Abstract
Methamphetamine (METH) is an addictive psychostimulant that induces damage to the dopamine terminals and the apoptosis of some neurons of the striatum. Our laboratory demonstrated using either a single bolus dose (30 mg/kg) or a binge (10 mg/kg 4× at 2-h intervals) of METH that pharmacological blockade of the substance P receptor (neurokinin-1) attenuates METH-induced damage to both the presynaptic dopamine terminals and the apoptosis of some neurons of the striatum. To determine the phenotype of striatal neuron ablated by METH, we combined TUNEL (Terminal Deoxyncleotidyl Transferase-Mediated dUTP Nick End Labeling) with immunofluorescence for selective markers of projection and interneurons. METH induces the loss of approximately 20% of the projection neurons. The cholinergic and γ-aminobutyric acid (GABA)-parvalbumin interneurons sustain losses of 30% and 50%, respectively. The somatostatin/neuropeptide Y (NPY)/nitric oxide synthase (NOS) interneurons are not impacted by METH. To investigate the mechanism by which substance P mediates METH-induced damage in this part of the brain, we ablated the striatal interneurons that express the neurokinin-1 receptor (NK-1R) with the selective neurotoxin substance P-SAP. Ablation of the NK-1R-expressing interneurons prevented METH-induced apoptosis in the striatum but was without effect on depletion of dopamine terminal markers. We propose that substance P mediates the apoptosis of some striatal neurons via the intrastriatal activation of nitric oxide synthesis. In contrast, substance P may mediate damage of the dopamine terminals via an extrastriatal mechanism involving the substantia nigra and cortical glutamate release.
Keywords: striatum, methamphetamine, neurotoxicity, apoptosis, substance P, neurokinin-1 receptor
INTRODUCTION
Methamphetamine (METH) use is rapidly increasing in the United States posing a health problem because this psychostimulant causes damage in the brain. Evidence of the neurotoxic effects of METH on dopamine neurons was reported by Kogan et al.1 and Seiden et al.2 METH also causes damage to serotonergic neurons.3,4 Unequivocal demonstration of the neuronal damage induced by METH came from immunocytochemical studies5,6 and the application of the silver stain.7 METH has been shown to cause inhibition of the enzyme tyrosine hydroxylase (TH)1 and inactivation8 and oligomerization9 of striatal dopamine transporters. In addition to the neurochemical and morphological damage inflicted on the dopaminergic and serotonergic terminals, METH induces neuronal cell loss. Exposure to METH causes the loss of some neurons in the striatum and the cortex of rodents.10-12 The damaging effects of METH in the brain may be mediated in part by the excessive release of gluta-mate. For example, antagonists of the NMDA (N-methyl-d-aspartate) subtype of glutamate receptor protect from METH-induced neural damage.13 The overflow of striatal dopamine elicited by METH and required for neural damage, can be attenuated by antagonists of the NMDA receptor.14 METH induces the overflow of glutamate in the striatum15 and agents that abrogate this increase also decrease METH-induced depletion of striatal dopamine content.16
The excessive overflow of dopamine induced by METH is detrimental to neurons because of the production of reactive oxygen species. METH has been implicated in the generation of superoxide radicals via the auto-oxidation of catecholamines.17,18 The auto-oxidation of dopamine is associated with increased activity of neuronal nitric oxide synthase and elevated levels of nitric oxide.19 Pharmacological inhibition of nitric oxide synthase or deletion of the gene for this enzyme in neurons protects the striatum from METH.20,21 In the striatum, the neuronal nitric oxide synthase is co-localized with the neurokinin-1 receptor (NK-1R) in 95% of the somatostatin interneurons.22 Because we have observed that pharmacological blockade of the NK-1R protects the striatum of mice from METH,23,24 we investigated the role of this receptor by ablation of the NK-1R-expressing interneurons of the striatum with the selective neurotoxin [sar,9 met(O2)11] substance p-saporin (SSP-SAP). We also report on the phenotype of striatal neuron lost in the aftermath of METH.
MATERIALS AND METHODS
Animals
Ten-week-old male ICR mice (Taconic, Germantown, NY) were housed individually on a 12-h light/dark cycle with food and water available ad libitum. All animals were habituated for 2 weeks prior to commencement of intraperitoneal (i.p.) drug administration or intrastriatal microinjection. All procedures of animal use were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Hunter College of the City University of New York.
Drug Preparation and Administration
METH (30 mg/kg of body weight) (Sigma, St. Louis, MO) was dissolved in phosphate-buffered saline (PBS), pH 7.6, and administered as a single i.p. injection to 10- or 13-week-old (3 weeks after intrastriatal microinjection) ICR mice (Taconic). The NK-1R antagonist, WIN 51,708 (Sigma/RBI, St. Louis), was dissolved in 45% 2-hydroxylpropyl-β-cyclodextrin (Sigma/RBI) and PBS (1:4) and administered i.p. 30 min prior to METH treatment. SAP (Advance Targeting Systems, San Diego, CA) and SSP-SAP (Advance Targeting Systems) were dissolved in PBS and administered by intrastriatal injection. After treatment, (24 h or 3 days post-METH; 3 weeks postintrastriatal microinjection) animals were either euthanized by decapitation or were first anesthetized with i.p. injections of ketamine/acepromazine (100 mg/kg, 3 mg/kg of body weight) and then perfused transcardially with 30 mL of PBS with heparin followed by 30 mL of 4% paraformaldehyde in PBS. Brains were then either dissected out and immediately placed on dry ice or postfixed in 4% paraformaldehyde in PBS overnight and cryoprotected in 30% sucrose in PBS at 4°C. Tissue was then frozen and stored at −80°C until use.
Autoradiographic Analysis of Dopamine Transporter
Fresh frozen 20 m coronal sections were dried in a dessicator and then incubated in 0.073 nM [125I]RTI-121- (2200 Ci/mmol; New England Nuclear, Boston, MA) buffered solution (137 mM NaCl, 2.7 mM KCl, 10.14 mM Na2HPO4, 1.76 mM KH2PO4, 10 mM NaI) for1hat room temperature. Nonspecific binding was determined using 10 uM GBR-12909 (Sigma). After incubation, sections were washed twice with chilled buffer for 20 min and then quickly rinsed with chilled distilled water. Slides were allowed to air-dry overnight and exposed on Hyperfilm MP (Amersham Pharmacia, Piscataway, NJ) together with a [125I] microscale. Binding of [125I]RTI-121 was quantified by densitometry with use of a computer-based NIH image analysis system.
TH Western Blot
Striatal tissue was dissected and homogenized with lysis buffer (50 mM Tris-HCL pH 7.4, 150 mM NaCl, 320 mM sucrose, 5 mM HEPES, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM DTT, 1% Inhibitor Cocktail [1.04 mM AEBSF, 0.8 uM aprotinin, 0.02 mM leupeptin, 0.04 mM bestatin, 0.015 mM pepstatin A, 0.014 mM E-64 (Sigma)]). Homogenates were centrifuged at 800 g for 5 min at 4°C. Supernatants were further centrifuged at 3000 g, and supernatants were then used for Western blot analysis. After protein concentration was determined by the Bradford assay, samples were denatured in Laemmli sample buffer containing 20% β-mercaptoethanol for 10 min at 85°C. Samples were then subjected to 10% SDS-PAGE gel, and proteins were transferred onto PVDF membranes. Membranes were blocked with 5% nonfat dry milk and probed with mouse anti-TH (1:1000, Chemicon, Temecula, CA) overnight at 4°C with gentle rocking. Membranes were washed with TBS and incubated with HRP-conjugated goat anti-mouse (1:1000; Santa Cruz Biotech, Santa Cruz, CA) for 1 h at room temperature. After a wash with TBS, proteins were detected with the use of the SuperSignal West Pico Chemilumescent Substrate (Pierce, Rockford, IL) and apposed onto Hyperfilm ECL film (Amersham Biosciences Corporation, Piscataway, NJ). For internal standards, membranes were stripped and reprobed with mouse anti-β-actin (1:10,000; Sigma). Densitometry was performed using a computerized image analysis system with NIH software. The relative density of each band was normalized against that of β-actin.
Intrastriatal Microinjections
Mice were anesthetized with inhaled isoflurane (2.5% for induction, 2% for maintenance) and their heads were placed in a stererotaxic frame (Model 5000; David Kopf Instruments, Tujunga, CA). A hole was drilled on the skull and a 25 gauge 2 uL Hamilton microinjection syringe was lowered into the striatum. Distance of injection sites from bregma was determined using a mouse brain atlas25: anteroposterior +0.5 mm; mediolateral ±2.0 mm; dorsventral −2.5 mm. The microinjection needle was left in position for 5 min prior to drug injection of 1.0 μL PBS (pH 7.4), 1.0 μL (4 ng/μL) of SAP (Advance Targeting Systems), or 1.0 μL (4 ng/μL) SSP-SAP (Advance Targeting Systems). Drugs were injected over a 10-min (~0.1μL/min) period and the needle was left in place for an additional 5 min before removal from the striatum.
TUNEL Histochemistry
The method is as previously described.26 In brief, fresh frozen 20 μm coronal sections were taken between bregma 0.38 mm ± 0.1 mm and fixed in 4% paraformaldehyd (PFA) for 30 min. After PBS wash, sections were immersed in 0.4% Triton-X-100 in PBS for 5–10 min at 70°C. Sections were washed and TUNEL (Terminal Deoxyncleotidyl Transferase-Mediated dUTP Nick End Labeling) reactions (Roche Applied Science, Indianapolis, IN) were applied directly onto sections and incubated for 1 h in a humidified chamber. After TUNEL staining, sections were counterstained with DAPI. Stained sections were washed in PBS and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). Images were taken using Molecular Dynamics CLSM Multiprobe 2001 scanning confocal system.
Double-Labeling: Immunocytochemistry of NK-1R and TH
Dual staining of the NK-1R and TH was processed on striatal 20 m coronal sections from 3 weeks postintrastriatal surgery. Nonspecific-binding sites are blocked with a combination of 5% normal goat serum (Vector Laboratories) and 5% normal donkey serum (Chemicon) in 0.2% Triton X-100 in PBS for 1 h. Sections were then incubated with a mixture of rabbit anti-NK-1R (1:1000; Chemicon) with 2% normal goat serum and mouse anti-TH (1:200; Chemicon) with 2% normal donkey serum in 0.2% Triton-X-100 in PBS overnight at 4°C. After washing with PBS, sections are incubated with a mixture of fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit (1:1000; Chemicon) with 2% normal goat serum and Cy3-conjugated goat anti-mouse (1:1000; Chemicon) with 2% normal donkey serum in 0.2% Triton-X-100 in PBS for 1 h. After washing with PBS, sections are mounted on glass slides and overlaid with a coverslip using Vectashield (Vector Laboratories). Sections were viewed with the Nikon Eclipse E400 epifluorescent microscope (Melville, NY) and photographed with a Hamamatsu digital camera C4742-95 (Bridgewater, NJ).
Double-Labeling: Immunocytochemistry of NK-1R and TUNEL Staining
Dual staining of the NK-1R and TUNEL staining were processed on striatal 20 m coronal sections from 3 weeks postintrastriatal surgery. Sections were rinsed in PBS and then immersed in heated 0.4% Triton-X-100 in PBS for 5 min and immediately washed in PBS. Staining of NK-1R and TUNEL labeling were then processed as described above. Sections were viewed with the Nikon Eclipse E400 epifluorescent microscope and photographed with a Hamamatsu digital camera C4742-95.
Cell Counts and Quantification
Coronal sections were taken from bregma 0.38 ± 0.1 mm. TUNEL-positive cells were quantified from 20-μm-thick coronal sections in an area of 0.26 mm2 for each region of interest in the caudate-putamen (CPu) (dorsal-medial [DM], dorsal-lateral [DL], ventral-medial [VM], and ventral-lateral [VL]).26
Statistical Analysis
Analysis was performed from mean ± SEM. Differences between groups were analyzed by ANOVA followed by post hoc comparison using Fisher's protected least significance test. The significance criterion is set at P < 0.05.
RESULTS
Pharmacological Blockade of the NK-1R Protects from METH
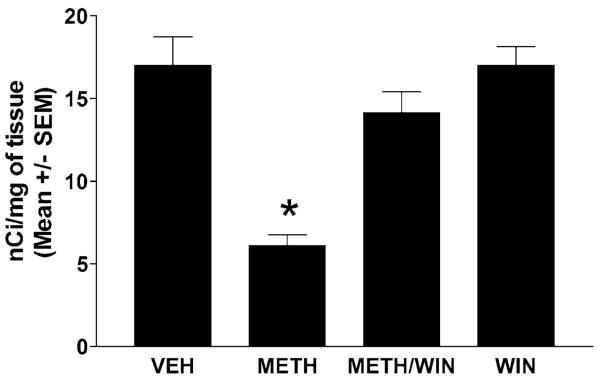
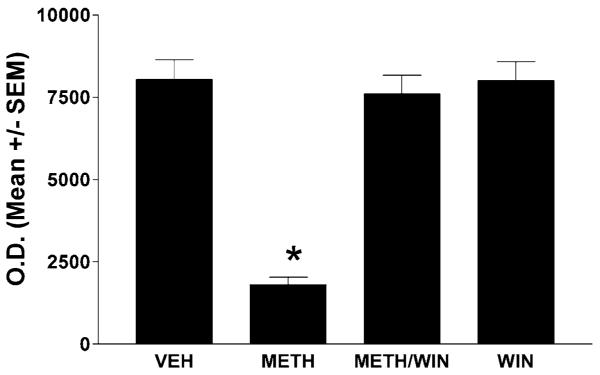
A bolus dose of METH (30 mg/kg, i.p.) induces significant loss of dopamine terminal markers: dopamine transporters (Fig. 1, receptor autoradiography) and TH (Fig. 2, Western blots) 3 days after the injection. To assess the role of the NK-1Rs in these depletions, we treated the mice 30 min before the METH bolus with a systemic injection of the nonpeptide NK-1R antagonist WIN-51,708 (5 mg/kg, i.p.). Pretreatment with WIN-51,708 dramatically attenuated METH-induced depletions of both striatal dopamine transporters and TH (Figs. 1 and 2, respectively) and also inhibited the induction of glial fibrillary acidic protein by METH in the striatal astrocytes (data not shown). These results demonstrate that blocking the NK-1R attenuates the METH-induced toxicity of the dopamine terminals of the striatum.
FIGURE 1.
The NK-1R antagonist WIN-51,708 (WIN) prevents METH-induced reduction of striatal dopamine transporter sites at day 3 post- METH (autoradiographic study). The mice (n = 6) received one injection of METH (30 mg/kg, i.p.). A separate group received WIN (5 mg/kg, i.p.) 30 min prior to METH. Binding of [125I]RTI-121 to striatal dopamine transporter sites was quantitated by image analysis of X ray autoradiograms. VEH, vehicle; SEM, standard error of the mean values. *P < 0.05 (ANOVA).
FIGURE 2.
Pharmacological blockade of the NK-1Rs with WIN-51,708 (WIN, 5 mg/kg) prevents METH-induced reduction of TH in the striatum at day 3 post-METH (Western blot analysis). The mice were treated as described in the legend to Figure 1. SEM, standard error of the mean values. *P < 0.05 (ANOVA).
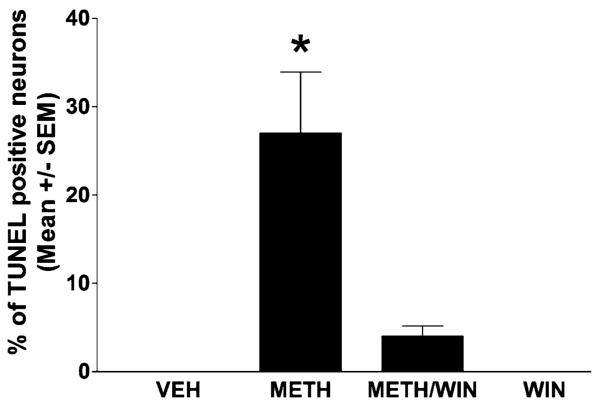
We have assessed METH-induced striatal apoptosis by the TUNEL assay. Unlike toxicity of the dopamine terminals that reaches a peak at day 3 post-METH, striatal apoptosis reaches a peak at 24 h after a bolus injection of METH (30 mg/kg).26 METH induces TUNEL staining in approximately 25% of the striatal neurons (Fig. 3). Treatment with WIN-51,708 (5 mg/kg) 30 min before METH attenuated this striatal apoptosis (Fig. 3). We have found that 5 mg/kg of WIN-51,708 is the most effective dose to attenuate both METH-induced apoptosis and dopamine terminal toxicity (data not shown). To estimate the number of striatal neurons undergoing apoptosis, we combined TUNEL and NeuN (a neuronal specific marker) immunofluorescence. An overlay of the two chromophores demonstrates that nearly all TUNEL-positive cells are also positive for NeuN, indicating that METH induces apoptosis principally in the striatal neurons (Neuroscience, submitted). These results indicate that in addition to preventing dopamine terminal loss, blocking NK-1Rs significantly attenuates the apoptosis of some striatal neurons.
FIGURE 3.
Pretreatment with WIN-51,708 (5 mg/kg, i.p.) attenuates METH-induced apoptosis in the striatum. METH was injected i.p. at a dose of 30 mg/kg. Apoptosis was assessed at 24 h post-METH by TUNEL. SEM, standard error of the mean values. *P < 0.05 compared to METH.
Because the striatum is composed of various kinds of neurons, to determine the phenotype of the neurons undergoing apoptosis, we combined TUNEL with immunofluorescence for DARPP-32 (dopamine- and cAMP-regulated phosphoprotein, of apparent Mr 32000) (projection neurons), choline acetyltransferase (cholinergic interneurons), somatostatin (somatostatin/ neuropeptide Y (NPY)/nitric oxide synthase (NOS) interneurons), and parvalbumin (γ-aminobutyric acid [GABA]-parvalbumin interneurons). We found that approximately 20% of the projection neurons are ablated by METH. Of the interneurons, 30% of the cholinergic and 50% of the GABA-parvalbumin is lost. Interestingly, the somatostatin/NPY/NOS interneurons are refractory to METH (Table 1).
TABLE 1.
Phenotype of striatal neurons killed by METHa
| Type of Neuron | % of total | Marker | % ablated |
|---|---|---|---|
| Striatonigral/striatopallidal | 90a | DARPP-32 | 20 |
| Cholinergic | <2b | ChAT | 30 |
| GABA-parvalbumin | 3-5c | Parvalbumin | 50 |
| Somatostatin/NOS/NPY | 1-2d | Somatostatin | 0 |
The mice received one injection of METH (30 mg/kg, i.p.) and were sacrificed 24 h after the injection. Serial sections of striatal tissue from controls and METH-treated animals were stained with antibodies against choline acetyltransferase, somatostatin, parvalbumin, and DARPP-32. The number of cells positive for any of these markers was counted in both groups. The amount of cell death is expressed as percent of the total number of neurons in a given population.
Note: DARPP-32, dopamine- and cAMP-regulated phosphoprotein, of apparent Mr 32000; ChAT, choline acetyltransferase
Wilson and Kawaguchi38
Bolam et al. 39
Kita et al. 40
Kawaguchi et al.34
Ablation of Striatal NK-1R-Expressing Interneurons with SSP-SAP
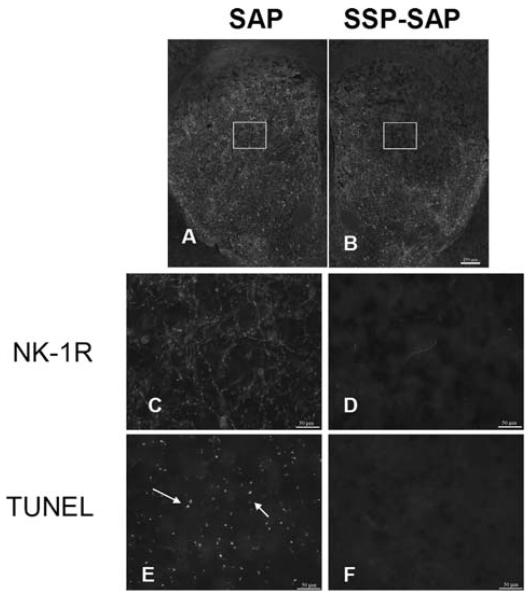
We asked if removal of the cells that express the NK-1Rs would have any effect on METH-induced striatal neuron apoptosis. For this study, a neurotoxin, SSP-SAP, which selectively eliminates NK-1R-bearing neurons was used. We first ensured that this neurotoxin was effective and selective by immunohistochemical methods. Figure 4 shows that there is a loss of NK-1R immunoreactivity in the side of the striatum that received the toxin, but no loss of NK-1R staining in the contralateral side, injected with SAP alone. To emphasize that all NK-1R-expressing interneurons are ablated in the core of the lesion, we showed that immunostaining for selective markers of these neurons, choline acetyltransferase and somatostatin, is absent on the side receiving the injection of SSP-SAP. In sharp contrast, after a similar SSP-SAP injection, the majority of the projection neurons (stained with DARPP-32 and representing approximately 90% of all striatal neurons) and GABA interneurons (GABA-parvalbumin) are spared (Neuroscience, submitted).
FIGURE 4.
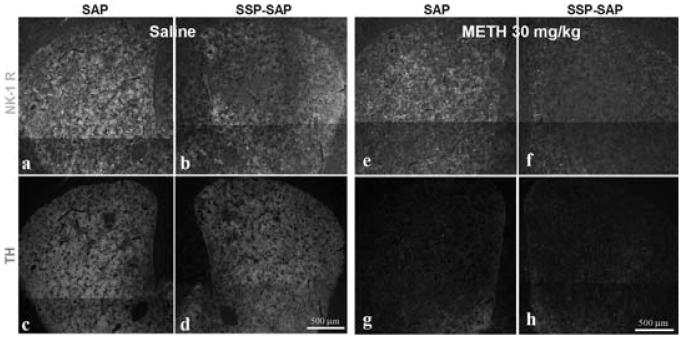
Intrastriatal injection of SSP-SAP (B) depletes immunostaining for the NK-1Rs (D) rendering the striatal tissue resistant to METH-induced apoptosis (TUNEL, F). The contralateral side that received SAP (A), retains NK-1R immunoreactivity (C) and remains vulnerable to METH (E). The mice (n = 6) received one intrastriatal injection of SSP-SAP or just SAP in the contralateral striatum. The animals received METH (30 mg/kg, i.p.) 3 weeks after the intrastriatal injection of SSP-SAP and were sacrificed 24 h after METH. NK-1Rs were visualized by immunofluorescence with an antibody conjugated to Cy3.
Next we administered a high dose of METH (30 mg/kg) to mice 3 weeks after they had received SSP-SAP. After 24 h they were euthanized and alternate serial sections were processed for TUNEL and for NK-1R immunostaining (on the same section). Figure 4 indicates that SSP-SAP had effectively eliminated NK-1R immunoreactivity. Importantly, in the absence of NK-1R-positive immunostaining, there was no detectable METH-induced apoptosis. In contrast, on the contralateral control side injected with SAP, there was little loss of NK-1R immunostaining but there was considerable apoptosis (Fig. 4).
In the light of these results, we hypothesized that ablation of the NK-1R-expressing interneurons would protect the dopamine terminals from METH. To test this hypothesis, we administered METH (30 mg/kg) to mice 3 weeks after the intrastriatal injection of SSP-SAP. After 3 days, the mice were sacrificed and serial sections through the striatum were processed for NK-1R and TH immunostaining. The SSP-SAP injection eliminated NK-1R immunostaining without affecting immunostaining for TH (Fig. 5). However, in sharp contrast to the apoptosis, removal of the NK-1R-expressing interneurons failed to preserve immunostaining for TH in the group receiving METH (Fig. 5).
FIGURE 5.
Ablation of striatal NK-1R-expressing interneurons does not prevent METH-induced depletion of TH. The mice (n = 6) received an intrastriatal injection of SSP-SAP into one side and SAP into the contralateral side. Then, 3 weeks later, the mice received a single injection of METH (30 mg/kg, i.p.) and were sacrificed 3 days after METH. Striatal sections were processed for NK-1R and TH immunofluorescence in the same section. Note that SSP-SAP abolishes NK-1R staining without affecting staining for TH (b and d). NK-1Rs were visualized with an antibody conjugated to FITC (a, b, e & f) and TH with Cy3 (c, d, g & h).
DISCUSSION
The data demonstrate that substance P signaling through the striatal NK-1R triggers the activation of a neurodegenerative cascade in the presence of METH. The apoptosis of some striatal neurons induced by METH requires the NK-1R-expressing interneurons because ablation of these neurons with SSP-SAP prevents the apoptosis. However, the toxicity of the dopamine terminals induced by METH can be attenuated by pretreatment with NK-1R antagonists,23 but interestingly, does not require the presence of the NK-1R-bearing interneurons. This observation suggests that substance P may affect the METH-induced glutamate overflow in the striatum.
In the striatum, various lines of evidence point toward a connection between substance P and glutamate transmission. First, our results show that substance P signals METH-induced damage of both the striatal dopamine terminals and some striatal neurons. Second, pharmacological studies demonstrate that blockade of the NMDA subtype of glutamate receptor protects the striatum from METH.13,27 Third, the activity of the striatal-nigral-cortical loop regulates METH-induced glutamate release in the striatum and dopamine toxicity.28 Finally, preprotachykinin-A knockout mice (lacking substance P) show resistance to kainic-acid-induced cell death in the hippocampus.29 We hypothesize that pharmacological blockade of the NK-1R will attenuate NMDA-induced cell death and dopamine terminal toxicity in the striatum because an NK-1R antagonist protects both pre- and postsynaptic sites from METH-induced damage. Substance P may have a dual role in signaling degeneration in the striatum in the presence of METH. For example, on the one hand, substance P may activate nitric oxide formation via an intrastriatal mechanism while, on the other hand, substance P may activate striatal glutamate release via an extrastriatal mechanism through the substantia nigra.
Identifying the phenotype of the striatal neurons killed by METH is very important because subpopulations of striatal neurons affect differently the output of the basal ganglia. Our data suggest that approximately half of the GABA-parvalbumin interneurons of the dorsal striatum are killed by METH. These interneurons have a high content of GABA30 and receive glutamatergic excitation from the cortex.31 Thus, in the aftermath of cortical transmission these interneurons exert inhibition on striatal neurons via GABA, and it is noteworthy that METH ablates nearly half of this population of interneuron in the dorsal striatum. We demonstrate here that another population of striatal interneuron impacted by METH is the cholinergic interneuron. These interneurons affect the output of the projection neurons. For example, cholinergic transmission augments striatal dopamine release32 and increases the responses of the medium spiny projection neurons to glutamate.33 Because the GABA-parvalbumin interneurons mediate inhibition and the cholinergic interneurons facilitate excitation, and because both populations show deficits in the aftermath of METH, it would appear that the two effects cancel each other out. However, the post-METH striatum has the interneuron balance skewed in favor of transmission by the somatostatin/NPY/NOS interneurons because the latter population is refractory to METH. The nitric oxide made by these interneurons is known to increase the release of glutamate and dopamine in the striatum.34
In addition to the loss of some interneurons, METH also induces the loss of some projection neurons. The substantia nigra and the globus pallidum are targets of striatal projection neurons that exert inhibition via GABAA receptors.35 Moreover, the recurrent collaterals within the striatum of the GABAergic medium spiny neurons provide the substrate for the mutual inhibition between these neurons.36,37 Thus, the ablation of some projection neurons in the aftermath of METH could be interpreted to result in attenuation of inhibition within the striatum and in the targets of the projection neurons. More work is needed to describe the functional state of striatal neurons in the aftermath of METH.
In conclusion, our results demonstrate a role for the striatal neuropeptide substance P and its receptor in the neurodegenerative cascade induced by METH affecting the striatal dopamine terminals and some striatal neurons. More work in needed to describe the mechanism by which substance P signals METH-induced striatal injury, the functional state of the striatum in the aftermath of METH, and recovery from METH.
ACKNOWLEDGMENTS
This work was supported by a “Specialized Neuroscience Research Program” grant NS41073 from the National Institute for Neurological Disorders and Stroke. Support for the research infrastructure at Hunter College has come from a “Research Centers in Minority Institutions” award to Hunter College.
REFERENCES
- 1.Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. Eur. J. Pharmacol. 1976;36:363–371. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- 2.Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J. Pharmacol. Exp. Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- 4.Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- 5.Axt KJ, Molliver ME. Immunocytochemical evidence for methamphetamine-induced serotonergic axon loss in the rat brain. Synapse. 1991;9:302–313. doi: 10.1002/syn.890090405. [DOI] [PubMed] [Google Scholar]
- 6.Ellison G, Eison MS, Huberman HS, et al. Long-term changes in dopaminergic innervation of caudate nucleus after continuous amphetamine administration. Science. 1978;201:276–278. doi: 10.1126/science.26975. [DOI] [PubMed] [Google Scholar]
- 7.Ricaurte GA, Guillery RW, Seiden LS, et al. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 8.Fleckenstein AE, Metzger RR, Wilkins DG, et al. Rapid and reversible effects of methamphetamine on dopamine transporters. J. Pharmacol. Exp. Ther. 1997;282:834–838. [PubMed] [Google Scholar]
- 9.Baucum AJ, II, Rau KS, Riddle EL, et al. Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J. Neurosci. 2004;24:3436–3443. doi: 10.1523/JNEUROSCI.0387-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pu C, Broening HW, Vorhees CV. Effect of methamphetamine on glutamate-positive neurons in the adult and developing rat somatosensory cortex. Synapse. 1996;23:328–334. doi: 10.1002/(SICI)1098-2396(199608)23:4<328::AID-SYN11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Eisch AJ, Marshall JF. Methamphetamine neurotoxicity: dissociation of striatal dopamine terminal damage from parietal cortical cell body injury. Synapse. 1998;30:433–445. doi: 10.1002/(SICI)1098-2396(199812)30:4<433::AID-SYN10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Deng X, Wang Y, Chou J, et al. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res. Mol. Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- 13.Sonsalla PK, Riordan DE, Heikkila RE. Competitive and non-competitive antagonists at N-methyl-D-aspartate receptors protect against methamphetamine-induced dopaminergic damage in mice. J. Pharmacol. Exp. Ther. 1991;256:506–512. [PubMed] [Google Scholar]
- 14.O'Dell SJ, Weihmuller FB, Marshall JF. Methamphetamine-induced dopamine overflow and injury to striatal dopamine terminals: attenuation by dopamine D1 or D2 antagonists. J. Neurochem. 1993;60:1792–1799. doi: 10.1111/j.1471-4159.1993.tb13405.x. [DOI] [PubMed] [Google Scholar]
- 15.Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- 16.Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- 17.Fridovich I. Superoxide radical: an endogenous toxicant. Annu. Rev. Pharmacol. Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 18.Fridovich I. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 1986;247:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- 19.Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem. Int. 1998;32:117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- 20.Itzhak Y, Ali SF. The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J. Neurochem. 1996;67:1770–1773. doi: 10.1046/j.1471-4159.1996.67041770.x. [DOI] [PubMed] [Google Scholar]
- 21.Imam SZ, Newport GD, Itzhak Y, et al. Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper-zinc superoxide dismutase. J. Neurochem. 2001;76:745–749. doi: 10.1046/j.1471-4159.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- 22.Li JL, Kaneko T, Mizuno N. Colocalization of neuronal nitric oxide synthase and neurokinin-1 receptor in striatal interneurons in the rat. Neurosci. Lett. 2001;310:109–112. doi: 10.1016/s0304-3940(01)02097-3. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Cadet JL, Angulo JA. Neurokinin-1 (NK–1) receptor antagonists abrogate methamphetamine-induced striatal dopaminergic neurotoxicity in the murine brain. J. Neurochem. 2002;83:613–622. doi: 10.1046/j.1471-4159.2002.01155.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Wang J, Cadet JL, et al. Histological evidence supporting a role for the striatal neurokinin-1 receptor in methamphetamine-induced neurotoxicity in the mouse brain. Brain Res. 2004;1007:124–131. doi: 10.1016/j.brainres.2004.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- 26.Zhu JPQ, Xu W, Angulo JA. Disparity in the temporal appearance of methamphetamine-induced apoptosis and depletion of dopamine terminal markers in the striatum of mice. Brain Res. 2005;1049:171–181. doi: 10.1016/j.brainres.2005.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.PU C, Vorhees CV. Protective effects of MK-801 on methamphetamine-induced depletion of dopaminergic and serotonergic terminals and striatal astrocytic response: an immunohistochemical study. Synapse. 1995;19:97–104. doi: 10.1002/syn.890190205. [DOI] [PubMed] [Google Scholar]
- 28.Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J. Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Cao Y, Basbaum AI, et al. Resistance to excitotoxin-induced seizures and neuronal death in mice lacking the preprotachykinin A gene. Proc. Natl. Acad. Sci. USA. 1999;96:12096–12101. doi: 10.1073/pnas.96.21.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolam JP, Clarke DJ, Smith AD, et al. A type of aspiny neuron in the rat neostriatum accumulates [3H]gamma-aminobutyric acid: combination of Golgi-staining, autoradiography, and electron microscopy. J. Comp. Neurol. 1983;213:121–134. doi: 10.1002/cne.902130202. [DOI] [PubMed] [Google Scholar]
- 31.Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- 32.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 33.Calabresi P, Centonze D, Gubellini P, et al. Endogenous ACh enhances striatal NMDA-responses via M1-like muscarinic receptors and PKC activation. Eur. J. Neurosci. 1998;10:2887–2895. doi: 10.1111/j.1460-9568.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi Y, Wilson CJ, Augood SJ, et al. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 35.Precht W, Yoshida M. Blockage of caudate-evoked inhibition of neurons in the substantia nigra by picrotoxin. Brain Res. 1971;32:229–233. doi: 10.1016/0006-8993(71)90171-5. [DOI] [PubMed] [Google Scholar]
- 36.Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J. Comp. Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- 37.Yung KK, Smith AD, Levey AI, et al. Synaptic connections between spiny neurons of the direct and indirect pathways in the neostriatum of the rat: evidence from dopamine receptor and neuropeptide immunostaining. Eur. J. Neurosci. 1996;8:861–869. doi: 10.1111/j.1460-9568.1996.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- 40.Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res. 1990;536:1–15. doi: 10.1016/0006-8993(90)90002-s. [DOI] [PubMed] [Google Scholar]