Abstract
Purpose of review
Exposure to non-inherited maternal antigens (NIMA) in fetal and neonatal life has lifelong immunological consequences. Though there is a plethora of evidence of effects of mother on the immune responses of her offspring, there is very little knowledge available on how exposure to NIMA can result in either tolerance or sensitization. Understanding the mechanism of NIMA effects will impact different fields of immunology including transplantation, autoimmunity, and tumor immunotherapy.
Recent findings
Following the discoveries of beneficial effects of NIMA exposure on clinical outcomes in solid organ and bone marrow transplantation, it has now been shown that the exposure to NIMA induces various types of T regulatory cells (TR) in fetus and adult, which may partially account for tolerance to allografts bearing the NIMA. Though all offspring are exposed to the maternal antigens, they exhibit a great variability in the NIMA effects, which can be explained by the variability in the extent of maternal microchimerism (MMc).
Summary
Exposure to NIMA can have tolerogenic or sensitizing effects on the offspring, resulting in acceptance or rejection of allografts expressing the NIMA. This variability may be partly explained by the level and distribution of maternal cells persisting in the offspring.
Keywords: Non-inherited maternal antigens, microchimerism, tolerance, T regulatory cells
1. Introduction
Graft survival is best when a kidney donor and the recipient are HLA identical, with graft half-lives of ≥20 years being common between HLA-identical siblings. The reasons for this remarkable success since the 1960’s, throughout all eras of immunosuppressive drug regimens, are still not entirely clear. Absence of anti-HLA antibody risk, and lower frequencies of T cell clones specific for donor antigens, may be 2 contributing factors. However, even without HLA differences, no competent transplant physician would recommend discontinuing all immunosuppression in HLA-identical sibling transplant recipients, since immunity to non-HLA “minor antigens” is known to be sufficient to cause rejection. We have recently speculated that the hidden secret of success of HLA-identical sibling transplants is not simply the lower frequency of T cells specific for minor H antigens only as compared to minor H plus HLA, but rather, an inherent tendency for siblings to develop low-avidity CD8 T regulatory cells specific for each others’ minor H antigens, due to exposure to maternal non-inherited minor H alleles [1]. When, instead of HLA-identical sibs, HLA haploidentical parents or siblings are considered as potential living related donors, immune-mediated graft rejection is a more frequent problem due to potential formation of anti HLA antibodies [2,3] and a high frequency of HLA allospecific T cells [4] that attack the donor tissue. To counteract this problem, immunosuppressive drugs are routinely administered lifelong to prolong graft survival. Unfortunately, these agents greatly increase the risk of systemic infections [5] and may encourage tumor growth [6,7]. So, it is the goal of transplant immunologists to find alternative ways to maintain a functional allograft without using immunosuppressive drugs that have undesirable side effects long term. Taking advantage of natural tolerance induced by HLA as well as minor H non-inherited maternal antigens (NIMA) is one of the more promising but still relatively unexplored approaches for this purpose.
More than fifty years ago, Owen et. al. showed that most Rh− women born of a Rh+ mother failed to produce antibody against Rh antigen when they gave birth to Rh+ children, whereas most Rh− women born of Rh− mothers did make anti-Rh [8]. Class et. al. [9] later found that HLA broadly-sensitized patients (> 90% + for antibody to leukocytes from a random panel of blood donors) commonly failed to produce antibody against mismatched non-inherited maternal HLA class I antigens (NIMA), but were fully capable of producing anti-HLA specific for non-inherited paternal antigens (NIPA control).
One possible mechanism behind the B cell hyporesponsiveness to NIMA is clonal deletion, an idea that was originally proposed to explain the finding that offspring exposed to foreign erythrocyte antigens in utero become lifelong blood cell chimeras [10]. Billingham et. al. [11] later showed when a mouse was exposed to allogeneic cells as a newborn it could accept a skin graft from the donor mouse strain as an adult. However, an alternative interpretation of these classic experiments is that the neonate is not clonally deleted for B and T cells recognizing the foreign (maternal or sibling) antigens, but rather that the acceptance of foreign tissue requires not only an initial exposure to a tolerogenic form of the antigen, but also establishment of a “tolerance network” involving host T regulatory cells and microchimerism.
2. Effects of exposure to NIMA
As discussed previously, exposure to NIMA in early life has life-long tolerogenic effects, which has been shown in both human and animal research.
3.1 NIMA effects in human transplantations
To evaluate NIMA effects in human solid organ transplantation, Burlingham et. al. [12] analyzed the outcome of primary renal transplants between siblings. In the study, haplotype-mismatched kidney graft survival was significantly better in NIMA- than in NIPA- mismatched donor-recipient pairs. Indeed, NIMA sibling haplotype-mismatched kidney graft survival was equal to that of HLA-identical sibling kidney transplants at 10 years [12]. The NIMA effect in solid organ transplants was echoed by studies of patients receiving haploidentical parental or sibling hematopoetic stem cell (HSC) transplants [13,14]. Graft-versus-host disease after bone marrow transplantation was significantly less in NIMA-mismatched siblings than in other haploidentical donor-recipient combinations [13]. Ichinohe et. al. [14] showed similar results in 35 patients who underwent non-T cell depleted hematopoietic stem cell transplantation from NIMA-mismatched donors. Interestingly, all 35 donors were selected on the basis of microchimerism; however, the extent and multi-lineage vs. unilineage form of microchimerism was not analyzed prior to transplantation.
3.2 NIMA effects in animal models
With a mouse F1 backcross breeding model (B6 × BDF1) originally described by Zhang and Miller [15], Andrassy et. al. [16] performed heterotopic heart transplantation, and showed that one could reproduce essential features of the clinical NIMA-tolerance effect found in human sibling kidney transplants. B6 (H2b/b) male mice were mated with BDF1 (H2b/d) female mice (NIMAd mating) to obtain NIMAd-exposed H2b-homozygous offspring. Conversely, BDF1 males were crossed with B6 females (control mating) to obtain NIPAd control mice (also H2b/b). Offspring (~50%) that had inherited the H-2d haplotype (H2b/d) were used as skin donors, while fully allogeneic DBA/2 strain mice were used as heart donors for transplantation experiments in homozygous H-2b offspring. This study was able to reproduce the modest skin graft prolongation in NIMA vs. NIPA controls reported previously [15]. However, to our surprise we found that 57% of NIMAd –exposed offspring exhibited allograft survival of > 180 days without conditioning, whereas the NIPA control F1 backcross offspring uniformly rejected the DBA/2 graft within 11 days. The surviving heart allografts in the NIMAd-exposed group were devoid of any vascular intimal hyperplasia, a common sign of chronic rejection, indicating that the tolerance induced by the NIMA exposure is not only resistant to acute rejection but also to chronic rejection.
Recently the impact of gender and strain differences on the NIMA effect was analyzed in mice. Only 25% of the female recipients in the NIMAd F1 backcross model were tolerant to DBA/2 heart allografts, which was significantly more than that of the female NIPAd control mice, but less than the 57% tolerance rate of NIMAd males [17*]. The weaker NIMA tolerance effect in female offspring may be due to estrogen-mediated enhancement of the CD4+ effector T cell responses [18], which could conceivably overcome T regulatory cell influences in cardiac graft rejection (see below). Another important variable influencing T effector: T regulatory cell balance in response to NIMA is the mouse strain from which the particular NIMAs are derived. Out of 6 different F1 backcross models in which H-2 homozygous offspring were developmentally exposed to H-2d, H-2k, or H-2b alloantigens, only those in which the offspring were developmentally exposed to the H-2d haplotype revealed a significant beneficial effect on heart allograft survival from a H-2 homozygous donor [DBA/2 or BALB/c]. NIMA exposure to H-2b or H-2k induced sensitization rather than tolerance to C3H or C57 BL/6 heart transplants [17*]. These differences may reflect differences in the maturity of the mouse immune system at birth—for example, the CBA (H-2k) does not have mature T cells until d.2 after birth, whereas C57 BL/6 mice have mature T cells at d.17–19 of gestation. In any case, the T and B cells in the offspring exposed to NIMA-H-2k or H-2b appear to be skewed toward sensitization/effector function, and away from tolerance/suppressor function.
Using the “tolerogenic” NIMAd F1 backcross model, a beneficial NIMA effect in mouse bone marrow transplantation has also been demonstrated [19]. In this study, BDF1 mice were transplanted with B6 bone marrow along with bone marrow cells from either the NIMAd-exposed or NIPAd control mice. The first group exhibited less graft versus host disease (GVHD) than the latter; furthermore, the NIMA effect was abolished by depleting TR cells (CD4+CD25+) from the NIMA-exposed donor splenocytes, showing that TR are indeed involved in NIMA-mediated GVHD prevention [19]. Molitor-Dart et. al. [20] showed that the NIMAd-exposed mice can exhibit bystander suppression of TT-mediated delayed type hypersensitivity (DTH) reaction in presence of maternal-type BDF1 antigen, while the NIPAd control mice did not. This bystander suppression could be blocked with anti-IL10 and anti-TGF-β antibody indicating a role of TR in the NIMA-mediated bystander suppression. When the splenocytes of NIMAd-exposed mice were injected i.v into a BDF1 host, they exhibited reduced lymphoproliferation and higher TGF-β expression on the CD4+ T cells than the control mice. When the NIMAd-exposed mice were transplanted with DBA/2 hearts, the regional lymph nodes and the allografts contained a high frequency of IL-10 and TGF-β-producing cells primarily in the CD4+CD25+ T cell population. The allografts were also enriched in LAP+ and Foxp3+ cells with relatively few CD8+ T cells.
A new study of human fetal lymph nodes has had a major impact on the field, shifting the focus of NIMA effects in humans to the T regulatory cell [21**] (For detailed review see Burlingham, WJ [22**]). This study showed suppressed proliferation of fetal T lymphocytes in response to maternal antigen presenting cells (APC), but not to unrelated APC. Fetal T cell proliferative responsiveness to NIMA was restored when CD25+ cells were depleted from the culture. A key finding from the study was that when T cells in the fetal lymph nodes proliferate in response to alloantigen, they acquire Foxp3 expression, and gain suppressive functions. The acquisition of Foxp3 and suppressive function was blocked by an agent that interferes with TGF-β signaling, indicating a role of TGF-β generated in the fetal [but not in adult] lymph node in the induction of the NIMA-specific TR.
NIMA effects are not only restricted in T lymphocytes, but also impact B lymphocytes [8,9]. Vernochet et. al. [23] investigated the effect of NIMA exposure on the development of B lymphocytes having different affinities for a NIMA MHC class I antigen. They used the B cell receptor 3–83 μδ transgenic mice as the male breeder with heterozygous mothers expressing H2Kd along with either H2Kk or H2Kb. The transgenic B cells recognized H2Kk and H2Kb MHC class I antigens (NIMA) with high and intermediate affinities, respectively. They found that when the B cells having higher affinity for the NIMA encountered the NIMA in fetal life, they were partially deleted in late gestation and the remaining transgenic B cells down-regulated their B cell receptors. The B cells also had impaired proliferative response and higher active caspase-3 (a central mediator of apoptosis) compared to the non-exposed controls indicating that exposure to high affinity NIMA leads to B cell apoptosis. In contrast, when the B cells encountered the NIMA to which they had lower affinity, they exhibit activated phenotypes including high proliferation, elevated CD69 expression, and preferential localization adjacent to T cell zones of the spleen. This suggests a mechanism whereby NIMA exposure could lead to sensitization or tolerance at the B cell level, depending on the strain sources of NIMA and the affinities of B cell receptors involved [17*].
3. Maternal Microcimerism (MMc)
What is the basis of the NIMA tolerance effects on T and B cells, leading to allograft survival? One possible answer is that many of us have already managed to accept a ‘mini-graft’ from our mothers in the form of widely disseminated maternal cells. Though fetal and maternal circulations are completely separated, fetal tissue is bathed with maternal blood in hemochorial placenta (e.g, mouse and human placenta) [24,25]. Trafficking of blood cells at the fetal maternal interface is bidirectional [26,27]. Fetal cells can cross the placenta and engraft into different organs of the mother, resulting in fetal microchimerism (FMc) [28–31]. Similarly maternal cells can also cross the placenta and engraft into different organs of the offspring resulting in maternal microchimersm (MMc) [32–35]. Hall et. al. [36] using in situ hybridization showed that 20% of male infants contain female (XX+) cells in their cord blood indicating the presence of maternal cells. MMc has since been detected by more sensitive PCR techniques in > 50% of offspring, in different organs including blood [37], spleen [38], brain [39], heart [38], lungs [38], liver [40], and pancreas [41]. Besides the transplacental migration of maternal cells, offspring can also acquire maternal cells during nursing after birth, which preferentially localize in the liver of the newborn [40].
3.1 Exchange of progenitor cells during pregnancy
Microchimerism has been demonstrated in a wide variety of cell types in peripheral blood and lymphoid organs including T cells [42–44**], B cells [43*], dendritic cells [44**,45], and macrophages [44**]. Khosrotehrani et. al. [43*] crossed Rag-deficient female mice with wild type male mice and recovered functional T and B cells of fetal origin from the spleen of the female mice during pregnancy. The finding of lymphoid and myeloid cells of maternal origin in adult offspring suggests the presence of microchimeric progenitor cells that differentiate into the various cell lineages throughout the life of the animal. Stevens et. al. [46*] showed presence of maternal hepatocytes in liver, renal tubular cells in kidney, and beta cells in pancreas in infants. To account for parenchymal Mc, transfer of stem cells across the placenta has been suggested by different researchers [30,47]. Bianchi et. al. [30], by a nested PCR, detected CD34+ male progenitor cells in the peripheral blood of 1/8 women who were pregnant with male offspring at least 27yrs before the blood sampling. Chen et. al. [48*] injected human mesenchymal stem cells into pregnant rats and showed that the human mesenchymal stem cells crossed the placenta and differentiated into different human mesenchymal cells in the organs of the rat offspring, indicating that mesenchymal stem cells can also cross the placenta, giving rise to multilineage Mc.
3.2 Possible beneficial effects of maternal-fetal cell exchange and microchimerism
Besides the reports relating Mc to autoimmune disease, Mc has also been shown to be associated with tissue regeneration and allograft tolerance.
3.2.1 Tissue regeneration
Cells with multilineage potential transferred across the maternal- fetal interface have tissue regeneration capability after differentiating into different cell lineages [49]. The first evidence came from a study by Starzl et. al. [50]. They studied patients with type IV glycogen storage disease characterized by deposition of amylopectin in the heart and type 1 Gaucher’s diseases characterized by deposition of glycocerebrocides in lymph nodes. The patients displayed significantly decreased depositions of the compounds and systemic donor Mc after liver transplants indicating the role of the Mc in cells of the myeloid lineage, ameliorating the enzyme deficiencies. Another study showed that children suffering from type I diabetes had higher level of MMc in blood and higher number of insulin-producing maternal beta cells in pancreas than control children [41]. If the maternal cells present in blood of the patients have multi-lineage potential, they can conceivably regenerate insulin-producing beta cells, as well as hematopoietic lineage cells. However this point remains unproven at present.
3.2.2 Induction of transplantation tolerance
The proposal first put forward by Starzl et. al. [51] that Mc is required in inducing transplantation tolerance has been controversial [52,53]. Burlingham et. al. [54] showed that cytotoxic T lymphocyte (CTL) unresponsiveness was linked with donor Mc in a patient who received donor-specific transfusion (DST) pretransplant and later became tolerant to a maternal kidney allograft. His PBMC, which contained approximately one donor cell in 104–105 cells showed very little donor specific CTL activity in primary MLR culture. Removal of the donor cells from the recipient PBMC failed to restore primary CTL response to the donor; however stimulation with donor stimulator cells in presence of IL-2 fully restored the anti-donor CTL activity. The anti-donor CTL activity was suppressed after addition of either fresh recipient PBMC or enriched donor cells isolated from the recipient PBMC. No inhibitory effect was seen with donor cell-depleted PBMC, indicating that the donor Mc is responsible for the reduced anti-donor CTL activity. This finding was supported by a study [55], in which B6 mice were engrafted with H8 cells that constitutively expressed H2Db-restricted GP33 epitope of lymphocytic choriomeningitis virus (LCMV). The microchimeric B6 mice elicited GP33-specific CTL unresponsiveness after LCMV challenge, which could be restored by experimental depletion of the microchimeric cells by injecting GP33-specific T cells into the B6 mice. These experiments together suggest that Mc can directly impact CTL responses in the host, and that removal of the rare foreign cells restores CTL activity/proliferation. However it does not account for how the Mc remains tolerogenic rather than sensitizing, to the host. For this we turn to the current focus in the NIMA field on Treg cell development and maintenance.
3.2.3 Induction of TR
Molitor-Dart et. al. [20] showed that exposure to NIMAd results in TGF-β1+ adaptive TR development in some but not all mice. This variability in TR induction could account for the variability in allograft tolerance, since only those NIMAd-exposed mice with strong mobilization of TGFβ1+ TR to the graft were able to accept heart allografts long-term [16]. To investigate whether the variability in the regulation induced through the exposure to the NIMA is due to the variability in the level of MMc, H-2Dd DNA level in 8–9 organs of H-2b homozygous offspring[n=36] of BDF1 mothers was determined by qPCR. All mice had previously been immunized with tetanus toxoid (TT). Spleen cell transfers into footpads of naïve B6 mice in the presence of a)PBS, b)TT, c)BDF1 cell sonicate, or d) BDF1 sonicate plus TT were performed and footpad swelling (DTH) responses were measured at 24 hrs. We found a significant linear correlation between the distribution of MMc (% of Dd + organs) and the % bystander suppression of TT-induced DTH reaction in the presence of maternal-type (BDF1) antigens [44**]. In addition, when injected into BDF1 hosts, CD4+ T cells of NIMAd-exposed mice having high levels of MMc proliferated less, and expressed significantly higher levels of surface TGF-β than CD4+ T cells of NIMAd-exposed mice having no detectable levels of MMc. This suggests a role of MMc in inducing and maintaining NIMA-specific TR; i.e. that the presence of maternal antigens is required for NIMA-specific TR. An alternative idea proposed by Mold et. al. [21**] is that NIMA-specific TR are long lived, and hence like other long-lived memory T cells, antigen-independent. It remains to be seen which hypothesis is correct.
4. Possible mechanisms behind NIMA effects
Several mechanisms behind NIMA effects have been proposed:
4.1 Clonal deletion and anergy
Maternal antigen presenting cells present in the fetal thymus and bone marrow can cause deletion of the NIMA-specific lymphocytes. Maternal thymic epithelial cells, which can serve as APCs in the thymus, have been detected in the NIMAd-exposed offspring [44**]. Vernochet et. al. [23] described partial deletion of B cells having high affinity for the NIMA; however, B cells having low affinity for the NIMA were not clonally deleted. Another study [56] showed central and peripheral deletion of donor specific T cells after establishing mixed chimerism in the recipient with high dose of bone marrow cells. However, low-doses of BMC induced a form of tolerance that was T-regulatory cell dependent, consistent with establishment of microchimerism[Mc] rather than mixed chimerism[56].. Whether deletion of antigen-specific T cells following establishment of Mc as has been reported by Bonilla et. al. [55] is the norm for complex antigens like the major and minor H NIMAs, or merely an artifact of the LCMV epitope- GP33 system remains to be seen. However it seems likely that anergy/deletion of T effector cells due to a form of ‘suppressive’ microchimerism in APC will be a consequence of the establishment of a dominant T regulatory cell population in the host. Alternatively, it is not the microchimeric APC themselves, but maternal antigen acquisition by host APC from these few Mc cells that drives the balance of TE and TR in favor of the latter.
4.2 Induction of NIMA-specific TR
As discussed before, exposure to NIMA in fetal and neonatal life induce NIMA-specific TR. There are several ways by which NIMA-specific TR can be induced.
4.2.1 Oral tolerance by nursing
Since oral tolerance is known to generate TGF-β-producing TR [57,58], oral exposure to maternal MHC antigens present in breast milk [59] may generate NIMA-specific TR cells, which may prevent deletion of maternal cells by NIMA-specific effector T cells resulting in high level of MMc (Fig. 1). Campbell et. al. [60] reported a poor outcome of maternal renal allografts in recipients who were not breast-fed, as compared with breast-fed recipients. Similarly, NIMAd-exposed mice foster nursed by B6 mothers not only lost tolerance to a NIMA-expressing heart allograft [16], but also exhibited significantly reduced levels of MMc; some even became sensitized to NIMA, as if effector T cells were now pre-dominant [44**]. Interestingly, when NIPAd control mice were nursed by BDF1 mothers, 2/7 offspring exhibited H2Dd Mc in their livers but not in other organs (Dutta et. al., unpublished data), consistent with the finding by Zhou et. al. [40] that maternal cells present in the breast milk mainly localize in livers of neonates after nursing. Aoyama et. al. [61**] showed that nursing alone could induce some protection against graft versus host disease (GVHD) in mother after bone marrow transplantation from donors exposed to maternal antigens only through nursing. However, for the maximum protection against GVHD, both the uterine and oral exposures to the maternal antigens were required.
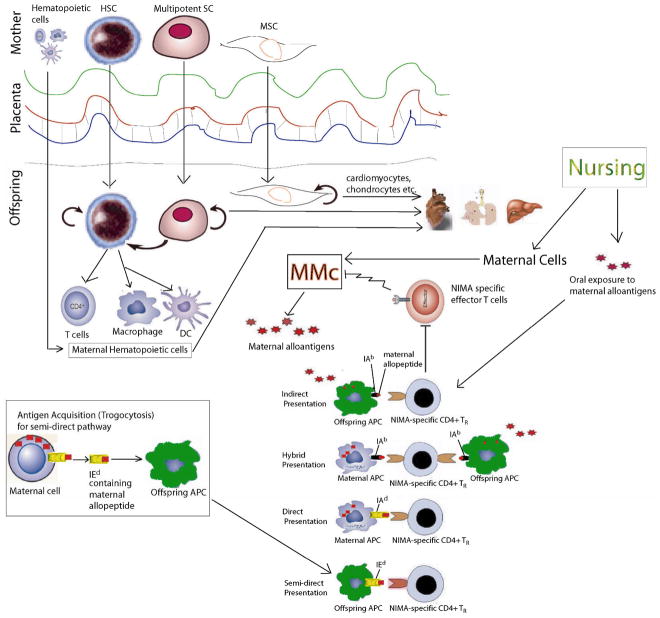
Figure 1. Proposed mechanism of NIMA-induced tolerance in H-2b homozygous offspring of BDF1 female × B6 male (F1 backcross) mice.
Multipotent and pluripotent maternal stem cells can cross the placenta and take residence in different organs of the offspring. The maternal stem cells can differentiate into hematopoietic (including tissue-resident class II+ APC) and parenchymal cell lineages resulting in maternal microchimerism (MMc). Offspring may also obtain maternal cells ingested in the colostrum/milk during nursing. The bottom part of the Figure depicts four different ways that maternal antigens can be presented to NIMA-specific CD4+ TR : 1) by offspring APCs that process and present soluble maternal antigen as allopeptides bound to I-Ab (indirect pathway) ;2) by maternal APC that that presents maternal allopeptide in context of allo MHC class II- direct presentation 3) by rare maternal APC that naturally express the same allopeptide on the shared [inherited maternal] MHC class II—note the possibility for dual or “hybrid” direct/indirect presentation; or 4) by offspring APCs that have acquired membrane from maternal cells via exosomes or by a process known as trogocytosis; these membrane fragments contain non-inherited maternal class I or class II, such as the I-Ed (the “semi-direct” pathway). NIMA-specific TR can block NIMA-specific TE from killing the maternal cells, thus preserving multilineage MMc lifelong.
4.2.2 Hybrid presentation of maternal antigens
We have recently shown MMc in CD11b, CD11c, and CD4-positive cells sorted from spleen and bone marrow [44**]. Presence of MMc in such MHC class II-positive cell populations may indicate the presence of direct pathway of maternal antigen presentation to the NIMA-specific TR cells. This suggests the possibility for inducing a NIMA-specific TR cell able to recognize maternal antigens via both direct and indirect pathways (hybrid presentation) (Fig. 1). TR cells having both indirect and direct allo-specificity have been shown to be especially potent in inducing tolerance to allografts [62**]. Induction of similar TR cells capable of dual recognition may help explain the long-term acceptance of a renal allografts that was HLA class I-mismatched, but HLA class II- closely matched [63*].
4.2.3 Maternal antigen acquisition by offspring cells
Low levels of MMc may not be enough for inducing NIMA-specific TR cells. There should be a way of amplifying the level of maternal antigens in the offspring. We found though lymphoid organs of the NIMAd-exposed mice, such as spleen had very low levels of MMc, they had higher levels of maternal antigen-positive cells than that of NIPAd control mice [44**] indicating the presence of indirect and ‘semi-direct’ [64] pathways of maternal antigen presentation to the NIMA-specific TR cells (Fig. 1). Molitor-Dart et. al. [17*] showed that NIMAk and NIMAb-exposed offspring that do not exhibit the NIMA-induced tolerance do not show maternal antigen acquisition, where as NIMAd-exposed offspring that exhibit the NIMA-induced tolerance do, indicating a role of maternal antigen acquisition in the NIMA-induced tolerance. The source of the maternal antigen may be the MMc present in non-lymphoid organs, which is then acquired by the offspring lymphoid cells. Antigen acquisition by a cell from another genetically non-identical cell is called trogocytosis [65]. Dendritic cells are one of the major cell types that perform trogocytosis [66] by ‘nibbling’ antigens from other cells with LFA-1 [67] and scavenger receptors [68]. T and B cells can also acquire antigens from other cells [69]. Antigen acquired by antigen presenting cells in this way can be presented to T cells, which is called ‘semi-direct’ antigen presentation [64]. Though trogocytosis is a random process, several biological significances of trogocytosis have been described [70], eg. clearance of CD8+ T cells which had acquired cognate MHC class I and peptide ligand by antigen-specific cytolysis, CD4+ T cells acquiring MHC class II and costimulatory molecules may act as antigen presenting cells [71]. Interestingly NK cells [72], CD4+, and CD8+T cells [73] acquiring HLA-G by trogocytosis can also acquire regulatory functions.
5. Conclusions
Although the exact mechanism of NIMA is not known, donor selection based on NIMA in solid organ transplantation could be valuable since HLA identical donors are very rare. Bone marrow and stem cell transplantations will also be benefitted from the better understanding of NIMA effects. The latest finding of our lab that the level of MMc is correlated with the NIMA effects indicates the necessity of the prescreening of the recipients for MMc along with screening assays that can accurately detect NIMA-specific TR/TE balance. The murine data from our lab, as well as preliminary studies in healthy humans [74] suggest that widespread/multilineage microchimerism in APC and parenchymal cells, but not unilineage Mc (for ex., in T cells only) [75**] or Mc in short-lived cell subpopulations (e.g. granulocytes) is correlated with a favorable Treg/Te balance.
References and recommended readings
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Burlingham WJ, Goulmy E. Human CD8+ T-regulatory cells with low-avidity T-cell receptor specific for minor histocompatibility antigens. Hum Immunol. 2008;69:728–731. doi: 10.1016/j.humimm.2008.08.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, Dantal J, Giral M, Blancho G, Cantarovich D, et al. Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005;16:2804–2812. doi: 10.1681/ASN.2004121130. [DOI] [PubMed] [Google Scholar]
- 3.Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, Tsai A, Lei HY. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74:1192–1194. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 4.Gallon L, Watschinger B, Murphy B, Akalin E, Sayegh MH, Carpenter CB. The indirect pathway of allorecognition. The occurrence of self-restricted T cell recognition of allo-MHC peptides early in acute renal allograft rejection and its inhibition by conventional immunosuppression. Transplantation. 1995;59:612–616. [PubMed] [Google Scholar]
- 5.Carstens J, Andersen HK, Spencer E, Madsen M. Cytomegalovirus infection in renal transplant recipients. Transpl Infect Dis. 2006;8:203–212. doi: 10.1111/j.1399-3062.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 6.Lopez MM, Valenzuela JE, Alvarez FC, Lopez-Alvarez MR, Cecilia GS, Paricio PP. Long-term problems related to immunosuppression. Transpl Immunol. 2006;17:31–35. doi: 10.1016/j.trim.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Saf. 2000;23:101–113. doi: 10.2165/00002018-200023020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. Evidence for Actively Acquired Tolerance to Rh Antigens. Proc Natl Acad Sci U S A. 1954;40:420–424. doi: 10.1073/pnas.40.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claas FH, Gijbels Y, van der Velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–1817. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 10.Owen RD. Immunogenetic Consequences of Vascular Anastomoses between Bovine Twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 11.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 12.Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, Brennan DC, de Fijter H, van Gelder T, Pirsch JD, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–1664. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 13.van Rood JJ, Loberiza FR, Jr, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, Champlin RE, Gale RP, Ringden O, Hows JM, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 14.Ichinohe T, Uchiyama T, Shimazaki C, Matsuo K, Tamaki S, Hino M, Watanabe A, Hamaguchi M, Adachi S, Gondo H, et al. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation between noninherited maternal antigen (NIMA)-mismatched family members linked with long-term fetomaternal microchimerism. Blood. 2004;104:3821–3828. doi: 10.1182/blood-2004-03-1212. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Miller RG. The correlation of prolonged survival of maternal skin grafts with the presence of naturally transferred maternal T cells. Transplantation. 1993;56:918–921. doi: 10.1097/00007890-199310000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Andrassy J, Kusaka S, Jankowska-Gan E, Torrealba JR, Haynes LD, Marthaler BR, Tam RC, Illigens BM, Anosova N, Benichou G, et al. Tolerance to noninherited maternal MHC antigens in mice. J Immunol. 2003;171:5554–5561. doi: 10.4049/jimmunol.171.10.5554. [DOI] [PubMed] [Google Scholar]
- 17*.Molitor-Dart ML, Andrassy J, Haynes LD, Burlingham WJ. Tolerance induction or sensitization in mice exposed to noninherited maternal antigens (NIMA) Am J Transplant. 2008;8:2307–2315. doi: 10.1111/j.1600-6143.2008.02417.x. The article reports that exposure to NIMA not only induces tolerance but also sensitization depending upon the breeding system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller D, Chen M, Vikingsson A, Hildeman D, Pederson K. Oestrogen influences CD4+ T-lymphocyte activity in vivo and in vitro in beta 2-microglobulin-deficient mice. Immunology. 1995;86:162–167. [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuoka K, Ichinohe T, Hashimoto D, Asakura S, Tanimoto M, Teshima T. Fetal tolerance to maternal antigens improves the outcome of allogeneic bone marrow transplantation by a CD4+ CD25+ T-cell-dependent mechanism. Blood. 2006;107:404–409. doi: 10.1182/blood-2005-07-3045. [DOI] [PubMed] [Google Scholar]
- 20.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, Torrealba JR, Bobadilla JL, Sollinger HW, Knechtle SJ, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–6761. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 21**.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. The autors reported that exposure to maternal antigens in human fetus induced TR in the lymph node, which is TGF-β-dependent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Burlingham WJ. A lesson in Tolerance-Maternal Instruction to Fetal Cells. The New England Journal of Medicine. 2009;360:1355–1357. doi: 10.1056/NEJMcibr0810752. This is a review of Mold et. al. and provides a new model for NIMA-induced tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernochet C, Caucheteux SM, Gendron MC, Wantyghem J, Kanellopoulos-Langevin C. Affinity-dependent alterations of mouse B cell development by noninherited maternal antigen. Biol Reprod. 2005;72:460–469. doi: 10.1095/biolreprod.104.035048. [DOI] [PubMed] [Google Scholar]
- 24.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 25.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 26.Lo YM, Lo ES, Watson N, Noakes L, Sargent IL, Thilaganathan B, Wainscoat JS. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88:4390–4395. [PubMed] [Google Scholar]
- 27.Rinkevich B. Human natural chimerism: an acquired character or a vestige of evolution? Hum Immunol. 2001;62:651–657. doi: 10.1016/s0198-8859(01)00249-x. [DOI] [PubMed] [Google Scholar]
- 28.Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93:2033–2037. [PubMed] [Google Scholar]
- 29.Aractingi S, Berkane N, Bertheau P, Le Goue C, Dausset J, Uzan S, Carosella ED. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898–1901. doi: 10.1016/S0140-6736(98)05121-6. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo ES, Lo YM, Hjelm NM, Thilaganathan B. Transfer of nucleated maternal cells into fetal circulation during the second trimester of pregnancy. Br J Haematol. 1998;100:605–606. doi: 10.1046/j.1365-2141.1998.0636a.x. [DOI] [PubMed] [Google Scholar]
- 32.Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, Nelson JL. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41–47. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Socie G, Gluckman E, Carosella E, Brossard Y, Lafon C, Brison O. Search for maternal cells in human umbilical cord blood by polymerase chain reaction amplification of two minisatellite sequences. Blood. 1994;83:340–344. [PubMed] [Google Scholar]
- 34.Scaradavou A, Carrier C, Mollen N, Stevens C, Rubinstein P. Detection of maternal DNA in placental/umbilical cord blood by locus-specific amplification of the noninherited maternal HLA gene. Blood. 1996;88:1494–1500. [PubMed] [Google Scholar]
- 35.Petit T, Dommergues M, Socie G, Dumez Y, Gluckman E, Brison O. Detection of maternal cells in human fetal blood during the third trimester of pregnancy using allele-specific PCR amplification. Br J Haematol. 1997;98:767–771. doi: 10.1046/j.1365-2141.1997.2603076.x. [DOI] [PubMed] [Google Scholar]
- 36.Hall JM, Lingenfelter P, Adams SL, Lasser D, Hansen JA, Bean MA. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood. 1995;86:2829–2832. [PubMed] [Google Scholar]
- 37.Loubiere LS, Lambert NC, Flinn LJ, Erickson TD, Yan Z, Guthrie KA, Vickers KT, Nelson JL. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab Invest. 2006;86:1185–1192. doi: 10.1038/labinvest.3700471. [DOI] [PubMed] [Google Scholar]
- 38.Lambert NC, Erickson TD, Yan Z, Pang JM, Guthrie KA, Furst DE, Nelson JL. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction: studies of healthy women and women with scleroderma. Arthritis Rheum. 2004;50:906–914. doi: 10.1002/art.20200. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan J, Land S. Influence of maternal-fetal histocompatibility and MHC zygosity on maternal microchimerism. J Immunol. 2005;174:7123–7128. doi: 10.4049/jimmunol.174.11.7123. [DOI] [PubMed] [Google Scholar]
- 40.Zhou L, Yoshimura Y, Huang Y, Suzuki R, Yokoyama M, Okabe M, Shimamura M. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology. 2000;101:570–580. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson JL, Gillespie KM, Lambert NC, Stevens AM, Loubiere LS, Rutledge JC, Leisenring WM, Erickson TD, Yan Z, Mullarkey ME, et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. Proc Natl Acad Sci U S A. 2007;104:1637–1642. doi: 10.1073/pnas.0606169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambert NC, Evans PC, Hashizumi TL, Maloney S, Gooley T, Furst DE, Nelson JL. Cutting edge: persistent fetal microchimerism in T lymphocytes is associated with HLA-DQA1*0501: implications in autoimmunity. J Immunol. 2000;164:5545–5548. doi: 10.4049/jimmunol.164.11.5545. [DOI] [PubMed] [Google Scholar]
- 43*.Khosrotehrani K, Leduc M, Bachy V, Nguyen Huu S, Oster M, Abbas A, Uzan S, Aractingi S. Pregnancy allows the transfer and differentiation of fetal lymphoid progenitors into functional T and B cells in mothers. J Immunol. 2008;180:889–897. doi: 10.4049/jimmunol.180.2.889. The article reports that fetal hematopoietic progenitor cells can differentiate into adult cells in the mother. [DOI] [PubMed] [Google Scholar]
- 44**.Dutta P, Molitor-Dart ML, Bobadilla J, Zhen Y, Torrealba JR, Roenneburg DA, Burlingham WJ. Microchimerism is strongly correlated with tolerance to non-inherited maternal antigens. Manuscript submitted. This is the first article that links Mc in adult offspring to NIMA-induced tolerance. [Google Scholar]
- 45.Cai J, Lee J, Jankowska-Gan E, Derks R, Pool J, Mutis T, Goulmy E, Burlingham WJ. Minor H antigen HA-1-specific regulator and effector CD8+ T cells, and HA-1 microchimerism, in allograft tolerance. J Exp Med. 2004;199:1017–1023. doi: 10.1084/jem.20031012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Stevens AMHH, Kiefer MM, Rutledge JC, Nelson JL. Chimeric Maternal Cells with Tissue-Specific Antigen Expression and Morphology are Common in Infant Tissues. Pediatr Dev Pathol. 2008;21 doi: 10.2350/08-07-0499.1. The article reports that maternal microchimeric cells express tissue-specific antigens indicating the presence of maternal pluripotent cells in the offspring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianchi DW. Fetomaternal cell traffic, pregnancy-associated progenitor cells, and autoimmune disease. Best Pract Res Clin Obstet Gynaecol. 2004;18:959–975. doi: 10.1016/j.bpobgyn.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 48*.Chen CP, Lee MY, Huang JP, Aplin JD, Wu YH, Hu CS, Chen PC, Li H, Hwang SM, Liu SH, et al. Trafficking of multipotent mesenchymal stromal cells from maternal circulation through the placenta involves vascular endothelial growth factor receptor-1 and integrins. Stem Cells. 2008;26:550–561. doi: 10.1634/stemcells.2007-0406. The article supports the idea that pluripotent maternal stem cells can cross the placenta, take residence in different organs of the fetus and differentiate into adult maternal cells. [DOI] [PubMed] [Google Scholar]
- 49.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. Jama. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 50.Starzl TE, Demetris AJ, Trucco M, Ricordi C, Ildstad S, Terasaki PI, Murase N, Kendall RS, Kocova M, Rudert WA, et al. Chimerism after liver transplantation for type IV glycogen storage disease and type 1 Gaucher’s disease. N Engl J Med. 1993;328:745–749. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, Kocova M, Ricordi C, Ildstad S, Murase N. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–877. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood K, Sachs DH. Chimerism and transplantation tolerance: cause and effect. Immunol Today. 1996;17:584–587. doi: 10.1016/s0167-5699(96)10069-4. discussion 588. [DOI] [PubMed] [Google Scholar]
- 53.Hamano K, Rawsthorne MA, Bushell AR, Morris PJ, Wood KJ. Evidence that the continued presence of the organ graft and not peripheral donor microchimerism is essential for maintenance of tolerance to alloantigen in vivo in anti-CD4 treated recipients. Transplantation. 1996;62:856–860. doi: 10.1097/00007890-199609270-00026. [DOI] [PubMed] [Google Scholar]
- 54.Burlingham WJ, Grailer AP, Fechner JH, Jr, Kusaka S, Trucco M, Kocova M, Belzer FO, Sollinger HW. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995;59:1147–1155. [PubMed] [Google Scholar]
- 55.Bonilla WV, Geuking MB, Aichele P, Ludewig B, Hengartner H, Zinkernagel RM. Microchimerism maintains deletion of the donor cell-specific CD8+ T cell repertoire. J Clin Invest. 2006;116:156–162. doi: 10.1172/JCI26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bemelman F, Honey K, Adams E, Cobbold S, Waldmann H. Bone marrow transplantation induces either clonal deletion or infectious tolerance depending on the dose. J Immunol. 1998;160:2645–2648. [PubMed] [Google Scholar]
- 57.Gonnella PA, Kodali D, Weiner HL. Induction of low dose oral tolerance in monocyte chemoattractant protein-1- and CCR2-deficient mice. J Immunol. 2003;170:2316–2322. doi: 10.4049/jimmunol.170.5.2316. [DOI] [PubMed] [Google Scholar]
- 58.Marth T, Strober W, Kelsall BL. High dose oral tolerance in ovalbumin TCR-transgenic mice: systemic neutralization of IL-12 augments TGF-beta secretion and T cell apoptosis. J Immunol. 1996;157:2348–2357. [PubMed] [Google Scholar]
- 59.Molitor ML, Haynes LD, Jankowska-Gan E, Mulder A, Burlingham WJ. HLA class I noninherited maternal antigens in cord blood and breast milk. Hum Immunol. 2004;65:231–239. doi: 10.1016/j.humimm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Campbell DA, Jr, Lorber MI, Sweeton JC, Turcotte JG, Niederhuber JE, Beer AE. Breast feeding and maternal-donor renal allografts. Possibly the original donor-specific transfusion. Transplantation. 1984;37:340–344. doi: 10.1097/00007890-198404000-00004. [DOI] [PubMed] [Google Scholar]
- 61**.Aoyama K, Koyama M, Matsuoka KI, Hashimoto D, Ichinohe T, Harada M, Akashi K, Tanimoto M, Teshima T. Improved outcome of allogeneic bone marrow transplantation due to breast-feeding-induced tolerance to maternal antigens. Blood. 2009 doi: 10.1182/blood-2008-05-155283. The article reports that both in-utero and oral exposure to the NIMA are important in NIMA-induced tolerance. Oral exposure to the NIMA can itself induce some degree of tolerance to NIMA. [DOI] [PubMed] [Google Scholar]
- 62**.Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, Stauss HJ, Bucy RP, Lombardi G, Lechler R. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. The article says TR having both indirect and direct allospecificity are very potent in inducing allograft tolerance. Similarly offspring TR that can be presented with antigens by both maternal APC (direct presentation) and offspring APC (indirect presentation) may be very potent in inducing NIMA tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Xu Q, Lee J, Keller M, Burlingham WJ. Analysis of indirect pathway CD4+ T cells in a patient with metastable tolerance to a kidney allograft Possible relevance to superior graft survival of HLA class II closely matched renal allografts. Transpl Immunol. 2009;20:203–208. doi: 10.1016/j.trim.2008.12.005. The article confirms importance of presence of TR having indirect and direct allospecificity in allograft tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 65.Daubeuf S, Puaux AL, Joly E, Hudrisier D. A simple trogocytosis-based method to detect, quantify, characterize and purify antigen-specific live lymphocytes by flow cytometry, via their capture of membrane fragments from antigen-presenting cells. Nat Protoc. 2006;1:2536–2542. doi: 10.1038/nprot.2006.400. [DOI] [PubMed] [Google Scholar]
- 66.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 67.Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179:1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 68.Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302–2309. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 69.Hudrisier D, Aucher A, Puaux AL, Bordier C, Joly E. Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J Immunol. 2007;178:3637–3647. doi: 10.4049/jimmunol.178.6.3637. [DOI] [PubMed] [Google Scholar]
- 70.Smyth LA, Afzali B, Tsang J, Lombardi G, Lechler RI. Intercellular transfer of MHC and immunological molecules: molecular mechanisms and biological significance. Am J Transplant. 2007;7:1442–1449. doi: 10.1111/j.1600-6143.2007.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caumartin J, Lemaoult J, Carosella ED. Intercellular exchanges of membrane patches (trogocytosis) highlight the next level of immune plasticity. Transpl Immunol. 2006;17:20–22. doi: 10.1016/j.trim.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 72.Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J. Trogocytosis-based generation of suppressive NK cells. Embo J. 2007;26:1423–1433. doi: 10.1038/sj.emboj.7601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, Carosella ED. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–2048. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 74.van Halteren AGS, Jankowska-Gan E, Joosten A, Blokland E, Pool J, Brand A, Burlingham WJ, Goulmy E. Naturally acquired tolerance and sensitization to minor histocompatability antigens in healthy family members. Blood (in review) 2009 doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75**.Chan WF, Razavy H, Luo B, Shapiro AM, Anderson CC. Development of either split tolerance or robust tolerance along with humoral tolerance to donor and third-party alloantigens in nonmyeloablative mixed chimeras. J Immunol. 2008;180:5177–5186. doi: 10.4049/jimmunol.180.8.5177. The article reports that presence of unilineage Mc generates split tolerance resulting in allograft rejection, whereas multilineage Mc can induce tolerance. [DOI] [PubMed] [Google Scholar]