Abstract
There is a growing appreciation of the profound effects that passive mechanical properties, especially the stiffness of the local environment, can have on cellular functions. Many experiments are conducted in a 2D geometry (i.e., cells grown on top of substrates of varying stiffness), which is a simplification of the 3D environment often experienced by cells in vivo. To determine how matrix dimensionality might modulate the effect of matrix stiffness on actin and cell stiffness, endothelial cells were cultured on top of and within substrates of various stiffnesses. Endothelial cells were cultured within compliant (1.0–1.5 mg/ml, 124±8 to 202±27 Pa) and stiff (3.0 mg/ml, 502±48 Pa) type-I collagen gels. Cells elongated and formed microvascular-like networks in both sets of gels as seen in previous studies. Cells in stiffer gels exhibited more pronounced stress fibers and ~1.5-fold greater staining for actin. As actin is a major determinant of a cell’s mechanical properties, we hypothesized that cells in stiff gels will themselves be stiffer. To test this hypothesis, cells were isolated from the gels and their stiffness was assessed using micropipette aspiration. Cells isolated from relatively compliant gels were 1.9-fold more compliant than cells isolated from relatively stiff gels (p<0.05). Similarly, cells cultured on top of 1700 Pa polyacrylamide gels were 2.0-fold more compliant that those cultured on 9000 Pa (p<0.05). These data demonstrate that extracellular substrate stiffness regulates endothelial stiffness in both three- and two-dimensional environments, though the range of stiffnesses that cells respond to vary significantly in different environments.
Keywords: Modulus, Biomechanics, Actin, Endothelial cell, Stiffness, Extracellular matrix
1. Introduction
In recent years, there has been a growing appreciation of the profound effects that passive mechanical properties, such as viscosity (Edwards et al., 1996), microstructure (Sieminski et al., 2002), and especially the stiffness (Discher et al., 2005; Peyton et al., 2007) of the local environment, can have on cellular functions relevant to development, homeostasis, and disease. For example, in fibroblasts substrate stiffness affects the rate (Pelham and Wang, 1997) and direction (Lo et al., 2000) of cell migration as well as focal adhesion (Pelham and Wang, 1997) and stress fiber formation (Halliday and Tomasek, 1995; Yeung et al., 2005). Neurons have increased branching densities when cultured on compliant substrates while glia cells, which are normally co-cultured with these neurons, do not survive on deformable substrates (Flanagan et al., 2002). Substrate stiffness influences the differentiation of mesenchymal stem cells with soft, intermediate, and stiff materials being neurogenic, myogenic, and osteogenic, respectively (Engler et al., 2006). In the latter study, it was shown that sensitivity to substrate stiffness required nonmuscle myosin II activity, which applies tension to and thereby stiffens cortical actin structures. Consistent with this idea, in the same study, it was shown that mesenchymal stem cells, C2C12 myoblasts, and hFOB cells cultured on collagen-laminated polyacrylamide gels with a substrate stiffness of ~1000, ~10,000, and ~40,000 showed a progressive increase in cell stiffness (Engler et al., 2006). More recently, it has been shown that for fibroblasts grown on fibronectin-coated polyacrylamide gels, the cells’ elastic moduli were equal to, or slightly lower than, those of their substrates for a range of substrate stiffness up to 20 kPa (Solon et al., 2007).
These studies employed cells cultured on top of protein-laminated polyacrylamide gels, where the cells interact with a two-dimensional surface. This system affords excellent control of the substrate stiffness but does not allow for cells to be cultured within a three-dimensional (3D) matrix, which for many cell types is more representative of their native environment. For these cell types, culture within three-dimensional extracellular matrix gels allows for a more realistic environment with the stiffness of the gels controlled by altering the gel concentration, crosslinker concentration, or the mechanical boundary conditions of the gels (Nehls and Herrmann, 1996; Roeder et al., 2002; Sieminski et al., 2004). For example, the stiffness of collagen gels regulates the morphology of embedded fibroblast with compliant gels favoring elaborate dendritic extensions. The proliferation and migration of mammary epithelial cells are regulated by the stiffness of the surrounding gels (Paszek et al., 2005). Endothelial cells within 3D collagen gels form microvascular networks, with the average length of the network and the average lumen area altered by the stiffness of the gel (Sieminski et al., 2004). Endothelial cells exhibit similar stiffness-induced alterations in network morphology when cultured in self-assembling peptide gels (Sieminski et al., 2007).
While there is ample evidence that substrate stiffness can affect cell function, very little is known about how a given response to changes in 2D substrate stiffness might correlate to cellular responses to changes in 3D substrate, an issue highlighted in a recent review on cellular responses to cellular stiffness (Peyton et al., 2007). Here, we investigate the effects of 2D and 3D substrate stiffness on endothelial cell stiffness. In addition, we explore substrate stiffness effects on actin, as actin is a major determinant of a cell’s mechanical properties, with both the abundance of actin and its tension likely playing important roles (Pourati et al., 1998). Endothelial cells are an ideal cell type for these studies as they reside in vivo in both types of environments: the endothelial monolayers lining conduit vessels that resemble the 2D in vitro environment and capillaries surrounded by basement membrane that resemble the 3D in vitro environment.
2. Materials and methods
2.1. Culture of cells on 2D and in 3D substrates
Bovine aortic endothelial cells (BAEC) were cultured on top of fibronectin-laminated PA gels with a stiffness of 1700 and 9000 Pa. Polyacrylamide gel stiffness was regulated while maintaining a constant level of fibronectin adsorbed to the surface as previously described (Yeung et al., 2005). Human umbilical vein endothelial cells (HUVEC) were from Cascade Biologics and cultured as previously described (Sieminski et al., 2004, 2005). To regulate the substrate stiffness in a 3D environment, HUVEC were suspended at 1 × 106 cells/ml in type-I rat-tail collagen gels at concentrations ranging from 1.0 mg collagen/ml (compliant gels) or 3.0 mg/ ml (stiff gels) as described before (Sieminski et al., 2005).
2.2. Characterization of gel stiffness
Cylindrical cell-free collagen gels were prepared. After polymerizing, gels were transferred to a RFS II fluids spectrometer (Rheometrics Inc.) and the shear modulus was measured in 3–6 gels at each concentration. From the shear modulus (G′), the elastic modulus (E) was calculated assuming that the gels are incompressible, a reasonable assumption given their high (>99%) water content and the short duration of the rheology measurements.
2.3. Measurement of cellular deformability by micropipette aspiration
Cellular stiffness was assessed using micropipette aspiration of substrate-attached cells. Micropipette aspiration has traditionally been conducted on cells not attached to the substrate (Hochmuth, 2000). We chose to measure cellular stiffness in substrate-attached cells to avoid changes in cellular stiffness due to alterations of cytoskeletal structure and tension that likely occur in non-attached cells. While it would be ideal to measure the stiffness of cells within three-dimensional gels, this is neither possible using micropipette aspiration, to our knowledge, nor has it been done using other techniques. Thus, we used micropipette aspiration to determine the stiffness of adherent cells recently isolated from compliant and stiff gels. Cells were removed from the collagen gel by adding 1 ml of 0.5 mg/ml collagenase type IV (Gibco), and incubating at 37 °C for ~15 min with continuous shaking. Cells were centrifuged, re-suspended in supplemented EBM-2, and plated on glass slides. Microaspiration measurements were performed on cells 1–3 h after plating; this provided adequate time for the cells to spread on the substrate but was the minimal time to acquire measurements from five independent cells from a single condition. We have previously detailed our adaptation of micropipette aspiration for use on substrate-attached cells (Byfield et al., 2004). Briefly, to facilitate the visualization of the aspirated membrane segment, cells were stained with DiIC18, a fluorescent membrane dye. The tip of the pipette was placed next to the cell membrane (Fig. 1A), a specific level of vacuum (P) was applied, and the length of the aspirated membrane (L) was observed. The steady-state membrane aspiration was then normalized to the pipette diameter (a), which ranged from 3 to 5 µm. The normalized aspiration length per mmHg vacuum (L/a)/P provided a convenient measure inversely related to cell stiffness.
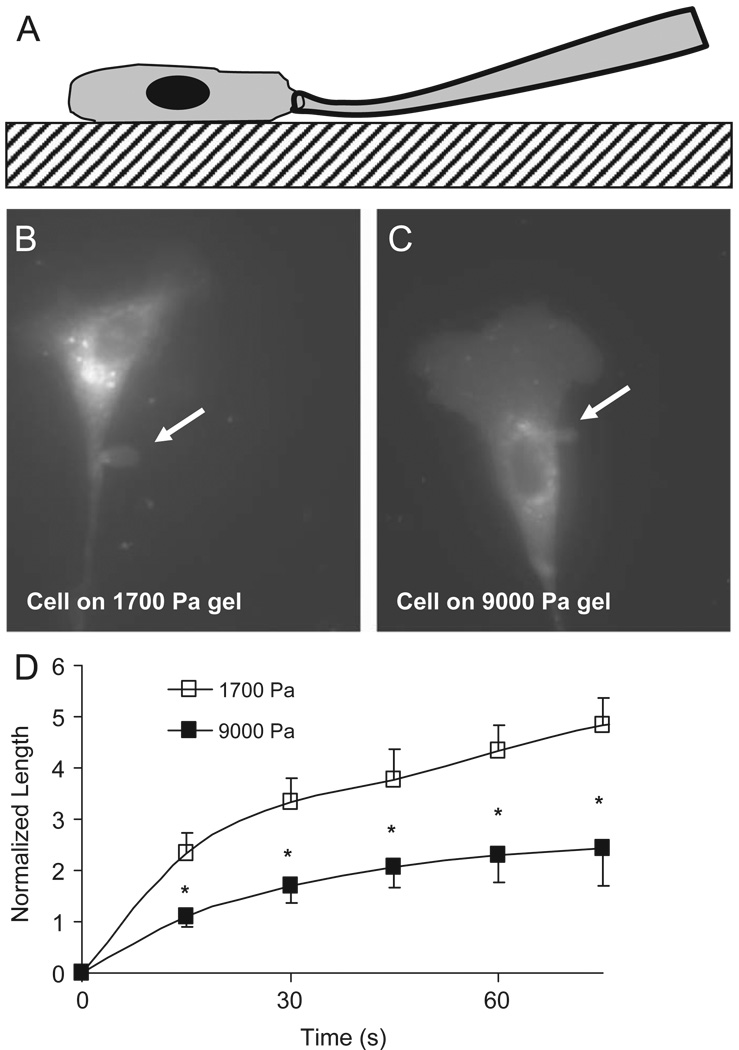
Fig. 1.
Microaspiration of adhered cells. The micropipette is gently pressed against the glass slide and slid into contact with the adhered cell (A). A vacuum is applied; aspirating a portion of the cell (arrow) into the pipette bore (B, C). The aspirated length is monitored as a function of time revealing that bovine aortic endothelial cells cultured on stiff substrates are more difficult to deform relative to those cultured on compliant gels (D).
2.4. Measurement of cellular deformability by atomic force microscope (AFM) microindentation
Individual cell stiffness was measured with a Novascan atomic force microscope (Novascan Technologies, Ames, IA) mounted on an inverted Nikon microscope (Tokyo, Japan) as described in the reference (Titushkin and Cho, 2007). A cantilever with borosilicate particle (10 µm, 0.12 N/m) (Novascan Technologies, Ames, IA) served as the cell indenter. The indentation was probed over the cytoplasmic area of the cell to avoid the nucleus and the cell edge. A total of 30–40 cells of each type and experimental condition were used, with 15 force–distance curves acquired from each cell. The force–distance curves were collected and analyzed according to the Hertz model, as listed as following:
F relates the loading force; δ is indentation depth; ν is the cellular Poisson’s ratio (assumed to be 0.5); R is the radius of the spherical indenter (5 mm); E is the local Young’s elastic modulus.
2.5. Visualization and quantification of actin
Collagen gels containing cells were fixed in 4% paraformaldehyde overnight. Collagen gels were cut into ~1 mm3 samples and stained with 0.165 µM AlexaFluor 546 Phalloidin (Invitrogen) for 24h according to manufacturer’s instructions. Samples were placed on glass slides and gently pressed with a glass coverslip. The cells in stained samples were then visualized using a Leica TSL SL confocal fluorescent microscope with a 63 × oil-immersion lenses at 543 nm excitation and 555–700 nm emission. Five representative maximal projection z-stacks from each sample were analyzed using ImageJ to determine the average brightness of cells within each image.
2.6. Statistics
Data are presented as average ± SEM. Means were compared using Student’s two-tailed t-test and paired t-test. p<0.05 is considered significant.
3. Results
Relative to endothelial cells on the less stiff polyacrylamide gels (1700 Pa), cells grown on the stiffer polyacrylamide gels (9000 Pa) were themselves stiffer (Fig. 1B–D). The (L/a)/P, a measure of how easy it is to deform a cell, for cells isolated from compliant gels was approximately twice that of cells isolated from stiff gels; 1.74±0.20 and 0.92±0.21 mmHg−1 (p<0.05), respectively.
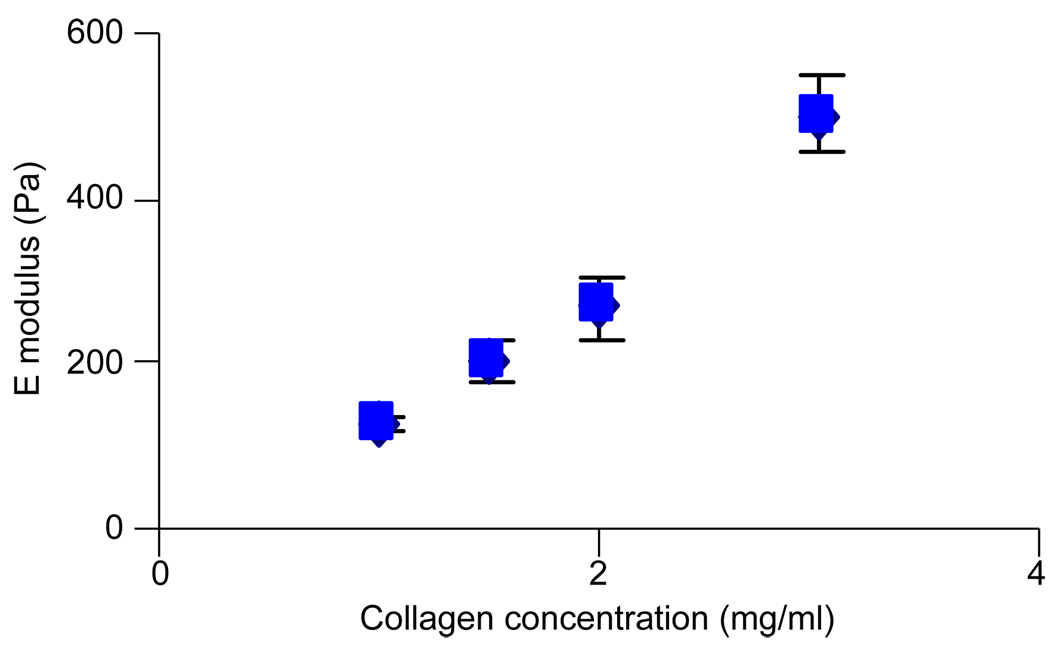
The elastic modulus of collagen gels increases as collagen concentration increases (Fig. 2). Our measured elastic moduli for type-I rat-tail collagen gels are in general agreement with those reported by others (Paszek et al., 2005; Leung et al., 2007; Sieminski et al, 2007). HUVEC cultured within collagen gels for 2 days in the presence of the phorbol ester, phorbol myristate acetate (PMA), and the pro-angiogenic growth factors bFGF and VEGF, each at 50 ng/ml, undergo morphogenesis and form capillary-like networks (Fig. 3A); in the absence of PMA, HUVEC do not form capillary-like networks (Sieminski et al., 2005). At 500 ng/ml, (10-fold concentrations greater than used here), PMA alters actin structure in endothelial cells while 100 ng/ml has no effect on actin structure (Liu and Sundqvist, 1995). Endothelial cells elongate in collagen gels of various stiffnesses. Qualitatively it appeared that the cells in stiff gels have more prominent stress fibers than those in compliant gels (Fig. 3B vs. A).
Fig. 2.
Increasing collagen concentration increases gel stiffness. Figure shows mean values and the SEM.
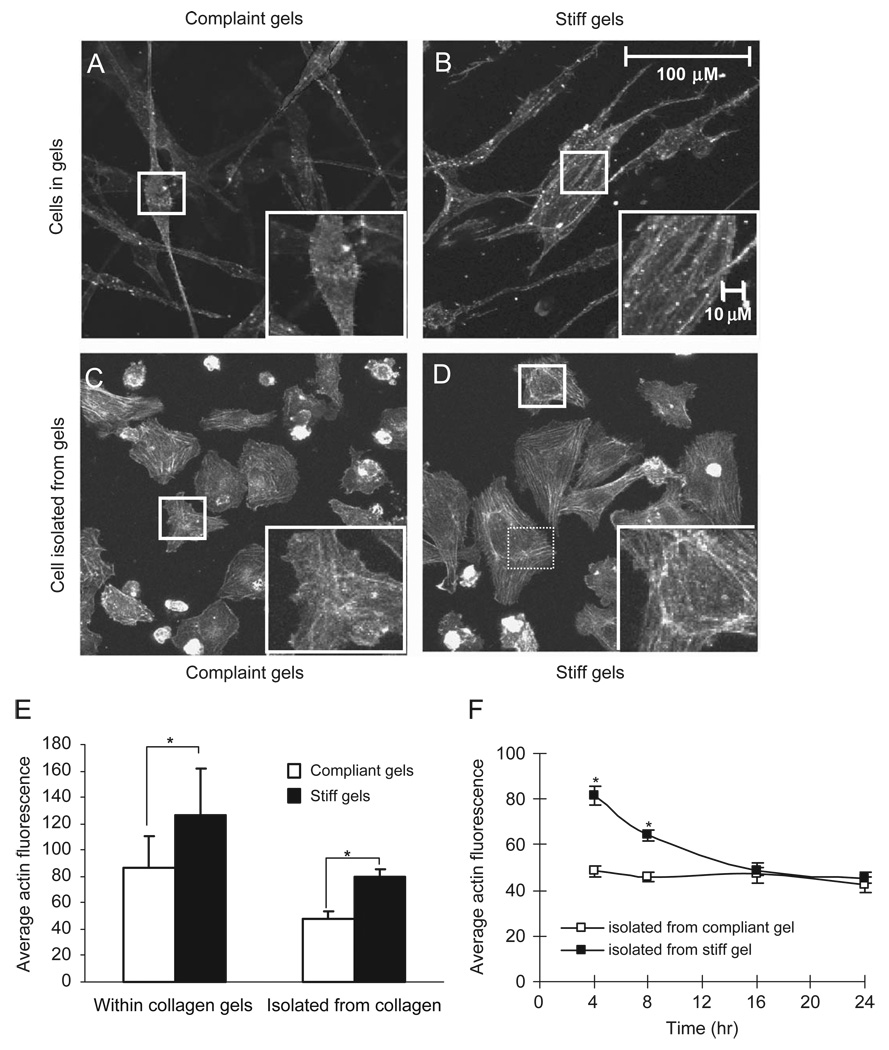
Fig. 3.
Qualitative (A–D) and quantitative (E, F) assessment of actin for endothelial cells. Cells within compliant gels (A) exhibit less intense actin staining and less prominent stress fibers as compared to cells within stiff gels (B). These differences in actin are preserved after the cells are isolated from compliant (C) or stiff (D) gels and plated on glass slides. Quantitative image analysis (E) confirm the qualitative observation. (F) The average actin fluorescence for cells isolated from stiff and compliant gels decreases as a function of time since plating of cells onto glass slides.
Consistent with qualitative observations, quantitative image analysis of cells fluorescently stained for actin revealed that the average staining intensity of HUVEC in stiffer 3D gels was 1.5-fold greater than those in relatively compliant gels (Fig. 3E). HUVEC isolated from compliant and stiff collagen gels and then plated onto tissue-culture-treated plastic (Fig. 3C and D) retained their difference in actin staining with cells isolated from stiff gels having 1.7-fold greater average staining intensity than those from compliant gels. The fold increases in average staining intensities between cells in stiff and compliant gels are similar whether the measurements are made on cells in or recently isolated from collagen gels (Fig. 3E). Within 1 day following plating onto glass slides, cells isolated from within collagen gels appear to adapt to their new mechanical environment and difference in the average actin staining due to the stiffnesses of the collagen gels they were previously cultured in disappear (Fig. 3F). Isolated cells tended to have lower average staining intensities than cells in the gel (Fig. 3E). It is likely that at least part of this decrease in average staining intensity is due to the fact that cells cultured on tissue-culture-treated plastic spread more than those in a gel (Fig. 3C and D vs. Fig. 3A and B), thus decreasing the average intensity by distributing a given signal over a greater area.
As actin is a major determinant of the mechanical stiffness of endothelial cells (Sato et al., 1990; Pourati et al., 1998), we reasoned that since endothelial cells in the relatively stiff 3D gels had more actin fibers, they would also be stiffer. A linear relationship was observed between the steady-state aspirated length and the vacuum pressure (Fig. 4A). Relative to cells isolated from stiff gels, cells isolated from compliant gels had larger aspirated lengths at a given pressure (Fig. 4B); i.e., cells from compliant gels were easier to deform and are less stiff. The average stiffness for the cells microaspirated ~1–2 h after plating was compared to those measured ~2–3 h after plating (a minimum of 1 h was allowed for the cells to adhere in all cases). The average stiffness corresponding to the two periods did not differ suggesting that cells were not significantly changing their stiffness in a time period comparable to that required to measure the stiffness in a sample set. In parallel experiments, the stiffness of HUVEC isolated from 1.5 mg/ml collagen gels and plated onto glass cover slips was assessed with AFM (Fig. 5). As observed with actin fluorescence, the average stiffness changed within the first 8 h, but had stabilized within 24 h.
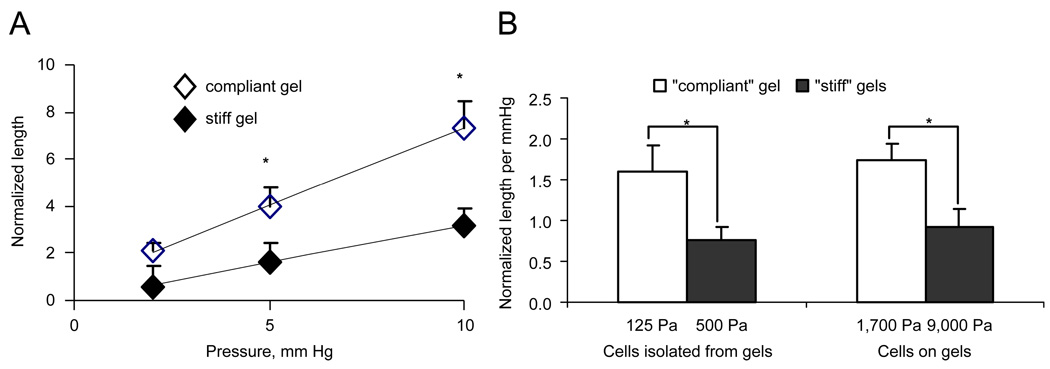
Fig. 4.
Micropipette aspiration data for endothelial cells isolated from relatively compliant (open symbols and bars) and relatively stiff (filled symbols and bars) gels. Panel A shows the steady-state normalized aspirated length (L/a) as a function of applied vacuum. Panel B shows the normalized aspirated length per mmHg with a vacuum of 5 mmHg for both cells isolated from gels as well as those cultured on gels.
Fig. 5.
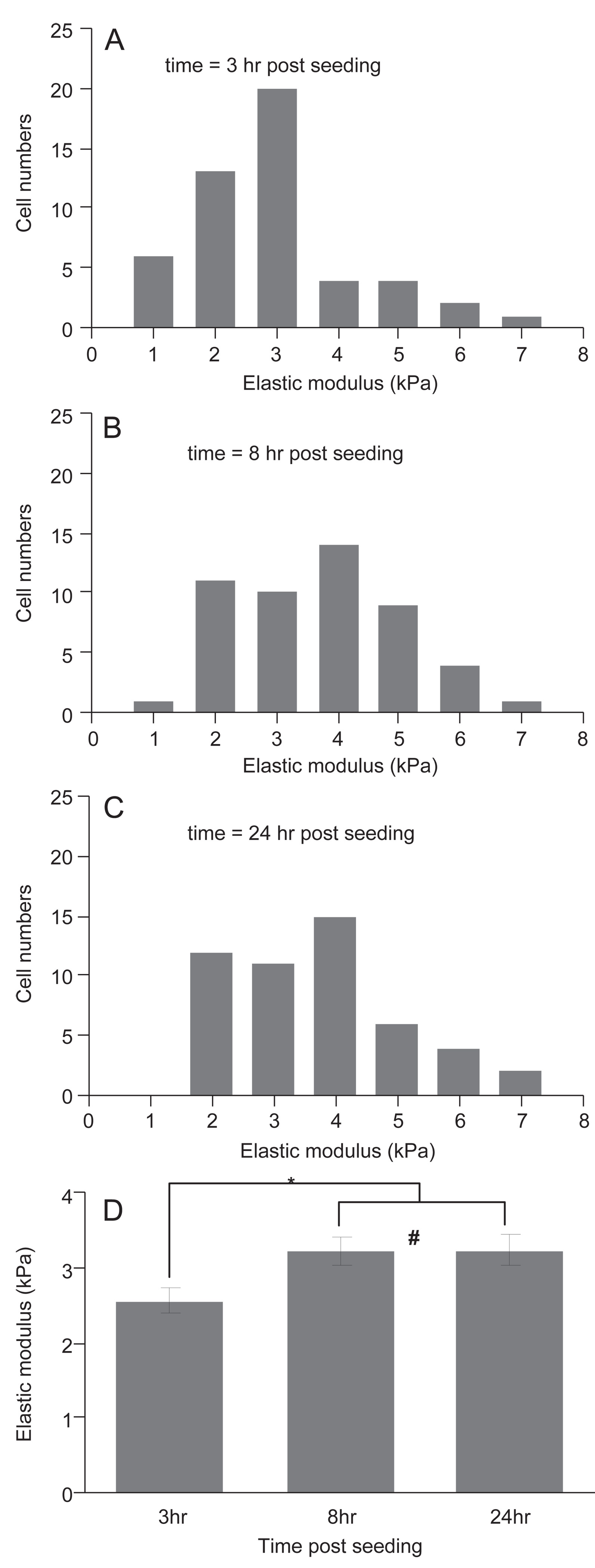
Elastic moduli of HUVEC following removal from a collagen gel and being plated onto glass. Histogram of elastic moduli of HUVEC measured by AFM at 3 h (A), 8 h (B), and 24 h (C) after seeding on glass slides. Average and SEM of elastic moduli as a function of time (D). * indicates p<0.02; # indicates p = 0.95.
4. Discussion
Endothelial cells alter their cell stiffness in response to the stiffness of the substrate they are cultured on top of. These observations are consistent with earlier studies of fibroblasts, mesenchymal stem cells, as well as immortalized osteoblast and myoblast cell lines (Engler et al., 2006; Solon et al., 2007). In addition, we report for the first time that the stiffness of the 3D matrix that cells are cultured within also alters the stiffness of the cells. Since technical limitations prevent the measurement of stiffness of the cells within the 3D gels, for these cells stiffness measurements were made on cells recently isolated from gels with different stiffnesses. It is reasonable to expect that the cell stiffness measured on cells recently isolated from gels reflects their stiffness within the gels for several reasons. First, quantification of actin staining, which was performed on cells still within the 3D gels, yielded differences in actin staining that were consistent with the trends observed in the stiffness measurements. The notion that changes in cell stiffness are accompanied by changes in the actin cytoskeleton is supported by a recent study with fiborblasts cultured on fibronectin-coated polyacrylamide gels of different stiffnesses (Solon et al., 2007). Second, cells isolated from gels of different stiffnesses initially had different stiffnesses and these differences remained stable for the several hours required to make microaspiration measurements. Finally, over a period of 8–16 h, the stiffness and actin of cells isolated from gels stabilized as they adapted to their new environment. The time scale of this adaptation is much slower than that required for our measurements of cell stiffness.
The choice of the stiffnesses for the 2D polyacrylamide gels was guided by the work of Yeung et al. showing that cells cultured on matrices with a stiffness of 1600 Pa or less lacked stress fibers or other actin bundles while cells on matrices with E of 3200 or greater stress fibers were abundant (Yeung et al., 2005). Yueng et al. also showed that endothelial cells attached to, but failed to spread on top of, gels with a modulus less than ~1000 Pa. Thus we chose a range of gel stiffnesses that would likely affect endothelial cells while still allowing the cells to exhibit normal behavior (Yeung et al., 2005) on top of their substrate. Given our data for endothelial cells on polyacrylamide gels and the published results of others showing cells respond to changes in stiffnesses on the order of 1000 or 10,000 s of Pa, one might initially suspect that changes across a similar range of stiffnesses would be required to stimulated changes in endothelial cells within gels. There are several reasons that we chose lower stiffnesses for the 3D gels. First, we (Sieminski et al., 2004) and others (Kanzawa et al., 1993) have previously demonstrated that endothelial cells within 3D gels alter their morphology to changes in this range of substrate stiffnesses. Second, others have shown that endothelial cells in significantly stiffer 3D gels (~1000 Pa) fail to form microvascular networks and remain essentially spherical (Sieminski et al., 2007). Thus, as in the 2D studies, in 3D studies, we chose a range of gel stiffnesses that were likely to affect endothelial cell behavior while still allowing the cells to exhibit normal behavior.
While endothelial cells showed very similar changes in their cellular stiffness in response to changes in either 2D substrate (cells on a gel) stiffness or 3D substrate (cells in a gel) stiffness (Fig. 4B), the magnitudes of the 2D substrate stiffnesses eliciting the affects were about an order of magnitude greater than the 3D substrate stiffnesses. The concept that matrix dimensionality may modulate matrix stiffness effects is consistent with concepts proposed by others and published data. Cukierman et al. suggested that matrix dimensionality and matrix stiffness both act to regulate cell-matrix adhesions (Cukierman et al., 2001). Studies looking at cellular responses to changes in either 2D substrate stiffness or 3D substrate stiffness also support the conclusion that the dimensionality of the substrate may modulate stiffness effects. For example, as we already noted, there are several studies investigating the effects of either 2D or 3D substrate stiffness on different endothelial cell function, yet those looking at cells within 3D gels saw responses at lower stiffnesses. Similarly, those studying fibroblasts in 3D collagen gels have seen effects attributed to stiffness at collagen concentrations in the range of those we used here (e.g., reviewed in (Grinnell, 2003)) while those studying matrix stiffness effects in fibroblasts on top of ECM-laminated polyacrylamide gels tend to explore higher stiffness from ~1000 to ~30,000 Pa (Lo et al., 2000; Jiang et al., 2006; Kostic and Sheetz, 2006).
In contrast to the notion that dimensionality is a key variable, other differences between the cells in collagen gel system and the cells on polyacrylamide gel system may account for the observed difference in the effectual stiffnesses. For example, ligand density, which will vary between the two systems we used, and stiffness are known to interact to regulate cellular responses in other systems (Engler et al., 2004). In addition, the apparent differences in the effectual stiffnesses we observe may not represent true differences in the endothelial cells’ response to their local environment but result from the difficulty characterizing the stiffness of a nonlinearly elastic material. Collagen gels, but not polyacrylamide gels, exhibit nonlinear elasticity with increased stiffness at greater strains (Storm et al., 2005). That is, in strain stiffening materials, the stiffness of collagen gels at larger strains is much higher than its stiffness at lower strains and thus it is possible that the stiffness that we report here for collagen, which is based on the linear elastic region observed at relatively low strains, may under represent the actual stiffness in the pericellular environment if larger strains are present. Conversely, the viscoelastic nature of collagen, opposed to the purely elastic nature of polyacrylamide, would enable collagen to creep over longer times, which could decrease stress thus lowering collagen’s effective stiffness. Regardless of the reasons for the real or apparent differences in the effectual stiffnesses in the 2D and 3D systems we employed here, these data further support the notion that changes in either 2D substrate stiffness or 3D substrate stiffness both can have significant effects on cells and show for the first time that cells alter their cellular stiffness in response to changes in the stiffness of their 3D environment. Further, these data illustrate that the magnitude of the stiffness initiating a response is strongly dependent on specific features of the environment, potentially including the dimensionality of the matrix, and suggest that care should be taken when extrapolating effectual stiffnesses obtained in one environment to another environment.
Acknowledgments
This work was funded by AHA 0655323B, NIH HL64388, HL073965(IL) and HL083298(IL). Paul Janmey suggested the potential role of the nonlinear elasticity of collagen in reconciling the apparent differences in effectual stiffnesses.
Footnotes
Conflict of interest statement
No conflicts.
References
- Byfield FJ, Aranda-Espinoza H, et al. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys. J. 2004;87(5):3336–3343. doi: 10.1529/biophysj.104.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, et al. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, et al. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Edwards D, Gooch KJ, et al. The nucleation of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 1996;93:1786–1791. doi: 10.1073/pnas.93.5.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A, Bacakova L, et al. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004;86(1 Pt 1):617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Ju YE, et al. Neurite branching on deformable substrates. Neuroreport. 2002;13(18):2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13(5):264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Halliday NL, Tomasek JJ. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp. Cell Res. 1995;217(1):109–117. doi: 10.1006/excr.1995.1069. [DOI] [PubMed] [Google Scholar]
- Hochmuth RM. Micropipette aspiration of living cells. J. Biomech. 2000;33(1):15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- Jiang G, Huang AH, et al. Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys. J. 2006;90(5):1804–1809. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzawa S, Endo H, et al. Improved in vitro angiogenesis model by collagen density reduction and the use of type III collagen. Ann. Plast. Surg. 1993;30(3):244–251. doi: 10.1097/00000637-199303000-00008. [DOI] [PubMed] [Google Scholar]
- Kostic A, Sheetz MP. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol. Biol. Cell. 2006;17(6):2684–2695. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LY, Tian D, et al. A new microrheometric approach reveals individual and cooperative roles for TGF-beta1 and IL-1beta in fibroblast-mediated stiffening of collagen gels. Faseb J. 2007;21(9):2064–2073. doi: 10.1096/fj.06-7510com. [DOI] [PubMed] [Google Scholar]
- Lo CM, Wang HB, et al. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79(1):144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SM, Sundqvist T. Effects of hydrogen peroxid and phorbol myristate acetate on endothelial transport and F-actin distribution. Exp. Cell Res. 1995;217(1):1–7. doi: 10.1006/excr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Nehls V, Herrmann R. The configuration of fibrin clots determines capillary morphogenesis and endothelial cell migration. Microvasc. Res. 1996;51(3):347–364. doi: 10.1006/mvre.1996.0032. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton SR, Ghajar CM, et al. The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem. Biophys. 2007;47(2):300–320. doi: 10.1007/s12013-007-0004-y. [DOI] [PubMed] [Google Scholar]
- Pourati J, Maniotis A, et al. Is cytoskeletal tension a major determinant of cell deformability in adherent endothelial cells? Am. J. Physiol. 1998;274(5 Pt 1):C1283–C1289. doi: 10.1152/ajpcell.1998.274.5.C1283. [DOI] [PubMed] [Google Scholar]
- Roeder BA, Kokini K, et al. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J. Biomech. Eng. 2002;124(2):214–222. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- Sato M, Theret DP, et al. Application of the micropipette technique to the measurement of cultured porcine aortic endothelial cell viscoelastic properties. J. Biomech. Eng. 1990;112(3):263–268. doi: 10.1115/1.2891183. [DOI] [PubMed] [Google Scholar]
- Sieminski AL, Hebbel RP, et al. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp. Cell Res. 2004;297(2):574–584. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Sieminski AL, Hebbel RP, et al. Improved microvascular network in vitro by human blood outgrowth endothelial cells relative to vessel-derived endothelial cells. Tissue Eng. 2005;11(9–10):1332–1345. doi: 10.1089/ten.2005.11.1332. [DOI] [PubMed] [Google Scholar]
- Sieminski AL, Padera R, et al. Systemic delivery of hGH using genetically modified tissue-engineered microvascular networks: prolonged delivery and endothelial survival with inclusion of non-endothelial cells. Tissue Eng. 2002;8(6):1057–1069. doi: 10.1089/107632702320934155. [DOI] [PubMed] [Google Scholar]
- Sieminski AL, Was AS, et al. The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation. Cell Biochem. Biophys. 2007;49(2):73–83. doi: 10.1007/s12013-007-0046-1. [DOI] [PubMed] [Google Scholar]
- Solon J, Levental I, et al. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007;93(12):4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm C, Pastore JJ, et al. Nonlinear elasticity in biological gels. Nature. 2005;435(7039):191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93(10):3693–3702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Georges PC, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure and adhesion. Cell Signaling Cytoskeleton. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]