Abstract
IL-17A and F regulate granulopoiesis and are produced by memory T cells. Recombinase activating gene (Rag)1−/− mice cannot produce mature T cells, but maintain normal neutrophil counts. Nude mice are neutropenic or have near-normal neutrophil counts, depending on the prevailing intestinal flora. Athymic nude mice can produce IL-17F, but not A. By contrast, thymi from Rag1−/− mice contain as much IL-17A as those from wild-type (WT) mice. IL-17A-producing cells are found in the double negative (DN)1 compartment of the Rag1−/− thymus, and express intracellular CD3. These cells colonize the spleen and MLN and secrete IL-17A in vitro following stimulation with IL-23, at a level similar to WT splenocytes. Adoptively transferred Rag1−/− or WT thymocytes correct neutrophil counts in neutropenic nude mice. We conclude that the development of IL-17A-producing T-lineage cells requires an intact thymic epithelium, but not V(D)J recombination.
Keywords: IL-17A, neutrophils, nude mice, Rag1−/−, granulopoiesis
IL-17A has pleiotropic effects and is important in the pathology of many disease processes including rheumatoid arthritis (1–3), experimental autoimmune encephalitis (4,5) and inflammatory bowel disease (6,7). IL-17A also controls neutrophil homeostasis (8–11) and elevated granulopoiesis induced by infections (12–16). IL-17A is produced by activated T cells with a memory phenotype (CD44high CD62Llow) (17). Three major subpopulations of IL-17A-producing T cells have been identified, which we have collectively termed neutrophil-regulatory T cells; CD4−CD8−αβlow, CD4+CD8−αβ+ (Th17 cells) and γδ+ T cells (10,18). γδ+ cells are the most common IL-17A-producing T cells in C57/BL6 WT mice, and CD4−CD8−αβlow are the most common in severely neutrophilic mice (15,18). Naïve CD4+ T cells develop into Th17 cells in the presence of IL-6, IL-21 transforming growth factor (TGF)-β1 and T cell receptor (TCR) stimulation, if the Th1 and Th2 cytokines IFN-γ and IL-4 are neutralized (5,19–23). Some IL-17A-producing T cells express CD8 (24). Beyond T cells, IL-17A has been shown to be produced by lymphoid tissue inducer (LTi) cells (25). The baseline serum concentration of IL-17A in WT mice, in the absence of inflammation, is below or near the detection limit of existing ELISA's (26,27).
Much less is known about IL-17F, which has been reported to be involved in inflammatory diseases of the airways (28). In the initial description of the IL-17F knockout mouse (Il17f−/−), it appeared that absence of IL-17F alone did not produce much of a spontaneous phenotype, but neutrophil counts and granulopoiesis were not investigated (Meeting of the Japanese Society for Immunology, Osaka 2006). When challenged, T cells from Il17f−/− mice showed reduced secretion of IL-17A in response to immunization with KLH and increased IgG2a production (29). Il17f−/− mice showed a striking lack of CCL2, CCL5 and CCL7 expression in a model of dextran sulfate-sodium-induced colitis (29). In a separate study, Ishigame et al. found that IL-17F was also produced by intestinal epithelial cells (30). In many situations, IL-17A and F are produced by the same cells, but both IL-17A and IL-17F single positive populations can exist (23,31).
T cell development occurs in phases of increasing lineage commitment (32). Some murine T cell deficiency models are neutropenic and have perturbed granulopoiesis, suggesting that basal granulopoiesis is regulated by mature T cells (33,34). One neutropenic model is the nude mouse, which has a mutation at the nude locus in the whn gene, also called Foxn1, resulting in an undeveloped thymic epithelium and almost no thymus-derived T cells (35). In nude and athymic mice, neutropenia is thought to occur due to an accumulation of immature myeloid and band cells in the bone marrow (BM), as neutrophils are unable to differentiate effectively (33,36). In other reports, athymic mice have been shown to have normal or even elevated blood neutrophil counts (37–39). The reason for this discrepancy is unknown. In contrast to nude mice, the number of blood neutrophils is normal in RAG-1 deficient mice, although these mice do not produce mature T cells owing to an absolute block during T cell development at the DN3 stage due to defective V(D)J rearrangement (40,41). Similar to Rag1−/− mice, Mybf/f cwLckCre mice also have a block at the DN3 to DN4 stage of thymocyte development (42) but no B cell defect. These mice have a normal phenotype, although their total thymus cellularity is reduced by 70% compared to littermate controls due to a lack of double-positive cells (42). Blood neutrophil numbers were not previously reported in Mybf/f cwLckCre mice (42).
Previous work has demonstrated that adoptive transfer of WT thymocytes can correct the neutropenia found in nude mice (33,43). These studies predated the discovery of the role of IL-17A and F in neutrophil homeostasis (10), so IL-17A and F-producing cells were not investigated. To test whether a lack of IL-17A and/or F-producing lymphocytes may be responsible for the neutropenia found in nude mice, we measured IL-17A and F at the mRNA and protein level in nude mice kept in two different vivaria at the University of Virginia (UVA) and the La Jolla Institute of Allergy and Immunology (LIAI), respectively. In both environments, nude mice were unable to produce IL-17A. Although nude mice were neutropenic at the Virginia facility, they were not neutropenic at LIAI. IL-17F can compensate for a deficiency in IL-17A with respect to controlling blood neutrophil counts (27). Recent work also suggests that the intestinal flora controls the production of IL-17A (44). Th17 cell differentiation in the lamina propria (LP) of the small intestine was found to require specific commensal bacteria, and Th17 polarization was inhibited by treating mice with selective antibiotics. Furthermore, mice from different sources had marked differences in their Th17 cell numbers and animals lacking Th17 cells acquired them after introduction of bacteria from Th17 cell-sufficient mice (44).
To test the role IL-17A-producing T cells and their precursors in neutrophil homeostasis, we transferred unfractionated or DN1 cell-sorted thymocytes from WT, or Rag1−/− mice into neutropenic nude mice. Following the adoptive transfer of WT thymocytes, IL-17A-producing T cells predominantly homed to the LP and were largely CD4+ Th17 cells. IL-17A was efficiently produced by cells lacking a functional TCR and without prior TCR engagement. IL-17A-producing cells were found in the thymus and periphery of WT, Rag1−/− and Mybf/f cwLckCre mice but not nude mice with a neutropenic phenotype. Collectively, our data suggest that the development of IL-17A-producing T-lineage cells requires input from the thymic epithelium, but not mature TCR in order to regulate granulopoiesis.
Materials and Methods
Animals
Rag1−/− (41), β2-integrin (Itgb2−/−; 45), C57BL/6nu/nu (nude; 35), Mybf/f cwLckCre and littermate controls (42) and WT C57BL/6 (CD45.2) or congenic C57BL/6 CD45.1 (Jackson Labs, Bar Harbor, ME) mice were used between 6 and 16 weeks of age. All mice were on a C57BL/6 background for at least ten generations. Mybf/f cwLckCre mice were of a mixed hybrid genetic background with the use of DNA taken from 129/SVJ library and IB10 mouse embryonic stem cells. The resulting Mybf/w embryonic stem cells were then injected into C57BL/6 blastocysts, and chimeric male and female mice were crossed to C57BL/6 mice to establish Mybf/w progeny (42). All animal experiments were approved by the Animal Care and Use Committee of the University of Virginia or LIAI. Both animal facilities are certified SPF. The LIAI facility has tested negative for mouse parvo virus (MPV), minute virus of mouse (MVM), parvovirus B19 (NS-1), mouse hepatitis virus (MHV), Theiler's murine encephalomyelitis virus (TMEV) and epizootic diarrhea of infant mice (EDIM) quarterly. LIAI did test positive for helicobacter sp and murine norovirus (MNV). The UVA facility (details).
Recombinant Proteins and Antibodies
The following monoclonal antibodies (all reagents from BD-PharMingen, San Diego, CA unless otherwise indicated) were used: biotin-conjugated lineage negative panel, PE-conjugated anti-IL-17A (TC11-18H10.1), purified or APC-conjugated anti-CD3ε (145-2C11), APC-CY7 or PerCP-conjugated anti-CD4 (RM4-5), APC-conjugated anti-CD8a (53-6.7), FITC, PB or PE-conjugated anti-CD11b (M1/70), APC-conjugated anti-CD19 (1D3), FITC-conjugated anti-Pan NK Cells (DX5), anti-CD16/CD32 (2.4G2; The Lymphocyte Culture Center, University of Virginia, USA), FITC-conjugated anti-CD24, Pe-CY7 conjugated CD25 (PC61), purified anti-CD28 (37.51), APC-CY7-conjugated anti-CD44 (IM7), PE-conjugated anti-CD45.1 (A20), FITC-conjugated anti-CD45.2 (104), PerCp-CY5.5-conjugated anti-CD69 (H1.2F3), FITC or APC-conjugated anti-GR-1 (RB6-8C5), APC-conjugated anti-NK1.1 (PK136), FITC or APC-conjugated anti-TCR β chain (H57-597), FITC-conjugated anti-γδ TCR (GL3), APC-conjugated anti-CD117 (2B8; eBioscience, San Diego, CA), APC-conjugated anti-CD127 (A7R34; eBioscience), PE-CY7- conjugated anti Sca1 (D7) (Biolegend, San Diego, CA), FITC-conjugated CD90.2 (Thy-1) (53-2.1), FITC-conjugated anti-integrin β7 chain (M293), APC-conjugated Ccr7 (4B12) and APC-conjugated anti-CCR9 (242503; R&D systems, Minneapolis, MN), APC-conjugated anti-L-selectin (MEL-14). PerCP-conjugated Streptavidin was used for the detection of the biotin-conjugated primary Ab.
Lymphocyte cell culture
Splenocytes were isolated as previously described (10) and cultured in RPMI-1640 containing 10 % FBS, 1× nonessential amino acids (Gibco), 10 mM HEPES, 2 mM L-glutamine (Gibco, Grand Island, NY), 1 mM sodium pyruvate (Gibco) and 1 % penicillin/streptomycin in the presence or absence of plate absorbed purified anti-CD3ε (10 μg/ml) and soluble anti-CD28 (10 μg/ml), 10 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml calcium ionophore (Sigma-Aldrich), IL-23 (20 ng/ml; R&D systems), IL-6 (100 ng/ml; R&D systems), and/or TGF-ß1 (1 ng/ml; PeproTech Inc., New Jersey) for 3 days.
Flow Cytometry
Single cell suspensions from the thymus, spleen, bone marrow and MLN tissues and LP lymphocytes were prepared as previously described (10,46) and treated with 10 ng/ml PMA (Sigma-Aldrich), 500 ng/ml calcium ionophore (Sigma-Aldrich) and GolgiStop (BD-Pharmingen) for 6 h. Fcγ III/II receptors were blocked with 0.5 μg anti-CD16/CD32 and the cell suspension was incubated with an optimal concentration of mAbs. Intracellular staining was performed using Fix & Perm® cell permeabilization reagents (Caltag Laboratories, Burlingame, CA) according to manufacturer's instructions. Flow cytometry analysis was performed on a Becton Dickinson FACS Calibur or LSRII (San Jose, CA) and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Gates were set by isotype controls. Alternatively, live thymocytes were stained and sorted for DN1 population using Becton Dickinson FACSVantage SE Turbo Sorter under aseptic conditions. IL-17A protein in the cell culture and thymus supernatants was measured by Quantikine M mouse IL-17A ELISA kit (R&D systems, Minneapolis, MN). Blood counts were taken via tail bleed into EDTA coated capillary tubes and analyzed by automatic analyzer (Hemavet 850, CDC Technologies Inc., Oxford, CT) and confirmed by Kimura-stained manual counts using a hemocytometer.
Adoptive transfer experiments
Single cell suspensions from thymi of Rag1−/− or CD45.1+ WT mice were created by straining tissues through a 70 μm nylon mesh. CD45.1+ or Rag1−/− thymocytes were depleted of myeloid cells using CD11b microbeads (Miltenyi Biotec Inc. Auburn, CA), and 107 cells suspended in 500 μl sterile PBS were injected i.v. into recipient nude mice under sterile conditions. Cell-sorted DN1 thymocytes were injected into nude mice recipients at 1.5 ×105 cells suspended in 500 μl sterile PBS. Nude mice were given autoclaved water supplemented with antibiotics (5 mM sulfamethoxazole, 0.86 mM trimethoprim (Sigma, St. Louis, MO). Neutropenic rescue experiments were analyzed 8–9 weeks post adoptive transfer. Homing experiments were analyzed at 1 week post transfer. For Rag1−/− homing experiments, the thymi from Rag1−/− mice were pooled (6–10 mice) and labeled with 2 μM CFSE (Molecular Probes, Eugene, OR) as previously described (47), before injection into nude mice. Single cell suspensions of pooled WT thymocytes (3–8 mice) were depleted of CD4+ and CD8+ cells with anti-CD4 and anti-CD8 conjugated beads (Miltenyi Inc. Auburn, CA) according to manufacturer's instructions in order to enrich for DN cell populations.
Immunohistochemistry
The MLN and spleen were removed from Rag1−/− mice. Immunohistochemistry was performed on 5 μm paraffin sections following heat-induced antigen retrieval with unmasking solution (Vector Laboratories, Burlingame, CA). Sections were probed with goat anti-mouse CD3 (M-20); Santa Cruz Biotechnology Inc, CA) and detected using Vectastain Elite Kit (Vector Laboratories). Positive cells were identified by staining with DAB (Dako, CA). Counterstaining was performed using Harris hematoxylin (Richard-Allen Scientific, Kalamazoo, MI.).
Statistical Analysis
Data were expressed as mean ± SEM. Statistical significance between groups was set at p<0.05 using a two-tailed t test or a non parametric Mann Whitney t test.
Results
TCR expression or engagement is not required for the production of IL-17A
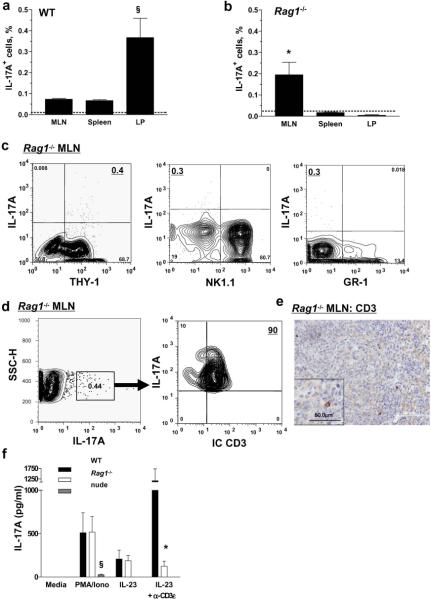
The number of blood neutrophils was assessed in WT mice and mice lacking mature T cells. WT and Rag1−/− mice were found to have normal circulating neutrophil numbers, which was confirmed in a second animal facility at LIAI (Table 1). A small reduction in total BM cellularity was found in Rag1−/− mice compared to WT mice (Supplemental Figure 1). C57BL/6nu/nu (nude) mice at Virginia showed an 80% reduction in neutrophil counts compared to WT mice and were considered neutropenic (Table 1). Serum IL-17A was not detectable in any mice used in the present study (Table 1). IL-17A-producing cells were readily detectable in the spleen, MLN (10) and lamina propria (LP) of WT mice and in the MLN of Rag1−/− mice following activation with a PMA/ionomycin (Figure 1a and b). The IL-17A-producing cells detected in the MLN of Rag1−/− mice were Thy-1+, NK1.1−, and GR-1− suggesting that they did not originate from myeloid progenitors (Figure 1c). Most IL-17A-producing cells found in the MLN of Rag1−/− mice expressed intracellular CD3 (Figure 1d–e), indicating that the IL-17A-producing cells in Rag1−/− mice are of T cell lineage. IL-17A-producing cells were not detectable in the MLN, spleen or LP of nude mice housed at UVA (Supplemental Figure 2).
Table 1.
Blood neutrophil counts and serum IL-17A levels for each mouse strain.
| Facility | Mice | Blood neutrophil counts (K/μl) | IL-17A (pg/mL) |
|---|---|---|---|
| UVA | Rag1 −/− | 1.5 ± 0.1 | ND |
| WT | 1.9 ± 0.2 | ND | |
| Nude | 0.4 ± 0.2 § | ND | |
| LIAI | Rag1 −/− | 1.7 ± 0.2 | not done |
| WT | 2.1 ± 0.2 | ND | |
| Nude | 2.8 ± 0.2* | ND | |
| Il17a −/− | 3.0 ± 0.2* | ND |
Mean ± SEM, n=4–9.
Significantly different from all other groups at the UVA facility (p<0.01).
Significantly different from Rag1−/− at the LIAI facility (p<0.05).
ND = not detectable (< 12 pg/ml).
Figure 1. Rag1−/−, but not nude mice contain IL-17A-secreting cells and have normal blood neutrophil numbers.
(a) IL-17A-producing cells were found in the MLN, spleen and LP of WT (n=3–7) and in the MLN of (b) Rag1−/− mice (n=3–7. Isotype control; dashed line). §Significantly different from MLN and spleen (p<0.05), *significantly different from spleen and LP (p<0.05). IL-17A-producing cells in the MLN of Rag1−/− mice were Thy-1+, NK1.1−, and GR-1− (c) and 90% of the IL-17A+ population in the Rag1−/− MLN expressed intracellular CD3 (d). CD3+ cells in the MLN of Rag1−/− mice were identified by immunoperoxidase staining (e). Unfractionated splenocytes from (f) WT, Rag1−/− or nude mice (n=4 each) were stimulated with IL-23 (20 ng/ml), PMA (10 ng/ml) and ionomycin (500 ng/ml) with or without plate-absorbed anti-CD3ε (α-CD3ε; 10 μg/ml) for 3 days. *Significantly different from WT mice with same treatment (p<0.01), §significantly different from all other mice with same treatment (p<0.01).
Although nude mice were consistently neutropenic at UVA, nude mice from the same source (Jackson laboratory) showed consistently elevated neutrophil counts at LIAI (table 1). These mice did not produce any IL-17A (data not shown), but did produce low levels of IL-17F (33.9 pg/ml ± 14.5). IL-17F but not IL-17A mRNA was detectable in the spleens of nude mice at LIAI (data not shown).
To test whether cells lacking a TCR were capable of secreting IL-17A, we stimulated splenocytes from WT, Rag1−/− and nude mice for 3 days in the presence of PMA/ionomycin, with and without IL-23 and plate-bound anti-CD3ε. IL-23 did not induce IL-17A secretion in cell supernatants from nude mice (Figure 1f), but WT and Rag1−/− splenocytes produced IL-17A protein at equal levels in response to PMA/ionomycin and IL-23. As expected, plate-bound anti-CD3ε enhanced IL-17A secretion from WT, but not Rag1−/− splenocytes. These findings show that TCR engagement is not required for the secretion of IL-17A in secondary lymphoid tissues.
IL-17A-producing Rag1−/− thymocytes can rescue neutropenia
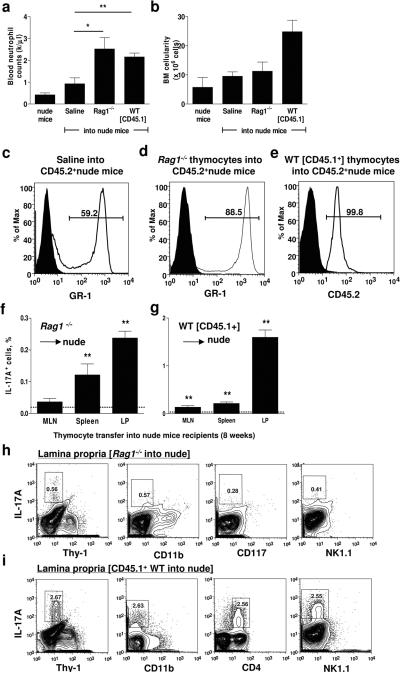
Mature T cells can regulate granulopoiesis as demonstrated by increased blood neutrophil numbers after engraftment of WT thymi or adoptive transfer of CD4+ T cells into nude mice (33). In these studies, it was assumed that functional TCR was required for this function, but this was not formally investigated. To directly test whether thymus-derived cells in Rag1−/− mice could also normalize neutrophil numbers in nude mice, myeloid cell-depleted (>99.9%) Rag1−/− thymocytes or CD45.1+ WT thymocytes (as a positive control) were adoptively transferred into nude mice. At 8 weeks, nude mice that received Rag1−/− or WT thymocytes had significantly elevated blood neutrophil counts (Figure 2a). Total BM cellularity remained unaltered in recipient nude mice receiving Rag1−/− thymocytes but was elevated in mice receiving CD45.1+ WT thymocytes (Figure 2b). Nude mice that received Rag1−/− thymocytes had significantly more GR-1+ BM neutrophils and neutrophil precursors compared to the saline control (compare Figure 2c and d). All neutrophils in the nude mice were CD45.2+ recipient-derived (99.8 ± 0.1 % CD45.2+), and not from contaminating donor-derived (CD45.1+) myeloid cells (Figure 2e). These results demonstrate that thymocytes lacking a functional TCR can regulate granulopoiesis to the same extent as WT thymocytes.
Figure 2. IL-17A-producing Rag1−/− thymocytes regulate granulopoiesis.
(a) Myeloid cell-depleted thymocytes from Rag1−/− mice or CD45.1+ WT mice (as positive controls) were adoptively transferred by i.v. injection into nude mice and blood neutrophil levels and (b) BM cellularity was measured after 8 weeks (n=4 each). (c) Gr-1+ cells in the BM of nude mice receiving saline (63 ± 6 %) or (d) Rag1−/− thymocytes (85 ± 2 %; p<0.05). (e) GR-1+ neutrophils and precursors in the BM (99.8%) were derived from the recipient (CD45.2+) and not the WT donor (CD45.1+). Solid fill indicates the isotype control. (f) IL-17A-producing cells were analyzed in the MLN, spleen and LP of nude mice injected with Rag1−/− or (g) WT thymocytes. Significantly different from isotype control (dashed line, **p<0.01). Eight weeks after the adoptive transfer of (h) Rag1−/− thymocytes or (i) CD45.1+ thymocytes into nude mice, IL-17A-producing cells in the LP were analyzed for Thy-1, CD11b, CD117, and NK1.1 expression or CD4 expression. Cells were gated on the lymphocyte population by forward and side scatter.
After the adoptive transfer of thymocytes, IL-17A-producing cells were found in the spleen and LP of nude mice receiving Rag1−/− thymocytes, although a lower proportion was detected than in nude mice receiving WT thymocytes (Figure 2f–g). The largest number of IL-17A+ cells was found in the LP of the adoptively transferred nude mice, suggesting that IL-17A-producing cells preferentially home to gut-associated lymphoid tissue. IL-17A-producing cells found in nude mice reconstituted with Rag1−/− thymocytes were Thy-1−, CD11b−, CD117−, and NK1.1− (Figure 2h). The majority of IL-17A-producing cells found in nude mice reconstituted with WT thymocytes also lost Thy-1 expression and were CD25−CD69+CD4+ T cells (Figure 2i and data not shown).
IL-17A-producing thymocytes home to gut-associated lymphoid tissue
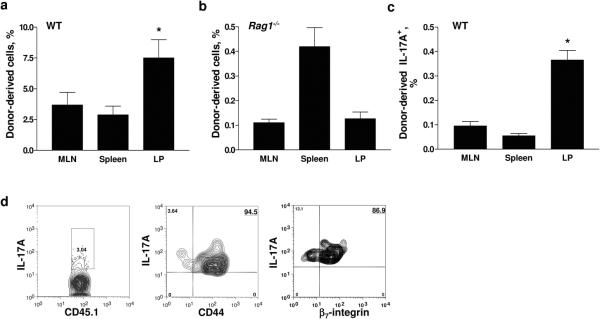
We reasoned that homing to gut-associated lymphoid tissues might be important in correcting neutrophil numbers after adoptive transfer. To compare the homing of WT CD45.1+ (Figure 3a) and Rag1−/− (Figure 3b) thymocytes, we injected CFSE-labeled Rag1−/− thymocytes into nude mice. After one week, the majority of Rag1−/−CFSE+ thymocytes were found in the spleen, with a lower number detected in the MLN and LP (Figure 3b). As expected (48), most IL-17A-producing donor-derived T cells from WT mice were found in the LP (Figure 3c). All of the transferred IL-17A-producing CD45.1+ T cells in the MLN were CD44hi, and 87% expressed β7 integrin (Figure 3d).
Figure 3. Thymocytes home to lymphoid tissues.
Thymocytes from (a) WT CD45.1+ mice (n=4) or (b) Rag1−/− mice (n=6–10 pooled per experiment) were adoptively transferred by i.v. injection into nude mice (n=2–4) and analyzed at one week. (c) IL-17A-producing Tn cells were measured in nude mice transferred with WT thymocytes (n=4). *p<0.05 from other organs. (d) CD44 and β7-integrin expression on IL-17A+ WT thymocytes that had homed to the LP of nude mice.
IL-17A-producing cells are found in the DN1 compartment of WT and Rag1−/− thymi
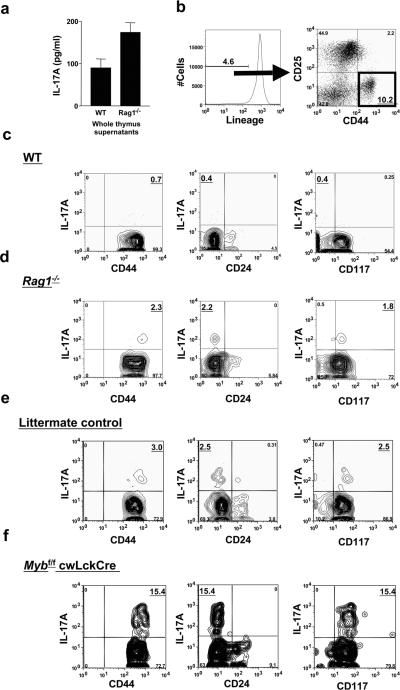
The ability of IL-17A-producing thymocytes from Rag1−/− mice to reconstitute normal granulopoiesis in nude mice suggests that normal thymic epithelium, but not TCR gene rearrangement is required for the differentiation of these cells. IL-17A protein was detectable in thymus supernatants from WT (90 ± 16 pg/ml) and Rag1−/− (174 ± 23 pg/ml) mice (Figure 4a). Among unfractionated thymocytes of WT mice, IL-17A-producing T cells were detectable at a low frequency (data not shown). These IL-17A-producing T cells were found among mature γδ+, CD4+ and CD8+ single positive and double positive cells (data not shown) and also in the DN1 compartment of WT mice (Figure 4b and c). Thymocytes were gated on the lineage-negative population and labeled with antibodies to CD44 and CD25 (Figure 4B). The DN1 IL-17A-producing cells were lin−CD25−, CD44hi, CD24−, CD117+/−, and CD127lo/+ (Figure 4b and c and data not shown). The proportion of IL-17A-producing thymocytes was enriched ~ 5-fold in the Rag1−/− compartment of WT thymi, possibly due to the decrease in total thymus cellularity seen in these mice. IL-17A-producing thymocytes in Rag1−/− mice were also CD25−, CD44hi, CD24− and CD117+ (Figure 4d), consistent with the DN1 stage of development.
Figure 4. IL-17A-producing cells are found in the DN1 compartment of the thymus.
(A) IL-17A protein was detectable in the supernatants of homogenized thymi of WT and Rag1−/− mice. (b–f) Unfractionated thymocytes were stimulated with PMA and ionomycin in the presence of GolgiStop for 5 h before staining, gated on the lymphocyte population by forward-side scatter and the DN population by the lineage negative (GR-1−, B220−, CD11b−, CD3−, CD4−, CD8−, Ter119−) population and then gated for DN (CD44+ CD25−). (b) In WT (c) and Rag1−/− (d) mice, IL-17A-producing cells were CD44hi and CD24−. Most IL-17A-producing cells in Rag1−/− mice were CD117+, whereas these cells in WT mice expressed mixed levels of CD117. IL-17A-producing CD44hiCD24−CD117+ thymocytes were found at higher levels in the DN1 compartment of Mybf/f cwLckCre (f) mice than their littermate controls, which are from a mixed genetic background (e).
Rag1−/− mice have a developmental block at the DN3 to DN4 transition but also have a defect in B cell development. To confirm that IL-17A-producing cells indeed arise prior to this block in a second model, we used Mybf/f cwLckCre mice. In these mice, Cre under the Lck promoter inactivates both alleles of the floxed c-Myb gene, which leads to a block at the DN3 to DN4 stage of thymocyte development (42). IL-17A-producing thymocytes in the Mybf/fcwLckCre mice were 5-fold elevated compared to the littermate controls (Figure 4e and f) (42). As in Rag1−/− mice, blood neutrophil counts were normal in Mybf/fcwLckCre mice (data not shown).
DN1 thymocytes can correct neutropenia in nude mice
To directly test whether DN1 thymocytes contained IL-17A-producing cells that were able correct neutropenia, we reconstituted nude mice with flow cytometry-sorted DN1 thymocytes (CD25−CD44hiCD24−) from WT CD45.1+ mice. These cells normalized blood neutrophil counts (Figure 5a) and were able to home to secondary lymphoid tissue with the largest proportion found in the LP (Figure 5b), where some of these cells expressed IL-17A (Figure 5c). Although a small population of CD45.1+ DN1 cells (1.0% ± 0.3) was found in the BM of the treated nude mice, they did not secrete IL-17A (Figure 5). Therefore, contact with thymic epithelium, but not V(D)J rearrangement, is needed for the development of IL-17A-producing cells and the regulation of granulopoiesis.
Figure 5. DN1 cells can regulate granulopoiesis.
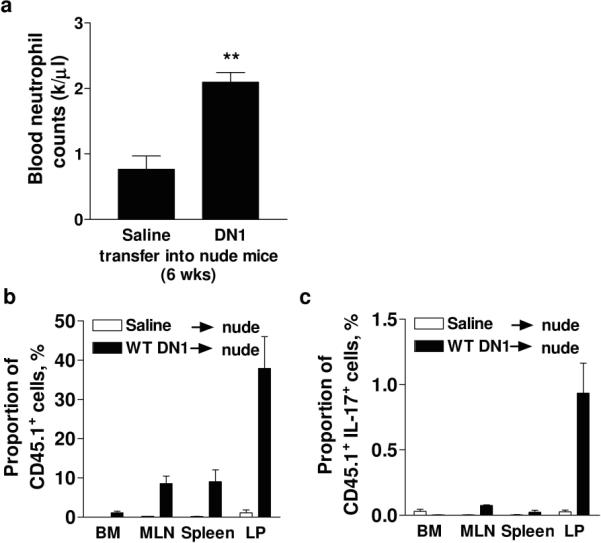
Cell-sorted WT CD45.1+CD24− DN1 thymocytes were adoptively transferred into nude mice and neutrophil counts assessed after 6 weeks (a). CD45.1+ cells were found predominantly in the LP (b) where some expressed IL-17A as detected by intracellular staining (c).
Discussion
We demonstrate that IL-17A can be released from thymus-derived cells without TCR gene rearrangement and therefore without TCR engagement. IL-17A-producing T cells were found in the DN1 compartment of WT mice, but also Rag1−/− and Mybf/f cwLckCre mice, which have a complete and partial block at the DN3 to DN4 stage of thymocyte development, respectively (41,42). IL-17A-producing cells were also detected in the MLN of RAG-1 deficient mice, where they expressed intracellular CD3, but not myeloid markers. This suggests that these cells are of T cell lineage. Unfractionated thymocytes from Rag1−/− mice were able to fully restore normal neutrophil levels in neutropenic nude mice, as were cells isolated from the DN1 compartment of WT mice. Taken together, our data suggest that thymus-derived T-lineage cells that lack a TCR can secrete IL-17A and regulate granulocyte homeostasis.
Perhaps the most surprising finding of our study is that nude mice are neutropenic in one SPF facility but not in another. This finding may explain previous reports of neutropenic (33) and neutrophilic nude mice (38,39). This difference in phenotype is most likely due to different commensal flora (44,49,50). While formal experiments to address the role of commensals were not part of this study, we found a striking discrepancy between the ability of nude mice to produce IL-17A and F at different facilities. Although IL-17A production was undetectable by RT-PCR, flow cytometry and plasma ELISA at the UVA and LIAI vivaria, plasma IL-17F was detectable in nude mice at LIAI, the facility where nude mice are not neutropenic. This finding supports the concept that IL-17F can compensate for IL-17A with respect to blood neutrophil numbers (27). The effect of vivarium housing on IL-17A production has been previously reported in WT mice, with mice imported from Taconic showing a 10-fold increase in Th17 cells in the LP compared to WT mice supplied from Jackson laboratories (44).
Although CD4+ T cells were the first cells to be identified as active secretors of IL-17A (17) and continue to receive the most attention, the most numerous IL-17A-producing T cells in normal mice are γδ+ T cells (10). A recent study has shown that naïve IL-17A-producing γδ+ T cells escape the thymus and are polarized to produce IL-17A (51). These CD122lo cells populated the thymus, lymph nodes and spleen. Taken together with a prior study (52), this finding suggests that positive selection is not required for these γδ+ T cells. IL-17A-producing γδ+ T cells can be derived from the fetal thymi of normal mice (53). These cells express CD25, but not CD122 and are detected in the thymus before CD25 is expressed. Although these authors did not investigate IL-17A expression in DN1 thymocytes, our data suggest that polarization towards IL-17A expression occurs earlier than previously reported (53). The primitive IL-17A-producing T-lineage cells colonize secondary lymphoid tissues of Rag1−/− mice, which lack mature T cells and are fully able to correct neutropenia in nude mice. Cells expressing intracellular CD3 have been previously described in lymphoid organs of Rag1−/− mice (54), although the function of these cells was not investigated. Here, we show that at least some of these cells produce IL-17A and support granulopoiesis in Rag1−/− mice.
FoxP3 is the defining transcription factor of regulatory T cells (55). In a recent study of FoxP3 expression in human thymocytes, FoxP3-expressing cells were found among DN1 thymocytes (56). This may reflect another example where T cell lineage commitment precedes TCR engagement. Regulatory and IL-17A-producing T cell lineages are related to each other (20,57) and both may undergo early lineage commitment before expressing a functional TCR. In the present study, we found that splenocytes from WT, and Rag1−/− mice were equipotent in their ability to secrete IL-17A in response to IL-23 in the absence of anti-CD3ε/CD28 stimulation. As expected, TCR ligation greatly augmented IL-17A release from WT cells but not Rag1−/− cells. This suggests a two-tiered model of IL-17A control: lower levels sufficient to maintain granulopoiesis may be secreted in response to cytokines such as IL-23, whereas large amounts, such as those seen in autoimmune diseases depend on TCR engagement.
The restoration of normal levels of circulating neutrophils in neutropenic nude mice reconstituted with myeloid-depleted WT or Rag1−/− DN1 thymocytes shows that homeostatic levels of IL-17A can be produced without the need for TCR expression or ligation. Elegant studies by Monteiro et al., 2000 showed that antigen activated CD4+T cells in the BM regulate granulopoiesis, but these studies predated the discovery of Th17 cells (33). Our Rag1−/− mice had normal circulating neutrophil counts (Table 1) and no block in myeloid progenitor cell differentiation in the BM (Supplemental Figure 1). Monteiro et al., 2000 showed that D011.10 RAG−/− mice (which only contain naïve CD4+T cells) were neutropenic and neutrophil counts were only restored following treatment with ovalbumin (33). This suggests that naïve T cells may actively suppress granulopoiesis in the D011.10 RAG1−/− mice. We hypothesize that under `normal' conditions, antigen-activated, IL-17A-producing CD4+ T cells regulate granulopoiesis in mice. In the absence of mature CD4+ T cells, other cells, possibly at the DN1 stage of development, are able to escape the thymus and regulate granulopoiesis without TCR engagement.
The fact that neutrophil levels in reconstituted nude mice become normal and not exaggerated suggests that IL-17A-producing cells from Rag1−/− mice are still subject to regulation similar to their WT counterparts. BM cellularity was also not altered between the reconstituted mouse groups. Previous studies have shown that adenovirus overexpression of IL-17A in WT mice also does not affect BM cellularity, although myeloid precursor colony forming units (CFU-GM/CFU-GEMM) were significantly raised (11). We have previously hypothesized that the regulation of granulopoiesis by the IL-17A-IL-23 cytokine axis is likely to occur in the gut-associated lymphoid tissue (10,18,58). Following adoptive transfer of unfractionated thymocytes, IL-17A+ cells from WT and Rag1−/− mice were found predominantly in the LP after 8 weeks. A small number of CD45.1+ cells were found in the BM, however, they did not secrete IL-17A. IL-17A regulates G-CSF secretion, most likely from BM stromal cells, and thus controls circulating neutrophil numbers (ref). As part of a negative feedback loop, IL-17A inhibits its own production and that of IL-17F, but IL-17F cannot serve this function (26, 27). One way to directly address the role of IL-17A in regulating blood neutrophil numbers by DN1 thymocyte transfer would be to adoptively transfer DN1 thymocytes from Il17a−/− mice. However, interpretation of any transfer experiments with these mice is hampered by the over exuberant production of IL-17F and consequently elevated neutrophil numbers in these mice (27), making this approach uniformative.
After adoptive transfer, IL-17A-producing CD45.1+ WT T cells lost Thy-1 expression and showed an organ distribution in recipient nude mice that was similar to the distribution of Thy-1+IL-17A-producing T cells in WT mice. However, the distribution of Rag1−/− IL-17A-producing cells transferred into nude mice followed a different pattern, with the largest population found in the spleen (after one week) and LP (after 8 weeks). This may reflect differences in the homing patterns between endogenous Rag1−/− cells able to escape thymic selection as seen in Rag1−/− mice and adoptively transferred bulk Rag1−/− thymocytes. Similar to the adoptively transferred CD45.1+ T cells, Rag1−/− thymocytes also lost Thy-1 expression on their cell surface. The mechanisms by which TCR− IL-17A-producing cells escape the thymus remain to be investigated.
The fact that nude mice fail to develop IL-17A-producing cells suggests that thymic epithelium is required for their development. Thymic epithelium is known to provide essential notch ligands needed for thymocyte development (59). Based on the present data, we speculate that IL-17A-producing T lineage cells may have originated in phylogeny before the advent of thymic selection. Rag1−/− mice express no functional TCR and therefore do not show thymic selection, yet these mice have IL-17A-producing cells in the thymus and elsewhere. IL-17 family members are found in all mammals and most vertebrates down to zebrafish (60) and even cartilaginous fishes (61,62). IL-17 receptor and a form of primordial IL-17 were found upregulated in skin cells following LPS-stimulation of lamprey (Lethenteron japonicum), which are jawless vertebrates (63,64). Interestingly, lampreys do not express Rag1 or Rag2 genes and are unable to undergo recombinatorial diversification. This suggests that the IL-17A-IL-17R system predates the advent of thymic selection and TCR expression (65). The precise orthologue relationships between different members of the IL-17 family of cytokines in different species remains to be investigated, so it is unclear whether the ancestral IL-17 may correspond to IL-17A, IL-17F or even another family member.
To investigate the role of V(D)J recombination, we used Rag1−/− mice for most of our experiments and show that their thymi produce copious amounts of IL-17A, their splenocytes respond to IL-23 by secreting IL-17A and their thymocytes home to the spleen and LP of nude mice, where they secrete sufficient amounts of IL-17A to restore normal blood neutrophil counts. This raises the question whether such cells exist in immunocompetent mice. We addressed this in two ways. First, DN1 thymocytes from WT mice also restored blood neutrophil counts in nude mice. Second, we found IL-17A-producing CD4−CD8− “double negative” T cells in secondary lymphoid organs of immunocompetent mice. Since these cells are extremely rare in normal WT mice (18), we used neutrophilic Itgb2−/− mice for these experiments. Two recent reports show that lymphoid tissue inducer (LTi)-like cells also produce IL-17A without TCR engagement. CD4+CD3−NK1.1−CD11b−Gr1−CD11c−B220− LTi cells can produce IL-17A in response to zymosan or IL-23 (25), and human fetal LTi cells are IL-17-producing precursors to RORC+ CD127+ natural killer-like cells (66). The cells described in the present report are not LTi cells, because they do not express CD4, but intracellular CD3, suggesting that they are of T cell lineage.
In conclusion, adoptive transfer of thymocytes from WT or Rag1−/− mice corrects neutropenia in nude mice. The maintenance of normal neutrophil levels in Rag1−/− mice together with normal IL-17A secretion from splenocytes in response to IL-23 indicates that V(D)J recombination is dispensable for the generation of IL-17A-producing cells. IL-17A-producers are found among DN1 thymocytes of normal WT mice and at elevated levels in Rag1−/− and Mybf/f cwLckCre mice. Although the mechanisms behind the thymic egress of these cells remain unknown, we show that DN1-derived T-lineage cells populate the secondary lymphoid organs of nude mice and produce enough IL-17A to correct neutropenia.
Supplementary Material
Acknowledgements
We thank Dr. M. Kronenberg for critically reading this manuscript and Anthony Bruce for expert animal husbandry.
This work was supported by grants from the National Institutes of Health HL73361 (K.L.), T32 GM 08715 (M.A.S.), Deutsche Forschungsgemeinschaft AZ428/2-1 (A.Z.) and VI508/1-1 (S.v.V),
Footnotes
Disclosure The authors have no competing financial interests.
Reference List
- 1.Lubberts E, Schwarzenberger P, Huang W, Schurr JR, Peschon JJ, van den Berg WB, Kolls JK. Requirement of IL-17 Receptor Signaling in Radiation-Resistant Cells in the Joint for Full Progression of Destructive Synovitis. J Immunol. 2005;175:3360–3368. doi: 10.4049/jimmunol.175.5.3360. [DOI] [PubMed] [Google Scholar]
- 2.Miossec P. IL-17 in rheumatoid arthritis: a new target for treatment or just another cytokine? Joint Bone Spine. 2004;71:87–90. doi: 10.1016/j.jbspin.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of Immune Induction of Collagen-Induced Arthritis in IL-17-Deficient Mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 4.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp.Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat.Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, Mckenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J.Clin.Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 Stimulates Granulopoiesis in Mice: Use of an Alternate, Novel Gene Therapy-Derived Method for In Vivo Evaluation of Cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 9.Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- 10.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, Bagby G, Nelson S, Kolls JK. Requirement of Endogenous Stem Cell Factor and Granulocyte-Colony-Stimulating Factor for IL-17-Mediated Granulopoiesis. J Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- 12.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting Edge: Roles of Toll-Like Receptor 4 and IL-23 in IL-17 Expression in Response to Klebsiella pneumoniae Infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp.Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect.Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 16.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident V{delta}1+ {gamma}{delta} T Cells Control Early Infiltration of Neutrophils after Escherichia coli Infection via IL-17 Production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol.Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 18.Ley K, Smith E, Stark MA. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol Res. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- 19.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat.Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-[beta] induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 22.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 24.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-Producing T Cells Are Important in Effector Functions for the Elicitation of Contact Hypersensitivity Responses. J Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp.Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith E, Stark MA, Zarbock A, Burcin TL, Bruce AC, Vaswani D, Foley P, Ley K. IL-17A Inhibits the Expansion of IL-17A-Producing T Cells in Mice through “Short-Loop” Inhibition via IL-17 Receptor. J Immunol. 2008;181:1357–1364. doi: 10.4049/jimmunol.181.2.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Vietinghoff S, Ley K. IL-17A Controls IL-17F Production and Maintains Blood Neutrophil Counts in Mice. J Immunol. 2009;183:865–873. doi: 10.4049/jimmunol.0804080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hizawa N, Kawaguchi M, Huang SK, Nishimura M. Role of interleukin-17F in chronic inflammatory and allergic lung disease. Clin Exp.Allergy. 2006;36:1109–1114. doi: 10.1111/j.1365-2222.2006.02550.x. [DOI] [PubMed] [Google Scholar]
- 29.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp.Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Croxford AL, Kurschus FC, Waisman A. Cutting Edge: An IL-17F-CreEYFP Reporter Mouse Allows Fate Mapping of Th17 Cells. J Immunol. 2009;182:1237–1241. doi: 10.4049/jimmunol.182.3.1237. [DOI] [PubMed] [Google Scholar]
- 32.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat Rev Immunol. 2008;8:9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monteiro JP, Benjamin A, Costa ES, Barcinski MA, Bonomo A. Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood. 2005;105:1484–1491. doi: 10.1182/blood-2004-07-2856. [DOI] [PubMed] [Google Scholar]
- 34.Broxmeyer HE, Bruns HA, Zhang S, Cooper S, Hangoc G, McKenzie ANJ, Dent AL, Schindler U, Naeger LK, Hoey T, Kaplan MH. Th1 Cells Regulate Hematopoietic Progenitor Cell Homeostasis by Production of Oncostatin M. Immunity. 2002;16:815–825. doi: 10.1016/s1074-7613(02)00319-9. [DOI] [PubMed] [Google Scholar]
- 35.Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 36.Zipori D, Trainin N. Defective capacity of bone marrow from nude mice to restore lethally irradiated recipients. Blood. 1973;42:671–678. [PubMed] [Google Scholar]
- 37.Aggio MC, Lozzio BB. Hematopoiesis of hereditarily asplenic-athymic (lasat) mice. Exp.Hematol. 1979;7:197–205. [PubMed] [Google Scholar]
- 38.Benestad HB, Strom-Gundersen I. Thymic hormones and syngeneic T-lymphocytes are not required for leukopoiesis in an in vivo culture system for mouse bone marrow cells. Exp.Hematol. 1984;12:319–325. [PubMed] [Google Scholar]
- 39.Zhdanov VV, Luk'yanova TA, Kirienkova EV. Mechanisms of hemopoiesis in athymic mice. Bull.Exp.Biol Med. 2002;133:450–452. doi: 10.1023/a:1019801502464. [DOI] [PubMed] [Google Scholar]
- 40.Forlow SB, White EJ, Thomas KL, Bagby GJ, Foley PL, Ley K. T cell requirement for development of chronic ulcerative dermatitis in E-and P-selectin-deficient mice. J Immunol. 2002;169:4797–4804. doi: 10.4049/jimmunol.169.9.4797. [DOI] [PubMed] [Google Scholar]
- 41.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 42.Bender TP, Kremer CS, Kraus M, Buch T, Rajewsky K. Critical functions for c-Myb at three checkpoints during thymocyte development. Nat Immunol. 2004;5:721–729. doi: 10.1038/ni1085. [DOI] [PubMed] [Google Scholar]
- 43.Monteiro JP, Bonomo A. Linking immunity and hematopoiesis by bone marrow T cell activity. Braz.J Med.Biol.Res. 2005;38:1475–1486. doi: 10.1590/s0100-879x2005001000004. [DOI] [PubMed] [Google Scholar]
- 44.Ivanov II, Frutos RL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host.Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp.Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivera-Nieves J, Olson T, Bamias G, Bruce A, Solga M, Knight RF, Hoang S, Cominelli F, Ley K. L-selectin, alpha 4 beta 1, and alpha 4 beta 7 integrins participate in CD4+ T cell recruitment to chronically inflamed small intestine. J Immunol. 2005;174:2343–2352. doi: 10.4049/jimmunol.174.4.2343. [DOI] [PubMed] [Google Scholar]
- 47.Galkina E, Tanousis K, Preece G, Tolaini M, Kioussis D, Florey O, Haskard DO, Tedder TF, Ager A. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J Exp.Med. 2003;198:1323–1335. doi: 10.1084/jem.20030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 49.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 50.Niess JH, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 51.Jensen KDC, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien Y. h. Thymic Selection Determines [gamma][delta] T Cell Effector Fate: Antigen-Naive Cells Make Interleukin-17 and Antigen-Experienced Cells Make Interferon [gamma] Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schweighoffer E, Fowlkes BJ. Positive selection is not required for thymic maturation of transgenic gamma delta T cells. J.Exp.Med. 1996;183:2033–2041. doi: 10.1084/jem.183.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ {gamma}{delta} T Cells As Fetal Thymus-Derived Naturally Occurring IL-17 Producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 54.Falk I, Potocnik AJ, Barthlott T, Levelt CN, Eichmann K. Immature T cells in peripheral lymphoid organs of recombinase-activating gene-1/-2-deficient mice. Thymus dependence and responsiveness to anti-CD3 epsilon antibody. J Immunol. 1996;156:1362–1368. [PubMed] [Google Scholar]
- 55.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 56.Tuovinen H, Kekalainen E, Rossi LH, Puntila J, Petteri Arstila T. Cutting Edge: Human CD4-CD8- Thymocytes Express FOXP3 in the Absence of a TCR. J Immunol. 2008;180:3651–3654. doi: 10.4049/jimmunol.180.6.3651. [DOI] [PubMed] [Google Scholar]
- 57.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 58.Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K. IL-23 Is Required for Neutrophil Homeostasis in Normal and Neutrophilic Mice. J Immunol. 2007;179:8274–8279. doi: 10.4049/jimmunol.179.12.8274. [DOI] [PubMed] [Google Scholar]
- 59.Radtke F, Wilson A, Mancini SJC, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 60.Gunimaladevi I, Savan R, Sakai M. Identification, cloning and characterization of interleukin-17 and its family from zebrafish. Fish.Shellfish.Immunol. 2006;21:393–403. doi: 10.1016/j.fsi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Litman GW, Anderson MK, Rast JP. Evolution of antigen binding receptors. Annu.Rev.Immunol. 1999;17:109–147. doi: 10.1146/annurev.immunol.17.1.109. [DOI] [PubMed] [Google Scholar]
- 62.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Mayer WE, Uinuk-Ool T, Tichy H, Gartland LA, Klein J, Cooper MD. Isolation and characterization of lymphocyte-like cells from a lamprey. Proc.Natl.Acad.Sci.U.S.A. 2002;99:14350–14355. doi: 10.1073/pnas.212527499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsutsui S, Nakamura O, Watanabe T. Lamprey (Lethenteron japonicum) IL-17 upregulated by LPS-stimulation in the skin cells. Immunogenetics. 2007;59:873–882. doi: 10.1007/s00251-007-0254-2. [DOI] [PubMed] [Google Scholar]
- 65.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu.Rev.Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 66.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.