patients contributing at each DSS level when estimating the ‘time to disability’ survival curves. Patients not reaching given DSS levels but followed for known periods were right censored. Proportional hazards assumption was checked by visual inspection of Schoenfeld residual plots and corresponding statistical tests. Analyses were replicated in ‘seen from onset’ and Middlesex County sub-populations in order to obtain further validation of results.
The Sylvia Lawry Centre drafted an analysis plan that was finalized with input from the study group. For consistency, two authors (AS and AD) then carried out the same analyses independently and blindly using the Statistical Package for the Social Sciences software (SPSS version 15); results from the two analyses were eventually reviewed, checked and partially extended at the Sylvia Lawry Centre where R software (Team RDC, 2008) was used.
Results
Table 1 lists clinical and demographic features of the 806 bout-onset patients. Secondary progressive (66.2%) and female (68.8%) patients predominated. The most common systems involved at onset were sensory (54.3%) and optic (21.5%); 66% of relapsing–remitting patients had converted to secondary progressive multiple sclerosis, slightly higher in males (188/252, 74.6%) than females (346/554, 62.5%). Estimated median time to secondary progressive onset was 15 years. For conversion rate to secondary progressive, patients were stratified by disease duration. After 25 years from onset, >80% of patients had developed secondary progressive (Supplementary Fig. 1). With the advantage of retrospect afforded by later visits, disability was rated between DSS 2 and DSS 4 (87%) with a median DSS level of 3 at secondary progressive onset (Kremenchutzky et al., 2006). At the end of the follow-up period, 657 patients (81.5%) had reached DSS 3, 543 patients (67.4%) DSS 6, 390 patients (48.4%) DSS 8 and 132 patients (16.4%) had reached DSS 10; the estimated median survival times were 10, 18, 28 and 63 years, respectively. There were no important differences among the epidemiological subgroups.
Table 1.
General features of the bout onset population
| Relapsing onset population | |
| No. of patients | 806 |
| Males, n (%) | 252 (31.2) |
| Females, n (%) | 554 (68.8) |
| Sex ratio (F/M) | 2.19 |
| Disease course (at the end of observation period; 1972–2000) | |
| Secondary progressive number (%) | 534 (66.2) |
| Relapsing–remitting number (%) | 272 (33.8) |
| Disease duration, years | |
| Mean (SE) | 24.4 (0.362) |
| Median | 23 |
| Age at disease onset, years | |
| Mean (SE) | 28.5 (0.316) |
| Median | 27 |
| Age at onset of progression, years | |
| Mean (SE) | 40.2 (0.447) |
| Median | 39 |
| DSS at onset of progression, years | |
| Mean (SE) | 2.9 (0.047) |
| Median | 3 |
| First inter-attack interval, years | |
| Mean (SE) | 3.8 (0.180) |
| Median | 2 |
| Systems involved at onset; no. of patients (%) | |
| Sensory | 438 (54.3) |
| Optic | 174 (21.5) |
| Brainstem | 167 (20.7) |
| Motor | 145 (17.9) |
| Cerebellar | 51 (6.3) |
| Bowel/bladder | 25 (3.1) |
| Kaplan–Meier estimates of the median time (years) from disease onset to: | |
| DSS 3 | 10 |
| DSS 6 | 18 |
| DSS 8 | 28 |
| DSS 10 | 63 |
| Onset of progression | 15 |
Table 2 groups patients by early clinical course. The majority (66.3%) had one neurological system involved at presentation. During the first 2 years, 1363 attacks were recorded. Attack frequency ranged from 1 to 8 with the mean relapse rate being 0.93 attacks/year. The first inter-attack interval ranged between 0 and 34 years; second attacks occurred after a median 2 years (Table 1). When reached, median interval from onset to DSS 3 was 8 years. Within the secondary progressive population, 1038 relapses were recorded from Year 3 to onset of progression with a mean relapse rate of 0.41 attacks/year. In 107 patients, no attack was registered after Year 2 before entering the secondary progressive phase. During the overall relapsing–remitting phase, 1882 attacks were documented with a mean relapse rate of 0.65 attacks/year.
Initial presentation
Polysymptomatic onset predicted neither time to convert to secondary progressive multiple sclerosis nor attainment of endpoints from disease onset or from onset of progression. Similar times to attain endpoints characterized patients with 1, 2 or ≥3 neurological systems involved at presentation. Brainstem was the only initial exacerbation location marginally related to shorter time to DSS 6 (P = 0.02) and DSS 8 (P = 0.001).
The development of secondary progressive multiple sclerosis
Inevitable imprecision in assigning onset of progression (secondary progressive), especially in those with concomitant relapses, was much attenuated by yearly consecutive assessments. For most patients, secondary progressive is implied by reaching DSS 3. Some 25% of the cohort reached DSS 3 through relapses but failed to progress for extended periods. In this minority and with hindsight, attaining DSS 3 was clearly not an indicator of secondary progression.
Early relapses and first inter-attack interval
The probability of developing secondary progressive multiple sclerosis was significantly affected by early relapses and first inter-attack interval assessed independently. More relapses in the first 2 years were related (HR = 1.1; P = 0.003) to a higher probability to convert to secondary progressive multiple sclerosis; relapses in Year 2 were marginally (P = 0.02) more predictive than relapses in Year 1. Latency between disease onset and onset of progression was significantly (P = 0.014) longer in groups with 1 versus ≥3 relapses during the first 2 years (Table 3).
Table 3.
Survival times from disease onset to onset of the progressive phase. Patients are stratified according to number of relapses in the first 2 years, first inter-attack interval and number of relapses from year 3 to onset of progression
| Time to onset of steady progression |
|||
|---|---|---|---|
| Mean years (median) | 95% CI | P-value | |
| Relapses Years 1 and 2 | |||
| 1 relapse | 19.9 (16) | 18.3–21.5 | 0.014 |
| 2 relapses | 16.7 (13) | 14.6–18.9 | 0.380 |
| ≥3 relapsesa | 15.1 (9) | 12.8–17.4 | |
| First inter-attack interval | |||
| 0–2 years | 18.1 (14) | 16.2–19.9 | 0.002 |
| 3–5 years | 17.3 (14) | 14.9–19.6 | 0.001 |
| ≥6 yearsa | 23.0 (20) | 20.7–25.2 | |
| Relapses Year 3-secondary progressive | |||
| 0 relapse | 8.2 (6) | 7.0–9.4 | <0.001 |
| 1–2 relapses | 10.8 (8) | 9.6–11.9 | 0.003 |
| ≥3 relapsesa | 13.6 (13) | 12.5–14.7 | |
a Reference category. Mean and median estimates obtained with Kaplan–Meier analysis. P-values were obtained through log rank test comparing the first two groups to the third one (reference category). Year 3-secondary progressive is period from end of Year 2 to onset of the progressive phase (secondary progressive).
CI = confidence interval.
The risk of entering the progressive phase also decreased modestly but significantly with the length of the first inter-attack interval: a longer interval was correlated with a lower probability of becoming secondary progressive multiple sclerosis (HR = 0.97; P = 0.007). Those with short (0–2 years) or intermediate (3–4 years) intervals entered the progressive phase in a significantly shorter time versus those with long (≥6 years) intervals (P = 0.002 and 0.001, respectively) (Table 3).
Relapsing–remitting phase
Fewer relapses from the third year to the onset of progression were modestly untoward (HR = 0.90; P < 0.001), predicting a shorter time to develop secondary progressive multiple sclerosis. This was also seen when patients were stratified according to total relapses during the relapsing–remitting phase (HR = 0.96; P = 0.02) (which includes the effect of early relapses). Both covariates yielded negative regression coefficients; greater risk of secondary progressive multiple sclerosis and shorter latency to onset of progression were inversely related to the number of attacks (Table 3). This was unlikely to be an artefact of how the onset of the progressive phase was defined, as there is an intrinsic opposite bias. Fewer attacks before progression correlated with identification of secondary progressive at lower mean DSS levels than when there were more attacks (Supplementary Fig. 2), indicating greater ease in pinpointing onset of progressive phase among such individuals.
Adjusted survival analysis
The survival model adjusted for the concomitant effect of all the variables analysed, yielding a larger impact of early relapses (HR = 1.25; P < 0.001) and first inter-attack interval (HR = 0.92; P < 0.001) on the probability of becoming secondary progressive than the univariate (Table 4). This effect increased proportionately to the number of Year 1 and 2 relapses and inversely to length of first inter-attack interval. The relationship between relapses during Year 3-secondary progression and latency to progression remained unchanged (HR = 0.85; P < 0.001); patients with more attacks in this time period were less likely to convert to secondary progressive and did so significantly later (Tables 3 and 4).
Table 4.
Multiple Cox regression survival analysis: risk of converting to secondary progressive multiple sclerosis according to number of attacks in the first 2 years, number of attacks from Year 3 to onset of progression and years (interval) between first and second attack
| Relapses Years 1–2 (n) | (RC = 0.221; P < 0.001) HR | Relapses Year 3-secondary progressive (n) | (RC = −0.159; P < 0.001) HR | Years between first and second attack | (RC = −0.088; P < 0.001) HR |
|---|---|---|---|---|---|
| 1 | 1.25 | 1 | 0.85 | 1 | 0.92 |
| 2 | 1.56 | 2 | 0.73 | 2 | 0.84 |
| 3 | 1.94 | 3 | 0.62 | 3 | 0.77 |
| 4 | 2.42 | 4 | 0.53 | 4 | 0.70 |
| 5 | 3.02 | 5 | 0.45 | 5 | 0.64 |
HR obtained through comparison with zero relapses or with 0-year interval between the first two attacks.
RC = regression coeffiicient.
The relationships among relapses in Years 1 and 2 and Year 3-secondary progression and the first inter-attack interval in Table 3 highlight Year 3-secondary progression as the outlier. Frequent Year 3-secondary progression relapses predicted modestly longer times to onset of the progressive phase while other variables more strongly predicted shorter times based on more frequent relapses (Table 3). Hazard ratios for conversion to secondary progression were tripled (HR = 3.02) by having had five attacks in Years 1 and 2 (Table 4). Similarly, risk of conversion was substantially influenced by first inter-attack interval, being a third less for an interval of 5 years (HR = 0.64) than for 1 year (HR = 0.92) (Table 4). In contrast, Year 3-secondary progression relapses were inversely and significantly related to risk of entering the secondary progressive phase, being nearly half in those with 5 attacks (HR = 0.45) versus those with a single attack (HR = 0.85) during the Year 3-secondary progressive period (Table 4).
Attainment of disability levels
Early relapses (Years 1 and 2)
Survival analysis confirmed the predictive value of Years 1 and 2 relapse number over a mean follow up of nearly three decades, encompassing hard outcomes including death. Years 1 and 2 relapses predicted attainment of all disability levels (DSS 6–8–10) from disease onset, from onset of progressive phase and from DSS 3 (Table 5 and Fig. 1A). The greater the relapse number, the higher the probability and the shorter the time for reaching endpoints. HRs graded from 1 to 5 attacks in Years 1 and 2 for reaching DSS 6 from disease onset were 1.23–2.78, for DSS 6 from onset of progression were 1.39–5.15 and for reaching DSS 6 from DSS 3 were 1.12– 1.75 (Table 6).
Table 5.
Survival times to DSS 6–8–10 from disease onset, from onset of progression and from DSS 3. Patients are stratified according to number of relapses in the first 2 years
| No. of relapses Years 1–2 | Mean years (median) | 95% CI | P-value | Mean years (median) | 95% CI | P-value | Mean years (median) | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Time from disease onset to DSS 6 | Time from disease onset to DSS 8 | Time from disease onset to DSS 10 | |||||||
| 1 relapse | 22.7 (20) | 21.1–24.1 | <0.001 | 33.2 (32) | 31.0–35.5 | <0.001 | 49.5 (63) | 46.7–52.3 | <0.001 |
| 2 relapses | 18.7 (16) | 16.9–20.4 | 0.010 | 28.9 (26) | 26.2–31.6 | 0.001 | 42.2 (–) | 39.7–44.7 | 0.001 |
| ≥3 relapsesa | 15.1 (10) | 12.5–16.7 | 20.4 (21) | 18.6–22.3 | 29.2 (32) | 27.6–30.7 | |||
| Time from onset of progressive phase to DSS 6 | Time from onset of progressive phase to DSS 8 | Time from onset of progressive phase to DSS 10 | |||||||
| 1 relapse | 6.1 (4) | 5.2–6.8 | <0.001 | 16.4 (14) | 14.5–18.2 | <0.001 | 32.7 (33) | 29.5–36.0 | 0.06 |
| 2 relapses | 5.3 (4) | 4.3–6.2 | <0.001 | 14.2 (13) | 12.5–15.9 | <0.001 | 32 (–) | 28.0–35.9 | 0.02 |
| ≥3 relapsesa | 2.5 (1) | 1.7–3.1 | 9.6 (9) | 8.2–11.1 | 22.4 (25) | 19.9–24.9 | |||
| Time from DSS 3 to DSS 6 | Time from DSS 3 to DSS 8 | Time from DSS 3 to DSS 10 | |||||||
| 1 relapse | 8.1 (6) | 7.2–8.9 | <0.001 | 18.2 (16) | 16.4–19.9 | 0.001 | 32.4 (34) | 29.9–34.9 | 0.06 |
| 2 relapses | 7.7 (6) | 6.7–8.8 | <0.001 | 17.3 (16) | 15.5–19.1 | 0.010 | 33.9 (–) | 30.9–36.8 | 0.02 |
| ≥3 relapsesa | 5.6 (4) | 4.4–6.4 | 13.7 (12) | 11.8–15.5 | 25.9 (30) | 23.7–28.1 | |||
a Reference category. Mean and median estimates obtained with Kaplan–Meier analysis. P-values obtained through Log Rank test comparing the first two groups (1 relapse, 2 relapses) to the third one (≥3 relapses) (reference category).
CI = confidence interval.
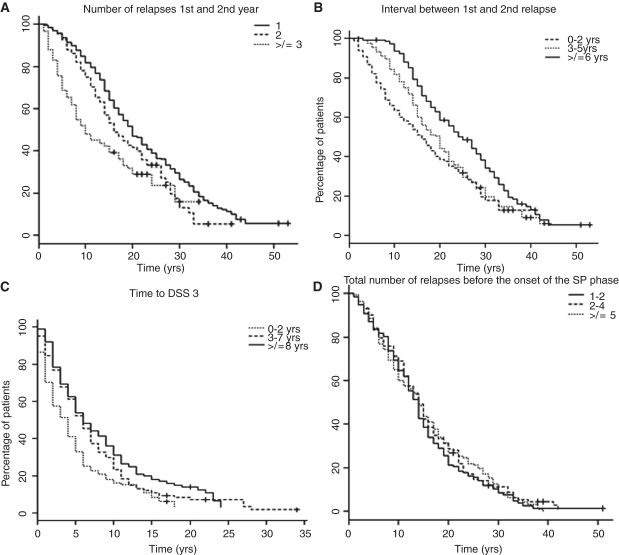
Figure 1.
Kaplan–Meier survival curves of time from disease onset to DSS 6 in patients grouped according to (A) total number of relapses in Year 1 and Year 2 (1 relapse; 2 relapses; ≥3 relapses). The estimated mean time from disease onset to DSS 6 was significantly shorter in those patients with a larger number of attacks in Years 1 and 2: 1 relapse group = 22.7 mean years, 2 relapses group = 18.7 mean years, ≥3 relapses group = 15.1 mean years. (B) First inter-attack interval (0–2 years; 3–5 years; ≥6 years). The estimated mean time from disease onset to DSS 6 was significantly shorter in those patients with a shorter interval between the first and the second attack. 0–2 years interval group = 18.2 mean years, 3–5 years interval group = 21.0 mean years, ≥6 years interval group=25.9 mean years. (C) Time from onset to moderate disability (DSS 3) (0–2 years; 3–7 years; ≥8 years). The estimated mean time from DSS 3 to DSS 6 was significantly shorter in those patients with a shorter interval between disease onset and moderate disability (DSS 3). 0–2 years interval group = 5.4 mean years, 3–7 years interval group = 7.4 mean years, ≥8 years interval group = 8.7 mean years. (D) total number of relapses before the onset of progression (1–2 relapses; 3–4 relapses; ≥6 relapses). The estimated mean times from disease onset to DSS 6 were remarkably similar in all three groups. 1–2 relapses group = 15.6 mean years, 3–4 relapses group = 15.7 mean years, ≥5 relapses group = 15.9 mean years.
Table 6.
Cox regression univariate analysis: risk of attaining DSS 6 from disease onset, from onset of progression and from DSS 3 according to the number of attacks in Years 1–2
| No. of relapses Years 1–2 | Time from disease onset to DSS 6 | Time from onset of progressive phase to DSS 6 | Time from DSS 3 to DSS 6 |
|---|---|---|---|
| HR (RC = 0.205; P < 0.001) | HR (RC = 0.328, P < 0.001) | HR (RC = 0.112, P < 0.002) | |
| 1 | 1.23 | 1.39 | 1.12 |
| 2 | 1.50 | 1.92 | 1.25 |
| 3 | 1.84 | 2.67 | 1.39 |
| 4 | 2.27 | 3.71 | 1.56 |
| 5 | 2.78 | 5.15 | 1.75 |
RC = regression coefficient.
The first inter-attack interval
First inter-attack intervals were predictive for all target endpoints from disease onset (Tables 7 and 8). Patients with longer times to second attack took significantly longer to reach DSS 6, 8 and 10 (Fig. 1B). Risk of attaining endpoints was also decreased proportionately to time between first and second attack; the negative regression coefficient indicates that a longer interval correlates with a lower probability of reaching disability milestones (Table 8). Shorter inter-attack intervals were less predictive than frequent early relapses.
Table 7.
Survival times to DSS 6–8–10 from disease onset, from onset of progression (onset of progressive phase) and from DSS 3. Patients are stratified according to time between first and second attack
| First inter-attack interval (years) | Mean years (median) | 95% CI mean | P-value | Mean years (median) | 95% CI mean | P-value | Mean years (median) | 95% CI mean | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Time from disease onset to DSS 6 | Time from disease onset to DSS 8 | Time from disease onset to DSS 10 | |||||||
| 0–2 | 18.2 (16) | 16.2–19.8 | <0.001 | 25.3 (25) | 23.6–26.8 | <0.001 | 39.1 (–) | 36.6–41.5 | 0.001 |
| 3–5 | 21.0 (20) | 18.9–23.0 | 0.005 | 32.5 (31) | 29.5–35.4 | 0.18 | 40.5 (–) | 37.8–43.0 | 0.01 |
| ≥6a | 25.9 (25) | 23.7–27.9 | 35.7 (33) | 32.6–38.6 | 52.6 (63) | 48.8–56.2 | |||
| Time from onset of progressive phase to DSS 6 | Time from onset of progressive phase to DSS 8 | Time from onset of progressive phase to DSS 10 | |||||||
| 0–2 | 4.0 (3) | 3.3–4.7 | <0.001 | 12.3 (11) | 11.0–13.4 | 0.01 | 27.9 (29) | 24.3–31.4 | 0.25 |
| 3–5 | 5.6 (3) | 4.4–6.7 | 0.24 | 17.7 (13) | 13.9–21.3 | 0.59 | 29.7 (25) | 25.2–34.2 | 0.17 |
| ≥6a | 6.6 (5) | 5.3–8.0 | 16.2 (14) | 13.5–18.8 | 34.4 (34) | 29.1–39.6 | |||
| Time from DSS 3 to DSS 6 | Time from DSS 3 to DSS 8 | Time from DSS 3 to DSS 10 | |||||||
| 0–2 | 6.9 (5) | 6.1–7.6 | 0.05 | 16.0 (15) | 14.5–17.3 | 0.10 | 30.6 (33) | 28.0–33.2 | 0.16 |
| 3–5 | 7.9 (5) | 6.6–9.2 | 0.48 | 22.3 (16) | 18.5–26.0 | 0.65 | 30.8 (25) | 26.4–35.1 | 0.06 |
| ≥6a | 8.4 (6) | 7.1–9.8 | 17.4 (16) | 15.5–19.2 | 33.3 (42) | 30.2–36.2 | |||
a Reference category. Mean and median estimates obtained with Kaplan–Meier analysis. P-values obtained through log rank test comparing the first two groups (0–2, 3–5 years) to the third one (≥6 years) (reference category).
CI = confidence interval.
Table 8.
Cox regression univariate analysis: risk of attaining DSS 6 from disease onset, from onset of progression and from DSS 3 according to the length (years) of the first inter-attack interval
| First inter-attack | Time from disease onset to DSS 6 | Time from onset of progressive phase to DSS 6 | Time from DSS 3 to DSS 6 |
|---|---|---|---|
| interval (years) | HR (RC = −0.052, P < 0.001) | HR (RC = −0.040, P < 0.001) | HR (RC = −0.013, P < 0.20) |
| 1 | 0.95 | 0.96 | 0.99 |
| 2 | 0.90 | 0.92 | 0.97 |
| 3 | 0.86 | 0.89 | 0.96 |
| 4 | 0.81 | 0.85 | 0.95 |
| 5 | 0.77 | 0.82 | 0.94 |
RC = regression coefficient.
A modest impact was also observed on attaining endpoints from onset of progressive phase. The shortest interval group (0–2 years) reached DSS 6 (P < 0.001) and DSS 8 (P = 0.01) more quickly (Table 7). The first inter-attack interval was only marginally significant for progressing from DSS 3 to DSS 6 and not for DSS 3–8 and 10 (Tables 7 and 8).
Multiple analysis of early relapse related measures
Multiple analysis of early relapses and first inter-attack interval left the impact of early relapses on disability from disease onset unchanged while the effect exerted by first inter-attack interval decreased but remained significant. Times to endpoints from onset of progression showed trends for the interval but were no longer significant. Inclusion of type and number of neurological systems involved at clinical onset in the multivariate model did not change the impact of early relapses and first inter-attack interval on outcomes.
Time to moderate disability (DSS 3)
We extended here the predictive effect of time to DSS 3 (Weinshenker et al., 1989b), advantaged by 81.5% of the population now ≥DSS 3. Patients with shorter intervals between disease onset and DSS 3 reached DSS 6 (Fig. 1C), DSS 8 and DSS 10 in modestly shorter times (HR = 0.97, P < 0.001; HR = 0.96, P < 0.001; and HR = 0.97, P = 0.04, respectively). The inclusion of those who reached DSS 3 via relapses and then remained stable for long periods undoubtedly diminished the impact. The size of predictive effect remained roughly constant for all endpoints analysed, while risks of accumulating disability increased inversely with time to DSS 3 (Table 9). Median times to DSS 6, 8 and 10 for this bout onset cohort were necessarily longer in the present analysis than at 12 years. Interim additions had predictably longer times to endpoint.
Table 9.
Univariate and multiple survival Cox regression analysis: risk of attaining DSS 6–8–10 from DSS 3 according to the length (years) of the interval between disease onset and the attainment of moderate disability (DSS 3)
| Time from DSS 3 to DSS 6 |
Time from DSS 3 to DSS 8 |
Time from DSS 3 to DSS 10 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | RC | HR (95% CI) | P-value | RC | HR (95% CI) | P-value | RC | HR (95% CI) | P-value |
| Time to DSS 3 | −0.029 | 0.97 (0.95–0.98) | <0.001 | −0.034 | 0.96 (0.95–0.98) | <0.001 | −0.029 | 0.97 (0.94–0.99) | 0.04 |
| Multiple analysis | |||||||||
| Time to DSS 3 | −0.034 | 0.96 (0.94–0.98) | <0.001 | −0.039 | 0.96 (0.93–0.98) | 0.001 | −0.021 | 0.97 (0.93–1.02) | 0.3 |
| Relapses Y1–Y2 | 0.082 | 1.08 (1.00–1.17) | 0.04 | 0.11 | 1.11 (1.01–1.23) | 0.02 | −0.021 | 0.97 (0.81–1.18) | 0.8 |
| Relapses Y1 | 0.045 | 1.04 (0.92–1.18) | 0.47 | 0.068 | 1.07 (0.92–1.24) | 0.37 | 0.014 | 1.01 (0.77–1.32) | 0.9 |
| Relapses Y2 | 0.134 | 1.14 (0.98–1.33) | 0.08 | 0.165 | 1.18 (0.99–1.40) | 0.05 | 0.066 | 0.93 (0.68–1.28) | 0.6 |
| First inter-attack | 0.024 | 1.02 (0.99–1.05) | 0.05 | 0.031 | 1.03 (0.99–1.06) | 0.05 | −0.012 | 0.98 (0.93–1.04) | 0.6 |
RC = regression coefficient; CI = confidence interval.
Adjusted survival analysis
The predictive effect of time to DSS 3 was independent of early relapses and first inter-attack interval, remaining unchanged in multiple analysis (Table 9). In contrast, when adjusted for time to reach DSS 3, the predictive effects of relapses in Years 1 and 2, and interval between first two attacks were diminished (Table 9). Nevertheless, total relapses in the first 2 years still exerted modestly significant effects on times from DSS 3 to DSS 6 (HR = 1.08, P = 0.04) and DSS 8 (HR = 1.11, P = 0.02) (Table 9).
Relapses from Year 3 to onset of progression
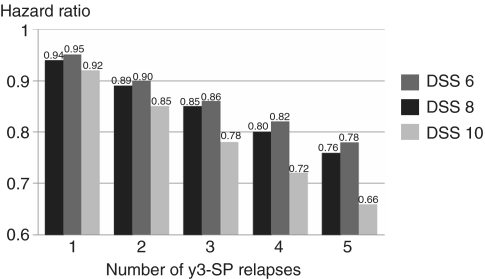
Relapses from Year 3 to secondary progression and disability outcomes yielded a negative regression coefficient. Patients with fewer relapses after Year 2 attained hard disability endpoints in shorter times from disease onset (Supplementary Table 1). The risk of accumulating severe disability decreased proportionately to relapses experienced in Year 3-secondary progression. Patients with 1–5 attacks in Year 3-secondary progression gave HR for times from disease onset to DSS 6 ranging from 0.94 to 0.76, to DSS 8 ranging from 0.95 to 0.78 and to DSS 10 ranging from 0.92 to 0.66 (Fig. 2). When grouped, patients with no attacks after Year 2 showed a statistically significant shorter time to reach DSS 6 (P = 0.003); no other significant differences were observed (Supplementary Table 1). Similarly, no effect of later relapses was detected on time to attain all endpoints from onset of progression or from DSS 3.
Figure 2.
Cox regression univariate analysis. Risk (y-axis) of attaining DSS 6 (black), 8 (dark grey) and 10 (light grey) from disease onset according to number of relapses experienced from Year 3 up to onset of progression. Hazard ratios are obtained through comparison with zero relapses. The y-axis expresses the variation of the hazard ratio according to the number of Year 3-secondary progression relapses (x-axis). A larger number of attacks was significantly related to a lower risk and a shorter time to attain the disability endpoints from disease onset. Year 3-secondary progression is period from end of Year 2 to onset of the progressive phase (secondary progression).
Total number of relapses in the relapsing–remitting phase
‘Total relapses’ combines the negative impact of more early relapses and the positive impact of more frequent late relapses. Unexpectedly they neutralized each other. Total number of relapses during the relapsing–remitting phase was neutral for time from onset to DSS 6, 8 and 10. Those grouped by low, intermediate or high number of total attacks, including Years 1 and 2 relapses, reached DSS 6 (Fig. 1D), 8 or 10 in very similar times (Table 10). However, more frequent relapses significantly predicted time from onset of progressive phase to DSS 6 (P < 0.001) and 8 (P = 0.004), influencing slope of progression (Table 10). Patients with frequent attacks during the relapsing–remitting phase reached endpoints more quickly following onset of progression, driven by Years 1 and 2 relapse frequency. This was not evident for times from DSS 3 to DSS 6, 8 and 10 (Table 10).
Table 10.
Survival times to DSS 6–8–10 from disease onset, from onset of progression and from DSS 3. Patients are stratified according to the total number of relapses during the relapsing remitting phase of the disease
| Total no. of relapses before progression | Mean years (median) | 95% CI mean | P-value | Mean years (median) | 95% CI mean | P-value | Mean years (median) | 95% CI mean | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Time from disease onset to DSS 6 | Time from disease onset to DSS 8 | Time from disease onset to DSS 10 | |||||||
| 1–2 relapses | 15.6 (14) | 13.9–17.3 | 0.66 | 26.2 (26) | 23.7–28.4 | 0.74 | 41.3 (45) | 37.9–43.3 | 0.60 |
| 3–4 relapses | 15.7 (14) | 14.0–17.4 | 0.98 | 25.8 (24) | 23.0–28.6 | 0.64 | 42.0 (48) | 38.2–45.3 | 0.17 |
| ≥5 relapsesa | 15.9 (14) | 14.3–17.5 | 26.6 (24) | 24.0–29.1 | 41.0 (–) | 38.3–43.2 | |||
| Time from onset of progressive phase to DSS 6 | Time from onset of progressive phase to DSS 8 | Time from onset of progressive phase to DSS 10 | |||||||
| 1–2 relapses | 6.9 (5) | 5.7–8.0 | <0.001 | 16.8 (14) | 14.8–18.7 | 0.004 | 32.4 (33) | 29.1–35.7 | 0.22 |
| 3–4 relapses | 4.6 (4) | 3.9–5.4 | 0.045 | 13.0 (12) | 11.4–14.6 | 0.48 | 27.7 (25) | 24.3–31.1 | 0.37 |
| ≥5 relapsesa | 3.7 (2) | 2.9–4.5 | 13.7 (11) | 11.4–15.8 | 26.9 (30) | 23.3–30.4 | |||
| Time from DSS 3 to DSS 6 | Time from DSS 3 to DSS 8 | Time from DSS 3 to DSS 10 | |||||||
| 1–2 relapses | 6.4 (5) | 5.4–7.3 | 0.79 | 16.6 (15) | 14.7–18.3 | 0.87 | 32.2 (34) | 29.1–35.3 | 0.89 |
| 3–4 relapses | 6.4 (5) | 5.3–7.4 | 0.91 | 15.6 (13) | 13.7–17.3 | 0.68 | 29.2 (30) | 26.1–32.2 | 0.10 |
| ≥5 relapsesa | 6.2 (5) | 5.3–7.1 | 16.6 (14) | 14.6–18.6 | 30.0 (33) | 27.4–32.5 | |||
a Reference category. Mean and median estimates obtained with Kaplan–Meier analysis. P-values obtained through log rank test comparing the first two groups (1–2, 3–4 relapses) to the third one (≥5 relapses) (reference category). CI = confidence interval.
Adjusted survival analysis
The concomitant impact of number of early relapses, first inter-attack interval and number of Year 3-secondary progression relapses on outcomes was assessed in a multiple model (Table 11).
Table 11.
Multiple survival Cox regression analysis: risk of attaining DSS 6 from disease onset, from onset of progression and from DSS 3 according to the concomitant effect of Years 1–2 relapses, first inter-attack interval and Year 3-secondary progressive relapses
| Time from disease onset to DSS 6 |
Time from onset of progressive phase to DSS 6 |
Time from DSS 3 to DSS 6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RC | HR (95% CI) | P-value | RC | HR (95% CI) | P value | RC | HR (95% CI) | P-value | |
| Total relapses Years 1 and 2 | 0.213 | 1.23 (1.13–1.35) | <0.001 | 0.282 | 1.32 (1.18–1.48) | <0.001 | 0.082 | 1.08 (0.99–1.18) | 0.06 |
| First inter-attack interval | −0.073 | 0.92 (0.90–0.95) | <0.001 | −0.010 | 0.98 (0.96–1.01) | 0.37 | −0.011 | 0.98 (0.96–1.01) | 0.33 |
| Relapses Year 3-secondary progressive | −0.088 | 0.91 (0.87–0.95) | <0.001 | 0.034 | 1.03 (0.99–1.07) | 0.09 | −0.032 | 0.96 (0.92–1.01) | 0.14 |
RC = regression coefficient; CI = confidence interval.
Accumulation of disability from disease onset
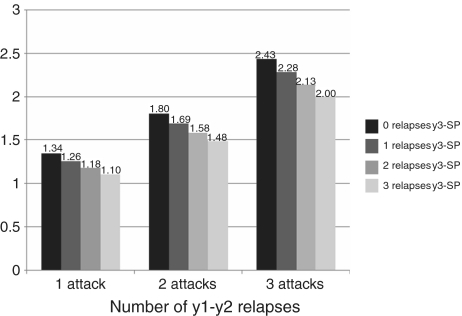
The risk of accumulating disability from disease onset was confirmed to increase proportionally with Years 1 and 2 relapse frequency and inversely with numbers experienced after Year 2; these effects are independent and were unchanged after multivariate analysis. More relapses from Year 3 to secondary progression paradoxically reduced combined risks of reaching endpoints (Fig. 3).
Figure 3.
Multiple survival Cox regression analysis. Risk (y-axis) of attaining DSS 6 from disease onset according to the combined effect of number of attacks in Years 1 and 2 (x-axis) and number of attacks from Year 3 up to onset of progression (0–1–2–3) in patients with secondary progressive multiple sclerosis. Hazard ratios are obtained through comparison with zero attacks. The y-axis shows the variation of the hazard ratio obtained by the combined effect of Years 1 and 2 relapses (x-axis) and Year 3-secondary progression relapses (each column) on the time to attain DSS 6 from disease onset. Patients at higher risk of disability have larger number of Years 1 and 2 relapses and smaller number of Year 3-secondary progression relapses. A larger number of attacks after Year 2 reduces the combined risk of attaining disability endpoints.
Accumulation of disability from onset of progression
The attainment of endpoints from onset of the progressive phase was significantly affected by prior early relapses and not by relapse frequency in Year 3-secondary progression. The predictive effect exerted by total relapses before progression on the accumulation of disability during secondary progression was shown to come exclusively from Years 1 and 2 relapses (Table 11). Multivariate analysis showed early relapses, first inter-attack interval and later relapses had no significant impact on times from DSS 3 to high disability levels (Table 11).
Discussion
The relationship between the relapsing and the progressive course of multiple sclerosis has remained ambiguous. On one hand, development of progression is the overwhelming determinant of outcome based on natural history studies; while on the other, relapses are what can be partially suppressed by currently available treatments. Widespread belief that accumulation of much unremitting disability results from successive exacerbations is not well-founded in multiple sclerosis, although it is a key pathway in neuromyelitis optica (Wingerchuk et al., 1999) and in Oriental multiple sclerosis (Kira, 2003). Some recollections of devastating relapses in multiple sclerosis were surely cases of neuromyelitis optica.
Biological mechanisms leading to the development of severe disability may be different from those responsible for attacks, as demonstrated by extensive neuropathological studies (Bjartmar et al., 2001; DeLuca et al., 2006; Trapp and Nave, 2008). Results from interferon and glatiramer acetate studies (IFNB Multiple Sclerosis Study Group, 1993, 1995; Johnson et al., 1995; Jacobs et al., 1996; European Study Group, 1998; PRISMS Study Group, 1998) and most clearly from the cladribine and the alemtuzumab studies (Beutler et al., 1996; Coles et al., 1999; Rice et al., 2000) brought this dichotomy to attention, failing to demonstrate a clear effect of relapse reduction on delaying progression. With alemtuzamab, 90% reduction of new gadolinium-enhancing MRI lesions and concomitant reduction in new relapses failed to prevent continued deterioration in disability (Coles et al., 1999), highlighting previously observed dissociation between inflammatory load and disability progression (Noseworthy et al., 1991). It remains possible that therapeutic reduction of early relapse rate might impact disease progression and long-term disability accumulation. Therapeutic monoclonal antibodies hold promise.
Relapse frequency and its surrogates, MRI T2 or gadolinium-enhancing lesions, still represent the most common outcome measures for evaluating treatment efficacy, notwithstanding that relapses came last among 12 clinical trial outcomes ranked for credibility two decades ago by a large group of multiple sclerosis clinical trialists (Noseworthy et al.,1989). Initial MRI lesion number and volume predicted conversion to clinically definite multiple sclerosis but late disability only modestly (Fisniku et al., 2008; The Optic Neuritis Study Group, 2008). Frequent early relapses associated with long-term disease evolution (Weinshenker et al., 1989b; Kantarci et al., 1998; Confavreux et al., 2003), but causality remains uncertain.
Late relapses had not shown indications they would influence unremitting disability, and certainly not after onset of the progressive phase (Kremenchutzky et al., 1999). Predictive effects of early relapses on disease progression (Weinshenker et al., 1989a, b; Eriksson et al., 2003) were reported not to apply once secondary progressive begins (Confavreux et al., 2003). Times for progressing from DSS 4 to higher disability levels (DSS 6 and 7) were independent of early relapses. However, early and later relapses were not separated and frequency of assessments and dropout rates were not enumerated (Confavreux et al., 2003).
Further evidence that secondary progressive is largely independent of preceding relapses or of those subsequent to its onset came from comparing progressive disease subtypes. Late outcomes were indistinguishable among those with none, one or many relapses preceding onset of progression, each subgroup having near identical ages when progression began. Common mechanisms in progressive multiple sclerosis subtypes were implied (Kremenchutzky et al., 1999, 2006). Neither the Lyons nor London, Ontario studies directly identified determinants of secondary progression probability, latency and slope that are of much practical importance.
The London Ontario database (Weinshenker et al., 1989a) now encompasses 28 000 patient-years of observation with most patients having reached hard disability outcomes. The low percentage of censored patients gives high reliability for survival estimates of later disability.
The role of relapses
Polysymptomatic presentation was strongly associated with a worse prognosis in patients with primary progressive multiple sclerosis (Cottrell et al., 1999). In contrast here, the number of neurological systems involved at disease onset did not independently influence time to secondary progressive or to disability endpoints. Similarly, degree of recovery from initial exacerbation did not influence long-term outcomes (Kremenchutzky et al., 2006).
Average attack frequencies in multiple sclerosis show marked variation within and between individuals over time (Weinshenker and Ebers, 1987). Prospective assessments yield greater frequencies (Fog and Linnemann, 1970; Patzold and Pocklington, 1982) and we confirm that attack rates lessen with time (Broman et al., 1981; Patzold and Pocklington, 1982; Myhr et al., 2001). Relapse rates were high during Years 1 and 2 (0.93 attacks/year) decreasing with disease duration. Overall, mean attack frequency during the relapsing–remitting phase (0.65 attacks/year) coheres with other studies (Confavreux et al., 1980: rate 0.86; Patzold and Pocklington, 1982: rate 1.1; Goodkin et al., 1989: rate 0.64), the higher rates being taken earlier in the disease overall or not population based, or not extending up to onset of progression. They conform to rates seen for placebo arms in relapsing–remitting multiple sclerosis trials. Table 2 gives the distribution of relapse frequencies in early and later relapsing–remitting multiple sclerosis prior to onset of the progressive phase.
Relapses and the probability and the latency of progression
We confirmed that neither the risk of entering the secondary progressive phase nor the latency of onset of progression were related to total attack number during the relapsing–remitting phase (Kremenchutzky et al., 2006). Patients with fewer total relapses prior to progression and with fewer relapses from Year 3 up to progression (Table 3) actually converted to secondary progressive multiple sclerosis significantly earlier. The size of this effect was larger for relapses from Year 3-secondary progression compared to the total number of attacks in the relapsing–remitting phase (including early relapses). These data should further discourage any direct causal relationship between clinical attack numbers and disability accumulation.
Given the predictive effects of frequent Years 1 and 2 relapses for shortened times to disability endpoints, we hypothesized that patients with higher relapse frequency in Years 1 and 2 must not only have increased probabilities of developing a progressive course but also shorter latencies from disease onset to progression. This was proven to be true, highlighting the key role of developing the progressive course. Interestingly, attacks from Year 2 exerted slightly greater predictive effects compared to attacks from Year 1; possibly Year 2 relapses are a marker for an inadequate immune regulatory response to events in Year 1.
A modest independent impact of the first inter-attack interval on probability and time to enter the secondary progressive phase was also observed. The predictive effect of Years 1 and 2 relapses and first inter-attack interval on the probability of entering the progressive phase became larger when we took into account the effect of Year 3-secondary progression relapses; five attacks versus none in the first 2 years tripled the risk (HR = 3.02) of developing secondary progressive multiple sclerosis. The relationship between relapses from Year 3 up to progression and latency to progression remained unchanged. Patients with more frequent attacks appeared to convert to secondary progressive multiple sclerosis significantly later; five attacks after Year 2 halved the risk of starting to progress. This is most unlikely to result from uncertainties in defining progressive onset in those still having relapses. Onset of progressive phase in those with fewer relapses was identified lower not higher on the DSS (Supplementary Fig. 2).
Relapses and disability outcomes
We confirmed and extended the independent predictive effect for hard disability outcomes of early relapses and first inter-attack interval, observed for lesser degrees of disability after 12 years of follow-up (Weinshenker et al., 1989b). With 16 years of additional follow up, Years 1 and 2 relapses influenced times to DSS 6, 8 and 10 from onset, from onset of the progressive phase and from DSS 3; predictive effects from disease onset were smaller than from onset of progressive phase. The analysis from onset of disease included patients with long relapsing–remitting phases or who never developed secondary progressive and therefore were less impacted by early relapses. Times from disease onset to DSS 6, 8 and 10 between 1 attack and ≥3 in Years 1 and 2 were substantially different i.e. 7.6, 12.8 and 20.3 years, respectively. Intervals between the first two attacks strongly associated with times from onset to DSS 6, 8 and 10 and from onset of progressive phase to DSS 6 and 8.
The risk of accumulating disability increased proportionally with number of attacks and inversely with time between the first and the second attack. Increased Years 1 and 2 relapses had the larger impact (Tables 6 and 8). In multiple analysis, the effect exerted by early relapses remained unchanged. The impact of first inter-attack interval diminished but remained significant implying that the predictive effect of a short interval between the first two attacks largely derives from having or not having the second relapse in Years 1 or 2.
Total relapses during the relapsing–remitting phase (including Years 1 and 2), exerted no significant effect on attainment of high disability levels from disease onset (Table 10). Times for reaching DSS 6–8–10 were remarkably equal, being 15, 26 and 41 ± 1 years for groups with high (≥5), intermediate (3–4) and low (1–2) numbers of attacks prior to onset of progressive phase. Times were nearly identical from DSS 3 to DSS 6, 8 and 10 based on numbers of relapses prior to progression (Table 10). The only indication that total relapses influenced any late outcome was seen in times from onset of progressive phase to DSS 6 and 8 but driven by Years 1 and 2, indicating a modest influence of these early relapses on slope of progression (Table 10).
It is important to put the relapse data in the general context provided by previous articles in this series. We had shown that among those with primary progressive multiple sclerosis, outcome did not differ by the presence (in 28%) or absence of relapses, and survival curves for those with relapsing–progressive multiple sclerosis were indistinguishable from those with progressive multiple sclerosis without relapses (Kremenchutzky et al., 1999). Disease course during the progressive phase was homogenous among multiple sclerosis progressive subtypes (Kremenchutzky et al., 2006). These findings, coherent in showing no impact of relapses on hard outcomes in progressive disease, left little rationale for considering relapses in the assessment of progressive disease or for unremitting changes in disability. These observations then permitted more focused examination of the relapsing–remitting phase in isolation. The data in this article are restricted to this phase but it will be apparent that, combined with what we have already examined, the two together total three decades of disease evolution, encompassing the relapsing–remitting and secondary progressive phases.
We have shown already that neither the location nor severity/degree of recovery characterizing the first attack nor a polysymptomatic onset were independently predictive of hard outcomes (Kremenchutzky et al., 2006). We show here that total relapses in relapsing–remitting phase are unrelated to hard outcomes (combined with the progressive results therefore, essentially the entire course of multiple sclerosis is spanned). These findings once again serve to discount or invalidate relapses in general, either as factors prognostic for hard outcomes in the relapsing–remitting phase overall or as therapeutic targets (but vide infra). However, the results for Year 3-secondary progressive showed an inverse relation to hard outcomes, enfeebling relapses in this time period as they fail, as do total relapses, to attain the basic starting premise for postulating a causal relationship.
The analysis of Year 3-secondary progression relapse number isolated the impact on outcome of these later relapses, frequently counted in clinical trials. Negative regression coefficients indicated that more attacks after Year 2 correlated with significantly lower risk (Fig. 2) and longer times to reach the endpoints from disease onset (Supplementary Table 1). Five versus zero attacks after Year 2 reduced the hazard of attaining DSS 6, 8 and 10 by 24% (HR = 0.76), 22% (HR = 0.78) and 34% (HR = 0.66), respectively (Fig. 2). This was unexpected but highly significant, counterbalancing the negative impact of early relapses on outcome. Some kind of as yet undetermined interaction between the development of progression and the suppression of relapses is strongly implied, possibly analogous to what occurs in primary progressive multiple sclerosis.
Because times for Year 3-secondary progression are necessarily variable, they are not comparable to the time fixed by definition for Years 1 and 2. Therefore we assessed variation in relapse frequency in serial 2-year intervals from Year 3 up to secondary progression, making comparisons within each time interval. For each 2-year interval past Years 1 and 2, and despite considerable variation in relapse frequency and slight indication of an impact beyond Year 2, no significant effect of relapse frequency for individual 2-year blocks could be found. We cannot easily explain the apparent and counterintuitive negative association of relapses with hard outcomes coming from Year 3-secondary progressive but the results, at the very least, serve to discredit relapse outcomes in this stage of disease. It seems likely that existing models relating relapse to disability have been too simple. All that remains viable in these contexts for relapse as an outcome is frequency in the first 2 years as that does predict hard outcome, modestly overall, but strongly for higher relapse frequencies.
The effect of early and later relapses on outcomes remained unchanged after multiple analysis. Patients at higher risk of accumulating disability from disease onset had more Years 1 and 2 relapses, shorter relapsing–remitting phases, yet fewer total Year 3-secondary progression attacks. The combined risk of attaining disability endpoints decreases consistently with increasing numbers of relapses after Years 1 and 2 (Fig. 3). Those with larger numbers of relapses in Years 1 and 2 seem unable to suppress or generate mechanisms evolving into progressive disability accumulation with further relapses being suppressed and/or masked by earlier development of the progressive phase.
These results re-emphasize that the predictive effect of relapses is more or less restricted to their frequency in Years 1 and 2 and within this window to those having three or more attacks. Nevertheless, the relapse rate characterizing the overall relapsing–remitting phase does not detectably contribute to the long-term accumulation of disability from disease onset (Table 10).
The findings for Years 1 and 2 relapses and first inter-attack interval may serve to explain the somewhat worse outcome seen for those seen at onset versus those coming to attention later (Weinshenker et al., 1989b). Those seen within a year of the first symptom are selected for having already had a second attack (and even additional ones) and therefore for short inter-attack intervals, an independent contributor to outcome.
It has been suggested that late progression is still related to inflammation caused by local compartmentalization of effector cells later in the disease (Meinl et al., 2008; Frischer et al., 2009). Although this would be an attractive way of linking relapsing–remitting and secondary progressive phases, this notion seems improbable. We have pointed out how progression has no predilection for initial or previous sites of exacerbation (Kremenchutzky et al., 2006), which might be predicted by this notion. Not only would previous sites have vulnerable partially damaged axons and oligodendrocyte loss but they would be loci where ‘compartmentalization’ would be expected to have a focal head start. There is no hint of this when secondary progressive supervenes and clinicians will know, for example, how rarely they see progressive blindness localized to the optic nerve affected with the first attack. A potential role for continued inflammation would have to be disconnected from relapsing–remitting inflammation to the extent that progression does not associate with the same concomitants as does the relapsing–remitting course. The widely differing prevalence of progressive disease in Caucasian versus Japanese Western multiple sclerosis hints at a genetic explanation for what must be a true dichotomy.
Relapses and the course of progression
The progressive phase was reported to be independent of preceding factors (Confavreux et al., 2000, 2003). Despite much individual variation, its age of onset and rate is remarkably homogeneous among progressive multiple sclerosis subtypes (Kremenchutzky et al., 2006). However, these studies did not address separately the role of early and later relapses on the evolution of the progressive course; potential effects on the probability of developing progressive multiple sclerosis or the latency of onset of progression were not examined.
We were able to address this aspect in two ways: (i) separately analysing the predictive effect of early and later relapses on the attainment of endpoints from onset of progressive phase; and (ii) analysing the predictive effect of early and later relapses on the time for progressing from DSS 3 to higher disability levels. The two approaches were methodologically different. The first analysis included only patients having entered secondary progression. The onset of progression could certainly be ambiguous and confounded by concomitant relapses but there was the advantage of long retrospect in this study, which typically clarified ambiguities at the time of evaluation. The second analysis also included patients still experiencing the relapsing–remitting phase, although >60% of secondary progressive patients in our population were deemed to have started to progress at DSS ≤ 3.
In mild contrast to previous reports (Confavreux et al., 2003), the slope of the progressive phase was modestly affected by early relapses. More frequent Years 1 and 2 relapses were independently related to significantly shorter times to attain DSS 6–8–10 from onset of progressive phase and the same independent predictive effect was observed on time to progress from DSS 3 to higher disability levels. Again, Year 3-secondary progression relapses had no impact on times to disability endpoints either from progression onset or from DSS 3.
The predictive effect of early relapses on long-term disability appears to be exerted primarily by increasing the probability of developing secondary progressive disease, shortening its latency and, to a lesser degree, by influencing the slope of progression. The impact of Years 1 and 2 relapses on the attainment of endpoints from progression was larger than the effect exerted from disease onset (Table 6). This probably is indirect, driven by increased probability of developing secondary progressive multiple sclerosis and by shortened latency of its onset. The analysis from onset of progressive phase excluded those patients who never entered the secondary progressive phase and therefore less impacted by Years 1 and 2 relapses.
Once progression has begun, there is consensus that outcome has been largely determined. In fact, early relapses and, similarly the first inter-attack interval, exerted a much smaller impact on times to disability from DSS 3 than on times to the same endpoints from onset of progressive phase (Tables 6 and 8). Although DSS 3 is composed mostly of those already progressing, a minority (25%) reached this level through relapses, remained stable and free of progression for long periods or never entered the secondary progressive phase, explaining this result. Those with more frequent attacks after Year 2 have longer latency to progression and paradoxically better outcomes, but again this operates via an effect on progression, albeit a beneficial one.
The evolution of the progressive phase then is largely driven by mechanisms independent of the inflammatory attack frequency characterizing the entire relapsing–remitting phase. Total relapses prior to secondary progressive and number of relapses from Year 3 to secondary progressive exerted no detectable independent effect on the attainment of hard disability endpoints from DSS 3 upward. In addition, multiple analysis accounting for early relapses, first inter-attack interval and later relapses showed little impact from any of the covariates on times for progressing from DSS 3 to higher DSS levels. These results highlight the landmark status of both time to DSS 3 and even more of onset of progressive phase as predictors of disability, further emphasizing the impropriety of later relapses as surrogates for long-term outcome.
Although frequent Years 1 and 2 relapses predict shorter times to DSS 6, 8 and 10, a causal relationship between such attacks and faster disease progression cannot and should not be assumed. A higher early relapse frequency could be concomitant to a predestined, more rapid clinical course. Time to DSS 3, known to predict time to DSS 6 (Weinshenker et al., 1989b) at 12 years follow-up, did predict risks for attaining DSS 6, 8 and 10, which increased inversely with length of interval between disease onset and DSS 3 (Table 9). The effect size remained unchanged after multiple analysis, thus independent of Years 1 and 2 relapses and first inter-attack interval.
The impact of Years 1 and 2 relapses on DSS 6, 8 and 10 lessened consistently when adjusted for initial disease progression (time to DSS 3) remaining modestly significant for times to DSS 6 and DSS 8 from DSS 3 (Table 9). DSS 3 and time to it necessarily encompasses most individuals becoming progressive over the period of observation. However, time to DSS 3, if reached, is often protracted while early relapses in Years 1 and 2 are, by definition, available early. Despite the practical predictiveness of Years 1 and 2 relapses, validation as an outcome still requires demonstration that suppression of these relapses translates into suppression of long-term disability.
These results, in sum, indicate that late disability is predetermined relatively early. Time to DSS 3 in multivariate analysis accounts for the effect of Years 1 and 2 relapses, probably by heralding the progressive course and the effect may have been underestimated by inclusion of relapse-mediated arrival at DSS3. This suggests that even frequent early relapses might be concomitant with, rather than causative of, poor outcome.
Conclusions
This geographically based, systematically ascertained study of some 28 000 patient-years of essentially untreated multiple sclerosis completes our assessment of the relationship of relapses to hard long-term outcome measures. For those entering the progressive phase, prior relapse frequency had been shown to be unassociated with time to DSS 6, 8 and 10. Relapse location, degree of recovery and polysymptomatic onset are similarly non-predictive. We show here that total relapses in the relapsing–remitting phase have no association with the same hard outcomes. Stratification by numbers of total relapses yielded almost identical times to outcomes. However, early relapse frequency does associate overall with what is destined to be a more rapid clinical course.
There were graded associations for all relapse frequencies but the 21.6% of patients having three or more attacks in the first 2 years largely drive this association. Here, substantial relapse frequency-related differences in times to hard disability outcomes were seen. Notwithstanding these findings, there were no indications that poor late outcomes result from relapse-determined accumulation of disability. Frequent relapses in the first 2 years were shown to associate with later disability by increased probability of entering the secondary progressive phase, by shorter latency of its onset and to a lesser degree by increased slopes of progression. In contrast, later relapse frequency could not be shown to have a deleterious effect, even within serial 2-year blocks subsequent to Years 1 and 2. If anything, these relapses associate with a more benign outcome but we acknowledge that relapse detectability could be masked by progression itself.
For reasons we cannot explain, shorter latency to enter the progressive phase is associated with significantly fewer relapses in the period from Year 3 to secondary progressive. This departure from what happens in Years 1 and 2 takes place soon after the second year of the relapsing–remitting phase. We have confirmed this by controlling for total relapses and showing it does matter if a single relapse is in Years 1 and 2 versus Year 3-secondary progression (data not shown). Total relapses in the relapsing–remitting phase had no detectable influence on the probability of developing secondary progressive or on its latency, complementing the very similar results seen once progression has begun. These results implicate a more complex relationship between relapses, disease duration and age, and disability accumulation than had been envisaged.
The findings here from nearly 30 000 patient-years of data have implications for current clinical practice, for interpreting trial results and for designing future ones. The dissociation between relapses and progression implies that relapses (except possibly during Years 1 and 2) are not a valid outcome surrogate for the late disability constituting the main social, medical and economic impact of multiple sclerosis. Therapeutic reduction/prevention of relapses should not be relied on to impact on later disability accumulation. Treatment of relapsing–remitting patients should be aimed at preventing or delaying features of the initial course, which associate with poor outcome later. Preventing, delaying or attenuating the progressive phase of the disease are the key therapeutic targets in multiple sclerosis.
Funding
Multiple Sclerosis Foundation of Canada, the Multiple Sclerosis Societies of Canada and the UK and Northern Ireland Fondazione Italiana Sclerosi Multipla (grant number 75/07/F1 and 2008/R/16 to A.S.); Bayer-Schering Pharma (to G.C.E.); Hertie and Porticus Foundations (to A.N. and M.D., partial).
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We thank Silke Seemüller for support in data management and Jackie Palace for comments on the manuscript.
Glossary
Abbreviations
- HR
hazard ratio
- DSS
Disability Status Scale
References
- Beutler E, Sipe JC, Romine JS, Koziol JA, McMillan R, Zyroff J. The treatment of chronic progressive multiple sclerosis with cladribine. Proc Natl Acad Sci USA. 1996;93:1716–20. doi: 10.1073/pnas.93.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar C, Kinkel RP, Kidd G, Rudick RA, Trapp BD. Axonal loss in normal-appearing white matter in a patient with acute multiple sclerosis. Neurology. 2001;57:1248–52. doi: 10.1212/wnl.57.7.1248. [DOI] [PubMed] [Google Scholar]
- Broman T, Andersen O, Bergmann L. Clinical studies on multiple sclerosis. I. Presentation of an incidence material from Gothenburg. Acta Neurol Scand. 1981;63:6–33. [PubMed] [Google Scholar]
- Coles AJ, Wing MG, Molyneux P, Paolillo A, Davie CM, Hale G, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol. 1999;46:296–304. doi: 10.1002/1531-8249(199909)46:3<296::aid-ana4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Aimard G, Devic M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients. Brain. 1980;103:281–300. doi: 10.1093/brain/103.2.281. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343:1430–8. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126(Pt 4):770–82. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- Cottrell DA, Kremenchutzky M, Rice GP, Koopman WJ, Hader W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain. 1999;122(Pt 4):625–39. doi: 10.1093/brain/122.4.625. [DOI] [PubMed] [Google Scholar]
- Cox DR, Oakes D. Analysis of survival data. London: Chapman & Hall; 1984. [Google Scholar]
- DeLuca GC, Williams K, Evangelou N, Ebers GC, Esiri MM. The contribution of demyelination to axonal loss in multiple sclerosis. Brain. 2006;129(Pt 6):1507–16. doi: 10.1093/brain/awl074. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler. 2003;9:260–74. doi: 10.1191/1352458503ms914oa. [DOI] [PubMed] [Google Scholar]
- European Study Group. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive multiple sclerosis. Lancet. 1998;352:1491–7. [PubMed] [Google Scholar]
- Fisniku LK, Brex PA, Altmann DR, Miszkiel KA, Benton CE, Lanyon R, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131(Pt 3):808–17. doi: 10.1093/brain/awm329. [DOI] [PubMed] [Google Scholar]
- Fog T, Linnemann F. The course of multiple sclerosis in 73 cases with computer-designed curves. Acta Neurol Scand Suppl. 1970;47:3–175. [PubMed] [Google Scholar]
- Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–89. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin DE, Hertsgaard D, Rudick RA. Exacerbation rates and adherence to disease type in a prospectively followed-up population with multiple sclerosis. Implications for clinical trials. Arch Neurol. 1989;46:1107–12. doi: 10.1001/archneur.1989.00520460093019. [DOI] [PubMed] [Google Scholar]
- Hader WJ, Elliott M, Ebers GC. Epidemiology of multiple sclerosis in London and Middlesex County, Ontario, Canada. Neurology. 1988;38:617–21. doi: 10.1212/wnl.38.4.617. [DOI] [PubMed] [Google Scholar]
- IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:655–61. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- IFNB Multiple Sclerosis Study Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology. 1995;45:1277–85. [PubMed] [Google Scholar]
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996;39:285–94. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–76. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- Kantarci O, Siva A, Eraksoy M, Karabudak R, Sutlas N, Agaoglu J, et al. Survival and predictors of disability in Turkish multiple sclerosis patients. Turkish Multiple Sclerosis Study Group (TUMSSG) Neurology. 1998;51:765–72. doi: 10.1212/wnl.51.3.765. [DOI] [PubMed] [Google Scholar]
- Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol. 2003;2:117–27. doi: 10.1016/s1474-4422(03)00308-9. [DOI] [PubMed] [Google Scholar]
- Kremenchutzky M, Cottrell D, Rice G, Hader W, Baskerville J, Koopman W, et al. The natural history of multiple sclerosis: a geographically based study. 7. Progressive-relapsing and relapsing-progressive multiple sclerosis: a re-evaluation. Brain. 1999;122(Pt 10):1941–50. doi: 10.1093/brain/122.10.1941. [DOI] [PubMed] [Google Scholar]
- Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129(Pt 3):584–94. doi: 10.1093/brain/awh721. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. A new scale for evaluating disability in multiple sclerosis. Neurology. 1955;5:580–3. doi: 10.1212/wnl.5.8.580. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Meinl E, Krumbholz M, Derfuss T, Junker A, Hohlfeld R. Compartmentalization of inflammation in the CNS: A major mechanism driving progressive multiple sclerosis. J Neurol Sci. 2008;274:42–4. doi: 10.1016/j.jns.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Myhr KM, Riise T, Vedeler C, Nortvedt MW, Gronning R, Midgard R, et al. Disability and prognosis in multiple sclerosis: demographic and clinical variables important for the ability to walk and awarding of disability pension. Mult Scler. 2001;7:59–65. doi: 10.1177/135245850100700110. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Vandervoort MK, Hopkins M, Ebers GC. A referendum on clinical trial research in multiple sclerosis: the opinion of the participants at the Jekyll Island workshop. Neurology. 1989;39:977–81. doi: 10.1212/wnl.39.7.977. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Vandervoort MK, Penman M, Ebers G, Shumak K, Seland TP, et al. Cyclophosphamide and plasma exchange in multiple sclerosis. Lancet. 1991;337:1540–1. doi: 10.1016/0140-6736(91)93226-y. [DOI] [PubMed] [Google Scholar]
- Patzold U, Pocklington PR. Course of multiple sclerosis. First results of a prospective study carried out of 102 MS patients from 1976–1980. Acta Neurol Scand. 1982;65:248–66. doi: 10.1111/j.1600-0404.1982.tb03084.x. [DOI] [PubMed] [Google Scholar]
- Phadke JG. Clinical aspects of multiple sclerosis in north-east Scotland with particular reference to its course and prognosis. Brain. 1990;113(Pt 6):1597–628. doi: 10.1093/brain/113.6.1597. [DOI] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352:1498–504. [PubMed] [Google Scholar]
- Rice GP, Filippi M, Comi G. Cladribine and progressive MS: clinical and MRI outcomes of a multicenter controlled trial. Cladribine MRI Study Group. Neurology. 2000;54:1145–55. doi: 10.1212/wnl.54.5.1145. [DOI] [PubMed] [Google Scholar]
- Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- The Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis. Arch Neurol. 2008;65:727–32. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–69. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Ebers GC. The natural history of multiple sclerosis. Can J Neurol Sci. 1987;14:255–61. doi: 10.1017/s0317167100026573. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989a;112(Pt 1):133–46. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain. 1989b;112(Pt 6):1419–28. doi: 10.1093/brain/112.6.1419. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.