Summary
Despite improvements in the detection and use of biomarkers, including epidermal growth factor receptor, ERCC1, and p16, the 5-year survival rate with non–small cell lung cancer remains at 15%. This suggests that additional biomarkers are needed to better prognosticate clinical course and guide therapeutic approaches. Previous studies showed that increased levels of aspartyl (asparaginyl)-β-hydroxylase and a highly related molecule, humbug, correlate with clinical course and survival with hepatic, biliary, pancreatic, and colon carcinomas. We now characterize the prognostic use of aspartyl (asparaginyl)-β-hydroxylase/humbug immunoreactivity in different subtypes of non–small cell lung cancer. Tissue microarrays including 375 paraffin-embedded non–small cell lung cancers (195 adenocarcinomas; 18 bronchioloalveolar carcinomas; 113 squamous cell carcinomas; and 49 large cell carcinomas) were immunostained with FB50 monoclonal antibody, which recognizes human aspartyl (asparaginyl)-β-hydroxylase/humbug. Immunoreactivity (intensity and distribution) in neoplastic cells were scored under code, and data were subjected to univariate and Cox multivariate analyses, adjusting for age, stage, and treatment. High levels of FB50 immunoreactivity were more often detected in adenocarcinomas (28% for adenocarcinoma, 17% for bronchioloalveolar carcinoma), compared with squamous cell carcinomas (10%) and large cell carcinomas (10%). Univariate analysis demonstrated inverse relationships between intensity of FB50 immunoreactivity and survival with squamous cell carcinoma (P = .004), and a strong trend with respect to large cell carcinoma (P = .057). Cox multivariate test showed that FB50 immunoreactivity (P = .025), clinical stage (P = .029), and tumor size (P = .0001) were all independent predictors of survival with squamous cell carcinoma. High levels of FB50 immunohistochemical staining correlate with poor prognosis in non–small cell lung cancer, particularly squamous cell carcinoma subtype. Therefore, FB50 immunoreactivity may be useful in defining patient subsets that are likely to benefit from adjuvant therapy.
Keywords: Aspartyl-β-hydroxylase, Lung carcinoma, Biomarkers, Tissue microarray, Cancer prognosis, Non-small cell carcinoma
1. Introduction
Worldwide, lung cancer is the leading cause of cancer deaths in men and women [1]. Despite improvements in early detection and treatment for the past 2 decades, prognosis remains poor among patients with non–small cell lung cancer (NSCLC) [2], in whom the 5-year survival rate is less than 15% because of high recurrence rates, irrespective of complete tumor resection [3]. The TNM staging system for NSCLC is currently the most accurate method of predicting survival; nonetheless, some tumor subtypes exhibit highly aggressive behavior with rapid progression to death despite favorable staging [4–6]. To make further improvements in survival, we need increased accuracy of clinical behavior of the tumors. An important approach has been to identify and characterize biomarkers in the tumors that could serve as prognostic indices. Examples of such biomarkers include epidermal growth factor receptor (EGFR), excision repair cross-complementing 1, and the p16 oncosuppressor gene [7–8]. Nonetheless, the quest for additional complementary prognostic biomarkers continues because the current 5-year survival in patients with NSCLC remains low.
Aspartyl (asparaginyl)-β-hydroxylase (ABH) and humbug are alternatively spliced protein products of the ABH messenger RNA [9]. ABH is a type 2 transmembrane protein and a member of the α-ketoglutarate–dependent dioxygenase family of proteins [9]. ABH catalyzes hydroxylation of specific aspartyl and asparaginyl residues in epidermal growth factor–like domains of proteins such as Notch, Jagged, and cellular adhesion molecules such as laminin and tenascin [9–12]. Humbug is a truncated homolog of ABH [13,14] and lacks ABH’s catalytic domain that has roles in intracellular calcium regulation [15,16] and cell motility [17], which is required for metastatic spread of malignant neoplasms.
ABH is abundantly expressed in malignant neoplasms, including those originating from liver, biliary, pancreas, or colon [18–23]. ABH has a demonstrated role in regulating cell motility [18,19,24], which is needed for tumor cell infiltration and metastatic spread. Normal and mature tissues generally express low levels of ABH, with the notable exception of placental trophoblasts [19], which are naturally highly motile and invasive. In addition, previous studies demonstrated that up-regulation of ABH and/or humbug correlates with poor cancer prognosis [21,23]. Because the monoclonal antibody FB50 binds to the N-terminus of ABH, which is virtually identical to humbug, both ABH and humbug are equally detected by immunohistochemical staining. Herein, we report the prognostic use of FB50 immunohistochemical staining for NSCLC [25].
2. Materials and methods
2.1. Tissue samples
We retrieved 375 surgically resected primary pulmonary carcinoma specimens from the archives of the Department of Pathology at Rhode Island Hospital, Providence, RI. The specimens were obtained between 1993 and 2003, and they were all formalin fixed and paraffin embedded. Included among the cases were 195 adenocarcinomas, 18 bronchioloalveolar carcinomas (BACs), 113 squamous cell carcinomas, and 49 large cell carcinomas. Clinical stage at the time of diagnosis and tumor resection was designated in accord with the American Joint Committee on Cancer Criteria [26]. Postoperative treatments included nothing other than follow-up, radiotherapy, chemotherapy, or both radiation and chemotherapy. Recurrence and survival data were obtained from the Rhode Island Tumor Registry. Case selection for inclusion in the study was based on the review of all hematoxylin and eosin–stained sections of the tumor to confirm the histopathologic diagnosis and determination that sufficient and adequate tissue was available to generate tissue microarrays (TMAs). Histologic subtyping of the tumors was performed using criteria set by the World Health Organization [27]. In addition to tumor subtype, we recorded the presence or absence of lymphovascular invasion.
2.2. TMA preparation
TMAs were constructed as previously described [28]. In brief, viable and representative areas of tumor on hematoxylin and eosin–stained sections were identified and marked by a pathologist. The corresponding region of the source block was cored, and then 1-mm cores were transferred to recipient master blocks using the Beecher Tissue Microarrayer (Beecher Instruments, Silver Spring, MD). Three to 6 cores of each specimen were simultaneously examined using the TMA approach. In addition, representative samples of tumor-free and relatively normal lung tissue were available from more than 50% of the resection specimens and included in the TMAs as controls. This study was approved by the institutional review board at the Rhode Island Hospital.
2.3. Immunohistochemical staining
Formalin-fixed paraffin-embedded multitumor microarray sections (5-μm thick) containing solid tumors and normal lung were immunostained to detect ABH/humbug. Samples of colonic adenocarcinoma were used as positive controls for FB50 immunoreactivity. TMA sections were deparaffinized, rehydrated, and then treated with 3% H2O2 for 10 minutes at room temperature to quench endogenous peroxidase activity. Antigen retrieval was then accomplished by treating the TMA sections with CC2 buffer according to the manufacturer’s protocol (Ventana, Tucson, AZ). The TMA sections were incubated overnight with FB50 (1:5000 dilution) monoclonal antibodies using the Ventana Discovery System (Ventana), and immunoreactivity was detected using the Omnimap labeling system (Ventana). The sections were lightly counterstained with hematoxylin, dehydrated, cleared, and mounted with cover glass. Negative controls included substitution of primary antibody with a nonreacting antibody of the same species. The intensity of FB50 staining was scored as negative (0), weak (+1), positive (+2), or strong (+3). The scores corresponding to overall distribution of FB50 immunoreactivity were averaged across the different tumor plugs per case. All sections were scored independently by 2 pathologists (M. Luu and L. Wang) in a blinded fashion, that is, without knowledge of the clinicopathologic features or clinical course. However, because the interobserved scoring was found to be concordant in 93% of cases, the few discordant scores were settled by joint review to achieve consensus.
2.4. Statistical analysis
Data were analyzed using the Pearson χ2 test or the Fisher exact test to assess associations between FB50 immunostaining levels and clinicopathologic parameters. We used Kaplan-Meier statistics to examine the influence of age, disease stage, treatment, and levels of FB50 immunoreactivity on clinical course/outcome, and compared subgroups using log-rank test for univariate analysis. The multivariate Cox proportional hazard model was applied using a stepwise forward method to detect independent predictors of survival. Two-tailed P values less than .05 were considered as statistically significant.
3. Results
3.1. Clinicopathologic features
Among the cases included in this study, the sex ratios were similar with 194 males (52%) and 181 females (48%) (Table 1). At the time of surgical resection, the mean (±SD) and range in age among males (76 ± 9.3, 42–92 years) were similar to the females (71 ± 11, 48–93). The mean follow-up period was 67 months (range, 1–137 months). Patients who were labeled as “unknown cause of death” have been eliminated from this analysis. The distribution of tumor types by histopathologic appearance was as follows: 195 adeno-carcinomas (52%), 18 bronchioloalveolar (5%), 133 squamous (30%), and 49 large cell carcinomas (13%). Initial evaluations revealed that 325 (87%) of the patients had stage I to II disease, whereas 50 (13%) had stage III to IV disease. Eighty-two patients (22%) had either N1 or N2 metastasis at the time of resection. None of the patients with BAC has died because of the disease.
Table 1.
Clinicopathologic characteristics
| Characteristic | No. of patients (N = 375) |
|---|---|
| Sex | |
| Male | 194 (52%) |
| Female | 181 (48%) |
| Age (y) | |
| Male, mean (±SD) | 76 (±9.3) |
| Female, mean (±SD) | 71 (±11) |
| Tumor type, total | 375 |
| Adenocarcinoma | 195 (52%) |
| Bronchioloalveolar | 18 (5%) |
| Squamous | 113 (30%) |
| Large cell carcinoma | 49 (13%) |
| Tumor stage | |
| I | 169 |
| II | 156 |
| III | 27 |
| IV | 23 |
3.2. FB50 Immunoreactivity in NSCLC
Nontumorous alveolar cells and interstitial cells in normal lung tissues were not immunoreactive with FB50. Very low levels (+1) of FB50 immunoreactivity were detected in alveolar macrophages (Fig. 1A). In the samples of lung carcinoma, FB50 immunoreactivity was localized throughout the cytoplasm, but in a distinct and prominent perinuclear pattern (Fig. 1). Among the tumor subtypes, 28% of adenocarcinomas (17% of bronchioloalveolar) (Fig. 1B), 10% of squamous cell carcinomas (Fig. 1C), and 10% of large cells (Fig. 1D) exhibited +2 (moderate) or +3 (strong) levels of FB50 immunoreactivity.
Fig. 1.
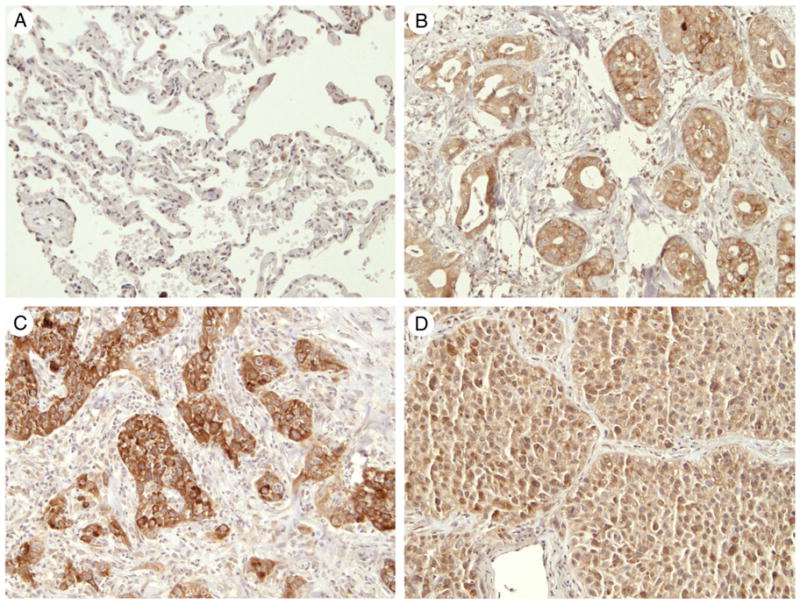
FB50 immunohistochemical staining in representative normal and cancerous lungs. (A) Normal lung showing no immunostaining of the alveolar and interstitial cells but weak staining of some alveolar macrophages, (B) positive immunostaining in adenocarcinoma tumor, (C) squamous cell carcinoma, and (D) large cell carcinoma (A-D, original magnification ×20).
3.3. Correlation of FB50 staining with clinical outcome in NSCLC
Intensities of FB50 immunoreactivity of +2 or +3 were significantly associated with overall higher disease stage (III and IV) for NSCLC (P = .027) and specifically with respect to adenocarcinoma (P = .002) and squamous cell carcinoma (P = .005) (Table 2). In contrast, levels of FB50 immunoreactivity were not correlated with sex or age. The impact of moderate or high levels FB50 immunoreactivity on cancer-related death was assessed using Kaplan-Meier survival analysis. Univariate analysis revealed a significant correlation between poor clinical outcome and high levels of FB50 immunoreactivity in patients with squamous cell carcinoma (P = .004), and a strong trend with respect to large cell carcinoma (P = .057) (Fig. 2). There was no correlation between the presence or intensity of FB50 immunoreactivity and survival with adenocarcinoma (P = .2). Cox multivariate modeling with adjustments for age, stage, and treatment demonstrated FB50 immunoreactivity (P = .025), clinical stage at the time of tumor resection (P = .029), and tumor size (P = .001) to be strong independent predictors of shortened survival in patients with squamous cell lung carcinoma. With adenocarcinoma, only tumor size (P = .002) was demonstrated to be an independent predictor of survival. There were no significant independent predictors of clinical course in patients with large cell carcinoma.
Table 2.
Results of FB50 staining in studied cohorts
| Variables | No. of cases | +2/+3 FB50, no. (%) | P |
|---|---|---|---|
| Histology | .004 | ||
| ADC | 195 | 55 (28%) | |
| BAC | 18 | 3 (17%) | |
| SCC | 113 | 11 (10%) | |
| LCC | 49 | 5 (10%) | |
| Stage (total of all subtypes) | 375 | 74 | .027 |
| I, II | 325 | 57 (18%) | |
| III, IV | 50 | 17 (34%) | |
| ADC, stage | 195 | 55 | .002 |
| I, II | 180 | 47 (26%) | |
| III, IV | 15 | 8 (53%) | |
| SCC, stage | 113 | 11 | .005 |
| I, II | 95 | 7 (7%) | |
| III, IV | 18 | 4 (22%) | |
| LCC, stage | 49 | 5 | .51 |
| I, II | 32 | 3 (9%) | |
| III, IV | 17 | 2 (12%) |
Abbreviations: ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma.
Fig. 2.
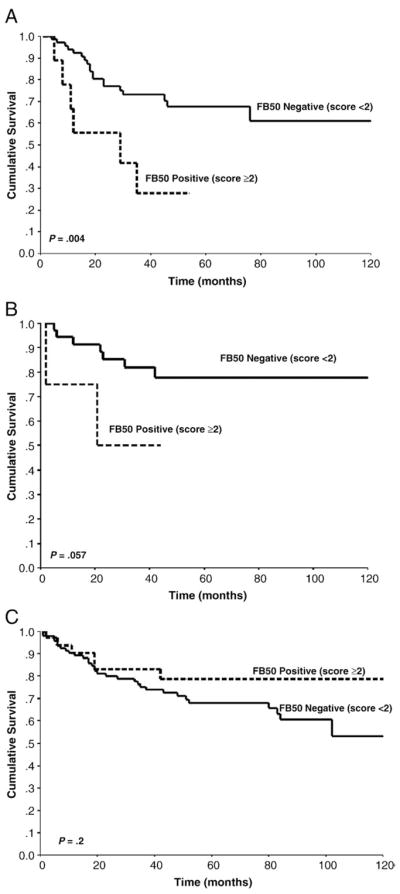
Correlation of FB50 immunostaining with survival in patients with squamous cell carcinoma (A), large cell (B), and adenocarcinoma (C).
4. Discussion
The aspartyl-(asparaginyl)-β-hydroxylase (ABH) and/or humbug genes have been documented to be overexpressed in a variety of malignant neoplasms, including those of colonic, pancreatic, biliary, or hepatic origin [18–23]. The present study demonstrates that in non–small cell lung carcinomas, ABH/humbug immunoreactivity is strikingly increased relative to normal lung parenchyma, and high levels of ABH/humbug immunoreactivity are negatively correlated with survival, particularly in squamous cell carcinoma. The finding that the presence and intensity of FB50 immunoreactivity significantly vary with stage and subtype of NSCLC is novel. The correlation between FB50 immunoreactivity, higher tumor stage, and poor prognosis observed in this study is similar to that seen in other neoplasms [21,23]. Although the significance of FB50 staining in different subtypes of NSCLC is still unknown, one potential explanation is that differential levels of FB50 immunoreactivity may reflect differences in molecular pathology of these tumor subtypes. For example, EGFR and KRAS expression are altered in lung adenocarcinomas, whereas TP53 and p16 expression are often perturbed in squamous cell carcinomas [29–30]. Indeed, recent evidence linking ABH/humbug overexpression to insulin/IGF-1 (insulin-like growth factor) signaling via the EGFR pathway may be key to our understanding of the differences observed herein [31]. Future studies will assess the molecular mechanisms and biologic consequences of ABH and humbug overexpression in NSCLC.
Previous studies demonstrated a role for ABH in invasion and metastatic spread of malignant neoplasms of hepatic, biliary, or pancreatic origin [18–22]. Correspondingly, analysis of surgical biopsy specimens revealed higher levels of FB50 immunoreactivity in neoplastic cells along infiltrating margins of cholangiocarcinomas and pancreatic carcinomas [21–22], whereas in vitro studies demonstrated that increased ABH expression leads to increased directional motility of cholangiocarcinoma and neuroblastoma cells, and small interfering RNA inhibition of ABH decreases directional motility [24,31–32]. With regard to the present work, antisense inhibition of ABH suppressed penetrative growth of human A549 lung carcinoma cells in an in vivo xenograft model [12]. The molecular pathways mediating the effects of ABH on motility and invasion involve transcriptional as well as posttranslational mechanisms. For example, increased signaling through insulin/IGF pathways stimulates ABH messenger RNA and protein expression by activating Erk/MAPK and phosphoinositide 3-kinase/Akt [31,33].
Notch and Jagged, which mediate various functions during development and neoplastic transformation, including cell migration, are substrates for ABH hydroxylase activity [9–10,34–36]. ABH protein mediates its effects on motility by physically interacting with and hydroxylating Notch and Jagged [33]. Consequently, increased levels of ABH protein result in increased levels of Jagged and Notch, nuclear translocation of Notch, and transcription of target genes such as HES-1 [33] and SNAIL, which mediates cell motility [37]. Thus, the oncogenic effects of increased ABH expression may involve dysregulation of the Notch signaling pathway, which is a well-documented phenomenon in a variety of cancer cells [38]. Although less is known about the role of humbug in cancer progression, its relationship has been established for hepatocellular carcinoma [33] and colon adenocarcinoma in that overexpression of humbug is correlated with a poor prognosis [23]. Humbug’s probable role in regulating intracellular calcium through ryanodine receptors in the endoplasmic reticulum [15–16] is likely critical to the assembly and disassembly of cellular cytoskeletal proteins and, consequently, the modulation of cell shape and adhesion. Indeed, recent in vitro studies showed humbug-transfected gastric cancer cells to migrate more robustly than the nontransfected counterparts [17]. Thus, overexpression of ABH and humbug may have a synergistic effect on the dynamic alterations in cell shape and adhesion, which are essential to the processes of invasion and metastatic spread of tumors.
In conclusion, FB50 staining specifically correlated with poor prognosis in patients with squamous cell carcinoma of the lung. Overexpression of ABH or humbug most likely reflects increased potential for tumor invasiveness and metastatic spread; thus, detecting increased levels of either protein using the FB50 antibody could serve as an important biomarker of aggressive NSCLC behavior and help predict need for adjuvant therapy, irrespective of tumor grade.
Footnotes
This study was supported by grant Center of Biomedical Research Excellence (COBRE) from the National Institutes of Health.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001;93:277–83. doi: 10.1093/jnci/93.4.277. [DOI] [PubMed] [Google Scholar]
- 3.Cox G, Jones JL, Andi A, Waller DA, O’Byrne KJ. A biological staging model for operable non–small cell lung cancer. Thorax. 2001;56:561–6. doi: 10.1136/thorax.56.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choy H, Pass H, Rosell R, et al. Lung cancer. In: Chang A, Ganz PA, Hayes DF, et al., editors. Oncology: an evidence-based approach. New York (NY): Springer Science and Business Media Inc; 2006. pp. 545–621. [Google Scholar]
- 5.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 6.Potti A, Mukherjee S, Petersen R, et al. A genomic strategy to refine prognosis in early-stage non–small-cell lung cancer. N Engl J Med. 2006;355:570–80. doi: 10.1056/NEJMoa060467. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rosell R. Weighing tumor biology in treatment decisions for patients with non–small cell lung cancer. J Thorac Onc. 2007;2:S68–76. doi: 10.1097/01.JTO.0000269737.05962.a0. [DOI] [PubMed] [Google Scholar]
- 8.Singhal S, Vachani A, Antin-Ozerkis D, Kaiser LR, Albelda SM. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non–small cell lung cancer: a review. Clin Cancer Res. 2005;11:3974–86. doi: 10.1158/1078-0432.CCR-04-2661. [DOI] [PubMed] [Google Scholar]
- 9.Dinchuk JE, Henderson NL, Burn TC, et al. Aspartyl beta-hydroxylase (Asph) and an evolutionarily conserved isoform of Asph missing the catalytic domain share exons with junctin. J Biol Chem. 2000;275:39543–54. doi: 10.1074/jbc.M006753200. [DOI] [PubMed] [Google Scholar]
- 10.Gronke RS, Welsch DJ, Van Dusen WJ, et al. Partial purification and characterization of bovine liver aspartyl beta-hydroxylase. J Biol Chem. 1990;265:8558–65. [PubMed] [Google Scholar]
- 11.Korioth F, Gieffers C, Frey J. Cloning and characterization of the human gene encoding aspartyl beta-hydroxylase. Gene. 1994;150:395–9. doi: 10.1016/0378-1119(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 12.Ho SP, Scully MS, Krauthauser CM, et al. Antisense oligonucleotides selectively regulate aspartyl beta-hydroxylase and its truncated protein isoform in vitro but distribute poorly into A549 tumors in vivo. J Pharmacol Exp Ther. 2002;302:795–803. doi: 10.1124/jpet.302.2.795. [DOI] [PubMed] [Google Scholar]
- 13.Feriotto G, Finotti A, Brevegleri G, Treves S, Zorzato F, Gambari R. Multiple levels of control of the expression of the human AβH-J-J locus encoding aspartyl-β-hydroxylase, junctin, and junctate. Ann NY Acad Sci. 2006;1091:184–90. doi: 10.1196/annals.1378.065. [DOI] [PubMed] [Google Scholar]
- 14.Hong CS, Kwon SJ, Kim DH. Multiple functions of junctin and junctate, two distinct isoforms of aspartyl-β-hydroxylase. Biochem Biophys Res Comm. 2007;362:1–4. doi: 10.1016/j.bbrc.2007.07.166. [DOI] [PubMed] [Google Scholar]
- 15.Treves S, Feriotto G, Moccagatta L, Gambari R, Zorzato F. Molecular cloning, expression, functional characterization, chromosomal localization, and gene structure of junctate, a novel integral calcium binding protein of sar(endo)plasmic reticulum membrane. J Biol Chem. 2000;275:39555–68. doi: 10.1074/jbc.M005473200. [DOI] [PubMed] [Google Scholar]
- 16.Treves S, Franzini-Armstrong C, Moccagatta L, et al. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J Cell Biol. 2004;166:537–48. doi: 10.1083/jcb.200404079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH. Overexpression of humbug promotes malignant progression in human gastric cancer cells. Oncol Rep. 2008;19:795–800. [PubMed] [Google Scholar]
- 18.Ince N, de la Monte SM, Wands JR. Overexpression of human aspartyl (asparaginyl) beta-hydroxylase is associated with malignant transformation. Cancer Res. 2000;60:1261–6. [PubMed] [Google Scholar]
- 19.Lavaissiere L, Jia S, Nishiyama M, et al. Overexpression of human aspartyl (asparaginyl) beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. J Clin Invest. 1996;98:1313–23. doi: 10.1172/JCI118918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de la Monte S, Tamaki S, Cantarini MC, et al. Insulin receptor substrate-1 regulates aspartyl-asparaginyl-beta hydroxylase and hepatocellular carcinoma invasiveness. J Hepatol. 2006;44:971–83. doi: 10.1016/j.jhep.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Maeda T, Taguchi K, Aishima S, et al. Clinicopathological correlates of aspartyl (asparaginyl) beta-hydroxylase over-expression in cholangiocarcinoma. Cancer Detect Prev. 2004;28:313–8. doi: 10.1016/j.cdp.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Palumbo KS, Wands JR, Safran H, King T, Carson RI, de la Monte SM. Human aspartyl (asparaginyl) beta-hydroxylase monoclonal antibodies: potential biomarkers for pancreatic carcinoma. Pancreas. 2002;25:39–44. doi: 10.1097/00006676-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Wang JY, de la Monte SM, Sabo E, et al. Prognostic value of humbug gene overexpression in stage II colon cancer. Hum Pathol. 2007;38:17–25. doi: 10.1016/j.humpath.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Maeda T, Sepe P, Lahousse S, et al. Antisense oligodeoxynucleotides directed against aspartyl (asparaginyl) beta-hydroxylase suppress migration of cholangiocarcinoma cells. J Hepatol. 2003;38:615–22. doi: 10.1016/s0168-8278(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 25.Wands JR, Lavaissiere L, Moradpour D, et al. Immunological approach to hepatocellular carcinoma. J Viral Hepat. 1997;4(Suppl 2):60–74. doi: 10.1111/j.1365-2893.1997.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 26.American Joint Committee on Cancer. Cancer staging handbook. 6. New York: Springer; 2002. [Google Scholar]
- 27.Travis WD, Brambilla E, Muller-Hermelink K, Harris CC, editors. World Health Organization. Lyon, France: IARC Press; 2004. Pathology and genetics: Tumours of the lung, pleura, thymus and heart; pp. 9–50. [Google Scholar]
- 28.Resnick MB, Routhier J, Konkin T, Sabo E, Pricolo VE. Epidermal growth factor receptor, c-MET, beta-catenin, and p53 expression as prognostic indicators in stage II colon cancer: a tissue microarray study. Clin Cancer Res. 2004;10:3069–75. doi: 10.1158/1078-0432.ccr-03-0462. [DOI] [PubMed] [Google Scholar]
- 29.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nature Medicine. 2007;7:778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 30.Wistuba II, Gazdar AF. Lung cancer neoplasia. Annu Rev Pathol. 2006;1:331–48. doi: 10.1146/annurev.pathol.1.110304.100103. [DOI] [PubMed] [Google Scholar]
- 31.Lahousse SA, Carter JJ, Xu XL, Wands JR, de la Monte SM. Differential growth factor regulation of aspartyl-(asparaginyl)-β-hydroxylase family genes in SH-Sy5y human neuroblastoma cells. BMC Cell Biol. 2006;7:41–62. doi: 10.1186/1471-2121-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sepe PS, Lahousse SA, Gemelli B, et al. Role of the aspartyl-(asparaginyl)-beta-hydroxylase gene in neuroblastoma cell motility. Lab Invest. 2002;82:881–91. doi: 10.1097/01.lab.0000020406.91689.7f. [DOI] [PubMed] [Google Scholar]
- 33.Cantarini MC, de la Monte SM, Pang M, et al. Aspartyl–asparagyl beta-hydroxylase overexpression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446–57. doi: 10.1002/hep.21272. [DOI] [PubMed] [Google Scholar]
- 34.Jia S, McGinnis K, Van Dusen WJ, et al. A fully active catalytic domain of bovine aspartyl (asparaginyl) beta-hydroxylase expressed in Escherichia coli: characterization and evidence for identification of an active-site region in vertebrate alpha-ketoglutarate–dependent dioxygenases. Proc Natl Acad Sci U S A. 1994;91:7227–31. doi: 10.1073/pnas.91.15.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahlback B, Hildebrand B, Linse S. Novel type of very high affinity calcium-binding sites in beta-hydroxyasparagine–containing epidermal growth factor-like domains in vitamin K-dependent protein S. J Biol Chem. 1990;265:18481–9. [PubMed] [Google Scholar]
- 36.Gronke RS, Van Dusen WJ, Garsky VM, et al. Aspartyl beta-hydroxylase: in vitro hydroxylation of a synthetic peptide based on the structure of the first growth factor-like domain of human factor IX. Proc Natl Acad Sci U S A. 1989;86:3609–13. doi: 10.1073/pnas.86.10.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timmerman LK, Grego-Bassa J, Raya A, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Development. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Reviews. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]


