Abstract
Background
Intermediate conductance Ca2+-dependent K+ channels (KCa3.1) have been proposed as therapeutic targets for numerous diseases. We recently characterized the endocytic fate of these channels; leading to the possibility that this can be pharmacologically manipulated, thereby altering the number of channels (N) at the plasma membrane.
Results & discussion
We demonstrate that plasma membrane-localized KCa3.1 can be rapidly (10 min) tagged with a fluorophore using a combination of a biotin ligase (BirA) acceptor peptide-tagged channel and an ER-localized BirA. Endocytosis of KCa3.1 was quantified using a 96-well plate format, demonstrating that the ubiquitin-activating enzyme E1 inhibitor UBEI-41, blocks the endocytosis of KCa3.1.
Conclusion
We describe a novel method for identifying modulators of KCa endocytosis and demonstrate this can be used to modulate N at the plasma membrane. It is anticipated that altering N will provide novel therapeutic strategies for targeting these channels in disease.
Since the cloning of KCa3.1 (previously called IK1 or SK4 [1,2]) and KCa2.x (previously called SK1–3 [3]), these channels have been confirmed as playing critical roles in a host of physiological responses, including a component of the afterhyperpolarization in neurons where they control action potential firing rate [4], smooth muscle excitability [5], T-cell activation [6], the endothelial-derived hyperpolarizing factor response, which controls vascular tone and hence blood pressure regulation [7], the regulatory volume decrease across red blood cells [8] and the maintenance of the basolateral membrane electrochemical potential difference that controls transepithelial fluid secretion during a Ca2+-mediated agonist response [9]. Based on this wide array of physiological functions, it has been proposed that activators and inhibitors of channel gating would be potentially useful in the treatment of autoimmune diseases [10], cardiovascular disease [7,11,12], autosomal dominant polycystic kidney disease [13], sickle cell anemia [14] and ataxia [15].
In 1996, our laboratory characterized 1-ethyl-2-benzimidazolinone (1-EBIO) as the first activator of KCa3.1 channels [16]. We subsequently demonstrated that chlorzoxazone (Parafon Forte DSC), a centrally acting smooth muscle relaxant, as well as its structural analogue, zoxazolamine, activated KCa3.1 and that in vivo administration resulted in a hyperpolarization of nasal potential difference in normal healthy volunteers [17]. These results lead us to speculate that KCa3.1 activators would be useful in chronic obstructive pulmonary disease (COPD) and cystic fibrosis [17]. Further structure activity studies resulted in the development of DCEBIO, a compound with a 100-fold greater potency for the activation of KCa3.1 [18]. These compounds, as well as the structurally similar neuroprotective drug, riluzole, were subsequently also shown to activate the related KCa2.x channels [19–22]. These compounds have been shown to produce an apparent shift in Ca2+ affinity to lower concentrations as well as an increase in current flow at saturating Ca2+, indicating they have Ca2+-dependent and Ca2+-independent effects on channel gating, respectively [21–23]. Based on a kinetic analysis, Fakler and colleagues concluded that this class of compounds stabilized the association between calmodulin and the calmodulin binding domain of the channel [23]. More recently, Christophersen and colleagues developed more potent activators of KCa2.x and KCa3.1 channels, including NS309 [24] as well as GW542573X, a compound that has 100-fold greater selectivity for KCa2.x than KCa3.1, as well as being the first true activator of these channels, due to its ability to increase channel activity in zero Ca2+ [25]. Finally, Wulff and colleagues have characterized SKA-31 as a new activator of KCa2.x and KCa3.1 channels; demonstrating an antihypertensive effect in mice, further confirming the therapeutic potential of modulators of these channels [26].
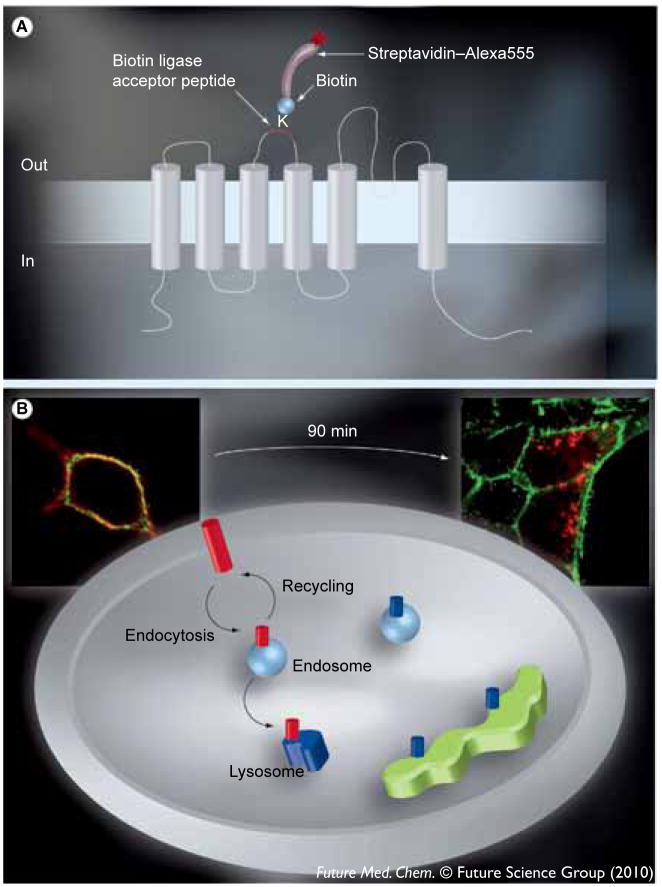
An alternate means of increasing current flow across the membrane and, hence, modifying the response of the cell/tissue to Ca2+-dependent agonists, is to increase the number of channels (N) at the plasma membrane. This could theoretically be accomplished by increasing the anterograde trafficking of channels to the plasma membrane or by decreasing the retrograde trafficking of channels out of the plasma membrane through alterations in the endocytic and/or recycling rates of the channels. We recently developed a strategy for monitoring the endocytosis and recycling of KCa3.1 and KCa2.3 based on the work of Ting and colleagues [27]. This involved inserting the biotin ligase acceptor peptide (BLAP) sequence into the second extracellular loop of each of these channels and confirming that this did not alter their fundamental gating properties. Using recombinant biotin ligase (BirA) we can then specifically label plasma membrane-localized channels with biotin, followed by a fluorophore-conjugated streptavidin. The relative position of the BLAP tag in KCa3.1 as well as the streptavidin-dependent tagging of the channel is schematically illustrated in Figure 1A. We previously demonstrated that KCa2.3 is rapidly endocytosed and recycled back into the plasma membrane with a time constant of approximately 5 min (with a total membrane half-life of approximately 13 h) [28]. In contrast, KCa3.1 is completely endocytosed from the plasma membrane within 90 min and targeted to the lysosome for degradation [Balut CM, Gao Y, Thibodeau PH, Murray SA, Devor DC. ESCRT-dependent targeting of plasma membrane localized KCa3.1 to the lysosomes. Manuscript Submitted].
Figure 1.
(A) The architecture of KCa3.1 relative to the plasma membrane and the position of the 17 amino acid Bacillus licheniformis β-lactamase (BLAP) epitope tag between the third and fourth transmembrane domains. KCa3.1 is a six transmembrane domain K+ channel in which the N- and C-termini are cytoplasmic. The lysine within the BLAP sequence is specifically labeled with biotin via the co-expressed BirA-KDEL in the endoplasmic reticulum. Plasma membrane-expressed channels are then specifically labeled with streptavidin–Alexa555 such that only plasma membrane localized channels are tagged with fluorophore. (B) The labeling of KCa3.1 and the plasma membrane with unique fluorophores. Following labeling of plasma membrane channels with streptavidin–Alexa555 (red) and the plasma membrane itself with WGA–Alexa488 (green) we can discriminate between plasma membrane-localized KCa3.1 and channels in either endosomes or lysosomes, as illustrated. Channels within the biosynthetic pathway are biotinylated, but not fluorescently tagged (shown in blue), and therefore not recognized in this assay. High-resolution images of individual cells are shown to further illustrate this protocol. As shown at time 0, KCa3.1 is localized exclusively to the plasma membrane. This colocalization is shown as yellow. By contrast, after 90 min at 37°C, KCa3.1 has been endocytosed (red endosomes). By examining the loss of colocalization we can determine the amount of endocytosis and whether this is affected by small molecules. Cellular images were captured with an Olympus IX-81 epifluorescence microscope equipped with a 60× oil objective. Multiple z-planes were captured, the images deconvolved and shown as a projection image.
Experimental
We recently characterized a labeling process for plasma membrane-localized KCa3.1 using BLAP-tagged channels in combination with recombinant BirA [28]. Briefly, cells were washed in PBS, followed by incubation in recombinant BirA for 30 min at room temperature, three PBS washes, incubation in streptavidin (10 μg/ml in PBS with BSA 1% ) at 4°C for 10 min and three washes in PBS with BSA 1% to remove unbound streptavidin. While this provides excellent labeling of channels, it takes nearly 50 min to complete and therefore needed to be streamlined for adaptation to a 96-well plate application. In light of this, we subcloned both BLAP-tagged KCa3.1 as well as BirA, in which a KDEL endoplasmic reticulum-retention motif (BirA-KDEL) had been added at the C-terminus (kindly provided in pDISPLAY), into the bicistronic plasmid, pBudCE4.1 (Invitrogen) behind the EF-1α and CMV promoters, respectively. BirA-KDEL was directly subcloned from pDISPLAY using Sal I and Hind III restriction sites. We then introduced a silent mutation into the Kpn I site that was transferred from the pDISPLAY vector during this cloning step. Finally, BLAP-KCa3.1 was PCR amplified from pcDNA3.1 and Kpn I, and Xho I restriction sites added in a single step and subcloned into pBudCE4.1 containing BirA-KDEL. Stable HEK293 cell lines were then selected using zeocin. With this approach, each subunit of the channel was biotinylated in the endoplasmic reticulum as it was synthesized via a covalent modification, resulting in a greater biotinylation efficiency compared with the addition of recombinant BirA. Cells expressing BirA-KDEL and KCa3.1-BLAP were seeded at a density of approximately 50% confluence (16 h prior to the experiment) into Nunc 96-well optical bottom black plates (Nalge Nunc International, Rochester, NY), which have been previously poly L-lysine coated. To ensure the highest biotinylation efficiency possible, 10-μM biotin was added to the growth media when the cells were seeded to eliminate the possibility that biotin supply is rate limiting.
To label plasma membrane channels, the growth media was removed, the cells were washed once with PBS with BSA 1% and the channels were labeled in a single step with streptavidin–Alexa555 (10 μg/ml in PBS with BSA 1%) at 4°C for 10 min followed by three washes in PBS with BSA 1% to remove unbound streptavidin. In this way, the total labeling time was reduced to approximately 12–15 min compared with the 50 min previously required. Cells treated in this way serve as controls (time = 0 min) in which all the channel is localized to the plasma membrane. To assess endocytosis of the channel, a subset of plates is returned to 37°C for 90 min in the absence or presence of the compound in order to be tested for its ability to inhibit endocytosis. We previously demonstrated that 90 min is sufficient to endocytose most of the labeled KCa3.1 in both HEK293 and endothelial cells [28, Balut CM, Gao Y, Thibodeau PH, Murray SA, Devor DC. ESCRT-dependent targeting of plasma membrane localized KCa3.1 to the lysosomes. Manuscript Submitted]. It is important to note that the binding of streptavidin to biotin is essentially irreversible, with a Ka of 1015 M−1, such that it will stay attached to the channel throughout its endocytosis and subsequent degradation. To adapt our endocytosis assay to an automated analysis system we labeled the plasma membrane with WGA–Alexa488 (wheat germ agglutinin, 5 μg/ml, Invitrogen) in PBS with BSA 1% at 4°C, either at time 0 or after 90 min. Importantly, this WGA-labeling step is carried out prior to the cells being fixed and permeabilized, such that only the plasma membrane was labeled. For this, the cells are either immediately incubated in WGA–Alexa488 (time 0) or the growth medium is first removed and the cells washed once in PBS with BSA 1% (time 90 min) prior to WGA–Alexa488 labeling. Subsequently, the cells are washed three times in PBS with BSA 1% and once in PBS at 4°C to remove unbound WGA. This protocol allowed us to unequivocally define the membrane fluorescently and thus colocalize the channel with the plasma membrane as a means of assessing endocytosis. Subsequent to WGA-Alexa488 labeling, the cells were fixed and permeabilized in paraformaldehyde 2% with Triton X-100 0.1% for 15 min and the nuclei labeled with DAPI for 30 s. A schematic depicting this protocol and the labeling of plasma membrane channels is shown in Figure 1. Also shown is a high-resolution image at both time 0 and 90 min to illustrate the labeling of both KCa3.1 with streptavidin–Alexa555 and the plasma membrane with WGA–Alexa488 (Figure 2). As is apparent, at time 0, all of the channel is resident in the plasma membrane and colocalizes with WGA (the plasma membrane marker). After 90 min at 37°C, the majority of KCa3.1 has been endocytosed resulting in less colocalization with WGA–Alexa488.
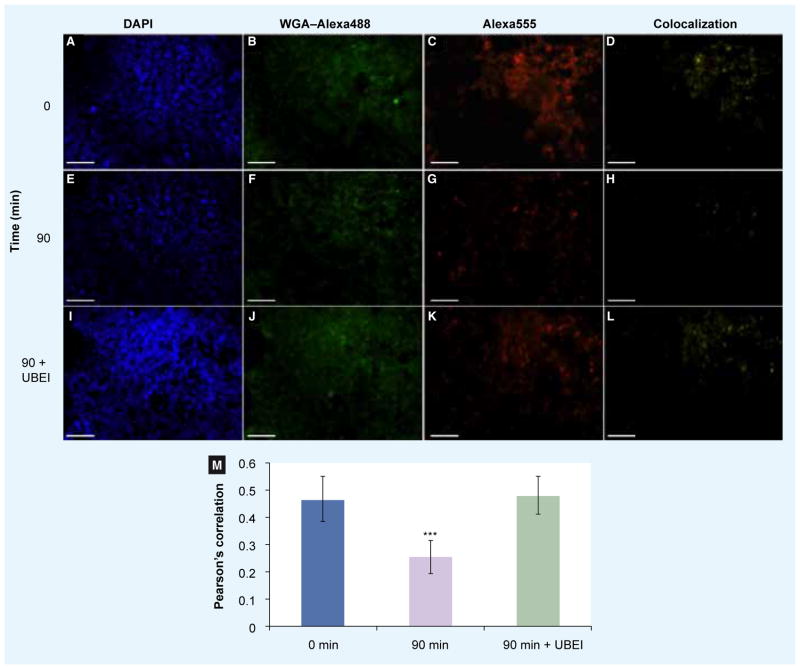
Figure 2. Endocytosis of plasma membrane-localized KCa3.1 can be measured using fluorescence imaging.
Plasma membrane-localized biotin ligase acceptor peptide-tagged KCa3.1 was specifically labeled with streptavidin–Alexa555 at 4°C (C, G & K). Subsequently, the cells were incubated for either 0 min (A–D), 90 min (E–H) or 90 min in the presence of UBEI-41 (I–L) at 37°C in an optical bottom 96-well plate. The plasma membrane was then labeled with WGA–Alexa488 (B, F & J) at 4°C. The cells were then fixed, permeabilized and the nuclei labeled with DAPI (A, E & I). Various fields for each well were imaged using an automated Nikon TiE epifluoresencence inverted microscope, captured and digitized using Volocity Acquisition v5.3.1. The colocalization channel (D, H & L) and Pearson’s correlation (M) for each field was calculated using the colocalization feature of Volocity Acquisition v5.3.1. N = 97, 88 and 59 imaged fields for 0, 90 and 90 min with UBEI, respectively. Error bars represent the standard deviation. ***p < 0.001.
Following labeling, individual cells within each well of the 96-well plate were imaged using a Nikon TiE inverted, widefield epifluoresence microscope with Plan–Neofluor objectives, a motorized six-position fluorescent filter cube turret with zero-pixel shift filters and a registered motorized stage with plate insert. The 12-bit grayscale images were captured using a Retiga 2000 camera (QImaging) and Volocity Acquisition software (v5.3.1, Perkin Elmer) that autofocused on the plasma membrane using the WGA–Alexa488 fluorescence, automatically capturing four to ten images per well. For these studies we utilized a 20× air objective with an numerical aperature of 0.5. Under the experimental conditions, both cellular autofluorescence and the autofluorescence associated with UBEI-41 were negligible and eliminated by thresholding prior to image collection. Fields that failed to autofocus or which were devoid of cells were ignored. Images were analyzed using the Volocity Quantification module using the colocalization analysis feature. Under the imaging conditions employed, there are 0.738 μm per pixel in both the x and y planes. Colocalization between the red and green channels is expressed as a Pearson’s correlation where a value of 1 indicates complete colocalization and 0 indicates no colocalization. A Pearson’s correlation coefficient was calculated for each experimental condition by averaging the coefficients for individual imaged fields. Initially, at time 0, KCa3.1 was exclusively localized to the membrane such that the Pearson’s correlation was high. However, given that KCa3.1 did not occupy all membrane space, colocalization will be less than 100% and was determined by expression level. Clearly, a high level of expression is necessary to obtain a large signal-to-noise level for these experiments, such that endocytosis can be clearly defined relative to time 0. This was obtained by having a stable cell line that expresses KCa3.1 in all cells driven by the EF-1α promoter coupled with high-efficiency labeling of the channel with biotin by utilizing the BirA-KDEL driven off a CMV promoter. The minimal starting colocalization, which will allow us to clearly define small-molecule modulators of endocytosis, has not been defined. Following endocytosis, colocalization of KCa3.1 with membrane fluorescence will be reduced such that modulators of this event can be defined.
Results & discussion
Initially we confirmed that there was no non-specific labeling with streptavidin–Alexa555 by transfecting either BirA-KDEL or BLAP-KCa3.1 alone (data not shown). We then determined whether these constructs would be useful for examining endocytosis in a 96-well plate format. An example of one experiment to demonstrate the utility of this method is shown in Figure 2. In Figure 2A–d, a single field from one well is shown at time 0. Figure 2A shows the DAPI fluorescence; Figure 2B depicts the fluoresence associated with WGA-Alexa488; Figure 2C shows the fluorescence associated with KCa3.1 and the pixels that colocalized as determined by Volocity Quantification are shown in Figure 2D. As is expected, at time 0, KCa3.1 is at the plasma membrane resulting in a Pearson’s correlation of 0.463 (Figure 2M, blue bar, N = 97). The fluorescence images from a single field from one well at time 90 min are shown in Figure 2E–H. As shown in Figure 2G, KCa3.1 has endocytosed from the plasma membrane at 90 min with reduced colocalization as shown in Figure 2H. As is apparent, colocalization between the plasma membrane and KCa3.1 has significantly decreased (P < 0.001, Students T-test), giving an average Pearson’s correlation of 0.251 (Figure 2M, purple bar, N = 88). As proof of concept, we determined whether we could reduce translocation of KCa3.1 using the ubiquitin-activating enzyme, E1 inhibitor (UBEI-41; Figure 2I–M). By inhibiting E1 for a short time (90 min), the ubiquitinylation cascade will be effectively eliminated, such that proteins that are transiently ubiquitinylated will no longer be modified. As endocytosis of numerous plasma membrane proteins is triggered by their ubiquitinylation [29] we questioned whether the endocytosis of KCa3.1 was similarly dependent upon this signaling cascade. Figure 2I–L depicts the same 90-min time period as described previously, except in the presence of 50-μM UBEI-41 (Biogenova). As shown in Figure 2K, endocytosis is dramatically reduced giving more colocalization (Figure 2L) with an average Pearson’s correlation of 0.481 (Figure 2M; green bar, N = 59). This level of colocalization is not different from the time 0 control (P > 0.5), indicative of this compound inhibiting endocytosis. Based on these results, we are carrying out secondary screens to define the role of ubiquitinylation in the endocytosis of KCa3.1.
Conclusion
Our results demonstrate that we can rapidly label plasma membrane KCa3.1 and determine its endocytic fate using a fluorescent membrane marker. To highlight the utility of this approach, we demonstrate that the ubiquitin-activating enzyme, E1 inhibitor, UBEI-41 blocks KCa3.1 endocytosis. These results further suggest that a high-throughput screen can be developed to identify small-molecule modulators of KCa3.1 endocytosis. Further, this methodology can be adapted to study protein–protein interactions involved in this process using an siRNA library approach. Finally, we are adapting this assay to identify compounds that block the recycling of KCa2.3, which will result in a decrease in plasma membrane colocalization.
Executive summary.
The intermediate conductance, Ca2+-activated K+ channel, KCa3.1 has been proposed as a therapeutic target in a wide array of diseases.
Modulating the number of channels at the plasma membrane (N) will directly affect the physiological response of a cell to an agonist or pharmacological stimulation.
KCa3.1 can be rapidly (12 min) labeled at the plasma membrane using a combination of biotin ligase acceptor peptide-tagged channels and endoplasmic reticulum-localized BirA.
Using WGA–Alexa488 as a membrane mask, we were able to delineate plasma membrane localized versus endocytosed channels in a 96-well assay.
Using this assay, we identify the ubiquitin-activating enzyme, E1 inhibitor, UBEI-41 as an inhibitor of KCa3.1 endocytosis.
This assay can be used to identify small-molecule modulators of KCa3.1 endocytosis, resulting in an increase in the number of channels at the plasma membrane and an increased physiological response.
Footnotes
For reprint orders, please contact reprints@future-science.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was supported by NIH grants (HL083060, HL092157) to Daniel C Devor and an American Heart Fellowship (0825542D) to Corina M Balut. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA. 1997;94(21):11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joiner WJ, Wang LY, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci USA. 1997;94(20):11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohler M, Hirschberg B, Bond CT, et al. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273(5282):1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 4.Bond CT, Herson PS, Strassmaier T, et al. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24(23):5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohya S, Kimura S, Kitsukawa M, Muraki K, Watanabe M, Imaizumi Y. SK4 encodes intermediate conductance Ca2+-activated K+ channels in mouse urinary bladder smooth muscle cells. Jpn J Pharmacol. 2000;84(1):97–100. doi: 10.1254/jjp.84.97. [DOI] [PubMed] [Google Scholar]
- 6.Ghanshani S, Wulff H, Miller MJ, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275(47):37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Brahler S, Kaistha A, Schmidt VJ, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119(17):2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. Demonstrates that both IK1 (KCa3.1) and SK3 (KCa2.3) are required for the endothelial derived hyperpolarizing factor response and that deletion of these channels causes hypertension in mice. [DOI] [PubMed] [Google Scholar]
- 8.Vandorpe DH, Shmukler BE, Jiang L, et al. cDNA cloning and functional characterization of the mouse Ca2+-gated K+ channel, mIK1. Roles in regulatory volume decrease and erythroid differentiation. J Biol Chem. 1998;273(34):21542–21553. doi: 10.1074/jbc.273.34.21542. [DOI] [PubMed] [Google Scholar]
- 9.Flores CA, Melvin JE, Figueroa CD, Sepulveda FV. Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+ channel KCNN4. J Physiol. 2007;583(Pt 2):705–717. doi: 10.1113/jphysiol.2007.134387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wulff H, Knaus HG, Pennington M, Chandy KG. K+ channel expression during B cell differentiation: implications for immunomodulation and autoimmunity. J Immunol. 2004;173(2):776–786. doi: 10.4049/jimmunol.173.2.776. [DOI] [PubMed] [Google Scholar]
- 11▪.Kohler R, Brakemeier S, Kuhn M, et al. Impaired hyperpolarization in regenerated endothelium after balloon catheter injury. Circ Res. 2001;89(2):174–179. doi: 10.1161/hh1401.093460. Demonstrates that KCa3.1 expression is induced following balloon angioplasty and is involved in the restenosis that occurs. [DOI] [PubMed] [Google Scholar]
- 12.Toyama K, Wulff H, Chandy KG, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118(9):3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albaqumi M, Srivastava S, Li Z, et al. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;74(6):740–749. doi: 10.1038/ki.2008.246. [DOI] [PubMed] [Google Scholar]
- 14.Brugnara C, Gee B, Armsby CC, et al. Therapy with oral clotrimazole induces inhibition of the Gardos channel and reduction of erythrocyte dehydration in patients with sickle cell disease. J Clin Invest. 1996;97(5):1227–1234. doi: 10.1172/JCI118537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shakkottai VG, Chou CH, Oddo S, et al. Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia. J Clin Invest. 2004;113(4):582–590. doi: 10.1172/JCI20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪▪.Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl- secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am J Physiol. 1996;271(5 Pt 1):L775–784. doi: 10.1152/ajplung.1996.271.5.L775. First characterization of an opener of KCa3.1 and 1-EBIO. [DOI] [PubMed] [Google Scholar]
- 17▪▪.Singh AK, Devor DC, Gerlach AC, Gondor M, Pilewski JM, Bridges RJ. Stimulation of Cl− secretion by chlorzoxazone. J Pharmacol Exp Ther. 2000;292(2):778–787. First to demonstrate the efficacy of a KCa3.1 opener in human subjects. [PubMed] [Google Scholar]
- 18▪▪.Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2001;296(2):600–611. Describes the synthesis of DCEBIO, a compound with a 100-fold increased affinity for activation of KCa3.1 compared with 1-EBIO. [PubMed] [Google Scholar]
- 19.Cao Y, Dreixler JC, Roizen JD, Roberts MT, Houamed KM. Modulation of recombinant small-conductance Ca2+-activated K+ channels by the muscle relaxant chlorzoxazone and structurally related compounds. J Pharmacol Exp Ther. 2001;296(3):683–689. [PubMed] [Google Scholar]
- 20.Cao YJ, Dreixler JC, Couey JJ, Houamed KM. Modulation of recombinant and native neuronal SK channels by the neuroprotective drug riluzole. Eur J Pharmacol. 2002;449(1–2):47–54. doi: 10.1016/s0014-2999(02)01987-8. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen KA, Schroder RL, Skaaning-Jensen B, Strobaek D, Olesen SP, Christophersen P. Activation of the human intermediate-conductance Ca2+-activated K+ channel by 1-ethyl-2-benzimidazolinone is strongly Ca2+-dependent. Biochim Biophys Acta. 1999;1420(1–2):231–240. doi: 10.1016/s0005-2736(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 22.Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2000;278(3):C570–581. doi: 10.1152/ajpcell.2000.278.3.C570. [DOI] [PubMed] [Google Scholar]
- 23.Pedarzani P, Mosbacher J, Rivard A, et al. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem. 2001;276(13):9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- 24.Strobaek D, Teuber L, Jorgensen TD, et al. Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1h-indole-2,3-dione 3-oxime) Biochim Biophys Acta. 2004;1665(1–2):1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 25▪.Hougaard C, Jensen Ml, Dale TJ, et al. Selective activation of the SK1 subtype of human small-conductance Ca2+-activated K+ channels by 4-(2-methoxyphenyl-carbamoyloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester (GW542573X) is dependent on serine 293 in the S5 segment. Mol Pharmacol. 2009;76(3):569–578. doi: 10.1124/mol.109.056663. First demonstration of a true activator of KCa2.x and KCa3.1 channels. That is, GW542573X activates these channels in the absence of cytosolic Ca2+ [DOI] [PubMed] [Google Scholar]
- 26▪▪.Sankaranarayanan A, Raman G, Busch C, et al. Naphtho[1,2-d]thiazol-2-ylamine (ska-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75(2):281–295. doi: 10.1124/mol.108.051425. Demonstrates that openers of KCa2 and KCa3.1 are capable of decreasing blood pressure, suggesting that increasing the activity of these channels may have therapeutic benefit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howarth M, Takao K, Hayashi Y, Ting AY. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc Natl Acad Sci USA. 2005;102(21):7583–7588. doi: 10.1073/pnas.0503125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪.Gao Y, Balut CM, Bailey MA, Patino-Lopez G, Shaw S, Devor DC. Recycling of the Ca2+-activated K+ channel, KCa2.3 is dependent upon RME-1, Rab35/EPI64c and an N-terminal domain. (In Press). First paper demonstrating the endocytic route for KCa2.3 and KCa3.1, suggesting this can be modulated for therapeutic benefit. [Google Scholar]
- 29.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315(5809):201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]