Abstract
Primary progressive aphasia is a clinical syndrome defined by progressive deficits isolated to speech and/or language, and can be classified into non-fluent, semantic and logopenic variants based on motor speech, linguistic and cognitive features. The connected speech of patients with primary progressive aphasia has often been dichotomized simply as ‘fluent’ or ‘non-fluent’, however fluency is a multidimensional construct that encompasses features such as speech rate, phrase length, articulatory agility and syntactic structure, which are not always impacted in parallel. In this study, our first objective was to improve the characterization of connected speech production in each variant of primary progressive aphasia, by quantifying speech output along a number of motor speech and linguistic dimensions simultaneously. Secondly, we aimed to determine the neuroanatomical correlates of changes along these different dimensions. We recorded, transcribed and analysed speech samples for 50 patients with primary progressive aphasia, along with neurodegenerative and normal control groups. Patients were scanned with magnetic resonance imaging, and voxel-based morphometry was used to identify regions where atrophy correlated significantly with motor speech and linguistic features. Speech samples in patients with the non-fluent variant were characterized by slow rate, distortions, syntactic errors and reduced complexity. In contrast, patients with the semantic variant exhibited normal rate and very few speech or syntactic errors, but showed increased proportions of closed class words, pronouns and verbs, and higher frequency nouns, reflecting lexical retrieval deficits. In patients with the logopenic variant, speech rate (a common proxy for fluency) was intermediate between the other two variants, but distortions and syntactic errors were less common than in the non-fluent variant, while lexical access was less impaired than in the semantic variant. Reduced speech rate was linked with atrophy to a wide range of both anterior and posterior language regions, but specific deficits had more circumscribed anatomical correlates. Frontal regions were associated with motor speech and syntactic processes, anterior and inferior temporal regions with lexical retrieval, and posterior temporal regions with phonological errors and several other types of disruptions to fluency. These findings demonstrate that a multidimensional quantification of connected speech production is necessary to characterize the differences between the speech patterns of each primary progressive aphasic variant adequately, and to reveal associations between particular aspects of connected speech and specific components of the neural network for speech production.
Keywords: primary progressive aphasia, progressive non-fluent aphasia, semantic dementia, logopenic progressive aphasia, speech production
Introduction
Primary progressive aphasia (PPA) is a clinical syndrome in which degeneration of language regions in the dominant hemisphere is associated with progressive deficits in speech and/or language function (Mesulam, 1982, 2001). PPA can be classified into variants based on linguistic and cognitive features (Hodges and Patterson, 1996; Neary et al., 1998; Gorno-Tempini et al., 2004); each variant has a different distribution of atrophy (Gorno-Tempini et al., 2004) and is associated with different likelihoods of particular underlying pathologies (Davies et al., 2005; Josephs et al., 2006, 2008; Knibb et al., 2006; Mesulam et al., 2008). There are three widely recognised variants of PPA: non-fluent variant PPA (also termed progressive non-fluent aphasia), semantic variant PPA (also termed semantic dementia) and logopenic variant PPA (also termed logopenic progressive aphasia) (Gorno-Tempini et al., 2004; Mesulam et al., 2010).
For several decades after the initial description of PPA (Mesulam, 1982), patients with PPA were typically categorized as ‘fluent’ or ‘non-fluent’, analogous to this basic distinction in vascular aphasia. This approach oversimplifies the complexity of the data, because fluency reflects multiple dimensions of speech production, including speech rate, phrase length, articulatory agility, syntactic structure and prosody (Goodglass et al., 1964). Changes in these dimensions do not always co-occur in either vascular aphasia (Benson, 1967) or in PPA (Grossman and Ash, 2004), and the term ‘fluency’ can be adopted variably. In particular, patients who would now be classified as having the logopenic variant have sometimes been called fluent and sometimes non-fluent by different researchers, depending on which aspects of fluency were considered (Gorno-Tempini et al., 2008). Moreover, patients with the non-fluent variant do not necessarily demonstrate the same features as vascular patients with Broca’s aphasia, who are also considered to be non-fluent (Graham et al., 2004; Patterson et al., 2006).
In short, a single ‘fluency’ measure has been insufficient to capture reliably the language production patterns characteristic of each PPA variant, which distinguish them from each other and from the various vascular aphasias. Rather, it is necessary to consider multiple aspects of connected speech production. Speech production in each PPA variant has been described both qualitatively (Snowdon et al., 1989; Weintraub et al., 1990; Hodges et al., 1992; Grossman et al., 1996; Hodges and Patterson, 1996; Neary et al., 1998; Mesulam et al., 2001; Kertesz et al., 2003; Gorno-Tempini et al., 2004, 2008) and quantitatively (Thompson et al., 1997; Orange et al., 1998; Rogers and Alarcon, 1998; Bird et al., 2000; Graham et al., 2004; Ash et al., 2006, 2009; Patterson and MacDonald, 2006; Knibb et al., 2009; Meteyard and Patterson, 2009), but no study has quantitatively compared all three variants, and most studies have examined only a few patients, only a single variant or only some aspects of speech production. It is not known which motor speech and linguistic features derived from a speech sample are most useful clinically for distinguishing between variants, and the anatomical correlates of different aspects of abnormal connected speech production in PPA are not known, except for reduced speech rate, which has been linked to atrophy of the left inferior frontal and adjacent superior temporal gyri (Ash et al., 2009; see also Amici et al., 2007b).
The current study had two goals. The first was to identify the patterns of speech production characteristic of each PPA variant by quantitatively analysing samples of connected speech and deriving a wide range of motor speech and linguistic measures, some of which contribute to the traditional concept of fluency, and others of which do not. The second goal was to identify the neural correlates of different kinds of production deficits that can be observed in PPA, irrespective of diagnosis by variant, in order to establish which specific features have anatomical localization power and can thus aid in not only syndromic, but also aetiological diagnosis.
Materials and methods
Patients
Patients with PPA, patients with behavioural variant frontotemporal dementia (bvFTD) and healthy age-matched control subjects were recruited through the Memory and Aging Center at UCSF. Normal controls and patients with bvFTD were included as healthy and non-aphasic neurodegenerative control groups, respectively. All participants gave written informed consent, and the study was approved by the institutional review board. Patients and controls received a comprehensive evaluation including neurological history and examination, neuropsychological testing and neuroimaging.
A diagnosis of PPA required progressive deterioration of speech and/or language functions, and that deficits be largely restricted to speech and/or language for at least two years. Patients were then diagnosed with a particular PPA variant based on diagnostic guidelines recently developed by an international group of PPA researchers at meetings of the World Federation of Neurology Research Group on Aphasia and Cognitive Disorders in Buenos Aires in August of 2006 and the American Academy of Neurology in Seattle in 2009 (Supplementary Table 1). Patients were diagnosed with bvFTD according to established criteria (Neary et al., 1998). Neuroimaging results were not used for diagnostic purposes and were not available to the speech-language pathologists examining patients, but were used only to rule out other causes of focal brain damage, including extensive white matter disease.
Further criteria for inclusion in this study were: (i) availability of a videotaped picture description from the Western Aphasia Battery (Kertesz, 1982); (ii) Mini-Mental State Examination of at least 10; (iii) fluent in English; (iv) not mute; and (v) sufficiently intelligible speech such that the intended target could be determined for the majority of words. The first three criteria were met for 53 patients with PPA, however two patients with the non-fluent variant were mute and so were excluded according to the fourth criterion, and one patient with the non-fluent variant had such severe apraxia of speech that the intended targets could not be determined for the majority of words she produced, so she was excluded based on the fifth criterion. Therefore, it is important to note that the non-fluent variant subjects included in this study comprised only a subset of those who met diagnostic criteria for this variant, i.e. those who still produced at least some intelligible speech. The final sample included 70 participants: non-fluent variant (n = 14); semantic variant (n = 25); logopenic variant (n = 11); bvFTD (n = 10); normal controls (n = 10). When patients had performed the picnic description multiple times in successive years, we used the earliest available recording, typically obtained during the patient’s first research visit.
Demographic, linguistic and neuropsychological measures for each group are shown in Table 1. The only demographic variable on which any patient group differed from controls or from another patient group was sex: patients with the non-fluent variant were disproportionately female. The three PPA groups did not differ significantly from one another in age, Mini-Mental State Examination score, Clinical Dementia Rating or years from first symptom.
Table 1.
Demographic and neuropsychological data on the participants
|
PPA |
||||||
|---|---|---|---|---|---|---|
| Non-fluent variant | Semantic variant | Logopenic variant | bvFTD | Normal controls | Omnibus significance | |
| Demographic | ||||||
| Age | 67.8 (8.1)b | 66.7 (6.0) | 63.5 (7.3) | 63.6 (9.0) | 68.5 (5.9) | ns |
| Sex (M/F) | 1/13 | 14/11a | 4/7 | 7/3 | 5/5 | ** |
| Handedness (R/L) | 14/0 | 20/5 | 10/1 | 10/0 | 9/1 | ns |
| Education | 15.9 (3.1) | 15.8 (2.5) | 16.6 (2.8) | 16.6 (2.3) | 17.0 (1.7) | ns |
| Status | ||||||
| MMSE (30)† | 25.9 (4.1)* | 22.0 (6.2)*** | 22.3 (6.2)** | 26.4 (3.9) | 29.5 (0.5) | *** |
| CDR total | 0.5 (0.4) | 0.8 (0.5) | 0.6 (0.2) | 1.4 (0.5)a,b,c | *** | |
| Age at disease onset | 61.5 (8.0) | 57.8 (6.1) | 57.5 (7.2) | 52.7 (11.1) | + | |
| Years from first symptom | 6.3 (1.9) | 8.9 (3.1) | 6.0 (2.8) | 10.9 (5.7)a,c | ** | |
| Language production | ||||||
| Boston naming test (15) | 12.4 (2.8) | 3.7 (3.4)***,a,c,d | 10.2 (4.3)* | 12.4 (2.1) | 14.3 (0.8) | *** |
| Phonemic fluency (D words) | 5.0 (3.5)***,d | 6.1 (3.9)***,d | 8.5 (4.6)*** | 11.9 (7.5)* | 17.6 (3.1) | *** |
| Semantic fluency (animals) | 10.2 (6.4)*** | 7.2 (5.4)***,d | 10.9 (3.9)*** | 14.8 (6.7)* | 22.0 (5.1) | *** |
| Speech fluency (WAB, 10) | 6.9 (2.2)b,d | 8.8 (1.1) | 8.5 (1.8) | 9.7 (0.5) | *** | |
| Repetition (WAB, 100) | 83.6 (12.3)b,d | 89.7 (11.1) | 78.8 (10.7)b,d | 94.3 (5.8) | ** | |
| Motor speech‡ | ||||||
| Apraxia of speech rating (MSE, 7)† | 1.7 (1.3)b,d | 0.0 (0.0) | 0.7 (1.6) | 0.0 (0.0) | *** | |
| Dysarthria rating (MSE, 7)† | 1.5 (2.5)b,c | 0.0 (0.0) | 0.0 (0.0) | 0.6 (1.1) | ** | |
| Language comprehension | ||||||
| Word recognition (WAB, 60)† | 59.4 (1.3) | 51.3 (10.1)a,d | 57.9 (2.9) | 59.8 (0.6) | *** | |
| Sequential commands (WAB, 80) | 68.6 (9.1) | 67.8 (19.5) | 63.1 (16.0) | 78.0 (3.5) | ns | |
| Syntactic comprehension (CYCLE, 55) | 45.0 (7.1) | 49.3 (7.3) | 39.4 (8.1)b,d | 48.5 (8.5) | ** | |
| Pyramids and Palm Trees—pictures (52) | 47.9 (4.0) | 37.7 (7.9)a,c,d | 49.1 (2.7) | 47.8 (5.4) | *** | |
| Reading | ||||||
| PALPA regular words (30)† | 29.1 (1.6) | 27.5 (3.0) | 29.2 (0.8) | 30.0 (0.0) | * | |
| PALPA exception words (30)† | 27.4 (3.5) | 19.6 (6.9)a,d | 26.7 (5.3) | 29.3 (1.1) | *** | |
| PALPA pseudowords (24)† | 20.3 (3.8) | 17.5 (3.9) | 20.1 (2.9) | 22.0 (2.7) | ns | |
| Visuospatial function | ||||||
| Modified Rey-Osterrieth copy (17) | 15.6 (1.2) | 15.5 (1.6) | 13.0 (3.2)*a,b,d | 15.3 (1.4) | 15.3 (0.9) | ** |
| Visual memory | ||||||
| Modified Rey-Osterrieth delay (17) | 10.2 (4.8) | 7.0 (4.7) | 5.5 (3.7)+ | 7.9 (5.8) | 11.0 (2.8) | * |
| Verbal memory | ||||||
| CVLT-MS trials 1–4 (40) | 23.7 (6.6) | 14.0 (6.6)a,d | 18.7 (7.6) | 21.9 (8.0) | *** | |
| CVLT-MS 30 s free recall (10) | 6.7 (1.7) | 2.5 (2.2)a | 4.7 (2.3) | 4.7 (3.3) | *** | |
| CVLT-MS 10 min free recall (10) | 6.2 (2.4) | 1.8 (2.3)a | 3.9 (2.6) | 3.7 (3.4)a | *** | |
| Executive function | ||||||
| Digit span backwards | 2.8 (1.2)***,b,d | 4.6 (1.3)* | 3.0 (1.0)***b,d | 4.8 (1.5) | 6.0 (1.3) | *** |
| Modified trails (lines per minute) | 12.8 (11.0)** | 21.1 (15.0) | 10.4 (10.6)** | 22.5 (16.6) | 34.3 (18.6) | ** |
| Calculation (5) | 4.3 (1.3) | 4.3 (0.8) | 2.8 (1.3)***a,b,d | 4.5 (1.0) | 4.8 (0.4) | *** |
Values shown are mean (SD). Note that values for patients with the non-fluent variant are not completely representative of this group because mute and severely apraxic patients were excluded from this study. ns = not significant; MMSE = Mini-Mental State Exam; CDR = Clinical Dementia Rating; WAB = Western Aphasia Battery; MSE = Motor Speech Evaluation (Wertz et al., 1984); CYCLE = Curtiss-Yamada Comprehensive Language Examination; PALPA = Psycholinguistic Assessments of Language Processing in Aphasia; CVLT-MS = California Verbal Learning Test-Mental Status. Asterisks denote significantly impaired relative to normal controls at *P < 0.05; **P < 0.01; ***P < 0.001; marginal significance: +P < 0.10. Superscript letters denote significantly impaired (or different, in the case of demographic data) relative to the anon-fluent variant, bsemantic variant, clogopenic variant and dbvFTD at P < 0.05.
†Tested with non-parametric statistics.
‡Of the 14 patients with the non-fluent variant of PPA, 11 had apraxia of speech and six had dysarthria. Of the 10 patients with the logopenic variant, two had apraxia of speech.
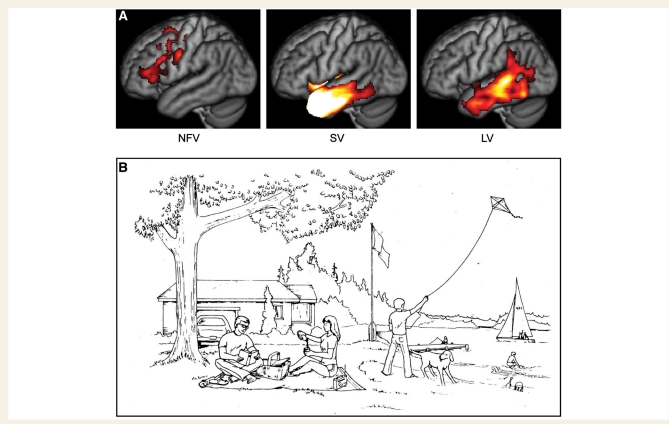
The three PPA variants each showed left-lateralized atrophy (Fig. 1A, Supplementary Table 2). The regional distribution of tissue loss was consistent with prior studies (Mummery et al., 2000; Gorno-Tempini et al., 2004). Note that only six patients (five with semantic variant and one with non-fluent variant) overlapped between the present study and a previous study from our group (Gorno-Tempini et al., 2004).
Figure 1.
Patient groups and stimulus material. (A) Regions of significant atrophy in each of the three PPA groups. These maps were derived by comparing each PPA patient group to 58 normal controls using voxelwise two-sample t-tests with independent variance, thresholded at voxelwise P < 10–5, with a minimum cluster size of 1000 mm3. NFV = non-fluent variant; SV = semantic variant; LV = logopenic variant. (B) The picnic scene from the Western Aphasia Battery (Kertesz, 1982), which patients were asked to describe. Copyright © 2006 NCS Pearson Inc. Reproduced with permission. All rights reserved.
Quantitative analysis of speech samples
The speech sample was the picnic picture description component of the Western Aphasia Battery (Fig. 1A), recorded and digitized as described in the ‘Supplementary methods’. Patients were instructed to ‘take a look at this picture, tell me what you see, and try to talk in sentences’. Speech samples were analysed with a procedure based on quantitative production analysis (QPA) (Saffran et al., 1989; Berndt et al., 2000). QPA provides an objective means of characterizing speech production in aphasia, with a particular focus on grammatical structure and lexical content. We followed the QPA procedures as described by Berndt et al. (2000) except where explicitly mentioned below. Most notably, we augmented QPA by coding speech-related phenomena (i.e. rate, speech sound errors) in more detail, and we also defined additional summary measures besides those defined in QPA.
Speech samples were transcribed with English orthography, except for phonological paraphasias and neologisms that were transcribed using the International Phonetic Alphabet. Non-narrative words such as direct responses to questions and coordinating conjunctions were excluded, and the remaining words were segmented into utterances. Utterances could comprise sentences, topic-comment structures (e.g. sailboat, in the water), or smaller phrases such as verb phrases or noun phrases. We placed utterance boundaries conservatively (creating shorter utterances) and in particular we assigned noun phrases in list-like constructions to separate utterances if they were preceded by a pause of >2 s. Each word was labelled for its grammatical category.
We calculated a set of measures (described below) that were aimed at quantifying four different aspects of the speech samples: (i) speech rate and speech sound errors; (ii) other disruptions to fluency; (iii) lexical content and (iv) syntactic structure and complexity. These measures overlap partly with those calculated in QPA.
Speech rate and speech sound errors
We recorded the total number of words produced, and the total duration of the sample. Word boundaries for word counts were determined orthographically, i.e. contractions such as they’re counted as one word. Filled pauses or false starts (see below) did not count as words. Excluded from the total duration were all periods during which the examiner was speaking, as well as any periods during which the patient was not attempting to speak (e.g. when the patient had stopped speaking but had not yet been prompted by the examiner to continue).
Speech rate was calculated by dividing the number of words by the duration of the speech sample. Maximum speech rate was calculated by coding the precise onset and offset of each word or sequence of connected words, and then by averaging the words per minute for the three most rapid sequences of 10 or more words.
Speech sound errors were classified as either distortions or phonological paraphasias. Distortions were defined as motor speech errors such as slurring or misarticulation that did not involve frank phonemic substitutions. For patients with dysarthria, every word could potentially be classified as a distortion, so for these patients we considered the patient’s most accurate speech as a baseline, and only coded as distortions words that were distorted above and beyond this baseline. Phonological paraphasias were defined as words where frank phonemic insertions, deletions or substitutions were evident. All phonemes in the word produced had to be non-distorted phonemes belonging to the English phoneme inventory. Phonological paraphasias were counted even if they were subsequently repaired. Speech sound errors that were not only distorted, but were also perceived to be closer to another phoneme than to the intended phoneme, were counted as distortions, and not as phonological paraphasias. This is because the distortion provides evidence for a motor speech component to the error, which may be sufficient to account for the apparent deviation from the intended phoneme without invoking an additional phonological mechanism.
Other disruptions to fluency
In this category, we included four further phenomena that contribute, along with speech rate and speech sound errors, to an overall impression of non-fluent speech: false starts, repaired sequences, filled pauses and incomplete sentences. These features occur regularly in normal speech, but are considerably more prominent in the speech of many aphasic patients.
False starts were defined as partial words, i.e. words that were abandoned, usually after just one or a few phonemes had been produced, whether the word was subsequently produced (e.g. pic- picnic) or not (e.g. h- um the man is reading a book). Filled pauses are words such as um, aah and hmmm. Repaired sequences were defined as sequences of one or more complete words, which were made redundant by subsequent repetitions, amendments, elaborations or alternative expressions, e.g. the kite is f- the boy is flying the kite.
Incomplete sentences were defined as sentences that were abandoned after the subject and verb had been produced (shorter abandoned sequences were counted as repaired sequences).
Lexical content
Each word was classified according to its part of speech. Nouns, most verbs, adjectives, numerals and some adverbs (those ending in -ly) were considered open class. All other words were counted as closed class, and the proportion of closed class words was derived. Unlike the procedures outlined in QPA, we counted the light verbs be, have and do as closed class, even when they were used as main verbs.
The proportion of pronouns (as a fraction of all nominals) was calculated by dividing the number of pronouns by the total number of nouns and pronouns. Similarly the proportion of verbs (as a fraction of major open class items) was calculated by dividing the number of verbs by the total number of nouns and verbs.
The frequency of each noun was obtained from the American National Corpus (http://www.americannationalcorpus.org) and the mean log frequency of nouns was calculated.
Syntactic structure and complexity
As mentioned above, all narrative words were segmented into utterances. The mean length of utterance is a simple and commonly used measure of complexity, but it is sensitive to the placement of utterance boundaries, which is not always straightforward.
The proportion of words in sentences was obtained by dividing the number of words in utterances that were sentences by the total number of words. This variable captures the classic agrammatic pattern of producing topic-comment structures, noun phrases and verb phrases instead of sentences, but it should be noted that sometimes patients, and even normal controls, produce phrases that are not sentences even though they are not agrammatic.
Syntactic errors were recorded when patients produced sentences or phrases that were ungrammatical. We further coded the proportions of nouns with determiners (in obligatory contexts) and verbs with inflections (in obligatory contexts). These types of errors are explicitly coded in QPA as they are particularly prevalent in agrammatic aphasia. Missing determiners and inflections were also coded as syntactic errors.
Embeddings were defined as sentences embedded within other sentences. The embedded sentence was required to have either an overt subject or an overt tense marking on the verb.
Semantic errors were recorded when patients produced sentences that were syntactically well-formed, but were either non-sensical or were semantically inappropriate for the context.
Inter-rater reliability
Of the 70 speech samples, 27 were transcribed and coded by one of the authors (S.M.W.) who has extensive experience with linguistic fieldwork and aphasic speech. The remaining 43 speech samples were initially transcribed and coded by another author (M.B.), who has an undergraduate degree in linguistics and experience with analysis of recorded discourse. These 43 speech samples were checked and revised by S.M.W.
The most difficult aspect of coding was the identification of distortions and phonological paraphasias. Therefore, the 25 speech samples from patients diagnosed with the non-fluent or logopenic variants were re-coded for these two measures by another author (M.H.), who is a licensed speech-language pathologist with many years experience working with patients with PPA and other aphasias. The correlation between the number of distortions recorded per patient across the two raters (S.M.W. and M.H.) was r = 0.93. The correlation for number of paraphasias was r = 0.96. Of all speech sound errors coded by both raters, the two raters agreed as to whether the errors were distortions or phonological paraphasias in 93% of cases. All discrepancies were resolved by consensus.
Statistical analysis
Statistical analysis was performed with R (http://www.r-project.org). For each measure, we compared the five groups—non-fluent variant, semantic variant, logopenic variant, bvFTD and normal controls—using ANOVAs where appropriate, or the Kruskal–Wallis non-parametric test for measures with significant floor or ceiling effects. If the omnibus test was significant, we examined the following planned contrasts: each of the four patient groups compared to normal controls, and the three PPA patient groups compared to each other. P-values were corrected for multiple comparisons with the default single step procedure implemented in the R program glht for ANOVAs (Hothorn et al., 2008), or the Steel procedure implemented in npmc for non-parametric tests (Munzel and Hothorn, 2001).
Voxel-based morphometry
Structural images were acquired on 1.5 T or 4 T scanners as described in ‘Supplementary methods’ online. Images were registered to each other and to Montreal Neurological Institute (MNI) space using SPM5 (Ashburner and Friston, 2005) and DARTEL (Ashburner, 2007). Modulated grey matter and white matter probability maps were scaled by Jacobians, smoothed with a Gaussian kernel of 8 mm full-width at half maximum, then summed together to obtain a map of brain parenchyma. Although voxel-based morphometry has been applied to white matter in many studies, the typical procedure is to analyse grey and white matter separately (e.g. Good et al., 2001). Instead, we added them, and then interpreted the anatomical basis of significant effects based on whether they fell in grey or white matter on the template.
Each speech/language measure was correlated voxelwise with the summed grey matter and white matter probabilities for the 60 patients (the 10 normal controls were not included). Outlier values were clipped at 1.5 SD from the mean of all patients, to avoid spurious correlations due to extreme values. Statistical maps were thresholded at voxelwise P < 0.005, and masked with the union of the four maps of atrophy in each patient group (including bvFTD) relative to controls (masks thresholded at voxelwise P < 0.01, uncorrected), to ameliorate the loss of power due to multiple comparisons by ruling out regions that were not atrophic in any patient group. This mask included most of the left cerebrum with the exception of the occipital lobe, and much of the right frontal lobe, temporal lobe and inferior parietal cortex (due to the fact that many patients had some degree of right hemisphere atrophy, especially those with bvFTD). Correction for multiple comparisons was achieved by permutation analysis. Statistical maps were calculated for 1000 random assignments of normally distributed behavioural scores to patients, with the maximum cluster size recorded each time. The fifth percentile maximum cluster size was 1297 mm3, so applying this as the minimum cluster size ensured corrected significance of P < 0.05.
Age, sex, total intracranial volume and scanner (1.5 or 4 T) were included as covariates in all analyses. Images were overlaid with MRIcron (http://www.sph.sc.edu/comd/rorden/mricron) on an average brain based on the grey and white matter templates.
Results
Speech rate and speech sound errors
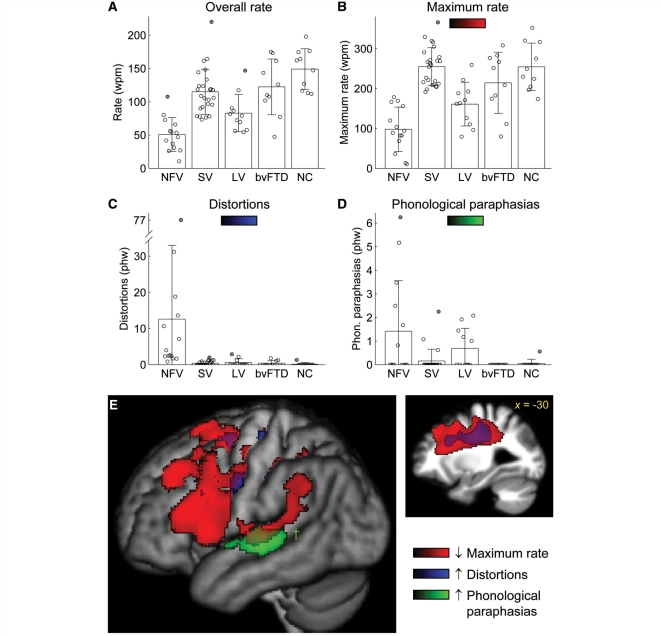
Only non-fluent patients differed significantly from normal controls in terms of number of words produced (less) and total speech sample duration (longer) (Table 2), however patients with bvFTD produced marginally fewer words than controls. Overall speech rate was significantly reduced in all PPA variants (but not in bvFTD), with the non-fluent patients slowest, followed by the logopenic patients and then the semantic variant patients (Fig. 2A, Table 2). Maximum speech rate, however, was reduced only in the non-fluent and logopenic patient groups, with the non-fluent patients significantly slower than the logopenic variant patients with the logopenic variant (Fig. 2B, Table 2). Maximum speech rate may be a more useful measure than overall speech rate because overall speech rate can be impacted by long pauses, which can reflect processes that are either linguistic (e.g. word-finding, sentence formulation) or non-linguistic (e.g. inattention). Note that mute patients were excluded from this study; if these patients had been considered to have zero words per minute, the mean rates for the non-fluent variant patients would be even lower.
Table 2.
Quantitative analysis of connected speech production
|
PPA |
||||||
|---|---|---|---|---|---|---|
| Non-fluent variant | Semantic variant | Logopenic variant | bvFTD | Normal controls | Omnibus significance | |
| Speech rate and speech errors | ||||||
| Total number of words | 85.9 (39.7)*b | 145.9 (64.6) | 117.7 (58.5) | 98.3 (25.3)+ | 156.4 (52.4) | *** |
| Total duration of narrative (s) | 104.8 (38.2)* | 79.3 (33.4) | 86.5 (40.9) | 52.1 (17.2) | 64.0 (20.1) | ** |
| Speech rate (wpm) | 50.9 (25.3)***b | 115.0 (34.0)* | 82.9 (28.0)***b | 122.4 (41.9) | 149.2 (30.5) | *** |
| Maximum speech rate (wpm) | 98.0 (55.7)***b,c | 255.0 (47.5) | 161.0 (54.6)**b | 214.4 (76.7) | 254.5 (59.6) | *** |
| Distortions (phw)† | 12.6 (20.4)***b,c | 0.4 (0.6) | 0.6 (1.1) | 0.4 (0.7) | 0.1 (0.4) | *** |
| Phonological paraphasias (phw)† | 1.4 (2.1) | 0.2 (0.5) | 0.7 (0.8) | 0.0 (0.0) | 0.1 (0.2) | ** |
| Other disruptions to fluency | ||||||
| False starts (phw) | 2.7 (3.4) | 2.3 (4.1) | 4.1 (2.8) | 1.4 (1.3) | 1.3 (0.8) | ns |
| Filled pauses (phw) | 10.3 (9.3)*b | 4.0 (3.4) | 10.0 (6.4)+b | 1.9 (2.6) | 4.0 (1.4) | *** |
| Repaired sequences (phw) | 3.7 (3.3) | 3.1 (2.3) | 6.0 (2.7)***b | 1.3 (1.2) | 1.6 (0.8) | *** |
| Incomplete sentences (phw)† | 0.3 (0.9) | 1.4 (1.2)**a | 1.2 (1.5) | 0.2 (0.4) | 0.2 (0.4) | *** |
| Lexical content | ||||||
| Closed class words (proportion) | 0.51 (0.17)+b,c | 0.68 (0.05)a | 0.64 (0.07)a | 0.62 (0.04) | 0.60 (0.05) | *** |
| Pronouns (proportion) | 0.14 (0.11)bc | 0.45 (0.14)***a,c | 0.31 (0.19)*a,b | 0.24 (0.07) | 0.14 (0.08) | *** |
| Verbs (proportion) | 0.30 (0.12)bc | 0.55 (0.09)***a | 0.46 (0.10)a | 0.41 (0.04) | 0.37 (0.06) | *** |
| Mean log frequency of nouns | 2.91 (0.21)b | 3.47 (0.23)***a,c | 3.13 (0.28)b | 3.13 (0.12) | 3.06 (0.14) | *** |
| Syntactic structure and complexity | ||||||
| Mean length of utterance | 5.4 (2.5)***b | 8.0 (2.1)*** | 7.4 (2.5)*** | 9.1 (1.8)+ | 12.0 (3.9) | *** |
| Words in sentences (proportion)† | 0.66 (0.38)* | 0.94 (0.06)* | 0.89 (0.21) | 0.92 (0.21) | 0.99 (0.04) | * |
| Syntactic errors (phw)† | 7.2 (10.6)+ | 0.8 (0.8) | 2.4 (1.3)**b | 0.3 (0.5) | 0.3 (0.4) | *** |
| Syntax principal component 1† | −1.4 (2.0)* | 0.4 (0.3)* | −0.2 (0.9)**b | 0.5 (0.7) | 0.7 (0.2) | *** |
| Nouns with determiners (proportion)† | 0.89 (0.17)* | 0.99 (0.03) | 0.97 (0.05) | 1.00 (0.00) | 1.00 (0.00) | *** |
| Verbs with inflections (proportion)† | 0.96 (0.07) | 1.00 (0.00) | 1.00 (0.00) | 1.00 (0.00) | 1.00 (0.00) | ** |
| Embeddings (phw) | 0.9 (1.4)b | 4.1 (2.3) | 1.9 (1.4)b | 1.9 (1.8) | 2.6 (1.1) | *** |
| Auxiliary complexity | 0.92 (0.38) | 0.85 (0.28) | 0.76 (0.25) | 0.70 (0.44) | 0.98 (0.35) | ns |
| Semantic errors (phw)† | 2.6 (3.3) | 1.3 (1.9) | 1.6 (2.7)+ | 0.3 (0.8) | 0.1 (0.2) | * |
Values shown are mean (SD). Note that values for patients with the non-fluent variant of PPA reflect only the subset of patients who could be included in this study, i.e. those with at least some ability to produce comprehensible speech. wpm = words per minute; phw = per hundred words; ns = not significant. Asterisks denote significantly impaired relative to normal controls at +P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001. Superscript letters denote significantly impaired relative to the anon-fluent, bsemantic and clogopenic variants at P < 0.05.
†Tested with non-parametric statistics.
Figure 2.
Speech rate and speech sound errors. (A) Overall speech rate in each of the five groups. Error bars show standard deviation. Each patient is represented by a circle, with outliers (>2 SD from the mean for the patient’s group) shaded grey. Within each patient group, patients are arranged from left to right in order of decreasing mini-mental state examination score, an approximate measure of disease progression. (B) Maximum speech rate. (C) Distortions. (D) Phonological paraphasias. (E) Voxel-based morphometry showing brain regions where atrophy was correlated with reduced maximum rate (red), increased numbers of distortions (blue) and increased numbers of phonological paraphasias (green). Statistical t maps were projected onto the lateral surface of the left hemisphere; also shown is a sagittal cut through the superior longitudinal fasciculus. Regions shown were statistically significant at P < 0.05 corrected for multiple comparisons based on cluster size, with the exception of the region associated with phonological paraphasias (dagger), which was only marginally significant (cluster size = 518 mm3, P = 0.012). wpm = words per minute; phw = per hundred words; NFV = non-fluent variant; SV = semantic variant; LV = logopenic variant; NC = normal controls.
Distortions were produced by every non-fluent patient without exception, often in substantial numbers (Fig. 2C, Table 2). In contrast, only a few patients in the other PPA groups or the bvFTD group produced occasional distortions. Most patients with the semantic variant and bvFTD who produced the occasional distortion were more advanced in the course of disease, as reflected by lower Mini-Mental State Examination scores.
Phonological paraphasias, in contrast, were produced by a subset of patients with the non-fluent variant and a subset of patients with the logopenic variant (Fig. 2D, Table 2). Although the omnibus Kruskal–Wallis test was significant, none of the follow-up tests were significant after correction for multiple comparisons. However, 6 of 14 patients with the non-fluent variant, and 5 of 11 patients with the logopenic variant produced phonological paraphasias, whereas only 3 of 25 patients with the semantic variant produced any, and two of these were late stage patients. Some examples of phonological paraphasias are shown here:
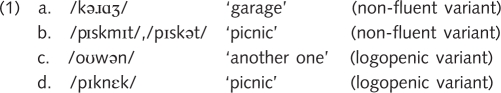
Note that phonological paraphasias such as these were not distorted, otherwise they would have been classified as distortions.
Neural correlates of speech variables
Reduced maximum speech rate was associated with atrophy of exclusively left-lateralized regions: the inferior frontal gyrus (pars opercularis and triangularis), ventral precentral gyrus, supplementary motor area, extensive white matter underlying these regions including the superior longitudinal fasciculus, and posterior language regions—the posterior superior temporal gyrus and the adjacent part of the supramarginal gyrus (Fig. 2E, red; Table 3). The regions correlated with overall speech rate (not shown) were similar to those correlated with maximum speech rate, but less extensive.
Table 3.
Voxel-based morphometry: neural correlates of motor speech and linguistic variables
| Speech/language variables and brain region(s) |
MNI coordinates |
Max T | Volume (mm3) | P | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Maximum speech rate | ||||||
| Left posterior IFG, supplementary motor area, underlying white matter, posterior STG, supramarginal gyrus | −28 | −1 | 35 | 6.07 | 87 488 | < 0.001 |
| IFG pars opercularis | −50 | 12 | 8 | 4.16 | ||
| IFG pars triangularis | −38 | 24 | 18 | 4.79 | ||
| SMA | −2 | 6 | 60 | 6.07 | ||
| Posterior STG | −52 | −26 | 6 | 4.42 | ||
| Distortions | ||||||
| Left SLF | −23 | −7 | 41 | 3.80 | 26 872 | 0.017 |
| Right SLF | 29 | −6 | 36 | 3.60 | 10 520 | 0.049 |
| Phonological paraphasias | ||||||
| Left STG | −58 | −24 | 2 | 3.79 | 4144 | 0.124 |
| False starts | ||||||
| Left posterior and anterior STG and ventral frontal operculum | −54 | −23 | 11 | 4.83 | 11 512 | 0.045 |
| Ventral frontal operculum | −50 | −2 | 0 | 4.83 | ||
| Posterior STG | −56 | −34 | 18 | 4.47 | ||
| Filled pauses | ||||||
| Left posterior STG | −56 | −30 | 8 | 4.71 | 16 112 | 0.028 |
| Repaired sequences | ||||||
| Left posterior STS, STG, inferior parietal lobule and angular gyrus | −47 | −50 | 17 | 6.01 | 34 152 | 0.011 |
| Posterior STS | −58 | −40 | 4 | 5.31 | ||
| Inferior parietal lobule | −36 | −44 | 42 | 3.96 | ||
| Angular gyrus | −38 | −74 | 36 | 6.01 | ||
| Pronouns | ||||||
| Left temporal pole, anterior ITG, MTG and STG, anterior fusiform gyrus, anterior parahippocampal gyrus, insula | −42 | −2 | −27 | 4.96 | 37 120 | 0.010 |
| Fusiform gyrus | −34 | −16 | −34 | 4.96 | ||
| Mid ITG | −44 | −34 | −28 | 3.81 | ||
| Insula | −42 | −6 | −2 | 3.47 | ||
| Right temporal pole, anterior ITG, anterior fusiform gyrus, anterior parahippocampal gyrus | 37 | 1 | −32 | 3.77 | 13 520 | 0.035 |
| Verbs | ||||||
| Left temporal pole, ITG, anterior MTG, anterior fusiform gyrus, anterior parahippocampal gyrus | −42 | −7 | −26 | 4.42 | 27 608 | 0.016 |
| Fusiform gyrus | −34 | −16 | −34 | 4.42 | ||
| Posterior ITG | −56 | −54 | −18 | 3.56 | ||
| Anterior STG | −58 | 10 | −12 | 4.07 | ||
| Frequency of nouns | ||||||
| Left temporal pole, ITG, anterior MTG and STG, anterior fusiform gyrus, anterior parahippocampal gyrus, insula | −43 | −12 | −22 | 6.60 | 78 584 | < 0.001 |
| Fusiform gyrus | −34 | −18 | −34 | 6.60 | ||
| Anterior STG | −42 | 16 | −22 | 6.26 | ||
| Posterior ITG | −56 | −52 | −20 | 4.64 | ||
| Insula | −42 | −4 | −4 | 5.05 | ||
| Right temporal pole, anterior ITG, anterior fusiform gyrus, anterior parahippocampal gyrus | 36 | 4 | −33 | 4.11 | 17 208 | 0.027 |
| Embeddings | ||||||
| Left posterior IFG, superior frontal sulcus and adjacent prefrontal regions, SMA | −29 | 18 | 31 | 4.43 | 18 448 | 0.027 |
| IFG pars opercularis | −46 | 8 | 2 | 3.36 | ||
| IFG pars triangularis | −42 | 22 | 20 | 3.74 | ||
| Superior frontal sulcus | −24 | 36 | 34 | 3.83 | ||
| SMA | 0 | 14 | 60 | 4.43 | ||
| Syntax principal component 1 | ||||||
| Left posterior IFG, SMA and underlying white matter | −26 | 8 | 36 | 4.02 | 17 624 | 0.027 |
| IFG pars triangularis | −38 | 26 | 14 | 3.63 | ||
| SMA | −6 | 8 | 52 | 4.02 | ||
P-values were corrected based on cluster extent. Cluster coordinates refer to the centre of mass, and for large clusters spanning multiple regions coordinates for peaks are also shown. MNI = Montreal Neurological Institute; IFG = inferior frontal gyrus; SLF = superior longitudinal fasciculus; SMA = supplementary motor area; STG = superior temporal gyrus; STS = superior temporal sulcus; MTG = middle temporal gyrus; ITG = inferior temporal gyrus.
The anatomical correlates of distortions and phonological paraphasias were much more specific. Distortions were correlated with volume loss in the white matter underlying left frontal cortex, especially the superior longitudinal fasciculus, and a smaller homologous region in the right hemisphere (Fig. 2E, blue; Table 3). Phonological paraphasias were correlated with damage to the left mid superior temporal gyrus (Fig. 2E, green; Table 3), although this cluster did not quite reach significance based on its size (volume = 518 mm3, P = 0.12).
Relationships between speech variables
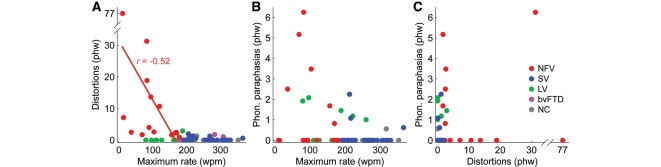
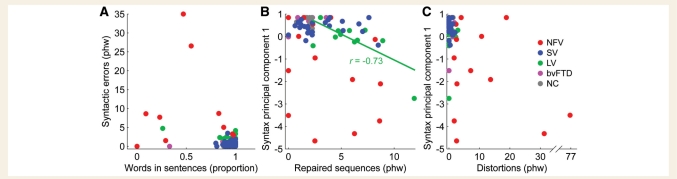
Increasing numbers of distortions were marginally associated with reduced speech rate in the non-fluent variant (r = –0.52; P = 0.058) (Fig. 3A), suggesting a common factor underlying these features for these patients. In contrast, there were no associations between phonological paraphasias and speech rate in either the non-fluent or logopenic variant (Fig. 3B), nor any correlation between distortions and phonological paraphasias in the non-fluent variant (Fig. 3C), which parallels the voxel-based morphometry result in suggesting that phonological paraphasias and distortions reflect damage to distinct regions and processes.
Figure 3.
Correlations between speech rate and two kinds of speech sound errors. (A) Correlations between maximum rate and distortions in each of the five patient groups. These variables were marginally correlated in non-fluent variant, as denoted by the fitted line. (B) Correlations between maximum rate and phonological paraphasias (none significant). (C) Correlations between distortions and phonological paraphasias (none significant). wpm = words per minute; phw = per hundred words; PC = principal component; Phon. = phonological; NFV = non-fluent variant; SV = semantic variant; LV = logopenic variant; NC = normal controls.
Other disruptions to fluency
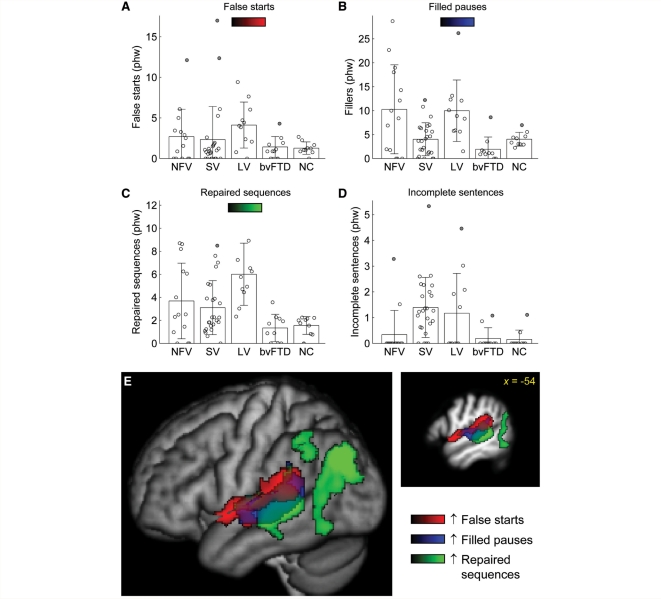
False starts were observed in all groups including controls (Fig. 4A, Table 2). Although most patients with the logopenic variant produced more false starts than any control, the effect of group was not significant.
Figure 4.
Other disruptions to fluency. (A) False starts. (B) Filled pauses. (C) Repaired sequences. (D) Incomplete sentences. (E) Voxel-based morphometry showing brain regions where atrophy was correlated with increased numbers of false starts (red), increased numbers of filled pauses (blue) and increased numbers of repaired sequences (green). phw = per hundred words; NFV = non-fluent variant; SV = semantic variant; LV = logopenic variant; NC = normal controls.
Patients with the non-fluent variant produced significantly more filled pauses than controls, and patients with the logopenic variant produced marginally more (Fig. 4B, Table 2). Both of these groups produced significantly more filled pauses than patients with the semantic variant, who did not differ from controls despite the severe lexical retrieval deficits present in this variant. This was because patients with the non-fluent and logopenic variants typically struggled to generate grammatical structures or find words (Example 2a), whereas patients with the semantic variant simply found alternative circumlocutory means of expression, even if they were semantically empty (Example 2b).
(2) a. the guy was aah flying a umm [pause] what do you call it? (logopenic variant)
b. oh and he’s doing something right there you know j- just like that yeah (semantic variant)
Many patients with PPA of all variants exhibited more repaired structures than any control, but only in patients with the logopenic variant was the number of repairs significantly greater (Fig. 4C, Table 2). In fact, there was no overlap between logopenic variant and normal control distributions. Below are examples of repaired sequences, with the repaired words underlined:
(3) a. This is a picture of a- th- that looks like summer (logopenic variant)
b. He also has a, a dog (logopenic variant)
c. The kite is f- the boy is flying the kite (non-fluent variant)
Incomplete sentences were most frequent in patients with the semantic variant and were also seen in the logopenic variant, although only the former group differed significantly from controls (Fig. 4D, Table 2). Despite their motor speech and grammatical difficulties, patients with non-fluent variant almost always completed their sentences.
Neural correlates of other disruptions to fluency
Although false starts did not differ significantly across groups, increased numbers of false starts were nevertheless significantly associated with atrophy of anterior and posterior perisylvian cortex, specifically the length of the superior temporal gyrus and the ventral frontal operculum (Fig. 4E, red: Table 3). Filled pauses and repaired sequences, however, were associated with tissue loss in posterior regions: the posterior superior temporal gyrus and superior temporal sulcus for filled pauses (Fig. 4E, blue; Table 3), and a similar region extending more ventrally and posteriorly into the angular gyrus and inferior parietal lobule for repaired sequences (Fig. 4E, green; Table 3). No brain regions were significantly associated with increased numbers of incomplete sentences.
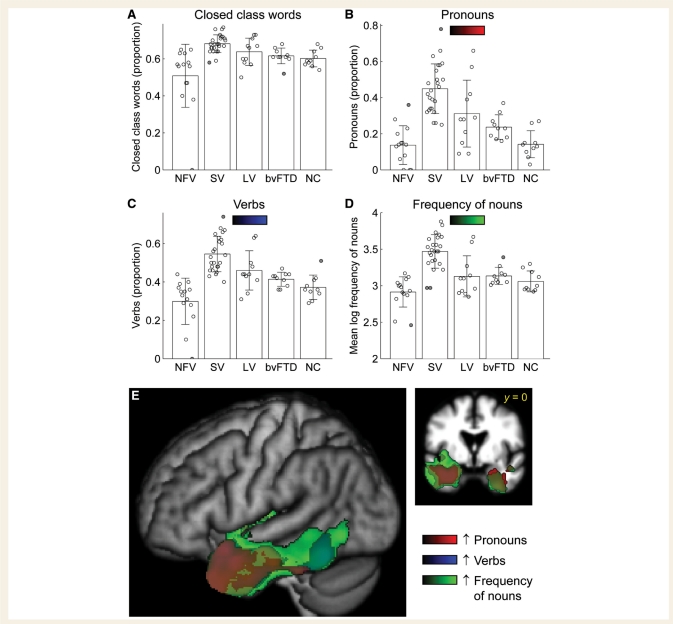
Lexical content
There was a significant omnibus group effect on the proportion of closed class lexical items, with patients with the non-fluent variant producing (marginally) less closed class items than controls, and patients with the semantic and logopenic variants producing (non-significantly) more (Fig. 5A, Table 2). It is noteworthy that while a subset of non-fluent patients (5 of 14) produced a lower proportion of closed class items than any control subject, the other non-fluent patients were in the normal range.
Figure 5.
Lexical content. (A) Closed class words (as a proportion of all words). (B) Pronouns (as a proportion of all nominals, i.e. pronouns and nouns). (C) Verbs (as a proportion of the two major open classes, i.e. verbs and nouns). (D) Mean log frequency of nouns. (E) Voxel-based morphometry showing brain regions where atrophy was correlated with greater proportions of pronouns (red), greater proportions of verbs (blue) and higher frequency nouns (green). NFV = non-fluent variant; SV = semantic variant; LV = logopenic variant; NC = normal controls.
Patients with the semantic variant produced a greater number of pronouns than controls (as a proportion of all nouns and pronouns) (Fig. 5B, Table 2), a greater number of verbs (as a proportion of all nouns and verbs) (Fig. 5C, Table 2) and the nouns that they did produce had a higher mean log frequency than controls or other patient groups (Fig. 5D, Table 2). The examples below show how individuals with the semantic variant often use action-oriented descriptions that entail the use of pronouns, verbs and high-frequency nouns, to work around lower frequency nouns that cannot be accessed:
(4) a. they- sh- they look like they’re handling s- some things while they’re sitting down there (referring to ‘book’, ‘cup’ and ‘thermos’) (semantic variant)
b. that may be where you take your boat to get onto the boat there (referring to ‘dock’) (semantic variant)
c. there’s um a place where they have their car (referring to ‘garage’) (semantic variant)
These effects were all specific to the semantic variant group, except that patients with the logopenic variant also showed a greater proportion of pronouns than controls, reflecting lexical retrieval problems that are less severe than those seen with the semantic variant.
Based on previous studies suggesting problems with verbs in non-fluent patients (Thompson et al., 1997; Hillis et al., 2002, 2004; Ash et al., 2009), we compared the proportions of verbs produced in non-fluent patients and normal controls with an uncorrected one-tailed t-test, and found a significantly reduced proportion of verbs in the non-fluent patients (P = 0.035). It should be noted that this effect was driven by only three patients who produced primarily isolated nouns or noun phrases; most patients with the non-fluent variant produced normal proportions of verbs.
Neural correlates of lexical content variables
Voxel-based morphometry revealed that volume loss in left anterior temporal cortex was associated with increased proportions of closed class words (not shown), pronouns (Fig. 5E, red; Table 3) and verbs (Fig. 5E, blue; Table 3) and increased frequency of nouns (Fig. 5E, green; Table 3). Less extensive anterior temporal regions in the right hemisphere were also associated with increased use of pronouns and a higher frequency of nouns. These results reflect the relatively homogeneous semantic variant patients who all showed atrophy in these regions and changes on these measures.
Syntactic structure and complexity
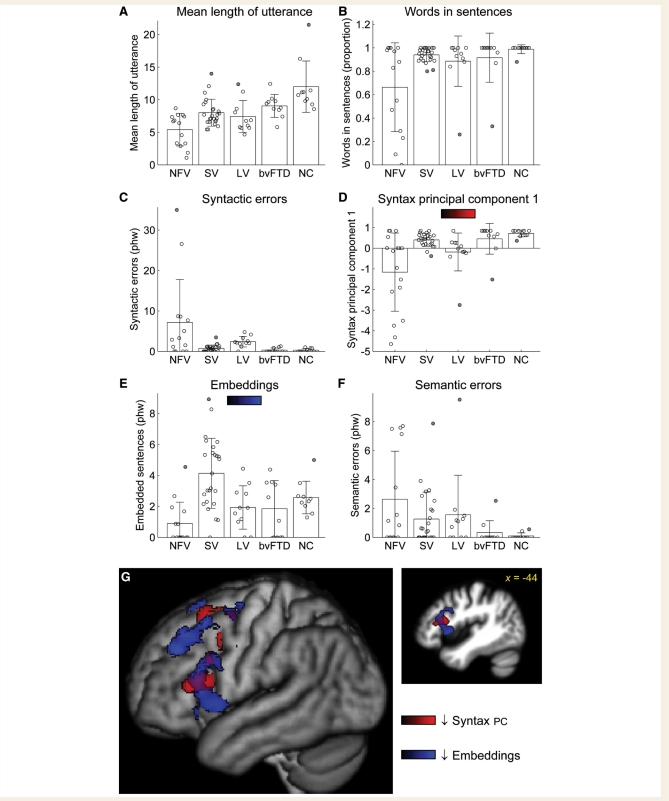
Mean length of utterance, a simple measure of complexity, was significantly reduced in all PPA groups, especially in the non-fluent variant; and even marginally in bvFTD (Fig. 6A, Table 2).
Figure 6.
Syntactic structure and complexity. (A) Mean length of utterances. (B) Words in sentences (as a proportion of all words). (C) Syntactic errors. (D) A summary syntax measure, comprising the first principal component derived from words in sentences and syntactic errors. (E) Embeddings. (F) Semantic errors, i.e. sentences that were syntactically correct but semantically anomalous or inappropriate. (G) Voxel-based morphometry showing brain regions where atrophy was correlated with lower scores on the composite syntactic measure indicating greater syntactic impairments (red) and reduced numbers of embeddings (blue). The syntax measures were not used independently for voxel-based morphometry because they each captured only a subset of patients with syntactic deficits. Phw = per hundred words; NFV = non-fluent variant; SV = semantic variant; LV = logopenic variant; NC = normal controls; PC = principal component.
All but one normal control subject produced all of their words in sentences, but the majority of patients with PPA of all variants produced some utterances consisting of topic-comment structures, or smaller phrases such as verb phrases or noun phrases (Fig. 6B, Table 2). The non-fluent and semantic variant groups differed significantly from controls, with six of the 14 patients with the non-fluent variant producing numerous non-sentence utterances. Usually failure to produce words in sentences reflects agrammatism (Examples 5a and b), but sometimes it can simply reflect failure to follow the task instructions to produce sentences.
(5) a. [the picnic basket]Noun Phrase //and [the man]Noun Phrase //and [the book]Noun Phrase //and [the woman has a wine]Sentence (non-fluent variant)
b. [dog on the ground]Topic-Comment //… [sailboat … in the water]Topic-Comment (non-fluent variant)
All of the patients with PPA produced syntactic errors, and the non-fluent and logopenic variant groups differed from controls in the number of errors produced (marginally in the case of the non-fluent variant) (Fig. 6C, Table 2). Many different types of syntactic errors were observed. Some had an ‘agrammatic’ quality, such as omission of determiners (Example 6a), omission of auxiliaries (Example 6b), omission of verbal inflections (Example 6c) and occasional garbled structures (Example 6d). Other errors had a ‘paragrammatic’ quality, i.e. ‘unacceptable juxtapositions of phrases and misuse of words’ (Goodglass et al., 1994). Examples of this are incorrect use of lexical aspect (Example 6e), incorrect argument structure (Example 6f), agreement errors (Example 6g) and various failures to correctly resolve syntactic dependencies (Examples 6h and i).
(6) a. the /mjæn/ is reading book (non-fluent variant)
b. the man … flying a kite (non-fluent variant)
c. the family is have a picnic (non-fluent variant)
d. a thongs off the man (non-fluent variant) (i.e. the man has taken his thongs/sandals off)
e. one of the guys is having a, um, something in the sky (semantic variant)
f. I know what they’re doing but I can’t think the words what they’re, doing (semantic variant)
g. a girl and a boy is having a /p
 kn
kn k/ (logopenic variant)
k/ (logopenic variant)h. something that you put things to eat from (logopenic variant)
i. there’s another man play, or, has a kite and he’s flying that (logopenic variant)
Although patients of all variants made agrammatic errors (i.e. frank omissions of functional words or morphemes), these were most frequent in patients with the non-fluent variant: non-fluent patients omitted at least one determiner, as opposed to only 5 of 25 semantic variant patients and 4 of 11 logopenic patients patients with the logopenic variant (Table 2). In contrast, syntactic errors in the semantic and logopenic variant patient groups were predominantly paragrammatic (as in Examples 6e–i above).
Neither of the two syntactic measures presented so far—words in sentences or syntactic errors—completely reflect a patient’s abilities to construct well-formed syntactic structures, because some patients attempt only very simple utterances (e.g. isolated nouns), and therefore do not make syntactic errors (Fig. 7A). Consequently, to obtain a summary measure of syntactic competence, we conducted principal components analysis on these two features. Patients who either produced many errors, or who produced many words that were not in sentences, could then be identified by low scores on the first principal component (Fig. 6D, Table 2). The non-fluent variant group had the lowest scores on this composite measure, though all three PPA groups were significantly impaired relative to controls. In the logopenic variants, but not in other variants, scores on the composite syntactic measure were correlated with the number of repaired sequences produced (r = 0.63, P = 0.040, Fig. 7B), suggesting that their syntactic abilities might reflect these constant reformulations, which create a barrier to resolving syntactic dependencies.
Figure 7.
Correlations between syntactic measures and other measures. (A) The relationship between words in sentences and syntactic errors, showing how some patients with the non-fluent variant of PPA make many errors, whereas others often fail to produce complete sentences. (B) Correlations between repaired sequences and the composite syntactic measure. Only in the logopenic variant group, there was a significant association between these variables (fitted line). (C) Correlations between distortions and the composite syntactic measure, showing that patients with the non-fluent variant could be impaired on one or both of these measures. phw = per hundred words; NFV = non-fluent variant; SV = semantic variant; LV = logopenic variant; NC = normal controls.
Number of embeddings is a good measure of patient ability to produce syntactically complex structures. There was a robust main effect of group on number of embeddings (Fig. 6E, Table 2). Although follow-up tests relative to controls were not significant after correction for multiple comparisons, patients with the non-fluent variant showed reduced numbers of embeddings, with most producing none at all; patients with the logopenic variant produced normal numbers of embeddings; and patients with the semantic variant actually produced more embeddings than controls. This is likely to reflect the intricate constructions that they often generate to work around lexical retrieval problems:
(7) a. there’s a, what appears to be, though you can’t see his face, a younger gentleman, who is closer to the lake, and he is um, flying a, a unit, that uh, is at the end of a, piece of uh, end of end of a, it’s it’s flying it, so he can control it (semantic variant)
b. seeing him fly this, I can not remember the name of what this is, that’s up in the air here (semantic variant)
These examples highlight the striking dissociation between impaired lexical access and preserved syntactic abilities in patients with the semantic variant.
Semantic errors (syntactically well-formed sentences that were either non-sensical or semantically inappropriate for the context) were observed in all PPA groups (Fig. 6F, Table 2). The omnibus test was significant but not the follow-up tests after multiple comparisons. Substitutions of semantically related items were the most common type of semantic error (Examples 8a–e).
(8) a. a temporary type of, radio (a portable radio) (semantic variant)
b. it’s got plants inside (meaning the radio runs on batteries) (semantic variant)
c. the other one that put all this stuff up (all this stuff for ‘kite’) (semantic variant)
d. the lake is filled with um sailboat and people waving and jet skis (there are no jet skis) (non-fluent variant)
e. I used to like to have to carry those too (referring to the kite, ‘carry’ substituted perhaps for ‘fly’) (logopenic variant)
Neural correlates of syntactic variables
Voxel-based morphometry revealed that scores on the composite syntactic measure, reflecting syntactic errors and/or failure to produce sentences, were correlated with tissue volume in the left posterior inferior frontal gyrus (pars triangularis), supplementary motor area, and the white matter underlying these structures (Fig. 6G, red; Table 3). Reduced numbers of embeddings were associated with atrophy of the left posterior inferior frontal gyrus (pars opercularis and orbitalis), superior frontal sulcus and adjacent prefrontal regions and the supplementary motor area (Fig. 6G, blue; Table 3). These sets of regions were largely overlapping.
Reduced mean length of utterance was associated with the left superior longitudinal fasciculus (not shown). Increased numbers of semantic errors were not associated with any brain regions, probably reflecting the many different types of errors that were classified as semantic.
Relationship between syntactic and motor speech deficits
Since agrammatism and motor speech impairments are both associated with non-fluent variant PPA (Gorno-Tempini et al., 2004), we wanted to determine whether these two types of impairments are associated in individual patients (Fig. 7C). Although the two patients who produced the most distortions also had severe syntactic impairments, these two features were dissociable, and overall the correlation was not significant (r = –0.37, P = 0.19). The brain regions associated with distortions (the left and right superior longitudinal fasciculus) overlapped partly with the regions linked to impaired syntax, but distortions were correlated with a wider expanse of white matter, whereas syntactic deficits but not distortions were associated with volume loss in the left inferior frontal gyrus (compare Fig. 2E, blue and Fig. 6G, red).
Discussion
This study has provided the most comprehensive quantitative description to date of connected speech production in all three variants of PPA, and the neural correlates of different aspects of abnormal connected speech in neurodegenerative aphasias. We found that connected speech has different properties in each PPA variant, and that these features can be informative in distinguishing the three variants from each other and from normal controls. We showed that specific speech and language variables correlated with atrophy in distinctive brain regions.
Connected speech characteristics in the three variants of primary progressive aphasia
Non-fluent variant primary progressive aphasia
Patients with non-fluent variant are by definition ‘non-fluent’ and are often described as agrammatic. Some patients become mute or so apraxic that even single words are unintelligible and we excluded several such patients. In our remaining mild-moderate sample, we confirmed that patients with the non-fluent variant are impaired on many of the dimensions that contribute to fluency. They had the slowest speech rate of any group, and invariably made at least some distortions. Some but not all patients also produced non-distorted phonological paraphasias, which are likely to reflect phonological encoding processes prior to articulation. Patients with the non-fluent variant produced a greater number of false starts, filled pauses and repaired sequences than normal controls, but most of these differences were not significant and many patients fell into the normal range. Several of these features—certainly rate and distortions, and probably many of the false starts—are probably consequences of apraxia of speech (Wertz et al., 1984; Ogar et al., 2007), however it should be noted that a motor speech evaluation that requires patients to produce sequences of increasing articulatory complexity is more sensitive for detecting apraxia of speech than a connected speech sample in which patients may choose their own words.
In terms of lexical content, a substantial minority of patients produced fewer closed class words and/or fewer verbs than controls, consistent with prior studies (Thompson et al., 1997; Hillis et al., 2002, 2004; Ash et al., 2009). However there were also many patients whose speech was normal in these respects, so these lexical features cannot be considered diagnostic for the non-fluent variant.
Many patients produced utterances that were not complete sentences, and most but not all patients produced syntactic errors. The fact that some patients produced no syntactic errors and others only a few demonstrates that most patients with the non-fluent variant are not frankly agrammatic in the way that patients with Broca’s aphasia are (Graham et al., 2004; Patterson et al., 2006; Knibb et al., 2009). However, the mean length of utterances and number of embeddings were reduced, consistent with previous studies (Thompson et al., 1997; Ash et al., 2006, 2009, Knibb et al., 2009), suggesting a reduction in ability to generate complex syntactic structures.
In sum, patients with the non-fluent variant were non-fluent when compared to the other variants of PPA, but it was our impression that most of the mild-moderate patients we studied were less impaired than the typical non-fluent vascular patient, both in terms of speech errors and syntactic structure.
Semantic variant primary progressive aphasia
Patients with the semantic variant produced fluent speech with a maximum rate that did not differ from controls, although overall speech rate was slightly reduced. Reduced speech rate in the semantic variant of PPA has been reported in some studies (Ash et al., 2006, 2009) but not in others (Bird et al., 2000; Patterson and MacDonald, 2006). Our findings suggest that because maximum speech rate was normal, any reductions are not due to motor speech or syntactic factors, but probably reflect higher-level discourse processes (Ash et al., 2006). Patients with the semantic variant produced negligible numbers of speech sound errors, and did not differ from controls in terms of false starts, filled pauses or repaired sequences.
They produced a greater proportion of closed class items relative to open class, pronouns relative to nouns and verbs relative to nouns, and the nouns they did produce were of higher frequency than other groups, consistent with prior studies (Bird et al., 2000; Patterson and MacDonald, 2006). These findings were highly robust; there was little overlap with controls on most of these measures.
Patients with the semantic variant made some but relatively few syntactic errors, consistent with recent reports (Patterson and MacDonald, 2006; Meteyard and Patterson, 2009), and their errors tended to be paragrammatic rather than agrammatic. Although mean length of utterance was reduced, patients with the semantic variant actually produced more embedded sentences than did controls, probably reflecting attempts to work around problems due to anomia. These findings provide a counterpoint to the claim that syntax is less elaborate in semantic variant patients than in controls (Patterson and MacDonald, 2006).
Overall, connected speech production was strikingly preserved in the semantic variant group, with the exception of lexical content. Preserved motor speech and syntactic function in these patients is likely to reflect the structural (Mummery et al., 2000) and functional (Mummery et al., 1999; Wilson et al., 2009a) integrity of dorsal language regions and the white matter pathways that connect them (Agosta et al., 2010).
Logopenic variant primary progressive aphasia
Patients with the logopenic variant have been categorized as fluent by some researchers but non-fluent by others (Gorno-Tempini et al., 2008). Our results revealed a mixed picture on the different dimensions of fluency, which accounts for the inherent difficulty of classifying patients on a single composite construct such as fluency. Patients with the logopenic variant produced speech at a rate that was intermediate between the non-fluent and semantic variant groups. Unlike non-fluent patients, logopenic patients produced few or no distortions; but like non-fluent patients, a subset of logopenic patients produced phonological paraphasias. In other words, not all patients with the logopenic variant made errors involving speech sounds, but when they did, the errors were typically frank phonemic errors (e.g. substitution of a different phoneme) rather than misarticulations, and therefore probably reflect phonological rather than motor speech impairments. Note that even in the patients who made no phonemic errors in this short speech sample, it is likely that at least some phonemic errors would be observed in a longer speech sample, or in other tasks such as confrontation naming. Logopenic patients also produced the greatest numbers of false starts, filled pauses and repaired sequences of any group, often giving rise to an overall impression of non-fluency.
In terms of lexical content, patients with the logopenic variant produced significantly more pronouns than normal controls, and somewhat more verbs, although their values for these measures were less extreme than for semantic variant patients. Also, there was no tendency for logopenic patients to produce nouns of higher frequency, as semantic variant patients did.
The speech of logopenic patients comprised mostly words in sentences, however all but one patient made at least one syntactic error. Similar to patients with the semantic variant, most of the syntactic errors made by logopenic patients were paragrammatic rather than agrammatic, and the relationship between repaired sequences and syntactic deficits suggested that these constant rewordings, combined with well-documented verbal working memory deficits (Gorno-Tempini et al., 2004, 2008), led to syntactic errors reflecting failures to resolve syntactic dependencies.
In summary, motoric aspects of speech were relatively preserved in the logopenic variant group, while deficits in other aspects of speech production combined to give rise to a variable overall impression of degree of fluency depending on the patient. Speech production in the logopenic variant of PPA resembles mild conduction aphasia in that there are at least islands of fluency, and any syntactic errors are paragrammatic rather than agrammatic (Goodglass et al., 1994). Although these groups both have phonological retrieval and assembly problems, these tend to result in word-finding pauses and rephrasings in the logopenic variant more often than they lead to phonological paraphasias or neologisms as seen in conduction aphasia.
Diagnostic utility of motor speech and linguistic features
PPA and its variants are typically diagnosed based on multiple sources of information including speech and language tests (including assessment of connected speech), neuropsychological testing, neurological examination and neuroimaging (Hodges and Patterson, 1996; Neary et al., 1998; Gorno-Tempini et al., 2004, 2010; Wilson et al., 2009c). Normally no single source of evidence is sufficient to make a diagnosis, so it is important to extract as much information as possible from each source. Here we consider which combinations of speech and language features derived from short connected speech samples are most clinically informative in distinguishing the three PPA variants from each other.
Discrimination between the non-fluent and semantic variants is typically straightforward since there is little to no overlap between these groups on a number of measures, including overall and maximum speech rate, number of distortions, proportion of pronouns, proportion of verbs and frequency of nouns.
The logopenic variant can sometimes be difficult to distinguish from the other two variants. The most informative feature for discriminating between the logopenic and non-fluent variants was the presence of distortions, which were seen in all patients with the non-fluent variant, but only 3 of 11 patients with the logopenic variant, reflecting the prevalence of significant motor speech impairments in the former group. This variable could be combined with others such as proportion of verbs (higher in the logopenic variant) or number of embeddings (also greater in the logopenic variant); maximum speech rate should also be considered, as well as the presence of other disruptions to fluency such as repaired sequences, which are generally more prevalent in patients with the logopenic variant. Agrammatism, like fluency, is a compound construct including features such as agrammatic (not paragrammatic) syntactic errors, non-sentence utterances, reduced mean length of utterance and lack of embeddings (Goodglass et al., 1993, 1994). All of these features were more prevalent in non-fluent patients than logopenic patients. While the presence of frankly agrammatic speech is indicative of the non-fluent variant, the absence of frank agrammatism is less informative, because many non-fluent variant patients have only very mild syntactic impairments, at least early in the course of disease.
Discrimination between the logopenic and semantic variants can be based on a number of variables, none of which provide complete separation alone, but which in combination can divide the two groups. These discriminative features include maximum speech rate (greater in the semantic variant), phonological paraphasias (more likely in the logopenic variant), filled pauses (more frequent in the logopenic variant), repaired sequences (more frequent in the logopenic variant), proportions of pronouns and verbs (higher in the semantic variant) and frequency of nouns (higher in the semantic variant).
Neural correlates of specific motor speech and linguistic features
This study is the first to associate particular motor speech and linguistic features derived from a connected speech sample with specific brain regions in patients with PPA, apart from a single overall measure of speech rate, which was linked to the left inferior frontal and adjacent superior temporal gyri (Ash et al., 2009). We found that maximum speech rate correlated with reduced tissue volume in both anterior and posterior language regions, which is not surprising because disturbances at any level of the language production system could lead to a slowdown of speech. The finding of a role for posterior regions in this study but not in Ash et al. (2009) probably reflects the fact that they did not include patients with the logopenic variant.
There was a clear distinction between the neural correlates of distortions and phonological paraphasias. Distortions were associated with reduced volume of white matter underlying the frontal lobe, particularly the superior longitudinal fasciculus. This is useful clinically, since such a distinct anatomical localization can provide clues about the underlying pathological cause of the clinical symptom. Frontal cortical and subcortical dorsal involvement is associated with corticobasal degeneration, which is often the underlying aetiology of the non-fluent variant of PPA (Josephs et al., 2006; Mesulam et al., 2008). Although little is known about the role of white matter damage in motor speech deficits, lesions to the superior longitudinal fasciculus in stroke patients have been associated with speech production (Dronkers et al., 1993; Bates et al., 2003; Ogar et al., 2006) and repetition deficits (Breier et al., 2008). Reduced fractional anisotropy in this tract has been associated with speech deficits in Rett syndrome (Mahmood et al., 2009), and dissection of a tumour border contacting the superior longitudinal fasciculus has been shown to cause speech arrest (Davtian et al., 2008).
In contrast, phonological paraphasias correlated with atrophy of the left posterior superior temporal gyrus, which is typically impacted in logopenic variant patients, due to the presumed underlying Alzheimer’s pathology (Josephs et al., 2008; Mesulam et al., 2008; Rabinovici et al., 2008), as well as in the non-fluent variant of PPA as the disease progresses (Rohrer et al., 2009). These findings suggest that speech sound errors that involve non-distorted substitutions, insertions or deletions of whole phonemes are more likely to reflect dysfunction of posterior language regions important for phonological representations and processes, whereas misarticulation errors result from damage to speech motor regions and underlying white matter.
Other phenomena that contribute to an overall impression of impaired fluency—false starts, repaired sequences, filled pauses—were associated mostly with posterior brain regions. These findings do not have a strong interpretation with respect to the neuroanatomical architecture of the language system, because these phenomena potentially reflect breakdown at several different levels of the speech production process. However, it may be clinically useful to note that these speech qualities are associated more with posterior dysfunction.
All variables reflecting lexical access were associated with anterior temporal regions, greater in the left than the right hemisphere, consistent with many previous studies (Damasio et al., 1996; Rosen et al., 2002; Grossman et al., 2004; Amici et al., 2007b). However, regions where atrophy was associated with some of these variables (specifically, increased proportion of nouns relative to verbs, and increased frequency of nouns) extended quite far posteriorly, encompassing the entire left inferior temporal gyrus and much of the left fusiform gyrus. Functional MRI studies have identified frequency effects in lexical access tasks in posterior but not anterior temporal regions (Graves et al., 2007; Wilson et al., 2009b), so it remains unclear which temporal lobe regions specifically are important for different aspects of lexical retrieval.
Syntactic structure and complexity were associated with tissue loss in widespread frontal regions. While syntactic comprehension has been associated with the left inferior frontal gyrus in neurodegenerative disease (Amici et al., 2007a), this is the first demonstration that structure and complexity of syntactic production also depend on left frontal cortex. These findings are not surprising, since production of complex syntactic structure has also been linked to left frontal cortex in stroke aphasia (Borowsky et al., 2007) and functional neuroimaging studies of normal controls (Blank et al., 2002; Indefrey et al., 2004).
Limitations
Our study has several notable limitations. Firstly, our speech samples were relatively short. Many of the patients did not produce the minimum of 150 narrative words recommended for QPA (Saffran et al., 1989; Berndt et al., 2000). In such small samples, most patients did not produce more than a few distortions, phonological paraphasias, syntactic errors or other features of interest, which means that our estimates of the prevalence of these features in the speech of any individual patient are not very accurate. Other studies of connected speech in PPA have successfully used short picture descriptions such as we used here (Bird et al., 2000; Graham et al., 2004; Patterson and MacDonald, 2006) and we were still able to observe many reliable differences between groups, but it would be preferable to elicit longer speech samples as several authors have done (e.g. Thompson et al., 1997; Ash et al., 2006, 2009; Knibb et al., 2009; Meteyard and Patterson, 2009).
Second, because we used picture description rather than narrative or conversational speech, we were unable to investigate organization of discourse beyond the level of the sentence. Previous studies have examined pragmatics (Orange et al., 1998) and discourse cohesion (Ash et al., 2006) in progressive aphasia, and impairments in narrative organization were associated with primarily right-lateralized atrophy to frontal and anterior temporal regions (Ash et al., 2006). Probably due to the fact that our measures focused on features at the sentence level or below, we found only left-lateralized neural correlates for most measures.
Third, the Western Aphasia Battery, including the picnic description, was one of the pieces of data contributing to the diagnosis of each patient, introducing a risk of circularity. However, this is only a minor concern since diagnoses were based on a comprehensive multidisciplinary evaluation of which the speech and language examination was only a small part.
Fourth, the correlations between motor speech and linguistic variables, and atrophy to specific brain regions identified with voxel-based morphometry, were to some extent driven by consistent associations between the anatomical characteristics of each variant and its specific set of typical features. In other words, for any given region associated with a speech/language feature, we cannot be sure if atrophy to that region directly causes the effect on the output, or whether atrophy to that region is associated with a particular patient group who show the deficit, perhaps due to atrophy of the region in question but maybe for some other reason. This is a limitation of many voxel-based morphometry studies. However, including patient group as a covariate is not a solution, since it would remove most of the variance that allows detection of brain-behaviour correlations.
Conclusion
The connected speech of patients with PPA has often been described simply as ‘fluent’ or ‘non-fluent’, but fluency is a complex construct encompassing features such as speech rate, phrase length, articulatory agility and syntactic structure, which do not always decline in parallel. We showed that a detailed multidimensional quantification of connected speech production is necessary to characterize the differences between the speech patterns of each PPA variant adequately. The motor speech and linguistic features derived from a simple picture description are clinically useful in differentiating between PPA variants and have clear anatomical localization power that can aid in the aetiological diagnosis of the neurodegenerative aphasias.
Funding
National Institutes of Health [NINDS R01 NS050915, NIDCD R03 DC010878, NIA P50 AG03006, NIA P01 AG019724]; State of California [DHS 04-35516]; Alzheimer’s Disease Research Center of California [03-75271 DHS/ADP/ARCC]; Larry L. Hillblom Foundation; John Douglas French Alzheimer’s Foundation; Koret Family Foundation; and McBean Family Foundation.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We thank Jean Gordon, David Wilkins and Kate Rankin for helpful discussions; Kate Possin for assistance with neuropsychological data; Robin Ketelle, Matthew Growdon, Jung Jang and Robert Nicholson for administrative support; and all of the patients, caregivers and volunteers for their participation in our research.
Glossary
Abbreviations
- bvFTD
behavioural variant frontotemporal dementia
- PPA
primary progressive aphasia
- QPA
quantitative production analysis
References
- Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, Dronkers NF, et al. Language networks in semantic dementia. Brain. 2010;133:286–99. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici S, Brambati SM, Wilkins DP, Ogar J, Dronkers NL, Miller BL, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci. 2007a;27:6282–90. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici S, Ogar J, Brambati SM, Miller BL, Neuhaus J, Dronkers NL, et al. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cogn Behav Neurol. 2007b;20:203–11. doi: 10.1097/WNN.0b013e31815e6265. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–13. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Vesely L, Gunawardena D, McMillan C, Anderson C, et al. Non-fluent speech in frontotemporal lobar degeneration. J Neuroling. 2009;22:370–83. doi: 10.1016/j.jneuroling.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–50. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Benson DF. Fluency in aphasia: correlation with radioactive scan localization. Cortex. 1967;3:373–94. [Google Scholar]
- Berndt RS, Wayland S, Rochon E, Saffran EM, Schwartz M. Quantitative production analysis: a training manual for the analysis of aphasic sentence production. Hove: Psychology Press; 2000. [DOI] [PubMed] [Google Scholar]
- Bird H, Lambon Ralph MA, Patterson K, Hodges JR. The rise and fall of frequency and imageability: noun and verb production in semantic dementia. Brain Lang. 2000;73:17–49. doi: 10.1006/brln.2000.2293. [DOI] [PubMed] [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJ. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–38. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Borovsky A, Saygin AP, Bates E, Dronkers N. Lesion correlates of conversational speech production deficits. Neuropsychologia. 2007;45:2525–33. doi: 10.1016/j.neuropsychologia.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29:483–7. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain. 2005;128:1984–95. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- Davtian M, Ulmer JL, Mueller WM, Gaggl W, Mulane MP, Krouwer HG. The superior longitudinal fasciculus and speech arrest. J Comput Assist Tomogr. 2008;32:410–4. doi: 10.1097/RCT.0b013e318157c5ff. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Redfern B, Shapiro JK. Neuroanatomic correlates of production deficits in severe Broca’s aphasia. J Clin Exp Neuropsychol. 1993;15:59–60. [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Christiansen JA, Gallagher R. Comparison of morphology and syntax in free narrative and structured tests: fluent vs. nonfluent aphasics. Cortex. 1993;29:377–407. doi: 10.1016/s0010-9452(13)80250-x. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Christiansen JA, Gallagher RE. Syntactic constructions used by agrammatic speakers: Comparison with conduction aphasics and normals. Neuropsychology. 1994;8:598–613. [Google Scholar]
- Goodglass H, Quadfasel FA, Timberlake WH. Phrase length and the type of severity of aphasia. Cortex. 1964;1:133–53. [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–34. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NL, Patterson K, Hodges JR. When more yields less: speaking and writing deficits in nonfluent progressive aphasia. Neurocase. 2004;10:141–55. doi: 10.1080/13554790409609945. [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gordon JK. A neural signature of phonological access: distinguishing the effects of word frequency from familiarity and length in overt picture naming. J Cogn Neurosci. 2007;19:617–31. doi: 10.1162/jocn.2007.19.4.617. [DOI] [PubMed] [Google Scholar]
- Grossman M, Ash S. Primary progressive aphasia: a review. Neurocase. 2004;10:3–18. doi: 10.1080/13554790490960440. [DOI] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E. Progressive nonfluent aphasia: language, cognitive, and PET measures contrasted with probable Alzheimer's disease. J Cogn Neurosci. 1996;8:135–54. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Ann Neurol. 2004;55:268–75. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cogn Neurosci. 2002;14:1099–108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc. 1996;2:511–24. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–63. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Hellwig F, Herzog H, Seitz RJ, Hagoort P. Neural responses to the production and comprehension of syntax in identical utterances. Brain Lang. 2004;89:312–9. doi: 10.1016/S0093-934X(03)00352-3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–98. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70:25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery. New York: Grune and Stratton; 1982. [Google Scholar]
- Kertesz A, Davidson W, McCabe P, Takagi K, Munoz D. Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsychol Soc. 2003;9:710–19. doi: 10.1017/S1355617703950041. [DOI] [PubMed] [Google Scholar]
- Knibb JA, Woollams AM, Hodges JR, Patterson K. Making sense of progressive non-fluent aphasia: an analysis of conversational speech. Brain. 2009;132:2734–46. doi: 10.1093/brain/awp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–65. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Bibat G, Zhan AL, Izbudak I, Farage L, Horska A, et al. White matter impairment in rett syndrome: diffusion tensor imaging study with clinical correlations. Am J Neuroradiol. 2010;31:295–9. doi: 10.3174/ajnr.A1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–19. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2010;66:1545–51. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meteyard L, Patterson K. The relation between content and structure in language production: an analysis of speech errors in semantic dementia. Brain Lang. 2009;110:121–34. doi: 10.1016/j.bandl.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJ, Vandenberghe R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122:61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Munzel U, Hothorn LA. A unified approach to simultaneous rank test procedures in the unbalanced one-way layout. Biom J. 2001;43:553–69. [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97:343–50. doi: 10.1016/j.bandl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21:S23–30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- Orange JB, Kertesz A, Peacock J. Pragmatics in frontal lobe dementia and primary progressive aphasia. J Neuroling. 1998;11:153–177. [Google Scholar]
- Patterson K, Graham NL, Lambon Ralph MA, Hodges JR. Progressive non-fluent aphasia is not a progressive form of non-fluent (post-stroke) aphasia. Aphasiology. 2006;20:1018–34. [Google Scholar]
- Patterson K, MacDonald MC. Sweet nothings: narrative speech in semantic dementia. In: Andrews S, editor. From inkmarks to ideas: current issues in lexical processing. Hove: Psychology Press; 2006. pp. 299–317. [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Alarcon NB. Dissolution of spoken language in primary progressive aphasia. Aphasiology. 1998;12:635–50. [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72:1562–9. doi: 10.1212/WNL.0b013e3181a4124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Saffran EM, Berndt RS, Schwartz MF. The quantitative analysis of agrammatic production: procedure and data. Brain Lang. 1989;37:440–79. doi: 10.1016/0093-934x(89)90030-8. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol. 1989;2:167–82. [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–321. [Google Scholar]
- Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–35. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of speech in adults: the disorder and its management. New York: Grune and Stratton; 1984. [Google Scholar]
- Wilson SM, Brambati SM, Henry RG, Handwerker DA, Agosta F, Miller BL, et al. The neural basis of surface dyslexia in semantic dementia. Brain. 2009a;132:71–86. doi: 10.1093/brain/awn300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Isenberg AL, Hickok G. Neural correlates of word production stages delineated by parametric modulation of psycholinguistic variables. Hum Brain Mapp. 2009b;30:3596–608. doi: 10.1002/hbm.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Ogar JM, Laluz V, Growdon M, Jang J, Glenn S, et al. Automated MRI-based classification of primary progressive aphasia variants. Neuroimage. 2009c;47:1558–67. doi: 10.1016/j.neuroimage.2009.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.