Abstract
Among many proinflammatory cytokines, interleukin-1β (IL-1β) is considered a key mediator of neuronal injury. However, in order to become activated, it must be processed and cleaved by a caspase-1 enzyme. In this study, we tested the neuroprotective effect of Ac-YVAD-CMK, a known selective caspase-1 inhibitor, in a mouse model of intracerebral hemorrhage (ICH). Sixty-six adult male CD-1 mice were subjected to collagenase-induced ICH. Ac-YVAD-CMK or vehicle was administered into the left lateral ventricle 20 min before ICH modeling. Brain edema and neurological functions were assessed at 24 and 72 h after the surgery. Expression of IL-1β, phosphorylated JNK, tight junction protein zona occludens 1 (ZO-1), and matrix metalloproteinase-9 (MMP-9) were measured by Western blot along with MMP-9 activity measured by zymography at 24 h after ICH. At 24 h after ICH, Ac-YVAD-CMK treatment significantly reduced brain edema and improved neurological functions. The neuroprotection was associated with downregulation of IL-1β, JNK, MMP-9, and an inhibition of ZO-1 degradation in brain. We conclude that Ac-YVAD-CMK protects the brain against ICH-induced injury, and the neuroprotective effect may result from anti-inflammation-induced blood–brain barrier protection.
Keywords: Collagenase, Intracerebral hemorrhage, Edema, IL-1β
Introduction
Intracerebral hemorrhage (ICH) is a devastating stroke subtype that accounts for roughly 15% of all strokes. It continues to be associated with a negative outcome with 40–50% of affected patients dying within the first 30 days and the rest with horrendous chronic disabilities [1]. Various experimental studies have looked at the possible underlying mechanisms behind the consequences of ICH. These include upregulation of pro-inflammatory cytokines, apoptotic death of surrounding hematomal cells, and direct brain tissue damage from the growing hematoma. Among many proinflammatory cytokines, interleukin-1β (IL-1β) is considered a key instigator of neuronal injury during central nervous system injuries [2].
One such consequence of IL-1β upregulation is the activation of matrix metalloproteinase-9 (MMP-9), a crucial protein responsible for the degradation of tight junction proteins, such as ZO-1, in the blood–brain barrier (BBB) [3]. The loss of key tight junction proteins in the endothelial lining facilitates capillary leakage leading to edema accumulation in the brain and subsequent damage. Recent experimental studies have linked MMP-9 induction by IL-1β through activation of the JNK pathway [4]. However, whether or not IL-1β is responsible for the downstream effects leading to BBB disruption and subsequent vasogenic edema remains unproven in ICH models.
In this study, we hypothesize that mice subjected to ICH brain injury will develop significant brain edema secondary to degradation of BBB tight junction proteins. This will occur through upregulation of the proinflammatory cytokine IL-1β, mediated through JNK activation and subsequent upregulation of MMP-9 s. We will attempt to prove the mechanism by blocking the activation of IL-1β with a known caspase-1 inhibitor, Ac-YVAD-CMK, that selectively inhibits the cleavage of inactive IL-1β, and thus should reduce the activation of IL-1β, JNK, and MMP-9 and decrease ZO-1 degradation.
Materials and Methods
All procedures for this study were approved by the Animal Care and Use Committee at Loma Linda University and complied with the NIH Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication 85-23, revised 1985) and with Guidelines for the Use of Animals in Neuroscience Research by the Society for Neuroscience.
Sixty-six CD-1 male mice, weighing 35–40 g, were randomly assigned to four groups: sham (n=20), vehicle [ICH + dimethyl sulfoxide (DMSO), n=20], low-dose (ICH + Ac-YVAD-CMK 50 ng/mice, n=6), and high-dose (ICH + Ac-YVAD-CMK 200 ng/mice, n=20) groups. Mice were housed in a 12-h light/dark cycle at a controlled temperature and humidity with free access to food and water. All neurological tests were performed during the light cycle.
Mouse ICH Model
We used the collagenase-induced ICH model as previously described in mice [5]. Briefly, mice were anesthetized with an intraperitoneal injection of ketamine/xylazine (100/10 mg/kg), and positioned prone in a stereotactic head frame (Kopf Instruments, Tujunga, CA). An electronic thermostat-controlled warming blanket was used to maintain the core temperature at 37°C±0.5°C. A midline scalp incision from the nasion to the superior nuchal line was made, exposing the calvarium. A cranial burr hole (1 mm) was then drilled 0.9 mm posterior to bregma and 1.5 mm lateral to midline. A 27-gauge needle was inserted stereotactically into the right basal ganglia approximately 4.0 mm in depth. The collagenase (VII-S, Sigma, St. Louis, MO; 0.05 U in 0.5 µl of saline) was infused into the brain over 2 min at a rate of 0.25 µl/min with a microinfusion pump (Harvard Apparatus, Holliston, MA). Sham-operated mice were subjected to needle insertion only. The needle was left in place for an additional 10 min after injection to prevent possible leakage of the collagenase solution. After removal of the needle, the skull hole was closed with bone wax, the incision was closed with sutures, and the mice were allowed to recover. To avoid postsurgical dehydration, normal saline was given to each mouse by subcutaneous injection in the amount of 2% of body weight immediately after surgery.
Drug administration and Intracerebral Ventricular Injections
As previously described [6], Ac-YVAD-CMK was administered intraventricularly 20 min before induction of ICH. The drug was dissolved in DMSO and further diluted in phosphate-buffered saline (PBS, final concentration of DMSO<0.2%). Low-dose (50 ng/mice, 1 µL) or high-dose (200 ng/mice, 1 µL) Ac-YVAD-CMK (Cayman Chemical Company, Ann Arbor, MI) was then injected into the right lateral ventricle (coordinates: 0 mm bregma, 1 mm lateral, and 2.5 mm ventral) using a 27-gauge needle. Vehicle group was treated with the same volume of DMSO diluted in PBS. Sham group only received needle insertion.
Neurobehavioral Tests
Sensorimotor Test
Neurological outcomes were assessed by a blind observer prior to euthanasia at 24 and 72 h using a modified 21-point sensorimotor scoring system as previously reported [7]. The scoring system consists of seven tests with scores of 1–3 for each test. These seven tests are as follows: (1) spontaneous activity, (2) symmetry in the movement of four limbs, (3) forepaw outstretching, (4) lateral turning, (5) climbing, (6) body proprioception, and (7) response to vibrissae touch.
Corner Turn Test
For the corner turn test, the mouse was allowed to walk down a corridor into a 30° corner. To exit the corner, the animal could turn either to the right or left. The mouse's choice of turn direction was noted. The number of right and left turns out of ten total attempts was recorded [8], and the percentage of right turn to all trials (ten trials) was calculated.
Beam Balance
Beam balance test was modified from previous description [9]. Beam (90 cm length and 1 cm diameter cylindrical) was mounted between two platforms. Mice were placed on each platform to run freely for 10 s before the first trial. Then, mice were positioned on the middle of the beam and allowed to move toward the platform. The performance of the mice was scored using a six-point scale assigned as follows: 0, fall off the beam within 40 s without moving; 1, did not move but held on for at least 40 s; 2, moved but not more than half the distance to the platform in 40 s and stays on for at least 25 s; 3, moved more than half the distance to the platform in 40 s and stays on for at least 25 s; 4, move onto either platform in 40 s or moved to the platform within 25 s but did not get onto the platform; and 5, moved onto either platform in 25 s. An average score of three trials was calculated.
Wire Hanging
The wire hanging test was used to measure motor strength [10]. An individual mouse was placed on top of a standard mouse cage wire lid where it was gently waved in the air three times to make the mouse firmly grip onto the wires. The lid was then immediately turned upside down and held at approximately 70 cm above the home cage for 5 min. Latency to fall was measured and averaged for three consecutive trials with 5 min inter-intervals between trials.
Brain Water Content
Brain water content was measured as previously described [5]. Briefly, mice were decapitated under deep anesthesia and brain immediately removed and divided into five parts: ipsilateral context, ipsilateral basal ganglia, contralateral context, contralateral basal ganglia, and cerebellum. The cerebellum was used as an internal control for brain water content. Tissue samples were then weighed on an electronic analytical balance (APX-60, Denver Instrument; Arvada, CO) to the nearest 0.1 mg to obtain the wet weight (WW). The tissue was then dried at 100°C for 24 h to determine the dry weight (DW). Brain water content (percent) was calculated as [(WW−DW)/WW]×100.
Western Blotting
Western blot analysis was performed as described previously [11]. Ipsilateral hemispheres (n=6 per group) were homogenized, and aliquots were used to determine the protein concentration of each sample using a detergent compatible assay (Bio-Rad, Philadelphia, PA). Protein samples (50 µg) were loaded on a Tris-glycine 10% gel, electrophorese, and transferred to a nitrocellulose membrane. Membranes were incubated overnight at 4°C with the primary antibodies: rabbit polyclonal anti-IL-1β (Abcam, Cambridge, MA; BioVision, Mountain View, CA), rabbit polyclonal anti-zonula occludens (ZO-1), rat monoclonal anti-MMP-9, and rabbit polyclonal anti-JNK (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoblots were processed with secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at 21°C, probed and then exposed to X-ray film. Blot bands were quantified using Image J software (NIH). β-Actin (Santa Cruz Biotechnology, Santa Cruz, CA) was blotted on the same membrane as a loading control because β-actin levels are reported to be unchanged in the brain [11, 12].
MMP Gelatin Zymography
To determine the effect of ICH on MMP-9 activity in the brain, deeply anesthetized rats were killed at 24 h after surgery. The ipsilateral hemispheres were homogenized in lysis buffer on ice. Homogenates were centrifuged, and total protein concentrations in the resulting supernatants were determined. For the MMP-9 zymography, we used a protocol published previously with modifications [5, 13]. Briefly, equal amounts (50 µg) of total protein extracts were prepared and separated by 10% Tris-glycine gel with 0.1% gelatin as substrate (Bio-Rad). After separation, the gel was renatured and incubated with developing buffer at 37°C for 48 h. After developing, the gel was stained with 0.5% Coomassie blue R-250 for 60 min and then destained. Gelatinolytic activity was determined as clear zones or bands at the appropriate molecular weights. MMP-9-positive controls from Chemicon were used as standards [5].
Statistical Analysis
Results are presented as mean ± SEM. Data were statistically analyzed with one-way analysis of variance (ANOVA) followed by Tukey multiple comparison post-hoc analysis. For beam balance and wire hanging test, Dunn's one-way ANOVA on ranks was applied. P<0.05 was considered statistically significant.
Results
Neurological Function and Behavioral Tests
At 24 h after ICH, marked functional deficits were seen in mice. Assessment by the 21-point scoring system, corner turn test, beam balance, and wire hanging tests resulted in no beneficial effect in the low-dose Ac-YVAD-CMK treatment group, whereas the high-dose Ac-YVAD-CMK treatment group showed significantly improved neurological function (Fig. 1). These results were consistent at 72 h after ICH (Fig. 2) with the high-dose Ac-YVAD-CMK treatment group showing significantly improved neurological function.
Fig. 1.
High-dose Ac-YVAD-CMK treatment improved neurological functions at 24 h after ICH. a Neurological score for sensorimotor function; b corner test performance expressed by the normalized laterality index; c beam balance; d wire hanging. Values are expressed as mean ± SEM. An asterisk represents significantly different by ANOVA on ranks #P<0.001 vs. sham group; *P<0.01 vs. Vehicle group. Low dose: ICH treated with Ac-YVAD-CMK at 50 ng/mice. High dose: ICH treated with Ac-YVAD-CMK at 200 ng/mice. Vehicle: ICH treated with DMSO
Fig. 2.
High-dose Ac-YVAD-CMK treatment improved neurological functions at 72 h after ICH. a Neurological score, b corner test performance expressed by the normalized laterality index, c beam balance, d wire hanging. Values are expressed as mean ± SEM. An asterisk represents significantly different by Dunn's ANOVA on ranks #P<0.05 vs. sham group; *P<0.05 vs. vehicle group
Brain Water Content
At 24 h, ICH mice groups demonstrated a marked increase in brain water content in both ipsilateral basal ganglia and cortex. Low-dose Ac-YVAD-CMK treatment did not reduce the brain water content at 24 h after ICH; however, the high-dose Ac-YVAD-CMK treatment did significantly reduce the water content in both ipsilateral cortex and basal ganglia (Fig. 3). Brain water content in the contralateral cortex, basal ganglia, and cerebellum were not different in Ac-YVAD-CMK-treated, vehicle-treated, and sham animals. These findings were consistent at brain water content measurements at 72 h.
Fig. 3.
Brain edema was measured by the changes in percentage of water content in mouse brain in all groups at 24 and 72 h after ICH. Water content in the ipsilateral basal ganglia and cortex increased after collagenase injection in all ICH mice. Brain water content was significantly reduced in high-dose Ac-YVAD-CMK-treated mice compared with that of vehicle-treated mice at 24 h (a) and 72 h (b) after ICH. ANOVA, P<0.05. No water content change was observed in contralateral basal ganglia and cortex and cerebellum in all groups, ANOVA, P>0.05. Ipsi-RC ipsilateral (right) cortex, Cont-LC contralateral (left) cortex, Ipsi-RB ipsilateral (right) basal ganglia, Cont-LB contralateral (left) basal ganglia, Cerebel cerebellum
Effect of Ac-YVAD-CMK on the Expression of IL-1β after ICH
The mature form of IL-1β was found to be at significantly higher levels in the ICH (vehicle) group than in the sham group at 24 h post-ICH (Fig. 4). High level Ac-YVAD-CMK treatment significantly reduced the levels of mature IL-1β, compared with vehicle group.
Fig. 4.
Western blots showed an increase in mature IL-1β protein at 24 h after ICH. The increase in mature IL-1β was significantly ameliorated by high-dose Ac-YVAD-CMK treatment. a Representative western blots; b densitometric analysis; n=6 animals per group, ANOVA, #P<0.01 vs sham group, *P<0.001 vs vehicle group
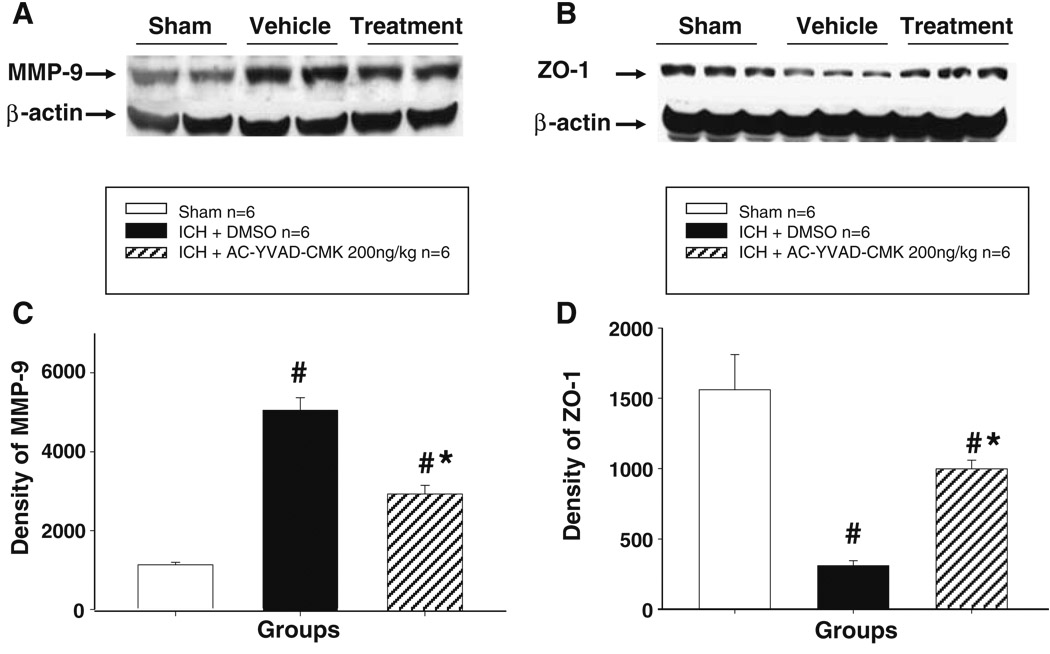
Effect of Ac-YVAD-CMK on the Expression and Activity of MMP-9
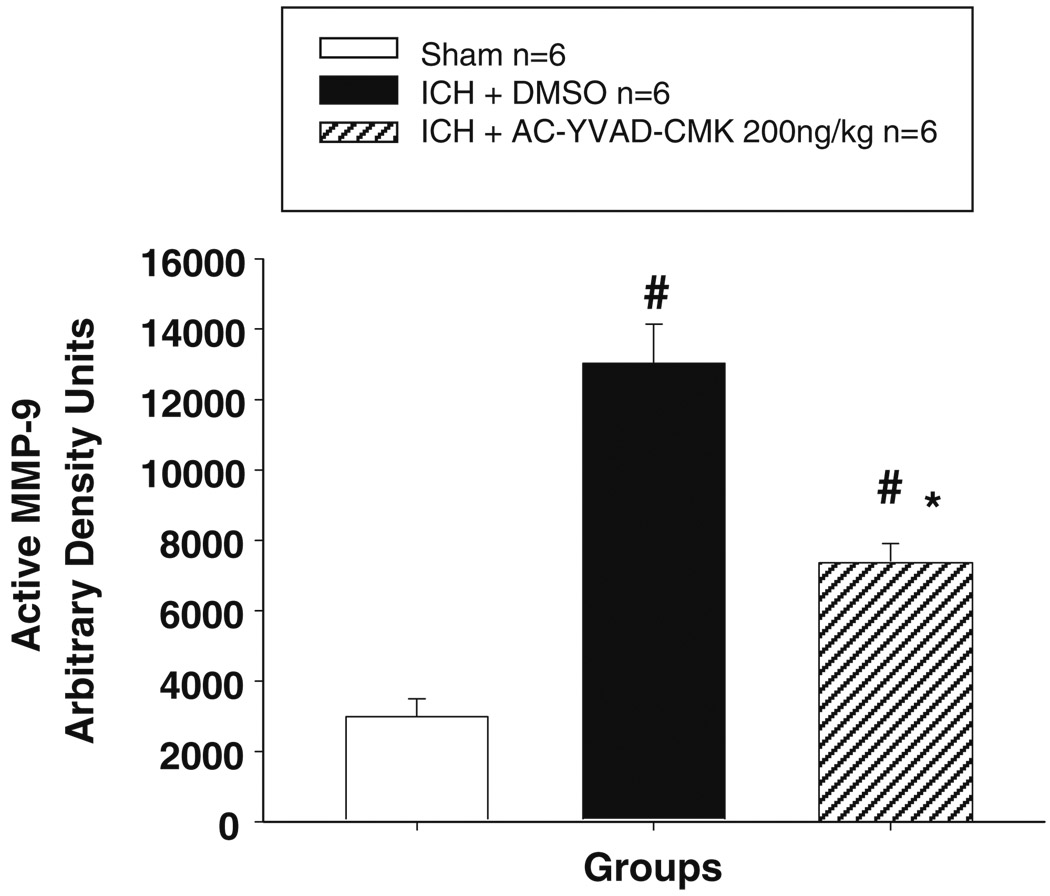
Consistent with previous reports, ICH produced a marked increase in MMP-9 expression as shown in Western blot (Fig. 6a, c) and an increase in the level of active form of MMP-9 as shown in zymography (Fig. 5). High-dose Ac-YVAD-CMK treatment significantly reduced the expression of MMP-9 and MMP-9 activity in the brain at 24 h after ICH.
Fig. 6.
Western blot analysis of MMP-9 and ZO-1. a Representative Western blots of MMP-9 protein expression at 24 h after ICH. MMP-9 expression was increase after ICH; high-dose Ac-YVAD-CMK treatment markedly reduced MMP-9 expression; b representative Western blots of ZO-1 protein at 24 h after ICH. ICH resulted in degradation of ZO-1. High-dose Ac-YVAD-CMK treatment significantly protected ZO-1 from degradation; c densitometric analysis demonstrated that the changes of MMP-9 were statistically significant. d Densitometric analysis demonstrated that the changes of MMP-9 were statistically significant; n=6 animals per group, mean ± SD, ANOVA, #P<0.001 vs. sham group; *P<0.001 vs. vehicle group
Fig. 5.
Densitometric analysis of gelatin zymography showed upregulation of MMP-9 activation at 24 h after ICH; high-dose Ac-YVAD-CMK treatment markedly reduced MMP-9 activity. n=6 animal per group, mean ± SEM, ANOVA, #P<0.05 vs. sham group; *P<0.05 vs. vehicle group
Effect of Ac-YVAD-CMK on ZO-1 Degradation
ZO-1 demonstrated significant degradation after ICH in the vehicle-treated group compared with sham group (Fig. 6b, d). Treatment with high-dose Ac-YVAD-CMK significantly inhibited the degradation of ZO-1 compared with vehicle-treated group.
Effect of Ac-YVAD-CMK on Activation of JNK
The phosphorylated JNK increased significantly in the vehicle-treated ICH group compared with sham group (Fig. 7). The levels decreased significantly with treatment by high-dose Ac-YVAD-CMK compared with vehicle group 24 h post-ICH.
Fig. 7.
a Representative western blots of phosphorylated JNK protein at 24 h after ICH. Phosphorylated JNK was markedly increased after ICH. This change was significantly ameliorated by high-dose Ac-YVAD-CMK treatment. b Densitometric analysis demonstrated that the changes of MMP-9 were statistically significant. n=6 animals per group, mean ± SD, ANOVA, #P<0.001 vs. sham group; *P<0.001 vs. vehicle group
Discussion
The results of the current study established a few significant findings. First, IL-1β processing and activation were seen in the ipsilateral cerebral hemisphere 24 h after ICH. Second, the caspase-1 inhibitor, Ac-YVAD-CMK, blocks the processing and activation of IL-1β and, in doing so, diminishes the consequences of ICH brain injury. Finally, the neuroprotective effects of Ac-YVAD-CMK were facilitated through inhibition of JNK phosphorylation, MMP activation, and the downstream blockage of tight junction protein ZO-1 degradation.
To the best of our knowledge, this is the first study describing the protective effects of an intraventricular administration of Ac-YVAD-CMK against brain injury after ICH.
Originally, we hypothesized that mice subjected to ICH brain injury would develop significant brain edema secondary to BBB degradation of tight junction proteins. The data collected from this study supports our hypothesis. In all ipsilateral brain content measurements, the increased levels of IL-1β, JNK, and MMP-9 witnessed after 24 h ICH were reversed almost equivalently to the level of the sham group with drug administration. Furthermore, the degradation of tight junction protein ZO-1 decreased significantly.
Cytokines are primary mediators of the inflammatory response, with IL-1β being one of the most extensively studied to date [2]. Normally found at low or undetectable levels in the brain, IL-1β can be rapidly upregulated by various experimental brain injuries. However, before mounting an appropriate response, IL-1β must be cleaved and processed by caspase-1.
By inhibiting caspase-1, we were able to demonstrate the neuroprotective effects of IL-1β inhibition, confirming previously reported data. In one study conducted by Rothwell et al. [14], they demonstrated the protective effects of IL-1 inhibition in ischemic brain injury models. In addition, Masada et al. reported on the attenuation of brain edema and thrombin-induced inflammation following the overexpression of IL-1ra via an adenovirus vector in autologous blood injection ICH model in rats [15].
To take the study a step further, we examined the mechanisms behind the pathogenesis of ICH brain injury by focusing on the IL-1β/MMP-9 interface. According to previous works [3], IL-1β cell stimulation activates MMP-9 secretion and allows for degradation of neurovascular matrix integrity, specifically, degradation of tight junction proteins that are responsible for interconnecting endothelial cells and preserving BBB impermeability. In our study, we observed that activated IL-1β and MMP-9 resulted in peripheral tight junction protein ZO-1 degradation, and administration of a caspase-1 inhibitor attenuated the breakdown during ICH. These findings support the hypothesis that IL-1β may in fact be a key mediator of MMP-9 activation and subsequent BBB disruption.
This study also found a strong indication that the caspase-1 inhibitor, Ac-YVAD-CMK, provided neuroprotection during brain injury via inhibition of JNK phosphorylation. According to previous studies [4], IL-1β appears to activate multiple signaling pathways, including the three major MAP kinases, ERK, JNK, and p38. Their roles in IL-1β have yet to be studied. However, it is thought that MMP-9 may in fact be upregulated by IL-1β via one of these signaling pathways, at least partly via the JNK pathway. In our study, we found that Ac-YVAD-CMK administration inhibited the phosphorylation of JNK, MMP-9 induction, and ZO-1 degradation, leading to the improvement of outcome.
In conclusion, the possibilities behind blocking the activation of IL-1β could be a new strategy in combating the effects of post-ICH brain injury sequela.
Acknowledgments
This study was supported by grants from NMTB to J. Tang.
Contributor Information
Bihua Wu, Department of Physiology and Pharmacology, School of Medicine, Loma Linda University, Loma Linda, CA 92354, USA; Department of Neurology, Affiliated Hospital, North Sichuan Medical College, Sichuan, China.
Qingyi Ma, Department of Physiology and Pharmacology, School of Medicine, Loma Linda University, Loma Linda, CA 92354, USA.
Nikan Khatibi, Department of Anesthesiology, Loma Linda University, Loma Linda, CA 92350, USA.
Wanqiu Chen, Department of Physiology and Pharmacology, School of Medicine, Loma Linda University, Loma Linda, CA 92354, USA.
Takumi Sozen, Department of Physiology and Pharmacology, School of Medicine, Loma Linda University, Loma Linda, CA 92354, USA.
Oumei Cheng, Department of Physiology and Pharmacology, School of Medicine, Loma Linda University, Loma Linda, CA 92354, USA.
Jiping Tang, Email: jtang@llu.edu, Department of Physiology and Pharmacology, School of Medicine, Loma Linda University, Loma Linda, CA 92354, USA.
References
- 1.Rincon F, Mayer SA. Novel therapies for intracerebral hemorrhage. Curr Opin Crit Care. 2004;10:94–100. doi: 10.1097/00075198-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Simi A, Tsakiri N, Wang P. Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans. 2007;35(part 5):1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- 3.Ruhul Amin AR, Senga T, Oo ML, Thant AA, Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: a role for the dual signaling pathways, Akt and Erk. Genes Cells. 2003;8:515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 4.Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, Yang CM. Interleukin-1beta induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-kappaB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol. 2007;211(3):759–770. doi: 10.1002/jcp.20992. [DOI] [PubMed] [Google Scholar]
- 5.Tang J, Liu J, Zhou C, et al. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94:1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- 6.Doyle KP, Yang T, Lessov NS, Ciesielski TM, Stevens SL, Simon RP, King JS, Stenzel-Poore MP. Nasal administration of osteopontin peptide mimetics confers neuroprotection in stroke. J Cereb Blood Flow Metab. 2008;28(6):1235–1248. doi: 10.1038/jcbfm.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo W, Bravo T, Jadhav V, Titova E, Zhang JH, Tang J. NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci Lett. 2007;414(3):228–232. doi: 10.1016/j.neulet.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 9.Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ, Schmid-Elsaesser R. Neurological impairment in rats after transient middle cerebral artery occlusion: a comparative study under various treatment paradigms. Brain Res. 2002;28:94–105. doi: 10.1016/s0006-8993(00)02100-4. [DOI] [PubMed] [Google Scholar]
- 10.Hong SM, Liu Z, Fan Y, Neumann M, Won SJ, Lac D, Lum X, Weinstein PR, Liu J. Reduced hippocampal neurogenesis and skill reaching performance in adult Emx1 mutant mice. Exp Neurol. 2007;206(1):24–32. doi: 10.1016/j.expneurol.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Jadhav V, Tang J, Zhang JH. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxicischemic model. Neurobiol Dis. 2008;31(3):433–441. doi: 10.1016/j.nbd.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Mori T, Jung JC, Fini ME, Lo EH. Secretion of matrix metalloproteinase-2 and -9 after mechanical trauma injury in rat cortical cultures and involvement of MAP kinase. J Neurotrauma. 2002;19:615–625. doi: 10.1089/089771502753754082. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell N. Interleukin-1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain Behav Immun. 2003;17(3):152–157. doi: 10.1016/s0889-1591(02)00098-3. [DOI] [PubMed] [Google Scholar]
- 15.Masada T, Hua Y, Xi G, Yang GY, Hoff JT, Keep RF. Attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist. J Neurosurg. 2001;95(4):680–686. doi: 10.3171/jns.2001.95.4.0680. [DOI] [PubMed] [Google Scholar]