Abstract
Trade-offs have long been a major theme in life-history theory, but they have been hard to document. We introduce a new method that reveals patterns of divergent trade-offs after adjusting for the pervasive variation in rate of resource allocation to offspring as a function of body size and lifestyle. Results suggest that preweaning vulnerability to predation has been the major factor determining how female placental mammals allocate production between a few large and many small offspring within a litter and between a few large litters and many small ones within a reproductive season. Artiodactyls, perissodactyls, cetaceans, and pinnipeds, which give birth in the open on land or in the sea, produce a few large offspring, at infrequent intervals, because this increases their chances of escaping predation. Insectivores, fissiped carnivores, lagomorphs, and rodents, whose offspring are protected in burrows or nests, produce large litters of small newborns. Primates, bats, sloths, and anteaters, which carry their young from birth until weaning, produce litters of one or a few offspring because of the need to transport and care for them.
Keywords: life-history theory, trade-off, litter size, offspring size, litter frequency, litter mass
Introduction
A synthetic conceptual framework that can account for the wide variation in mammal life histories has remained elusive, despite decades of vigorous theoretical investigation (e.g., Charnov 1991, 2001; Kozlowski and Weiner 1997; Oli 2004; Dobson 2007), meticulous collection and analysis of data (e.g., Gaillard et al. 1989; Promislow and Harvey 1990; Purvis and Harvey 1995; Jones and MacLarnon 2001; Charnov and Ernest 2006; Bielby et al. 2007), and a rich literature documenting how females allocate resources to reproduction (Charnov et al. 2007). It has long been recognized that the mass-specific rate of biomass production scales allometrically with adult female body mass, M, as approximately M−1/4 to M−1/3. This is similar to the scaling of mass-specific metabolic rate, which fuels the growth and development of offspring through gestation and lactation (Brown et al. 2004). Recently, we have shown that productivity differs between taxonomic and lifestyle groups of mammals in predictable ways (Sibly and Brown 2007). A lifestyle is a way of making a living that is made possible by a unique combination of anatomical, physiological, and behavioral traits. Productivity increases when adaptations exploit abundant, reliable food supplies, and it decreases when adaptations reduce predation. The evolution of these combinations appears to be relatively conservative, so lifestyles are typically deeply rooted in clades and widely shared within taxonomic groups. Evidence of their adaptive significance comes from their independent and convergent evolution in distantly related lineages. These lifestyle adaptations represent a second major axis of life-history variation, orthogonal to the pervasive effect of body mass (Brown and Sibly 2006; Dobson 2007; Sibly and Brown 2007). Here we consider how much, how often, and why production is allocated to individual offspring and evidenced in the fundamental life-history trade-offs.
Traditionally, both theoretical and empirical analyses of life histories have focused on hypothesized trade-offs: for example, between survival and reproduction, between “fast” and “slow” life histories, between juvenile and adult survival, and between the numbers and sizes of offspring. Many attempts to analyze these trade-offs have not explicitly considered the fundamental allometries of production and survival. For example, there is necessarily a negative correlation between production and survival: smaller animals with higher birth rates must have correspondingly higher death rates. Similarly, for animals of the same size, adaptations that increase production necessarily result in increased death rates (reduced survival) as a result of “ecological compensation” (Sibly and Calow 1986, 1987; Sutherland et al. 1986).
Several recent analyses of life histories have explicitly considered allometric correlates of body size (e.g., Gaillard et al. 1989; Charnov 1993; Bielby et al. 2007; Dobson 2007; Sibly and Brown 2007). These have called attention to other hypothesized trade-offs such as that between number and size of offspring or between juvenile and adult survival, which are not direct consequences of the allometry of production but instead depend on how production is allocated among different components of the life history. Such trade-offs should be evidenced as negative relationships in the residual variation that remains after accounting for the allometry of production within and between taxonomic and lifestyle groups. They can be empirically evaluated most powerfully and realistically by manipulating the relevant variables, such as in field experiments that manipulate clutch size and nest predation in birds (e.g., Fontaine and Martin 2006) or allocation to egg yolk in reptiles (e.g., Sinervo and Huey 1990). Similar experiments with eutherian mammals are more difficult because females retain developing embryos within the body during gestation and nourish them during lactation. Nevertheless, these unique features of mammalian life history offer opportunities to develop and test a more general and comprehensive theoretical framework.
Here we consider two potential trade-offs in how production is allocated to reproduction: (1) between the number of offspring in a litter and the size of the offspring and (2) between the number of litters and the biomass of each litter produced over a reproductive season. Our approach differs from that of most recent analyses in that it is explicitly mechanistic. We focus on variation among species, taxa, and lifestyle groups in the rate of mass-specific production and how this energy is allocated within and among litters.
Theoretical Framework and Predictions
Conservation of mass and energy constrains how resources are divided among multiple functions, so allocating more to one function means that less is available for allocation elsewhere. This “principle of allocation” has long been a fundamental assumption of life-history theory (Cody 1966). We use the principle twice here. First, productivity, measured as reproductive biomass produced per year, is assumed to be the product of litter mass and litter frequency, as in equation (3a). Second, litter mass is the product of offspring size and number, as in equation (3b).
Resources are assumed to be allocated so as to maximize the Darwinian fitness of the life history, which we define as the per-copy rate of increase of a gene for a specified set of life-history traits (Charlesworth 1980; Sibly and Curnow 1993). Darwinian fitness is given by an analog of the Euler-Lotka equation. The simplest life history that embraces the complexity we need has two stages—juvenile and adult—and for each we require measures of survivorship and duration. We distinguish the stages by subscripts (j for juvenile and a for adult) and let S and t denote survivorship and durations, respectively. Thus Sj; and Sa represent juvenile and adult survivorship, tj is the age at first breeding, and ta is the interval between breeding attempts, each of which results in n offspring. Then the Euler-Lotka equation defining fitness, F, is
| (1) |
(Sibly and Calow 1986). The central aim of life-history theory is to find the life-history parameters n, tj, ta, Sj, and Sa that maximize F subject to constraints imposed by the principle of allocation, that is, equations (3a) and (3b). Our immediate objective here is to find optimal offspring mass, but this depends on its effects on life-history variables. The simplest possibility is that offspring size affects only n, being inversely proportional as a result of the principle of allocation. Alternatively, it may also affect juvenile survivorship and/or age at first reproduction, the first being more important here; this is illustrated in figure 1B. In figure 1A and 1C, offspring size has no effect on juvenile survivorship, and the optimal strategy is to produce offspring as many and as small as possible. In figure 1B and 1D, offspring size has a marked positive effect on juvenile survivorship, but there are diminishing returns, so the optimal strategy is to produce offspring of intermediate size. Thus, everything else being equal, natural selection favors higher birth rates and hence many small offspring (fig. 1A, 1C). Everything else is not always equal, however, and larger offspring can be adaptive if juvenile survivorship increases with offspring size (fig. 1B, 1D). Additionally, everything else being equal, natural selection favors producing many small litters rather than a few large ones so as to avoid the chance that the mother dies or the litter is discovered by a predator before it can be weaned. Again, however, circumstances of lifestyle and ecology, such as restrictive seasonal breeding opportunities, can override this tendency.
Figure 1.
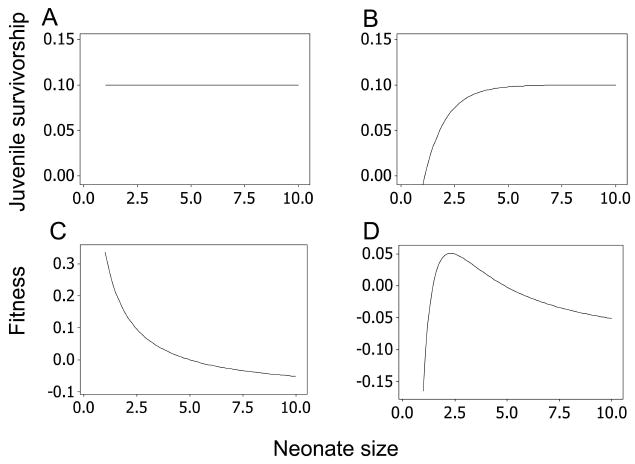
How neonate size may affect juvenile survivorship (A, B) and Darwinian fitness (C, D). Graphs plotted using equation (1) with parameter values n × neonate size = 10, tj, = ta = 1, Sa = 0.9.
In testing predictions we “corrected for” the variation in production with body mass and across different taxonomic and lifestyle groups by fitting parallel-line models, as in figure 2. Each line (color coded in fig. 2) corresponds to a different functional or taxonomic group. This procedure is justified theoretically and empirically for the data of figure 2A in Sibly and Brown (2007), which shows how variation in production rate orthogonal to the body size axis is due to lifestyle. Because both body size and lifestyle affect production, both may affect its components, so these also were analyzed using parallel-line models as detailed below. Parallel-line models are appropriate because our main interest is in comparing the heights of the lines (as quantified by the intercepts, i.e., normalization constants of the allometric equations). Following Sibly and Brown (2007) for a life-history trait w, we regressed log w on log M to obtain a regression equation of form
Figure 2.
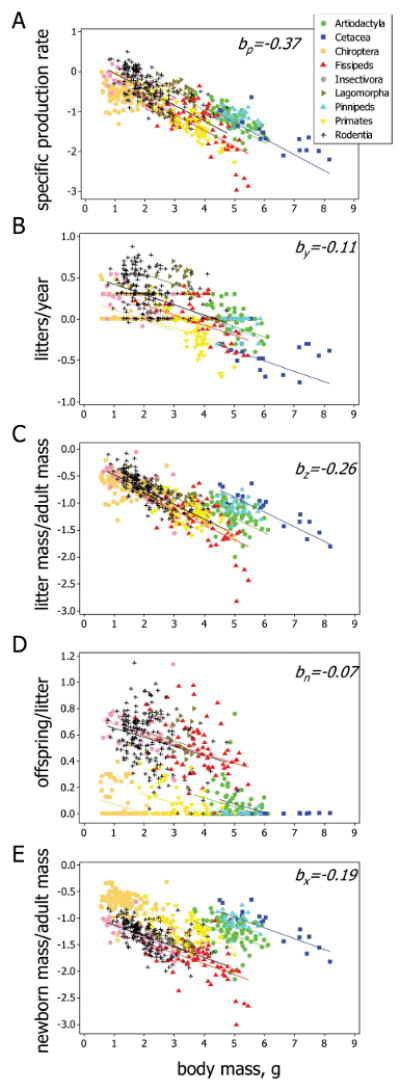
Variation in productivity (A) and the components of reproduction (B–E) as a function of body size. Productivity is measured as specific production rate, y−1 defined as the product of (litter mass)/(adult mass) and litter frequency (litters per year). (Litter mass)/(adult mass) is the product of offspring per litter and (newborn mass)/(adult mass). All scales are logarithmic to base 10. Symbols as in A throughout. The lines in each panel have the same slopes and are color coded according to taxon. The regression coefficients (slopes) are shown at the top right of each panel. The four outlying data points for fissipeds to the right of A, C, and E are bears of the family Ursidae. For clarity, only taxonomic/lifestyle groups with ≥10 species are shown.
| (2) |
where wi is a normalization constant (equivalent to a y-intercept) specific to the ith taxonomic or lifestyle group, bw is the regression coefficient of trait w and is assumed to remain constant across all groups, and M is adult female body mass. Let x denote (neonate mass)/(adult body mass), n be offspring per litter, z be (litter mass)/(adult body mass), y be the number of litters produced each year, and q be mass-specific production. Because (litter mass)/(adult body mass), z, is defined as the product x × n, and because mass-specific production, q, is defined as the product z × y, we have
| (3a) |
| (3b) |
and combining these equations with equations of the form of equation (2), we have, using obvious notation,
| (4a) |
| (4b) |
Equating coefficients, we have
| (5a) |
| (5b) |
and
| (6a) |
| (6b) |
Thus, our modeling approach is predicated on the assumption that each of the life-history traits should scale allometrically with body mass, as in equation (2). Our method of obtaining the normalization constants of specified taxonomic or lifestyle groups is shown in figure 3A. To analyze for trade-offs between pairs of traits that are due to the principle of allocation, the normalization constants for the two traits are plotted against each other, as shown in figure 3B. In the simple case illustrated in figure 3B, there is no variation between the three lifestyle groups in the quantity of resource, z, being allocated. In more complicated cases, it is necessary to allow for variation between lifestyle groups in their z normalization constants, and for this reason strategies with the same values of z are indicated by dashed brown lines in figures 4 and 5. Where desired, allowance for variation in z values can be achieved by moving points perpendicular to the z contours and assembling them on a common reference contour, as shown in figure 3C. The relative positions of the standardized points are the same irrespective of which z contour is chosen for standardization. This procedure allows analysis of trade-offs after standardization for the quantity of resource available for allocation.
Figure 3.
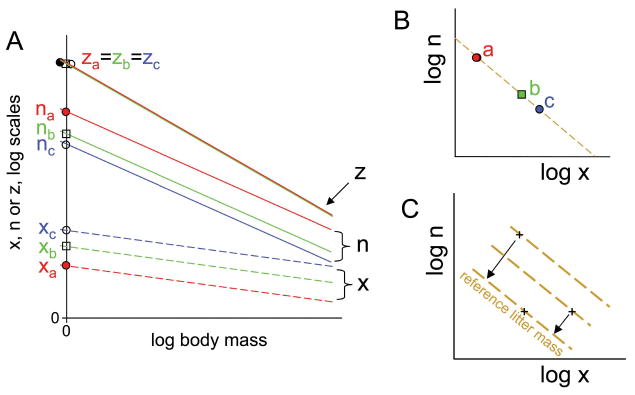
Schematic illustration of our analytical methods. A, First, the allometry of each trait is analyzed in a log-log plot (as in fig. 2). Here we show three hypothetical traits, x, n, and z, in relation to body mass, indicated by dashed, solid, and dotted lines, respectively, for each of three different lifestyle groups, a, b, and c, colored red, green, and blue. The variable z represents (litter mass)/(adult mass), n represents offspring per litter, and x represents (offspring mass)/(adult mass), so for each lifestyle and for each adult mass, z = n × x and log z = log n + log x (see “Methods”). At any body mass, a, b, and c all have the same value of trait z, but a has a higher value than b or c for trait n and a lower value for trait x. The key characteristic of each lifestyle group is the relative height of its trait lines, which are indexed by their y-intercepts, here called normalization constants. B, To analyze for trade-offs between traits, the normalization constants are plotted against each other, and a trade-off between traits x and n is revealed by the negative slope. In this example, all three lifestyle groups have the same normalization constants for trait z, so their normalization constants for traits n and x lie on a straight line, shown in brown, and the labeled points satisfy the equation log z = log n + log x. In this case, the amount of resource being allocated, z, does not differ between the lifestyle groups when allometry of body mass is accounted for. C, Generally the quantity of resource being allocated differs between lifestyle groups, so the points lie on different lines. We correct for this variation by projecting trait values onto a standard reference line, as shown here.
Figure 4.
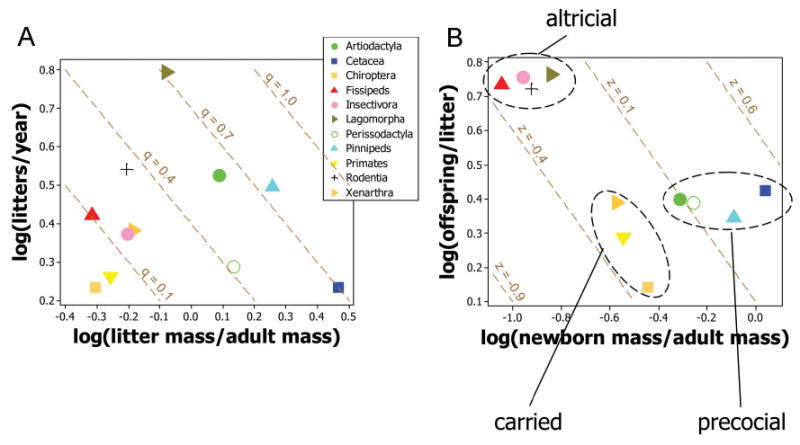
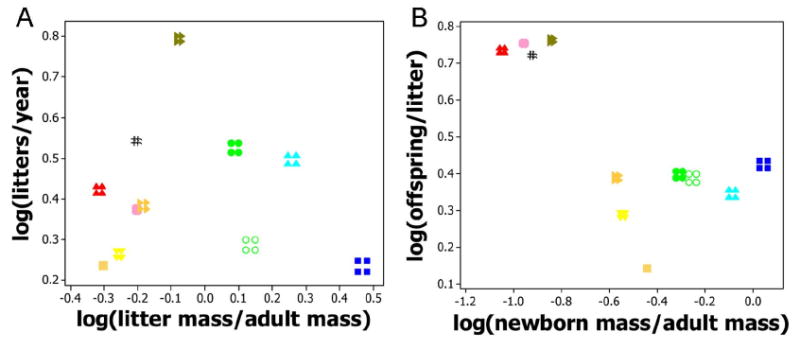
Scatterplots analyzing the two trade-offs between number of litters per year and litter mass (A) and between number of offspring per litter and offspring size (B) by plotting the normalization constants of the main mammal taxonomic/lifestyle groups. Numerical values of normalization constants are given, together with their standard errors (which are generally <0.05) in table A1. Ellipses enclose the classes of mammalian life histories referred to in the text. A, Litters per year as a function of (litter mass)/(adult mass). Dashed brown lines connect strategies having the same values of specific production rate (q) and satisfy equation (6a). B, Offspring per litter as a function of (newborn mass)/(adult mass). Dashed brown lines indicate strategies with the same values of (litter mass)/(adult mass) (z) and satisfy equation (6b) (see fig. 3 for a rationale). Logarithms are to base 10.
Figure 5.
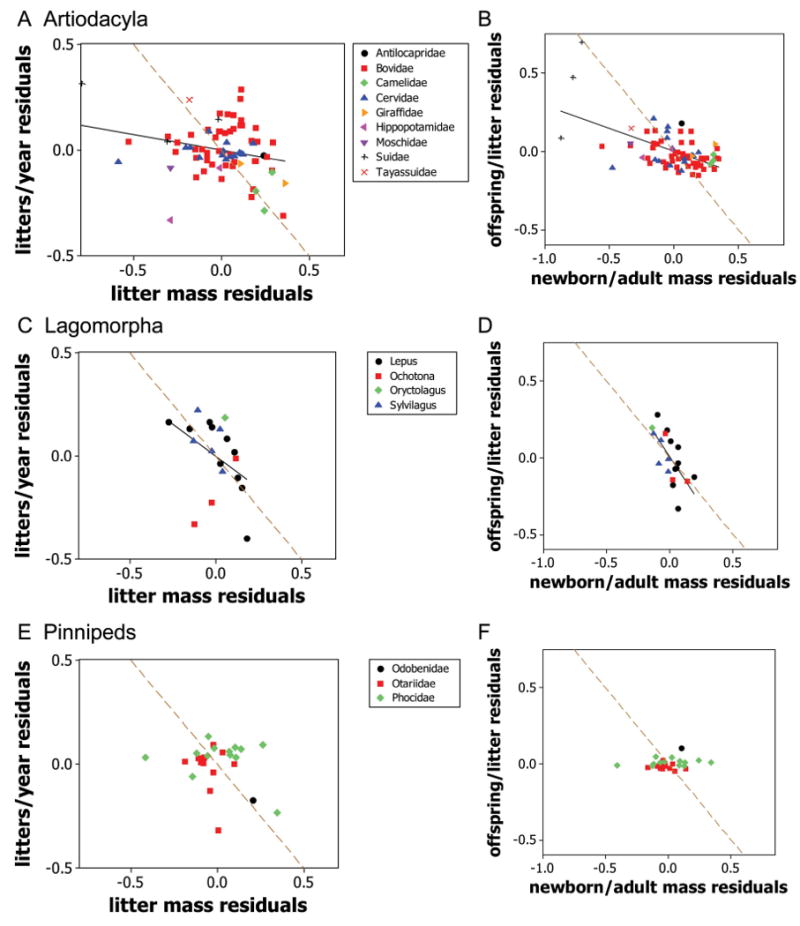
Scatter diagrams analyzing the two major trade-offs by plotting residuals within Artiodactyla (A, B), Lagomorpha (C, D), and Pinnipedia (E, F). A, C, E, Litters per year as a function of (litter mass)/(adult mass). B, D, F, Offspring per litter as a function of (newborn mass)/(adult mass). Residuals are calculated for each species from figure 2 as the vertical distance of the species from the lines of the same color in figure 2. Thus, residuals represent the difference between log10 life-history traits and the values expected from the species' body mass for members of the species' taxonomic/lifestyle group. Solid black lines are fitted regressions and are shown where correlations are significant (P < .05; table 1). Dashed brown lines connect strategies with the same resource allocation as in figure 4.
This conceptual framework allows us to predict theoretically and evaluate empirically how natural selection, responding primarily to the sensitivity of juvenile survivorship to neonate size, as in figure 1, has shaped the life histories of eutherian mammals. We now use this framework to make bold statements about the allocation strategies of different taxonomic and lifestyle groups and about the environmental conditions that have shaped the trade-offs. These statements represent plausible testable hypotheses that are consistent with current information on mammal life histories. Our hypotheses/predictions are:
A trade-off between number and size of offspring in a litter will be evidenced as a negative correlation among the normalization constants of the lifestyle groups. Groups that produce larger offspring should have smaller litters.
Artiodactyls, perissodactyls, cetaceans, and pinnipeds should give birth to a relatively small number of large, precocial offspring. Their offspring are born unprotected on the ground or in the sea. Offspring survival depends critically on offspring size, as in figure 1B, because large, well-developed offspring are better able to escape predators and require less time to mature. Additionally, thermoregulation is enhanced by the larger size and better insulation of the precocial condition.
Primates, bats, sloths, and anteaters should also have a few large offspring. These mammals mostly carry their young, which reduces risk of predation but limits the number because newborn offspring must be sufficiently developed to hold on and to thermoregulate outside the protective microclimate of a nest or burrow. Additionally, only a small number of offspring can be closely attended while the mother forages, interacts with conspecifics, and escapes from predators.
Insectivores, fissiped carnivores, lagomorphs, and rodents should produce large litters of relatively small altricial neonates. This should be true in particular for representatives of these groups that rear their dependent young in burrows or nests, so that survival from birth to weaning is not greatly affected by offspring size (see fig. 1A).
Putting together predictions 2–4, most mammals should separate into two classes: those producing either a few large, precocial offspring (artiodactyls, perissodactyls, cetaceans, pinnipeds, primates, bats, and xenarthrans) or many small, altricial offspring (insectivores, fissipeds, lagomorphs, and rodents).
The negative correlations predicted in hypothesis 1 should also be observed in the residuals for species within lifestyle groups after accounting for the effects of body size. So, for example, caviomorphs (guinea pigs and relatives) within the rodents, and hares within the lagomorphs, which give birth to precocial neonates, should produce litters of fewer, larger offspring. The sea otter, which differs from other fissiped carnivores in that it gives birth at sea, where risk of predation and costs of thermoregulation are high, should also produce litters of a few large, precocial neonates.
A trade-off between allocation per litter and number of litters per reproductive season should be evidenced as a negative correlation among the normalization constants of the various taxonomic/lifestyle groups. Groups that produce more litters per year should allocate less production to each litter.
Methods
We used recent compilations of mammalian life-history data for placental, nonvolant mammals (Ernest 2003) and for Chiroptera (K. E. Jones, unpublished data). These data sets record offspring per litter, litters per year, neonate and weaning masses, and adult body mass. Analyses were conducted for 628 species, representing 366 genera, 88 families, and 11 orders, for which data on offspring per litter, litters per year, neonate mass, and adult body size were available for at least five species per order. We did not consider monotremes or marsupials, which are long-divergent lineages with dramatically different reproductive biologies: egg laying and pouch rearing, respectively. The availability of data dictates that we use the mass of offspring at birth to assess the predicted trade-off between the size and number of offspring in a litter. We are aware that female mammals typically allocate much more production to lactation than to gestation, but neonate mass is a constant ratio of weanling mass within lifestyle groups and this ratio varies only from 0.10 to 0.30 among lifestyle groups (Sibly and Brown 2007). Data manipulation and statistical analyses were performed using Minitab 15.1, and parallel lines of the form of equation (2) were fitted to the data of figure 2 using general linear modeling.
Results
Mass-specific production rate and the other life-history variables for 628 species of eutherian mammals are plotted as a function of adult body mass on logarithmic axes in figure 2. Figure 2A shows specific production rate, our best estimate of annual resource investment in reproduction. Figure 2B and 2C shows how this is allocated among the litters that are produced each year to determine litter frequency (fig. 2B) and mass (fig. 2C). Figure 2D and 2E shows how litter mass is divided among offspring according to their number. Notice that the parallel-lines model generally fits the data well (fitting nonparallel-lines models increases the adjusted R2 value by only 2%, 3%, 1%, 0%, and 0% for fig. 2A–2E, respectively; tables A1, A3).
Values of the normalization constants and results of ANOVAs are given for the parallel-lines model in table A1, showing that the normalization constants differ markedly among the taxonomic/lifestyle groups for each trait (P ≪ .001). Normalization constants for the different groups based on taxonomy and lifestyle are plotted in figure 4, and residuals for species within these groups are plotted in figures 5 and A1.
These analyses can now be used to evaluate the predictions above.
1. A trade-off between the number and size of offspring in a litter should be evidenced as a negative correlation among the normalization constants for the taxonomic/lifestyle groups. Figure 4B shows that these traits are indeed negatively correlated (r9 = −0.73, P = .01). To control for lack of independence between closely related species, we repeated these analyses using genus and family means and found similar relationships (r9 = −0.72 and −0.79 for genus and family, respectively; P = .01; fig. A2).
2, 3. Two groups should have a relatively small number of large precocial offspring: (i) artiodactyls, perissodactyls, cetaceans, and pinnipeds, whose young are born unprotected in the open, and (ii) primates, bats, sloths, and anteaters, which carry their young from birth until weaning. These predictions are supported. After standardization for the rate of production using the method in figure 3, there were differences between the precocial, the carried, and the altricial groups (one-way ANOVA: F2,8 = 71.4, P < .001). The precocial and the carried groups of figure 4B are farther to the right along a common z contour than the altricial group (Dunnett's multiple comparison tests: P < .001). Using genus and family means gave similar results (P < .001; data in fig. A2), and the results are robust to errors in the allometric regression coefficients (data in fig. A3).
4. Insectivores, fissiped carnivores, lagomorphs, and rodents, whose offspring are protected in burrows or nests, should have many small, altricial offspring. These groups do indeed produce large litters of small offspring, as shown in figure 4B (statistics as in evaluation of predictions [2] and [3]). Outliers tend to be species such as caviomorph rodents and hares, which give birth to well-developed young in exposed environments (see [6], below).
5. Putting together predictions (2)–(4), most mammals should separate into two classes, with litters containing either a few large, precocial offspring (artiodactyls, perissodactyls, cetaceans, pinnipeds, primates, bats, and xenarthra) or many small, altricial offspring (fissipeds, insectivores, lagomorphs, and rodents). This is indeed the observed pattern, as shown in figure 4B.
6. The negative correlations predicted in (1) should also be observed among species residuals within lifestyle groups after the effects of body size have been accounted for. Scatterplots of residuals are shown in figures 5, A1, and correlation coefficients are given in table 1. If the predictions were perfectly supported, then the data would lie along the dashed brown lines in figures 5, A1. Prediction (1) suggests that, after accounting for variation due to body size, species in the same taxonomic/lifestyle group that produce more offspring per litter might be expected to produce offspring of smaller body size. This prediction is supported in most groups (plots in right-hand columns of figs. 5, A1; table 1) and is observed most clearly in the lagomorphs (fig. 5D). Note that, in groups in which there is usually only one offspring per litter, only limited variation is possible. This accounts for the unusual distributions observed in the plots for cetaceans, pinnipeds, and, to a lesser extent, artiodactyls, bats, and primates (figs. 5, A1). Caviomorph rodents and sea otters (Enhydra lutris) produce litters of relatively few, large, precocial neonates, as predicted (fig. A1), but there is only limited support from hares (genus Lepus; fig. 5D).
7. A trade-off between allocation per litter and number of litters per reproductive season will be evidenced as a negative correlation among the normalization constants of the various taxonomic/lifestyle groups. This prediction is not supported overall (r9 = −0.05, not significant; fig. 4A). Any evidence for the trade-off is obscured by the variation in productivity, p, among the lifestyle groups, which results in variation perpendicular to the q contours.
Table 1.
Correlation coefficients r and associated P values for the correlations between the residuals of allocation per litter (z) and number of litters per reproductive season (y) and of size of offspring (x) and their number (n)
| Order | No. species | rzy | P | rxn | P |
|---|---|---|---|---|---|
| Artiodactyla | 75 | −.253 | .029 | −.572 | .000 |
| Cetacea | 18 | .070 | .783 | −.553 | .017 |
| Chiroptera | 105 | .172 | .079 | −.299 | .002 |
| Fissipeds | 71 | .383 | .001 | −.302 | .011 |
| Insectivora | 28 | −.089 | .654 | −.238 | .223 |
| Lagomorpha | 19 | −.411 | .080 | −.682 | .001 |
| Pinnipeds | 25 | −.221 | .288 | .195 | .349 |
| Primates | 81 | −.191 | .088 | −.289 | .009 |
| Rodentia | 190 | .238 | .001 | −.656 | .000 |
However, when variation in productivity is corrected for using the standardization procedure of figure 3, there were differences between the precocial, the carried, and the altricial groups (one-way ANOVA: F2,8 = 7.1, P = .02). The precocial mammals are farther to the right along a common q contour than the altricial group (Tuke's multiple comparison test: P < .05). Using genus and family means gave similar results (P < .05 for genus, P < .07 for family; data in fig. A2), and the results are robust to errors in the allometric regression coefficients (data in fig. A3). Mammals that carry their offspring are intermediate between the precocial and the altricial mammals but are not significantly different from either. If this same trade-off holds within lifestyle groups, species that produce more litters per year should allocate less biomass to a litter. There is little support for this prediction in most groups (plots in left-hand columns of figs. 5, A1; table 1), with any trade-off being obscured by wide variations in productivity among species.
Discussion
We begin by emphasizing that we regard our predictions as plausible testable hypotheses and that the above data and analyses are only preliminary support for the predictions. We accept that additional analyses using improved techniques and more and better data would be desirable. For instance, for pragmatic reasons, we adopted parallel-lines models to identify differences between lifestyle groups in figure 2, even though in some cases nonparallel-lines models increase the proportion of variance explained. Our method allows unambiguous quantitative comparisons of trait values among groups across the entire range of body sizes. Alternative methods that allow slopes to vary give differences in trait value among groups that vary with body size. Additional theoretical and empirical work is required to assess the extent to which the framework that we have presented provides additional insights into the observed variation in mammalian life histories.
There is a long, rich literature on life-history theory (e.g., MacArthur 1962; Charlesworth 1980; Charnov 1982). There is also a rich literature of accumulating data on components of the life histories of diverse organisms, including mammals (e.g., Gaillard et al. 1989; Promislow and Harvey 1990; Purvis and Harvey 1995; Jones and MacLarnon 2001; Charnov and Ernest 2006; Bielby et al. 2007). Much of this literature is phenomenological. It provides adaptive interpretations of patterns of variation in terms of trade-offs, but it does not provide a conceptual framework based on specified evolutionary mechanisms and constraints. By contrast, our theory provides an explicitly mechanistic account of the evolution of mammal life histories. These life histories are powerfully constrained by the ability of females to acquire resources and convert them into reproductive biomass. The rate of production depends first on body size and second on lifestyle, as shown in figure 2A in Sibly and Brown (2007; see also Brown and Sibly 2006). Mass-specific productivity decreases as body size increases because of unavoidable increases in the costs of transporting resources around larger bodies. Productivity also depends on lifestyle, however, and this has two important components: diet and mortality. When body size is allowed for, mammals with more reliable and abundant foods have higher rates of production, whereas mammals with reduced mortality rates have lower productivity (Brown and Sibly 2006; Sibly and Brown 2007).
Our analyses focus on the allocation of productivity to offspring between and within litters. The factor of primary importance is how preweaning mortality varies with offspring size (fig. 1). Adaptive responses to mortality-size relationships have resulted in the frequently observed precocial and altricial strategies, which segregate at opposite ends of the trade-off between number and size of young in a litter (fig. 4B). At one extreme, survival of offspring born unprotected by a nest or burrow depends critically on their abilities to escape predation and to thermoregulate, which in turn depend on size and developmental state at birth, as in figure 1B. In these mammals, offspring number is traded for size, so that females produce a few large, precocial offspring, and offspring size is further increased by reducing litter frequency to increase litter mass. Thus, selection increases offspring size in both trade-offs so that some species produce only a single large offspring, once per year. At the other extreme, juvenile survival is relatively secure because offspring are protected in burrows or nests, so the strategy is to produce many small, altricial offspring. This is adaptive because, other things being equal, more is better (i.e., results in higher fitness; fig. 1C), and other things are more or less equal because survival before weaning is not greatly affected by offspring size. Litters are frequent, and, concomitantly, litter mass is small, thereby minimizing the number of offspring that die if the mother abandons them or dies before weaning. A third distinct strategy is exhibited by mammals that carry their young from birth until weaning. Their offspring are not particularly large or precocial, but they do have adaptations to cling to the mother as she engages in all activities. There are few offspring per litter primarily because of the difficulty of transporting and caring for more dependent offspring.
Mammals offer special challenges in developing and testing life-history theory. For one thing, maternal investment in gestation and lactation makes it much more difficult to perform the direct experimental manipulations of number and size of offspring that are possible in other groups such as birds and reptiles (e.g., Sinervo and Huey 1990; Fontaine and Martin 2006). Additionally, our results suggest that, to account for the observed trade-offs in allocation of production, the single most important factor is predation on juveniles and the way this varies with neonate size. Unfortunately, few reliable data on the mortality-size relationship are available, due to the inherent difficulties in measuring pre- and postweaning mortality of free-living wild mammals (e.g., see Sibly et al. 1997). Here we present a theoretical framework that overcomes some of these limitations by using a new method to analyze resource-allocation trade-offs. Our framework corrects for variation in both body mass and rate of production (fig. 3) to reveal patterns of divergence along trade-off axes. The usefulness of the method is particularly clear in figure 4A, where the divergence between altricial and precocial mammals is not apparent until variation in productivity is accounted for. This framework allows us to go beyond earlier treatments in identifying the particular trade-offs and lifestyles associated with the altricial, the precocial, and the offspring-carrying strategies. The trade-off between offpring size and offspring number in figure 4B has been shown previously (Read and Harvey 1989; Charnov and Ernest 2006), as has the finding that precocial neonates are heavier than altricial neonates (Martin 1984). When a lifestyle group is constrained to produce altricial or precocial neonates, there are additional consequences and opportunities for selection and adaptation (Martin 1984; Martin and McLarnon 1985; Harvey and Read 1988; Derrickson 1992).
Our analysis shows how ecological relationships have led to the evolution of life-history trade-offs. When the pervasive constraint of the allometry of production and the effects of lifestyle have been accounted for, how preweaning mortality depends on offspring size is the primary factor determining the trade-offs in allocation of resources to reproduction. Further work is needed to assess similarities and differences among species within and among taxonomic and lifestyle groups (e.g., fig. 5) due to the interplay between phylogenetic evolutionary relationships and environmental conditions.
Acknowledgments
We thank K. E. Jones for supplying the bat data, E. L. Charnov and members of the University of New Mexico/Santa Fe Institute Scaling Group and the Integrating Macroecological Pattern and Processes across Scales (IMPPS)/National Science Foundation (NSF)–funded Research Coordination Network (RCN; DEB-0541625) for helpful discussions, and S. Beissinger and two reviewers for comments. This is IMPPS RCN publication 2 and was supported by a Royal Society Travel Grant to R.M.S. and an NSF grant (DEB-0083422) and a Packard Interdisciplinary Science Grant to J.H.B.
Appendix: Normalization Constants and Allometric Regression Coefficients of Production Ratesti and Life-History Characters
Table A1.
Normalization constants of production rates and life-history characters (±SEs)
| Order | No. species | Production rate per adult mass, qi | Litters per year, yi | Litter mass per adult mass, zi | Offspring per litter, ni | Newborn mass per adult mass, xi |
|---|---|---|---|---|---|---|
| Artiodactyla | 75 | .614 ± .040 | .526 ± .026 | .088 ± .028 | .400 ± .021 | −.312 ± .031 |
| Cetacea | 18 | .701 ± .076 | .234 ± .049 | .467 ± .053 | .425 ± .040 | .042 ± .058 |
| Chiroptera | 105 | −.067 ± .052 | .235 ± .033 | −.303 ± .036 | .142 ± .028 | −.445 ± .040 |
| Fissipeds | 71 | .106 ± .035 | .421 ± .023 | −.315 ± .025 | .734 ± .019 | −1.048 ± .027 |
| Insectivora | 28 | .172 ± .061 | .375 ± .039 | −.203 ± .043 | .756 ± .033 | −.959 ± .047 |
| Lagomorpha | 19 | .716 ± .063 | .794 ± .040 | −.078 ± .044 | .763 ± .033 | −.842 ± .048 |
| Perissodactyla | 9 | .422 ± .094 | .288 ± .061 | .134 ± .066 | .389 ± .050 | −.255 ± .072 |
| Pinnipeds | 25 | .755 ± .059 | .496 ± .038 | .259 ± .041 | .346 ± .032 | −.087 ± .045 |
| Primates | 81 | .008 ± .034 | .264 ± .022 | −.256 ± .024 | .289 ± .018 | −.545 ± .026 |
| Rodentia | 190 | .339 ± .038 | .543 ± .025 | −.205 ± .027 | .721 ± .020 | −.925 ± .029 |
| Xenarthra | 7 | .197 ± .099 | .382 ± .063 | −.185 ± .069 | .389 ± .053 | −.573 ± .075 |
| F10, 616 | 36.6 | 38.4 | 29.1 | 152.8 | 104.9 | |
| Adjusted R2 | 73% | 56% | 69% | 75% | 68% |
Note: Parameters qi, yi, zi, ni, and xi are as in equations (6). Normalization constants measure the vertical displacement of the regression lines, that is, their y-intercepts at 1 g in figure 2 (see fig. 3; eq. [2]). The penultimate row gives F statistics from ANOVAs comparing the normalization constants. The critical value at the 0.001% significance level is 4.2.
Table A2.
Fitted regression coefficients with their SEs
| Parameter | Regression coefficient | SE |
|---|---|---|
| bq | −.3664 | .017 |
| by | −.1076 | .011 |
| bz | −.2587 | .012 |
| bn | −.0673 | .009 |
| bx | −.1914 | .013 |
Note: Parameters are as in equations (5).
Table A3.
Statistics for a comparison of parallel-lines and nonparallel-lines models
| Parameter | F10, 606 | P (parallel-lines model) | Adjusted R2 (%) |
|---|---|---|---|
| Production rate per adult mass, qi | 5.3 | .000 | 75 |
| Litters per year, yi | 6.6 | .000 | 59 |
| Litter mass per adult mass, zi | 2.8 | .002 | 70 |
| Offspring per litter, ni | 1.2 | NS | 75 |
| Newborn mass per adult mass, xi | 1.8 | NS | 69 |
Note: NS = not significant.
Figure A1.
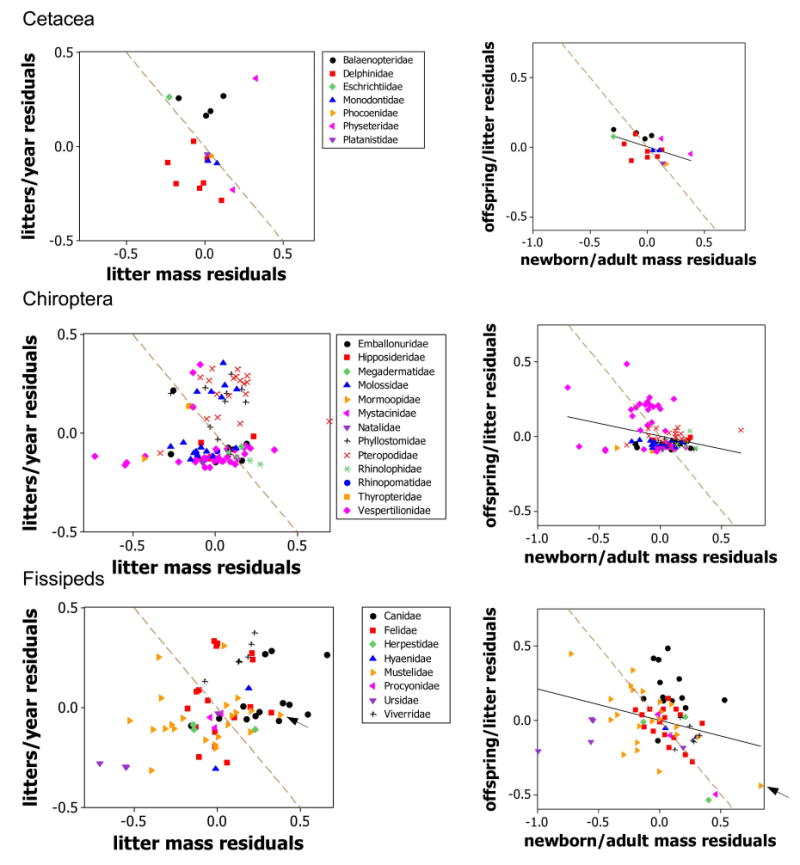
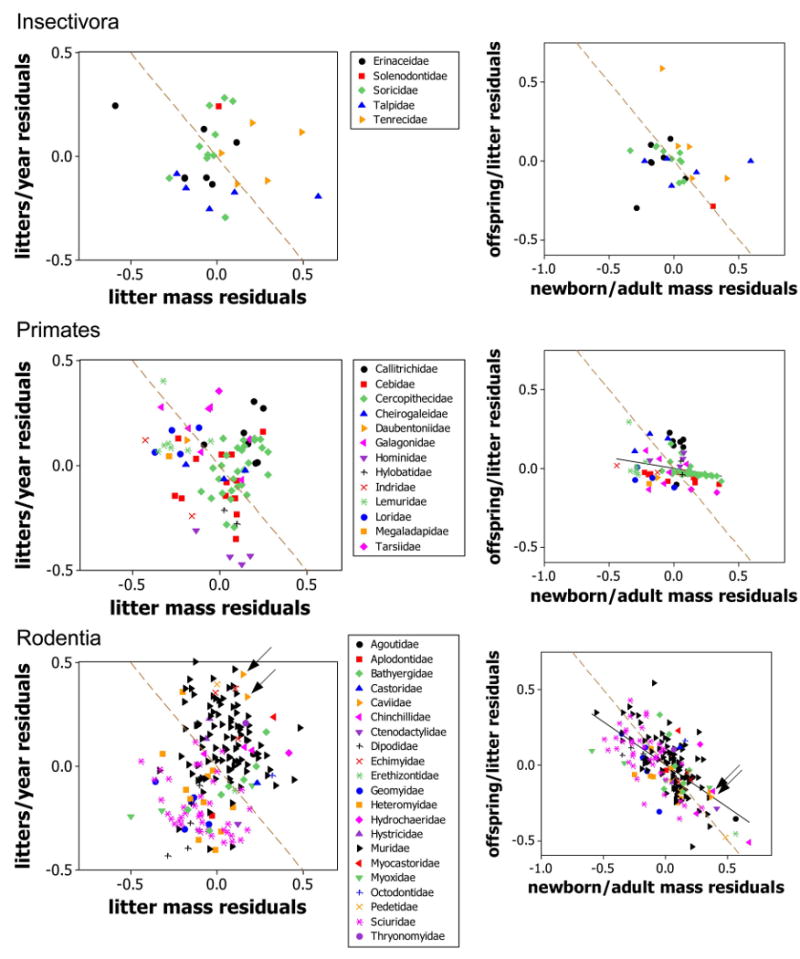
Scatter diagrams like those in figure 5 for the taxonomic/lifestyle groups not shown in that figure. One point did not fit in the left-hand column, a bear (Fissipeds, Ursidae) with coordinates (−1.20, −0.03). Dashed brown lines connect strategies with the same value of the resource being allocated. Thus, the equation of the line in the left-hand panels is (z – z̄) + (y – ȳ) = 0; here (z – z̄) represents the residual of z and (y – ȳ) the residual of y. The line goes through the point (0, 0) because the mean of the residuals of each trait is 0. Similarly, the line in the right-hand panels has the equation (x – x̄) + (n – n̄) = 0. The caviomorphs are arrowed within the rodents and the sea otter within the fissipeds (see main text).
Figure A2.
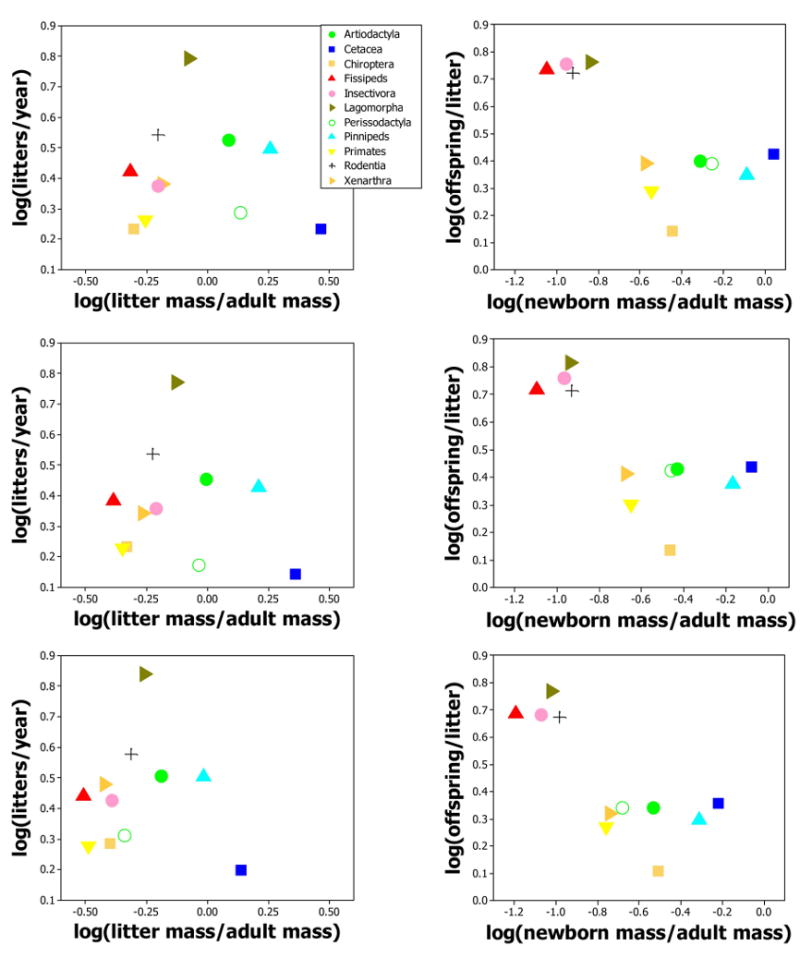
Scatterplots as in figure 4 (top) together with repeats of the analyses using genus means (middle) and family means (bottom).
Figure A3.
Scatterplots as in figure 4, with allometric regression coefficients varied by ± 2 SE.
Literature Cited
- Bielby J, Mace GM, Bininda-Emonds ORP, Cardillo M, Gittleman JL, Jones KE, Orme CDL, Purvis A. The fast-slow continuum in mammalian life history: an empirical re-evaluation. American Naturalist. 2007;169:748–757. doi: 10.1086/516847. [DOI] [PubMed] [Google Scholar]
- Brown JH, Sibly RM. Life-history evolution under a production constraint. Proceedings of the National Academy of Sciences of the USA. 2006;103:17595–17599. doi: 10.1073/pnas.0608522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- Charlesworth B. Evolution in age-structured populations. Cambridge University Press; Cambridge: 1980. [Google Scholar]
- Charnov EL. The theory of sex allocation. Princeton University Press; Princeton, NJ: 1982. [Google Scholar]
- Charnov EL. Evolution of life-history variation among female mammals. Proceedings of the National Academy of Sciences of the USA. 1991;88:1134–1137. doi: 10.1073/pnas.88.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov EL. Life history invariants. Oxford University Press; Oxford: 1993. [Google Scholar]
- Charnov EL. Evolution of mammal life histories. Evolutionary Ecology Research. 2001;3:521–535. [Google Scholar]
- Charnov EL, Ernest SKM. The offspring-size/clutch-size trade-off in mammals. American Naturalist. 2006;167:578–582. doi: 10.1086/501141. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Warne R, Moses M. Lifetime reproductive effort. American Naturalist. 2007;170:E129–E142. doi: 10.1086/522840. [DOI] [PubMed] [Google Scholar]
- Cody ML. A general theory of clutch size. Ecology. 1966;20:174–184. doi: 10.1111/j.1558-5646.1966.tb03353.x. [DOI] [PubMed] [Google Scholar]
- Derrickson EM. Comparative reproductive strategies of altricial and precocial eutherian mammals. Functional Ecology. 1992;6:57–65. [Google Scholar]
- Dobson FS. A lifestyle view of life-history evolution. Proceedings of the National Academy of Sciences of the USA. 2007;104:17565–17566. doi: 10.1073/pnas.0708868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest SKM. Life history characteristics of placental non-volant mammals. Ecology. 2003;84:3402. [Google Scholar]
- Fontaine JJ, Martin TE. Parent birds assess nest predation risk and adjust their reproductive strategies. Ecology Letters. 2006;9:428–434. doi: 10.1111/j.1461-0248.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- Gaillard JM, Pontier D, Allainé D, Lebreton JD, Trouvilliez J, Clobert J. An analysis of demographic tactics in birds and mammals. Oikos. 1989;56:59–76. [Google Scholar]
- Harvey PH, Read AF. How and why do mammalian life histories vary? In: Boyce MS, editor. Evolution of life histories of mammals. Yale University Press; New Haven, CT: 1988. pp. 213–232. [Google Scholar]
- Jones KE, MacLarnon A. Bat life histories: testing models of mammalian life-history evolution. Evolutionary Ecology Research. 2001;3:465–476. [Google Scholar]
- Kozlowski J, Weiner J. Interspecific allometries are byproducts of body size optimization. American Naturalist. 1997;149:352–380. [Google Scholar]
- MacArthur RH. Some generalized theorems of natural selection. Proceedings of the National Academy of Sciences of the USA. 1962;48:1893–1897. doi: 10.1073/pnas.48.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RD. Scaling effects and adaptive strategies in mammalian lactation. Symposia of the Zoological Society of London. 1984;51:81–117. [Google Scholar]
- Martin RD, McLarnon AM. Gestation period, neonatal size and maternal investment in placental mammals. Nature. 1985;313:220–223. [Google Scholar]
- Oli MK. The fast-slow continuum and mammalian life-history patterns: an empirical evaluation. Basic and Applied Ecology. 2004;5:449–463. [Google Scholar]
- Promislow DEL, Harvey PH. Living fast and dying young: a comparative-analysis of life-history variation among mammals. Journal of Zoology. 1990;220:417–437. [Google Scholar]
- Purvis A, Harvey PH. Mammal life-history evolution: a comparative test of Charnov's model. Journal of Zoology. 1995;237:259–283. [Google Scholar]
- Read AF, Harvey PH. Life-history differences among the Eutherian radiations. Journal of Zoology. 1989;219:329–353. [Google Scholar]
- Sibly RM, Brown JH. Effects of body size and lifestyle on evolution of mammal life histories. Proceedings of the National Academy of Sciences of the USA. 2007;104:17707–17712. doi: 10.1073/pnas.0707725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibly RM, Calow P. Physiological ecology of animals. Blackwell Scientific; Oxford: 1986. [Google Scholar]
- Sibly RM, Calow P. Ecological compensation: a complication for testing life-history theory. Journal of Theoretical Biology. 1987;125:177–186. doi: 10.1016/s0022-5193(87)80039-5. [DOI] [PubMed] [Google Scholar]
- Sibly RM, Curnow RN. An allelocentric view of life-history evolution. Journal of Theoretical Biology. 1993;160:533–546. doi: 10.1006/jtbi.1993.1034. [DOI] [PubMed] [Google Scholar]
- Sibly RM, Collett D, Promislow DEL, Peacock DJ, Harvey PH. Mortality rates of mammals. Journal of Zoology (London) 1997;243:1–12. [Google Scholar]
- Sinervo B, Huey RB. Allometric engineering: an experimental test of the causes of interpopulational differences in performance. Science. 1990;248:1106–1109. doi: 10.1126/science.248.4959.1106. [DOI] [PubMed] [Google Scholar]
- Sutherland WJ, Grafen A, Harvey PH. Life history correlations and demography. Nature. 1986;320:88. [Google Scholar]


