Abstract
Polysaccharide (PS)- and protein-specific murine IgG responses to intact Streptococcus pneumoniae (Pn) are both dependent upon CD4+ T cell help, B7-dependent costimulation, and CD40/CD40-ligand interactions. However, the primary PS-, relative to protein-specific, IgG response terminates more rapidly, requires a shorter period of T cell help and B7-dependent costimulation, and fails to generate memory. In light of the critical role for ICOS/ICOS-ligand interactions in sustaining T cell-dependent Ig responses and promoting germinal center reactions, we hypothesized that this interaction was non-essential for PS-specific IgG responses to Pn. We now demonstrate that ICOS-/-, relative to WT, mice elicit a normal PS-specific IgG isotype response to Pn, despite marked inhibition of both the primary and secondary IgG anti-protein (i.e. PspA, PspC, and PsaA) response. A blocking anti-ICOS-ligand mAb injected during primary Pn immunization inhibits both the primary anti-protein response and the generation of protein-specific memory, but has no effect when injected during secondary immunization. In contrast to Pn, both PS- and protein-specific IgG responses to a pneumococcal conjugate vaccine are inhibited in ICOS-/- mice. ICOS-/- mice immunized with intact Pn or conjugate exhibit nearly complete abrogation in germinal center formation. Finally, although mice that lack the adaptor molecule SAP resemble ICOS-/- mice (and can exhibit decreased ICOS expression), we observe that the PS-, as well as protein-specific IgG responses to both Pn and conjugate are markedly defective in SAP-/- mice. These data define a novel T cell-, SAP-, and B7-dependent, but ICOS-independent, extrafollicular pathway of Ig induction.
Keywords: Rodent, Bacterial, Antibodies, Transgenic/Knockout mice, Vaccination
Introduction
Systemic immunization with intact Streptococcus pneumoniae, capsular type 14 (Pn14) elicits an IgG response specific for a number of pneumococcal proteins, including pneumococcal surface protein A (PspA), as well as the capsular polysaccharide (PPS14) and the phosphorylcholine determinant (PC) of the cell wall C-polysaccharide (C-PS, teichoic acid). The protein- and PS-specific IgG responses to intact Pn are each dependent on CD4+ T cells, B7/CD28-dependent costimulation, and CD40-CD40-ligand interactions, whereas the PS-specific IgM response is T cell-independent (TI) (1-3). Nevertheless, relative to the protein-specific response, the primary IgG anti-PS response to intact Pn peaks earlier, requires a shorter period of T cell help and B7-dependent costimulation, and in contrast to the anti-protein response, fails to elicit a boost in serum PPS14-specific IgG titers upon secondary immunization. PPS14 expressed by intact Pn14 behaves as a distinct immunogen relative to either isolated, soluble PPS14, or PPS14 covalently linked to PspA (PPS14-PspA) [soluble conjugate]. Thus, the IgG response to isolated PPS14 is TI, whereas immunization with PPS14-PspA induces CD4+ T cell-dependent, PPS14-specific, in addition to PspA-specific, memory (4, 5) The mechanism underlying the divergent immunologic behavior between intact PN14 and soluble pneumococcal conjugate is unresolved.
Inducible costimulator (ICOS) is a member of the CD28 family that is induced on CD4+ T cells upon TCR crosslinking and CD28-mediated signaling, whereas CD28 is constitutively expressed (6, 7). The respective cognate ligands, ICOS-L and B7-1/B7-2, expressed on APC are constitutively expressed but can be upregulated by inflammatory stimuli. In this regard, CD28 is critical for initiation of CD4+ T cell activation (8-10), whereas ICOS plays a key role in the subsequent T cell effector response (11, 12). Genetic disruption or blockade of the B7/CD28 or ICOS/ICOS-L pathways inhibits both type 1 and type 2 CD4+ T cell-dependent humoral immune responses, although secondary, relative to primary, responses are impacted to a greater degree by loss of ICOS signaling (13-15). ICOS-mediated costimulation is critical for the development of the germinal center (GC) reaction (16), in part through induction of CD40L on the CD4+ T cell (13, 14) and the development of CD4+CXCR5+ follicular-helper T cells (TFH) (17, 18), and is thus is a key regulator of immunologic memory. Genetic deficiency in ICOS in humans leads to the common variable immunodeficiency syndrome (CVID), characterized by hypogammaglobulinemia and an almost complete lack of memory B cells (19).
CD4+ T cells, as well as CD8+ and NK T cells, NK cells, and some B cells, express a cytoplasmic adaptor protein, SAP, which is encoded by the gene SH2D1A, and is critical for cell signaling via SLAM family proteins expressed on the cell surface (20-22). Genetic deficiency of SAP in both mice and humans (X-linked lymphoproliferative syndrome [XLP]) results in immunologic phenotypes, some of which are similar, to loss of ICOS/ICOS-L interactions (18, 23). Thus, although SAP-/- CD4+ T can undergo initial proliferation and activation similar to WT CD4+ T cells, they can exhibit decreased ICOS expression, and in vivo show markedly impaired induction of GC and memory B cells (23-26). In this regard, the induction of GC and memory B cells is inhibited in SAP-/- mice. Notably, immunization of SAP-/- mice with TD antigens (soluble NP-KLH or SRBC) resulted in dramatic defects in GC formation, primary and secondary antigen-specific IgG (IgG1 most dramatically reduced, then IgG2b/IgG2a, then IgG3), but not IgM, production, and dramatic defects in the generation of long-lived plasma cells in the BM (26). In adoptive transfer studies both Th1 and Th2 SAP-/- T cells failed to promote an Ig response. Patients with XLP likewise exhibit hypogammaglobulinemia and a marked reduction in memory (CD27+) B cells, associated with reduced T cell expression of ICOS (24, 27). Collectively, these data raise the possibility that the failure of SAP-/- CD4+ T cells to upregulate ICOS might contribute to the defective humoral immunity in the SAP-deficient host. However, more recent data demonstrate that SAP-/- CD4+ T cells can upregulate ICOS, but fail to provide critical signals to B cells due to a selective defect in T cell-B cell adhesion (28).
Not surprisingly, deficiency in either SAP or ICOS/ICOS-L is associated with defective TD humoral immunity and host protection against a number of microbial pathogens. As mentioned above, the PS-specific TD humoral response to intact Pn14 is unusual in that the primary IgG response and requirement for CD4+ T cells and B7-dependent costimulation is relatively short-lived. This is associated with a failure to generate PS-specific memory, despite a critical role for B7/CD28 and CD40/CD40L costimulation for the primary response (1-3). Since ICOS induction follows initial TCR- and CD28-mediated CD4+ T cell activation, peaking ~48h following immunization (12, 29), we hypothesized that the PS-, but not protein-specific, IgG response to intact Pn14 would be ICOS-independent, whereas both responses would be ICOS- and SAP-dependent in response to pneumococcal conjugate. However, it remains unclear how SAP would affect the PS-specific responses to Pn14. In this report we demonstrate that indeed, the PS-specific IgG response to intact Pn14, represents a novel B7-dependent, ICOS-independent TD response. However, despite its ICOS-independence, the PS-specific IgG response to Pn14 in markedly inhibited in SAP-/- mice, strongly suggesting that SAP plays a key role at the earliest stages of T cell help for humoral immune responses, independent of ICOS.
Materials and Methods
Mice
SAP-/- mice (30) were backcrossed to C57BL/6 mice for 8-10 generations. CD28-/- mice (15) [C57BL/6 background] (B6.129S2-Cd28tm1Mak/J, Cat# 002666) and ICOS-/- mice (13) [C57BL/6 background] (B6129P2-ICOStm1mak/J, Cat# 004859) were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6, and BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). Female mice were used between 7-10 wks of age. These studies were conducted in accordance with the principles set forth in the Guide for Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, revised 1996, and were approved by the Uniformed Services University of the Health Sciences institutional animal use and care committee.
Reagents
Recombinant pneumococcal surface protein A (PspA) was expressed in Sacharomyces cerevisiae BJ3505 and purified as previously described (31). Recombinant PsaA and PspC (CbpA) were expressed as N-terminal His6-fusion proteins in recombinant E. coli and purified by Ni-NTA affinity chromatography, as previously described (32). Purified S. pneumoniae capsular polysaccharide type 14 (PPS14) was purchased from American Type Culture Collection (Manassas, VA). PC-KLH (keyhole limpet hemocyanin) was synthesized as previously described (1). The resulting conjugate had a substitution ratio of 19 PC/KLH molecule. Soluble conjugates comprising PspA covalently linked to either C-polysaccharide (C-PS-PspA) or PPS14 (PPS14-PspA) were synthesized as previously described (3). A blocking mAb against mouse B7RP-1 (ICOS-ligand) [clone HK5.3, rat IgG2a] was generated as previously described (33). The HK5.3 mAb and a control rat IgG2a anti-E coli β-galactosidase mAb (clone GL117) were purified from culture SN using protein G chromatography.
Preparation of S. pneumoniae, capsular type 14 (Pn14)
A frozen stock of Pn14 was thawed and sub-cultured on BBL pre-made blood agar plates (VWR International, Bridgeport, NJ). Isolated colonies on blood agar were grown in Todd Hewitt broth (Becton Dickinson, Sparks, MD) to mid-log phase, collected, and heat killed by incubation at 60°C for 1h. Sterility was confirmed by subculture on blood agar plates. After extensive washings, the bacterial suspension was adjusted with PBS to give an absorbance reading at 650 nm of 0.6 which corresponded to 109 CFU/ml. Bacteria were then aliquoted at 1010 colony-forming units (CFU)/ml and frozen at –80°C until their use as antigen for mouse immunizations. The Pn14 stock was tested for endotoxin using the Limulus Amebocyte Lysate assay (QCL-1000) from BioWhittaker (Walkersville, MD). This assay demonstrated that mice injected with 2×108 CFU equivalents of heat-killed Pn14 receive <20 pg of endotoxin.
Immunizations
Mice (7 per group) were immunized i.p. with 2 × 108 CFU heat-killed Pn14 in saline or i.p. with 1 μg (weight of polysaccharide) each of PPS14-PspA + C-PS-PspA adsorbed on 13 μg of Alum (Allhydrogel 2% [Brenntag Biosector, Denmark]) mixed with 25μg of a stimulatory 30 mer CpG-containing oligodeoxynucleotide (CpG-ODN) (34), and similarly boosted. Serum samples for measurement of anti-PPS14, anti-PC, anti-PspA, anti-PsaA, and anti-PspC Ig isotype titers were prepared from blood obtained through the tail vein.
Measurement of serum antigen-specific Ig isotype titers
Immulon 4 ELISA plates (Dynex Technologies, Inc., Chantilly, VA) were coated (50μL/well) with PC-KLH (5 μg/ml), PPS14 (5 μg/ml), PspA, PsaA, or PspC (5 μg/ml) in PBS overnight at 4°C. Plates were washed 3X with PBS + 0.1% Tween 20 and were blocked with PBS + 1% BSA for 1 h at 37°C. Threefold dilutions of serum samples, starting at a 1/50 serum dilution, in PBS + 1% BSA were then added overnight at 4°C and plates were washed 3X with PBS + 0.1% Tween 20. Alkaline phosphatase-conjugated polyclonal goat anti-mouse IgM, IgG, IgG3, IgG1, IgG2b, or IgG2a Abs (200 ng/ml final concentration) in PBS + 1% BSA were then added, and plates were incubated at 37°C for 1 h. Plates were washed 5X with PBS + 0.1% Tween 20. Substrate (p-nitrophenyl phosphate, disodium; Sigma, St. Louis, MO) at 1 mg/ml in TM buffer (1 M Tris + 0.3 mM MgCl2, pH 9.8) was then added for color development. Color was read at an absorbance of 405 nm on a Multiskan Ascent ELISA reader (Labsystems, Finland). Serum Ig titers were calculated as follows: A standard curve was generated using 3-fold dilutions of a positive serum sample, starting with an initial 1/50 serum dilution. The signal (in fluorescent units) from the most dilute sample of the standard curve that was still above background was randomly assigned a titer of “1” and all signals from consecutively less dilute samples were assigned the numbers 3, 9, 27 etc. For each experimental sample, a dilution was chosen which generated a signal within the linear part of the standard curve. The value extrapolated from the standard curve was then multiplied by the inverse of that dilution in order to generate the final inverse titer.
Detection of germinal centers
Mice were injected i.p. with either Pn14 or conjugate. Spleens were removed 12 d after immunization and incubated at least 6 hours in 15 ml of PLP buffer (0.05 M PBS containing 0.1 M L-lysine (pH 7.4), 2 mg/ml NaIO4, and 10 mg/ml paraformaldehyde). The fixed samples were washed in PBS, and dehydrated in 30% sucrose in PBS. Tissues were snap-frozen in Tissue-Tek (VWR, West Chester, PA). Twenty to thirty μm thick frozen sections were cut and stained with anti-mouse B220-Pacific Blue (clone RA36B2, Cat# 558108), anti-mouse CD3-PE (clone 17A2, Cat# 555275) [BD Bioscience, San Jose, CA], peanut agglutinin (PNA)-Alexa Fluor 488 from Arachis hypogaea, (Cat# L21409, Invitrogen, Carlsbad, CA). Immunofluorescence imaging was performed with a Zeiss Pascal Laser Scanning Confocal Microscope. Separate images were collected for each fluorochrome and overlaid to obtain a multicolor image. Final image processing was performed with ImageJ software (National Institutes of Health, Betheda, MD) and Adobe Photoshop. Spleens from four mice per group (4 sections per spleen) were analyzed for GC number and area, for a total of ~12 mm2 of splenic area per group.
Flow cytometric analysis
Splenic and peritoneal cells from WT and knockout mice (three mice per group [WT, SAP-/-, and ICOS-/-]; four CD28-/- mice), were harvested, and B cell subsets from each individual spleen were enumerated by flow cytometry as follows: splenic marginal zone B [MZB] (CD21highCD23low/neg), follicular B [FB] (CD21intermediateCD23high), and B-1 (B220intermedCD5+), peritoneal B-1a (B220+CD11b+CD5+), B-1b (B220+CD11b+CD5-), and B-2 (B220+CD11b-CD5-). The following mAbs, purchased from Pharmingen (San Diego, CA) were used: FITC-rat IgG2b,κ anti-mouse CD21/35 (clone 7G6) PE-rat IgG2a,κ anti-mouse CD23 (clone B3B4), FITC-rat IgG2a,κ anti-mouse CD45R/B220 (clone RA3-6B2), biotin-rat IgG2a,κ anti mouse CD5 [Ly-1] (clone 53-7.3) + streptavidin-PE-Texas Red, and PE-rat IgG2b,κ anti-mouse CD11b (clone M1/70). Cells were analyzed on a “BD-LSR-II” flow cytometer (BD Biosciences, San Jose, CA) using 488 and 635 lasers.
Statistics
Serum Ig isotype titers were expressed as geometric means ± standard errors of the means (SEM) of the individual serum Ig isotype titers. Splenic and peritoneal B cell subset numbers and percentages were determined by flow cytometry and expressed as arithmetic means of the numbers obtained from individual mice +/- SEM. Significance in both cases was determined by the Student t test. P values of <0.05 were considered statistically significant. All experiments were performed twice, except for flow cytometry, which was performed once.
Results
The IgG anti-protein and anti-polysaccharide response to Pn14 is CD28-dependent
Immunization i.p. with intact, heat-killed Streptococcus pneumoniae, capsular type 14 (Pn14) induces an IgM response specific for the capsular polysaccharide (PPS14) and the phosphorylcholine (PC) determinant of the cell wall C-polysaccharide (C-PS, teichoic acid) and/or membrane lipoteichoic acid (LTA) that is T cell-independent (TI) (1, 3). In contrast the IgG anti-PPS14 and IgG anti-PC responses are dependent on CD4+ T cells and CD40/CD40L interactions. In a previous study we also demonstrated, using the unencapsulated variant of Pn2 (R36A), that the IgG anti-PC response was dependent on B7-2 and CD28 (2). Pn14 also elicits IgG anti-protein responses (e.g. pneumococcal surface protein A [PspA], pneumococcal surface adhesin A [PsaA], and pneumococcal surface protein C [PspC]) (35). In particular, detailed analysis of the IgG anti-PspA protein response to Pn14 and/or R36A also demonstrated dependence on CD4+ T cells, B7-2/CD28 costimulation, and CD40/CD40L interactions. In light of our current use of Pn14 in this study to determine a potential role of ICOS costimulation in mediating Ig responses, we first wished to determine whether the TD IgG anti-PPS14, as well as IgG anti-PC and anti-PspA responses to Pn14 were dependent upon CD28-dependent costimulation. Thus, CD28-/- and WT mice were immunized with Pn14 and boosted 14 days later. Serum titers of antigen-specific IgM and IgG were measured on day 21. As illustrated in Figure 1A, the IgG anti-PspA response to Pn14 was completely abrogated in CD28-/- mice, similar to what we previously observed using R36A (2). Whereas the TI IgM anti-PPS14 and IgM anti-PC responses were unaffected in CD28-/- mice, both the TD IgG anti-PPS14, as well as the TD IgG anti-PC responses were significantly, though partially, reduced relative to WT mice (Figure 1A). The partial reduction in the IgG anti-PC response to Pn14 in CD28-/- mice was similar to what we previously observed using R36A (2). Similar results were observed from sera obtained on day 7 and/or day 14 (data not shown).
Figure 1. The IgG anti-protein and anti-polysaccharide response to Pn14 is CD28-dependent.
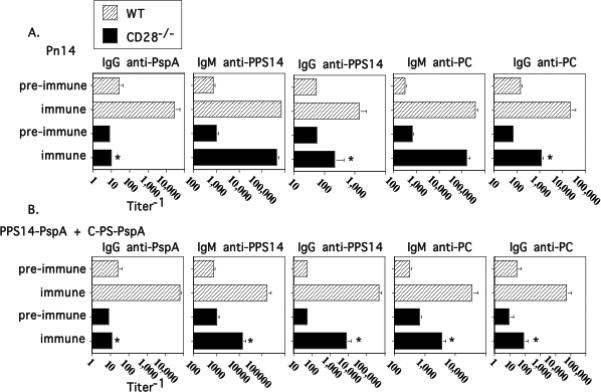
WT (C57BL/6) and CD28-/- mice (7 per group) were immunized i.p. and then boosted on day 14 with either (A) Pn14 in saline or (B) PPS14-PspA + C-PS-PspA in alum/CpG-ODN. Serum titers of antigen-specific IgM and IgG are illustrated for day 0 (“pre-immune”) and day 21 (“immune”). *significant, p<0.05 between WT and CD28-/- mice.
Covalent linkage of an immunogenic carrier protein to a polysaccharide antigen (conjugate) converts the IgG anti-PS response from TI-2 to TD, the latter now dependent on CD4+ T cells, B7-dependent costimulation, and CD40/CD40L interactions (36, 37), similar to what we observed for intact Pn14 (1). Of interest, whereas the IgM anti-PS response to Pn14 is TI, the IgM, as well as the IgG, anti-PS response to conjugate is partially TD. In contrast to a pneumococcal conjugate (i.e. PPS14-PspA + C-PS-PspA in alum/CpG-ODN), the primary IgG anti-PPS14 and IgG anti-PC response to Pn14 develops with more abbreviated kinetics and does not generate PS-specific immunologic memory. We thus wished to further determine the CD28 dependence of the conjugate-induced Ig response. As illustrated in Figure 1B, the IgG anti-PspA response to conjugate was completely abrogated in CD28-/- mice, similar to that observed with Pn14 immunization. In contrast to Pn14, both the IgM and IgG anti-PPS14 and anti-PC responses to conjugate were partially, but not completely, abrogated in CD28-/- mice. Of interest, the IgG anti-PPS14 and IgG anti-PC responses to conjugate were more strikingly reduced in CD28-/- mice than the same responses to Pn14 (significant, p<0.05). These data thus demonstrate absolute and relative differences in the CD28 dependence of the Ig response to Pn14 versus conjugate.
The IgG anti-PPS14 and anti-PC response to Pn14, in contrast to pneumococcal conjugate, is ICOS-independent
Numerous studies have documented the critical role of ICOS/ICOSL interactions in mediating TD IgG responses (11-14). In light of the CD28 dependence of both the IgG anti-PPS14 and anti-PC responses to Pn14, previously shown to be also dependent on CD4+ T cells and CD40/CD40L interactions (1, 3), we wished to determine whether or not they were also ICOS-dependent. Mice genetically deficient in ICOS (ICOS-/-) and WT mice were immunized i.p. with Pn14 and boosted 6 weeks later. WT mice elicited a detectable primary IgG anti-PspA response and, following secondary immunization, substantial boosting in PspA-specific IgG serum titers (Fig. 2A). As reported previously (35), serum titers of PspA-specific IgG3, IgG1, IgG2b, and IgG2a were all induced during the primary, and boosted upon secondary immunization (data not shown). In contrast, both the primary and secondary IgG anti-PspA response was essentially abrogated in ICOS-/- mice (Fig. 2A), reflected by a lack of induction of all 4 PspA-specific IgG isotypes (data not shown). In addition to the IgG anti-PspA response to Pn14, the primary and secondary IgG responses specific for two additional Pn14-expressed proteins, PsaA and PspC were also abrogated in ICOS-/- mice (Fig. 2C). However, in marked contrast to the IgG anti-protein response, ICOS-/- mice elicited IgM and IgG anti-polysaccharide (PPS14 and PC) responses to Pn14 that were essentially similar to that observed in WT mice (Fig. 2A).
Figure 2. The IgG anti-PPS14 and anti-PC response to Pn14, in contrast to pneumococcal conjugate, is ICOS-independent.
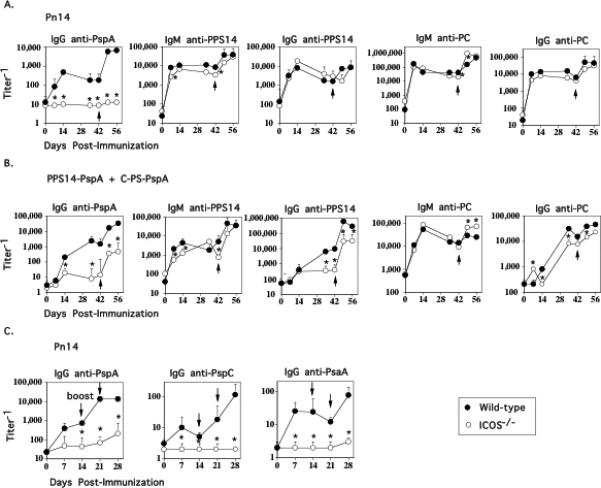
WT (C57BL/6) and ICOS-/- mice (7 per group) were immunized i.p. and then boosted on day 42 with either (A) Pn14 in saline or (B) PPS14-PspA + C-PS-PspA in alum/CpG-ODN. C) WT (C57BL/6) and ICOS-/- mice (7 per group) were immunized i.p. with Pn14 in saline and then boosted on days 14 and 21. Serum titers of antigen-specific IgM and IgG are illustrated. *significant, p<0.05 between WT and ICOS-/- mice.
We next wished to determine whether induction of the PPS14- and PC-specific, as well as the PspA-specific Ig responses is inhibited in ICOS-/- mice upon immunization with conjugate. Similar to Pn14, the primary and secondary IgG anti-PspA response to conjugate was markedly inhibited in ICOS-/- mice, although modest boosting (significant, p<0.05) was observed in ICOS-/- mice following secondary immunization (Fig. 2B). In addition, the primary IgG anti-PPS14 was essentially abrogated in ICOS-/- mice, whereas a partial, though significant, reduction in the IgG anti-PC response was also observed (Fig. 2B). Similar to the IgG anti-PspA response, the secondary IgG anti-PPS14 response was also significantly lower than that observed in WT mice, although significant boosting was observed in both strains. No significant boosting of the IgG anti-PC response was observed in either WT or ICOS-/- mice. The IgM anti-PPS14 and anti-PC responses to conjugate were largely ICOS-independent (Fig. 2B) although these responses are also partially T cell-dependent (data not shown). Thus, the IgG anti-polysaccharide responses to Pn14 and pneumococcal conjugate exhibit different requirements for ICOS costimulation despite both being dependent on CD4+ T cells, B7-dependent costimulation and CD40/CD40L interactions.
ICOS costimulation for induction of the IgG anti-PspA response to Pn14 is critical during primary, but not secondary immunization
In order to confirm our observations in ICOS-/- mice and further determine when (i.e. during primary and/or secondary response) ICOS costimulation was necessary for the IgG anti-PspA response, we injected mice with a neutralizing anti-ICOSL mAb (HK5.3) either at the time of the primary and/or secondary immunization with Pn14. As illustrated in Fig. 3, injection of anti-ICOSL mAb only at the time of primary immunization with Pn14 completely inhibited the primary IgG anti-PspA response. Following secondary immunization with Pn14, 6 weeks later in the absence of anti-ICOSL, a partial though significant reduction in the secondary IgG anti-PspA response was also observed indicating that ICOS was necessary both for the primary response and for the generation of memory. Of note, no effect on the primary IgG anti-PspA response was observed when Pn14 was first injected 6 weeks after injection of anti-ICOSL alone, indicating that the mAb was effectively cleared after 6 weeks (data not shown). Similar results were observed when anti-ICOSL was injected both at the time of primary and secondary immunization with Pn14 (Fig. 3). However, when mice were first primed with Pn14 in the absence of anti-ICOSL and then boosted in the presence of anti-ICOSL, no effect of the mAb on the secondary IgG anti-PspA response was observed (Fig. 3). Similar to what was observed in ICOS-/- mice, anti-ICOSL had no significant effect on the IgM or IgG anti-PPS14 or anti-PC responses to Pn14 (Fig. 3). Thus, ICOS costimulation appears to be critical for induction of both the primary IgG anti-protein response and the generation of memory, but not for the elicitation of the memory response following secondary immunization. The partial, though substantial, inhibition of the IgG PspA-specific secondary response using anti-ICOSL at the time of the primary versus the complete inhibition observed in ICOS-/- mice might reflect incomplete or relatively short-lived neutralization of ICOSL following a single injection of mAb.
Figure 3. ICOS costimulation for induction of the IgG anti-PspA response to Pn14 is critical during primary, but not secondary immunization.
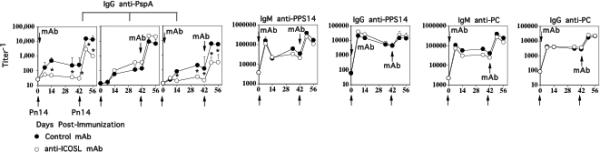
BALB/c mice (7 per group) were immunized i.p. with Pn14 in saline and boosted on day 42. Anti-ICOSL mAb (clone HK5.3) or a control rat IgG2a anti-E coli β-galactosidase mAb (clone GL117) [1 mg per mouse] were injected on day 0 and/or day 42. Serum titers of antigen-specific IgM and IgG are illustrated. *significant, p<0.05 between control mAb and anti-ICOSL mAb.
Germinal center formation in ICOS-/- mice is largely abrogated in response to either Pn14 or conjugate
ICOS costimulation of CD4+ T cells has been shown to be critical for induction of GC and thus the generation of memory (16). Although the IgG anti-PPS14 and IgG anti-PC responses to Pn14 are dependent on CD4+ T cells, CD28 costimulation, and CD40/CD40L interactions they fail to generate memory, in contrast to the induction of PS-specific memory to conjugate (4, 5). These data suggest the possibility that the TD IgG anti-PS responses to Pn14 and conjugate are extrafollicular and follicular, respectively. To explore this further, we measured the number and size of GCs in WT and ICOS-/- mice following immunization with either Pn14 or conjugate (Figure 4). Naïve WT and ICOS-/- mice have no detectable GCs in an area of 12mm2. Whereas GCs are strongly induced in WT mice, 10 days following primary immunization with either Pn14 (GCs=44/12mm2, GC total area=0.57mm2/12mm2) or conjugate (GCs=29/12mm2, GC total area=0.47mm2/12mm2), they are essentially abrogated, in response to both immunizations, in ICOS-/- mice. These data thus indicate that the IgG anti-PS responses to Pn14, which are normal in ICOS-/- mice, can indeed occur in the absence of GC formation, and thus are likely to represent a completely TD extrafollicular response in WT mice, consistent with the absence of PS-specific memory generation. In contrast, the inhibition of both the IgG anti-PS response and GC formation in response to conjugate in ICOS-/- mice are consistent with this response being follicular in nature in WT mice, associated with the generation of PS-specific memory.
Figure 4. Germinal center formation in ICOS-/- mice is largely abrogated in response to either Pn14 or conjugate.
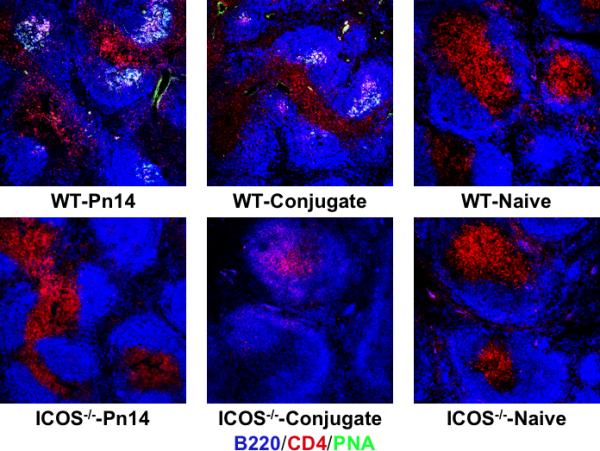
WT (C57BL/6) and ICOS-/- mice (4 mice per group) were immunized i.p. with either Pn14 in saline or PS14-PspA + C-PS-PspA in alum/CpG-ODN. Spleens were removed on day 10 and stained with PNA-Alexa Flour 488 (green), anti-B220-Pacific Blue (blue), and anti-CD4-PE (red). Four sections from each of 4 spleens per group were analyzed for GC number and area for a total spleen area of 12mm2 (see “Results”).
Protein- and polysaccharide-specific IgG responses to both Pn14 and conjugate are defective in SAP-/- mice, whereas the IgM anti-polysaccharide response is differentially regulated in response to the two immunogens
The adaptor protein SAP has previously been shown to be critical for a CD4+ T cell-dependent humoral immune response. In particular, both the primary and secondary IgG, but not IgM, responses to a number of TD antigens are strongly reduced in SAP-/- mice, associated with a defect in the formation of GCs (26). In light of data demonstrating decreased ICOS expression on SAP-/- CD4+ T cells to induce ICOS (24, 26), it has been postulated that decreased ICOS costimulation could lead at least in part, to the defective IgG responses. If true, we hypothesized that the IgG anti-PS response to Pn14, which is ICOS-independent, would also be SAP-independent, whereas SAP would play an important role in the Ig response to conjugate. However, other data argues that SAP-/- CD4+ T cells can express normal levels of costimulatory molecules including ICOS, but fail to interact properly with B cells, suggesting that SAP would be required for all T-dependent responses, including the IgG anti-PPS14 response to Pn14 (28). To evaluate these possibilities SAP-/- mice were immunized and boosted with either Pn14 or conjugate. As illustrated in Figure 5A/B, as expected, both the primary and secondary IgG anti-PspA responses to Pn14 and conjugate were markedly inhibited in SAP-/- mice, as they were in both CD28-/- and ICOS-/- mice, although these mice did elicit a very weak secondary response to conjugate. Surprisingly, although the IgG anti-PPS14 and IgG anti-PC responses to Pn14 were normal in ICOS-/- mice, they were either completely abrogated (anti-PPS14) or significantly reduced (anti-PC) in SAP-/- mice (Figure 5A). In contrast, the primary IgM anti-PPS14 and IgM anti-PC responses to Pn14 peaked earlier in SAP-/- mice but then declined below WT levels, whereas SAP-/- mice exhibited higher secondary and tertiary titers following boosting. In contrast to Pn14, immunization of SAP-/- mice with conjugate resulted in striking reductions in both IgM and IgG anti-PPS14 and anti-PC responses, even greater than that observed in ICOS-/- mice (Figure 5B). These data thus demonstrate that SAP plays a role in CD4+ T cell-dependent Ig responses that is at least partially or completely independent of ICOS.
Figure 5. Protein- and polysaccharide-specific IgG responses to both Pn14 and conjugate are defective in SAP-/- mice, whereas the IgM anti-polysaccharide response is differentially regulated in response to the two immunogens.
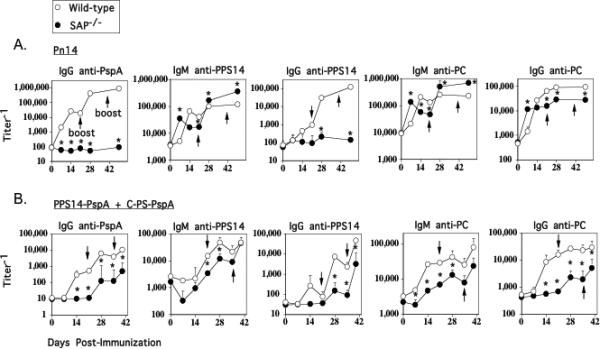
WT (C57BL/6) and SAP-/- mice (7 per group) were immunized i.p. with either (A) Pn14 in saline (boosted on day 21) or (B) PPS14-PspA + C-PS-PspA in alum/CpG-ODN (boosted on days 21 and 35). Serum titers of antigen-specific IgM and IgG are illustrated. *significant, p<0.05 between WT and SAP-/- mice.
Analysis of splenic and peritoneal B cell subsets in WT, SAP-/-, ICOS-/-, and CD28-/- mice
Splenic MZB and B-1 cells, and peritoneal B-1a and B-1b cells have been implicated in polysaccharide-specific Ig isotype responses, whereas splenic FB cells are thought to be responsible for most Ig responses to blood-borne protein antigens (38, 39). Thus, in a final set of studies, we wished to determine whether changes in B cell subset development could account, at least in part, for the differences in antigen-specific Ig isotype induction in SAP-/-, ICOS-/-, and/or CD28-/- relative to WT mice. As illustrated in Table 1, although some significant (p<0.05) changes in the percentages and/or numbers of certain B cell subsets are observed in SAP-/-, ICOS-/-, and CD28-/-, relative to WT mice, these differences are modest, and fail to explain the alterations observed in Ig isotype induction which are summarized in Table 2.
Table 1. Quantitation of splenic and peritoneal B cell subsets in WT, SAP-/-, ICOS-/-, and CD28-/- mice.
Spleen and peritoneal cells from individual mice (WT, SAP-/-, and ICOS-/- mice [three per group]; CD28-/- mice [4 mice]) were analyzed by flow cytometry as indicated in “Materials and Methods”. Data is expressed as arithmetic mean +/- SEM.
| |
Total cells (× 106) |
% (No. ×106) of cells in subset |
||
|---|---|---|---|---|
| FB |
MZB |
B-1 |
||
| Spleen | ||||
| WT | 69.16 ± 1.02 | 27.48 ± 0.03 (19.00 ± 0.02) | 3.78 ± 0.15 (2.61 ± 0.10) | 3.43 ± 0.05 (2.37 ± 0.03) |
| SAP-/- | 65.89 ± 8.90 | 32.55 ± 1.06* (21.45 ± 0.70*) | 3.75 ± 0.04 (2.47 ± 0.02) | 3.45 ± 0.04 (2.27 ± 0.02*) |
| ICOS-/- | 75.04 ± 14.29 | 37.70 ± 0.20* (28.29 ± 0.15*) | 4.16 ± 0.01* (3.12 ± 0.01*) | 3.57 ± 0.05 (2.68 ± 0.04*) |
| CD28-/- | 69.98 ± 1.18 | 26.83 ± 1.15 (18.78 ± 0.80) | 3.72 ± 0.25 (2.59 ± 0.17) | 3.80 ± 0.18 (2.66 ± 0.13) |
| |
% (No. ×106) of cells in subset |
|||
|---|---|---|---|---|
| B-1a |
B-1b |
B-2 |
||
| Peritoneum | ||||
| WT | 5.31 ± 0.41 | 3.19 ± 0.63 (0.17 ± 0.03) | 3.30 ± 0.85 (0.18 ± 0.05) | 3.25 ± 0.75 (0.17 ± 0.04) |
| SAP-/- | 6.26 ± 3.86 | 2.71 ± 1.33 (0.17 ± 0.08) | 6.60 ± 0.66* (0.41 ± 0.04*) | 6.39 ± 1.17* (0.40 ± 0.07*) |
| ICOS-/- | 2.62 ± 0.41 | 3.40 ± 1.17 (0.09 ± 0.03) | 2.75 ± 0.51 (0.07 ± 0.01*) | 9.00 ± 1.17* (0.24 ± 0.03) |
| CD28-/- | 5.44 ± 0.96 | 6.78 ± 0.88* (0.37 ± 0.05*) | 5.22 ± 0.71 (0.28 ± 0.04) | 3.85 ± 0.07 (0.21 ± 0.01) |
significance (p<0.05) relative to WT mice, by Student t test.
Table 2. Summary of results.
Knockout relative to wild-type mice (“=” no overall differences between knockout and wild-type)
| CD28-/- | ICOS-/- | SAP-/- | |
|---|---|---|---|
| Pn14 | |||
| IgG anti-protein |
|
|
|
| IgM anti-PPS14 | = | = |
|
| IgG anti-PPS14 |
|
= |
|
| IgM anti-PC | = | = |
|
| IgG anti-PC |
|
= |
|
| Conjugate | |||
| IgG anti-protein |
|
|
|
| IgM anti-PPS14 |
|
= |
|
| IgG anti-PPS14 |
|
|
|
| IgM anti-PC |
|
= |
|
| IgG anti-PC |
|
|
|
Discussion
Most studies on the regulation of PS-specific Ig responses have utilized isolated PS antigens, with or without haptenation (40). On the basis of these studies, the concept arose of PS antigens being either TI-1 or TI-2, depending on the presence or absence of an associated TLR ligand, respectively. Although non-zwitterionic PS antigens fail to associate with MHC-II antigens and thus are unable to recruit classical, cognate CD4+ T cell help (41-43), it was demonstrated that the covalent attachment of an immunogenic protein to the PS antigen (i.e. conjugate vaccine) could convert the PS antigen from a TI-2 to an apparently classical TD antigen (36, 37, 44). Thus, PS-specific B cells internalizing conjugate could present peptide from the PS-linked protein to CD4+ T cells to receive cognate help for Ig induction. Although studies of isolated PS antigens have been highly informative, minimal attention has been paid to how anti-PS responses are regulated when PS antigens are presented to the immune system in a physiological context, i.e. when expressed by an intact pathogen. The expression by the pathogen of immunogenic proteins, TLR and NOD ligands, scavenger receptor ligands, and the particulate nature of the pathogen itself might confer unique immunologic properties upon the associated PS antigen. In particular, we hypothesized that perhaps pathogen-associated PS antigens were indeed classical TD antigens, similar to conjugate vaccines, specifically on the basis of their association with protein antigens.
Our previous studies have refuted this hypothesis, at least regarding the regulation of the anti-PS response to the intact, Gram-positive extracellular bacterium, Streptococcus pneumoniae (Pn). Thus we demonstrated that PS antigens associated with intact Pn are unique immunologically, in that they exhibit both TD and TI properties. Thus, similar to conjugate vaccine, the anti-PS response to Pn is CD4+ T cell-, B7/CD28-, and CD40/CD40L-dependent, and generates IgG containing all 4 isotypes, in addition to IgM (i.e. TD properties). However, the anti-PS response to Pn, in contrast to conjugate vaccine, exhibits more abbreviated primary kinetics and a failure to generate PS-specific memory (i.e. TI properties), associated with a shortened period of dependence on CD4+ T cell help and B7-dependent costimulation (1-5). Thus, the anti-PS response to Pn, in contrast to conjugate vaccine, is not classically TD. Although it might thus be assumed that PS and protein antigens in a conjugate vaccine behave in an immunologically similar manner, this also may not be entirely true. Thus, PS in contrast to protein antigens contain repeating identical antigenic epitopes capable of mediating multivalent BCR crosslinking suggesting a differential requirement for BCR signaling in response to these two types of linked immunogen. In this regard, we previously demonstrated that anti-PS responses to conjugate, as well as intact Pn, are more heavily dependent on BCR-mediated signaling via Bruton's tyrosine kinase (Btk), relative to the anti-protein response (5). Moreover, recent, unpublished data from our laboratory, suggest that the nature of the association of the polysaccharide with the immunogenic proteins expressed by the intact bacteria versus the conjugate vaccine, may be a critical factor in determining the ability of the immunogen to generate a PS-specific IgG memory response.
In this study we have extended our observations on the relative immunologic properties of PS antigens associated with intact Pn versus conjugate vaccine by investigating the further role of ICOS costimulation, and the signaling adaptor protein, SAP, shown to be critical for CD4+ T cell help for germinal center formation (23, 24). We demonstrate that the TD IgG anti-PS responses to intact Pn and conjugate vaccine are ICOS-independent and ICOS-dependent, respectively, despite both responses being CD28 dependent. In contrast the anti-protein responses to both Pn and conjugate are strongly ICOS-dependent. For the generation and elicitation of protein-specific memory in response to Pn, ICOS was required only at the time of the primary, but not secondary, immunization with Pn, where it also was found to be necessary for the induction of the primary IgG response. These data are in contrast to our earlier observation of a requirement for B7-dependent costimulation for the IgG anti-PspA response during both the primary and secondary immunizations to Pn, although the dependence on B7 costimulation for the secondary response was of much briefer duration (2). Of note, ICOS-/- mice failed to generate a GC response to either Pn or conjugate, indicating that the IgG anti-PS response to Pn could occur normally in the absence of GC development. Collectively, these observations, and those in SAP-/- mice, discussed below, cannot be accounted for by the modest and selective alterations in B cell subset percentages and/or absolute numbers in these knockout strains.
Previous studies have demonstrated that CD4+ T cells genetically deficient in SAP fail to provide help for germinal center formation and show impaired ICOS induction (17, 18, 23, 24). However, we show that although the IgG anti-PS response to Pn is ICOS-independent and can occur normally in the absence of GC formation, it is still markedly inhibited in SAP-/- mice. Further, IgM and IgG anti-PS responses to conjugate were significantly more defective in SAP-/- as opposed to ICOS-/- mice. SAP is expressed by cell types other than CD4+ T cells, such as CD8+ and NK T cells, NK cells, and some B cells (20-22), and thus might be influencing the IgG anti-PS response to Pn through one or more of these latter cell types. However, the overall enhancement of the TI IgM anti-PS response to Pn in SAP-/- mice favors a specific role for SAP in CD4+ T cells in this model system. This idea is further supported by the markedly decreased IgM and IgG anti-PS responses to conjugate, both of which are dependent on CD4+ T cells, in SAP-/- mice. Consistent with our data, it was previously demonstrated that immunization of SAP-/- mice with TD antigens (soluble NP-KLH or SRBC) resulted in dramatic defects in GC formation, primary and secondary antigen-specific IgG (IgG1 most dramatically reduced, then IgG2b/IgG2a, then IgG3), although not IgM, production (26). The basis for our contrasting observation of decreased PS-specific IgM production in response to conjugate in SAP-/- mice is unknown, but could reflect intrinsic differences between PS versus protein antigens. The importance of SAP for the PS-specific Ig response to Pn suggests that there is a critical requirement for SAP and the associated SLAM family receptors in regulating the earliest stages of T cell help for B cells, prior to, or at least partially independent of, ICOS signals. While these conclusions appear to differ from those obtained in a recent study, which concluded that SAP expression in T cells was required only late (after 5 days) in response to a T-dependent protein antigen (45), the differences are likely to result from the distinct types of antigens examined. Indeed, the data here support the recent demonstration that SAP-/- CD4+ T cells have a selective defect in adhesion to B cells (28), which would be required for the early interactions between T cells and B cells mediating the PS-specific IgG response to Pn (2, 3, 46).
Collectively, these data strongly suggest that the IgG anti-PS response to Pn in WT mice is extrafollicular, most likely leading to a predominantly rapid plasma cell response to the exclusion of memory B cell generation, that is nevertheless dependent on CD4+ T cell help and CD40/CD40L interactions for its optimal induction (47-50). This would then account for the abbreviated primary kinetics, the shorter duration of CD4+ T cell help and B7-dependent costimulation, and the lack of dependence on ICOS costimulation. In contrast, the IgG anti-PS response to conjugate vaccine is likely to largely reflect a follicular response, associated with GC formation and the development of PS-specific memory that is dependent on ICOS costimulation of CD4+ T cells (51, 52). Plasma cells generated in the extrafollicular response undergo extensive apoptosis, likely limiting primary Ig induction (53). In this regard we recently demonstrated that transgenic (Tg) expression of the anti-apoptotic proteins Bcl-2 or Bcl-xL selectively within B cells leads to a dramatic enhancement of the primary IgG anti-PS, but not anti-protein, response to Pn, without inducing PS-specific memory (54). In contrast Tg and WT B cells elicited similar IgG anti-PS and IgG anti-protein responses to conjugate. Similarly, injection of an agonistic anti-CD40 mAb enhanced the primary IgG anti-PS response to Pn, but not to conjugate (data not shown), suggesting that exogenous CD40 activation of B cells also served to inhibit apoptosis of Pn-induced extrafollicular plasma cells, thus prolonging the response, and dramatically increasing the peak serum titers of PS-specific IgG.
Although PS antigen in both intact Pn and conjugate are associated with protein and thus might be expected to generate an IgG anti-PS response that is classically CD4+ T cell-dependent (i.e. GC formation and memory), there are several key difference between these two immunogens that might account for the observed dichotomy in the two responses. Firstly, intact Pn is particulate and contains scavenger receptor ligands that may both promote extended trapping, particularly by macrophages, within the marginal zone, and thus preferential engagement of PS-specific marginal zone B cells (MZB) (55, 56). Although MZB appear to be more effective APC than follicular B cells (FB) for CD4+ T cell activation (57) they appear to be programmed to favor, although not absolutely, plasma cell differentiation over memory B cell generation (58, 59). In contrast, soluble conjugate vaccine may preferentially traffic into the B cell follicles where it can engage PS-specific FB cells, which are more prone to becoming memory B cells. Indeed, using Lsc-/- mice, which exhibit a marked defect in MZB migration from the marginal zone following immunization (60), we recently provided strong evidence that the IgG anti-PS response to intact Pn14 versus conjugate derive from MZB and FB, respectively (54). Secondly, PS antigens expressed by intact Pn are not typically linked to Pn proteins through a covalent linkage, whereas PS and protein are covalently attached in the conjugate vaccine. During APC processing and/or trafficking of the immunogen (61-64), it is possible that PS and protein antigens in Pn, but not conjugate, become disassociated prior to eventual contact with PS-specific B cells, thus limiting the extent of specific cognate CD4+ T cell help for the anti-PS response. Thirdly, the degree of BCR crosslinking in response to antigen, which likely differs between protein and PS antigens, could influence whether the Ig response is of the predominantly extrafollicular plasma cell type (higher BCR strength) or alternatively progress through a GC reaction (lower BCR strength) (65) Finally, the nature of the association of capsular PS with Gram positive versus Gram-negative bacteria is distinct and could impart different immunologic properties to PS antigens expressed by these two classes of pathogen.
Acknowledgments
This study was supported by N.I.H. grants 1R01 AI49192 (CMS) and the U.S.U.H.S. Dean's Research and Education Endowment Fund (CMS) and by funding from the intramural program of the National Human Genome Research Institute (PLS).
Abbreviations used in this paper
- PS
polysaccharide
- Pn
intact Streptococcus pneumoniae
- Pn14
intact Streptococcus pneumoniae, capsular type 14
- PPS14
capsular polysaccharide, serotype 14
- PC
phosphorylcholine determinant of teichoic or lipoteichoic acid
- PspA
pneumococcal surface protein A
- PspC
pneumococcal surface protein C
- PsaA
pneumococcal surface adhesin A
Footnotes
Opinions and assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
References
- 1.Wu ZQ, Vos Q, Shen Y, Lees A, Wilson SR, Briles DE, Gause WC, Mond JJ, Snapper CM. In vivo polysaccharide-specific IgG isotype responses to intact Streptococcus pneumoniae are T cell dependent and require CD40- and B7-ligand interactions. J Immunol. 1999;163:659–667. [PubMed] [Google Scholar]
- 2.Wu ZQ, Khan AQ, Shen Y, Schartman J, Peach R, Lees A, Mond JJ, Gause WC, Snapper CM. B7 requirements for primary and secondary protein- and polysaccharide- specific Ig isotype responses to Streptococcus pneumoniae. J Immunol. 2000;165:6840–6848. doi: 10.4049/jimmunol.165.12.6840. [DOI] [PubMed] [Google Scholar]
- 3.Khan AQ, Lees A, Snapper CM. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J Immunol. 2004;172:532–539. doi: 10.4049/jimmunol.172.1.532. [DOI] [PubMed] [Google Scholar]
- 4.Sen G, Chen Q, Snapper CM. Immunization of aged mice with a pneumococcal conjugate vaccine combined with an unmethylated CpG-containing oligodeoxynucleotide restores defective immunoglobulin G antipolysaccharide responses and specific CD4+-T-cell priming to young adult levels. Infect Immun. 2006;74:2177–2186. doi: 10.1128/IAI.74.4.2177-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan AQ, Sen G, Guo S, Witte ON, Snapper CM. Induction of in vivo antipolysaccharide immunoglobulin responses to intact Streptococcus pneumoniae is more heavily dependent on Btk-mediated B-cell receptor signaling than antiprotein responses. Infect Immun. 2006;74:1419–1424. doi: 10.1128/IAI.74.2.1419-1424.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol. 2002;14:384–390. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- 7.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 8.Lucas PJ, Negishi I, Nakayama K, Fields LE, Loh DY. Naive CD28-deficient T cells can initiate but not sustain an in vitro antigen-specific immune response. J Immunol. 1995;154:5757–5768. [PubMed] [Google Scholar]
- 9.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- 11.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 12.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 13.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, Odermatt B, Ho A, Itie A, Horan T, Whoriskey JS, Pawson T, Penninger JM, Ohashi PS, Mak TW. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 14.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 15.Shahinian A, Pfeffer K, Lee K, Kundig T, Kishihara K, Wakeham A, Kawai K, Ohashi P, Thompson C, Mak T. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 16.Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. J Immunol. 2001;166:3659–3662. doi: 10.4049/jimmunol.166.6.3659. [DOI] [PubMed] [Google Scholar]
- 17.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 18.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 19.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, Kroczek RA, Peter HH. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 20.Al-Alem U, Li C, Forey N, Relouzat F, Fondaneche MC, Tavtigian SV, Wang ZQ, Latour S, Yin L. Impaired Ig class switch in mice deficient for the X-linked lymphoproliferative disease gene Sap. Blood. 2005;106:2069–2075. doi: 10.1182/blood-2004-07-2731. [DOI] [PubMed] [Google Scholar]
- 21.Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, Buckler AJ, Wise C, Ashley J, Lovett M, Valentine MB, Look AT, Gerald W, Housman DE, Haber DA. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95:13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikhalap SV, Shlapatska LM, Berdova AG, Law CL, Clark EA, Sidorenko SP. CDw150 associates with src-homology 2-containing inositol phosphatase and modulates CD95-mediated apoptosis. J Immunol. 1999;162:5719–5727. [PubMed] [Google Scholar]
- 23.Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005;203:180–199. doi: 10.1111/j.0105-2896.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 24.Ma CS, Hare NJ, Nichols KE, Dupre L, Andolfi G, Roncarolo MG, Adelstein S, Hodgkin PD, Tangye SG. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115:1049–1059. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 26.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malbran A, Belmonte L, Ruibal-Ares B, Bare P, Massud I, Parodi C, Felippo M, Hodinka R, Haines K, Nichols KE, de Bracco MM. Loss of circulating CD27+ memory B cells and CCR4+ T cells occurring in association with elevated EBV loads in XLP patients surviving primary EBV infection. Blood. 2004;103:1625–1631. doi: 10.1182/blood-2003-07-2525. [DOI] [PubMed] [Google Scholar]
- 28.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beier KC, Hutloff A, Dittrich AM, Heuck C, Rauch A, Buchner K, Ludewig B, Ochs HD, Mages HW, Kroczek RA. Induction, binding specificity and function of human ICOS. Eur J Immunol. 2000;30:3707–3717. doi: 10.1002/1521-4141(200012)30:12<3707::AID-IMMU3707>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 30.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Sen G, Snapper CM. Endogenous IL-1R1 signaling is critical for cognate CD4+ T cell help for induction of in vivo type 1 and type 2 antipolysaccharide and antiprotein Ig isotype responses to intact Streptococcus pneumoniae, but not to a soluble pneumococcal conjugate vaccine. J Immunol. 2006;177:6044–6051. doi: 10.4049/jimmunol.177.9.6044. [DOI] [PubMed] [Google Scholar]
- 32.Ogunniyi AD, Woodrow MC, Poolman JT, Paton JC. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect Immun. 2001;69:5997–6003. doi: 10.1128/IAI.69.10.5997-6003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwai H, Kozono Y, Hirose S, Akiba H, Yagita H, Okumura K, Kohsaka H, Miyasaka N, Azuma M. Amelioration of collagen-induced arthritis by blockade of inducible costimulator-B7 homologous protein costimulation. J Immunol. 2002;169:4332–4339. doi: 10.4049/jimmunol.169.8.4332. [DOI] [PubMed] [Google Scholar]
- 34.Sen G, Flora M, Chattopadhyay G, Klinman DM, Lees A, Mond JJ, Snapper CM. The critical DNA flanking sequences of a CpG oligodeoxynucleotide, but not the 6 base CpG motif, can be replaced with RNA without quantitative or qualitative changes in Toll-like receptor 9-mediated activity. Cell Immunol. 2004;232:64–74. doi: 10.1016/j.cellimm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Khan AQ, Chen Q, Wu ZQ, Paton JC, Snapper CM. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect Immun. 2005;73:298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guttormsen HK, Wetzler LM, Finberg RW, Kasper DL. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect Immun. 1998;66:2026–2032. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guttormsen HK, Sharpe AH, Chandraker AK, Brigtsen AK, Sayegh MH, Kasper DL. Cognate stimulatory B-cell-T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect Immun. 1999;67:6375–6384. doi: 10.1128/iai.67.12.6375-6384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes-Carvalho T, Foote J, Kearney JF. Marginal zone B cells in lymphocyte activation and regulation. Curr Opin Immunol. 2005;17:244–250. doi: 10.1016/j.coi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol. 2001;13:195–201. doi: 10.1016/s0952-7915(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 40.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 41.Kalka-Moll WM, Tzianabos AO, Bryant PW, Niemeyer M, Ploegh HL, Kasper DL. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149–6153. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- 42.Harding CV, Roof RW, Allen PM, Unanue ER. Effects of pH and plysaccharides on peptide binding to class II major histocompatiblity complex molecules. Proc. Natl. Acad. Sci. USA. 1991;88:2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishioka GY, Lamont AG, Thomson D, Bulbow N, Gaeta FC, Sette A, Grey HM. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J Immunol. 1992;148:2446–2451. [PubMed] [Google Scholar]
- 44.Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamperschroer C, Roberts DM, Zhang Y, Weng NP, Swain SL. SAP enables T cells to help B cells by a mechanism distinct from Th cell programming or CD40 ligand regulation. J Immunol. 2008;181:3994–4003. doi: 10.4049/jimmunol.181.6.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu ZQ, Shen Y, Khan AQ, Chu CL, Riese R, Chapman HA, Kanagawa O, Snapper CM. The mechanism underlying T cell help for induction of an antigen-specific in vivo humoral immune response to intact Streptococcus pneumoniae is dependent on the type of antigen. J Immunol. 2002;168:5551–5557. doi: 10.4049/jimmunol.168.11.5551. [DOI] [PubMed] [Google Scholar]
- 47.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y-J, Zhang J, Lane PJL, Chan EY-T, MacLennan ICM. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 49.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garside P, Ingulli E, Merica R, Johnson J, Noelle R, Jenkins M. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 51.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 52.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992;176:679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chattopadhyay G, Khan AQ, Sen G, Colino J, Dubois W, Rubtsov A, Torres RM, Potter M, Snapper CM. Transgenic Expression of Bcl-xL or Bcl-2 by Murine B Cells Enhances the In Vivo Antipolysaccharide, but Not Antiprotein, Response to Intact Streptococcus pneumoniae. J Immunol. 2007;179:7523–7534. doi: 10.4049/jimmunol.179.11.7523. [DOI] [PubMed] [Google Scholar]
- 55.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 56.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 57.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 58.Phan TG, Gardam S, Basten A, Brink R. Altered migration, recruitment, and somatic hypermutation in the early response of marginal zone B cells to T cell-dependent antigen. J Immunol. 2005;174:4567–4578. doi: 10.4049/jimmunol.174.8.4567. [DOI] [PubMed] [Google Scholar]
- 59.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J Exp Med. 2003;198:1923–1935. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubtsov A, Strauch P, Digiacomo A, Hu J, Pelanda R, Torres RM. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 2005;23:527–538. doi: 10.1016/j.immuni.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 62.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 63.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 64.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 65.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]


