Abstract
Bioflavonoids are human dietary components that have been linked to the prevention of cancer in adults and the generation of specific types of leukemia in infants. While these compounds have a broad range of cellular activities, many of their genotoxic effects have been attributed to their actions as topoisomerase II poisons. However, the activities of bioflavonoids against the individual isoforms of human topoisomerase II have not been analyzed. Therefore, we characterized the activity and mechanism of three major classes of bioflavonoids, flavones, flavonols, and isoflavones, against human topoisomerase IIα and IIβ. Genistein was the most active bioflavonoid tested, and stimulated enzyme-mediated DNA cleavage ~10–fold. Generally, compounds were more active against topoisomerase IIβ. DNA cleavage with both enzyme isoforms required a 5-OH and a 4′-OH and was enhanced by the presence of additional hydroxyl groups on the pendant ring. Competition DNA cleavage and topoisomerase II binding studies indicate that the 5-OH group plays an important role in mediating genistein binding, while the 4′-OH moiety contributes primarily to bioflavonoid function. Bioflavonoids do not require redox cycling for activity and function primarily by inhibiting enzyme-mediated DNA ligation. Mutagenesis studies suggest that the TOPRIM region of topoisomerase II plays a role in genistein binding. Finally, flavones, flavonols, and isoflavones with activity against purified topoisomerase IIα and IIβ enhanced DNA cleavage by both isoforms in human CEM leukemia cells. These data support the hypothesis that bioflavonoids function as topoisomerase II poisons in humans and provide a framework for further analysis of these important dietary components.
Bioflavonoids (i.e., phytoestrogens) are a diverse group of polyphenolic compounds that are constituents of many fruits, vegetables, legumes, and plant leaves (1–6). They are an integral component of the human diet and represent the most abundant natural source of antioxidants (1–4, 6–8).
It is believed that the dietary intake of bioflavonoids provides a number of health benefits to adults (1–6, 9–12). Epidemiological studies suggest that these compounds help protect against cancer, cardiovascular disease, osteoporosis, age-related diseases, and inflammation (1–6, 9–12). Despite the beneficial effects of bioflavonoids, they also display cytotoxic and genotoxic properties. To this point, the ingestion of these compounds by pregnant women has been linked to the development of specific types of infant leukemia (13–17). The majority of these leukemias feature aberrations involving the mixed lineage leukemia gene (MLL) at chromosomal band 11q23 (13, 14, 16, 17).
The mechanistic basis for the physiological actions of bioflavonoids is not known, as they have a variety of effects on human cells. Beyond their antioxidant properties, many of these polyphenols are potent inhibitors of tyrosine kinases (5, 18–23), act as either agonists or antagonists of estrogen receptors, or alter sex hormone production and metabolism (1, 11, 24–27). Furthermore, bioflavonoids display anti-proliferative and pro-apoptotic affects, decrease the expression or function of several proteins that are involved in cell-cycle progression, and inhibit both the NF-κB and Akt signaling pathways (5, 6, 11, 28–30). Finally, a number of these compounds are potent topoisomerase II poisons (16, 31, 32). It has been suggested that at least some of the cellular effects of polyphenols, including their clastogenic properties, are mediated through effects on topoisomerase II (13, 15, 16). To this point, the sensitivity of cells to the isoflavone genistein has been correlated to the activity of the type II enzyme (33).
Type II topoisomerases are ubiquitous enzymes that remove knots and tangles from the genetic material and are required for a number of critical nuclear processes (34–40). Humans encode two isoforms of topoisomerase II, α and β (34–42). While these two isoforms display similar enzymological properties, they differ significantly in their physiology and cellular functions. Topoisomerase IIα is essential to the survival of all proliferating cells (36, 37, 43–47). Levels of the protein increase dramatically during periods of growth and are regulated over the cell cycle, peaking at G2/M (36, 37, 43–47). The α isoform plays important roles in DNA processes related to proliferation, and is required for DNA replication and chromosome segregation (36, 37, 43–47). In contrast, the physiological roles of topoisomerase IIβ are poorly understood. Expression of this isoform is independent of proliferative status or the cell cycle, and the protein appears to be present in all tissue types (35, 36, 45, 48). Despite its wide tissue distribution, topoisomerase IIβ is not essential at the cellular level and cells that lack the protein show no known phenotype (49–51). However, mice that are genetically deficient in this isoform suffer severe neurological abnormalities during embryogenesis (51).
Type II topoisomerases modulate the topological state of DNA by generating transient double-stranded breaks in the backbone of the genetic material (36–40, 52, 53). To maintain genomic integrity during this cleavage event, the enzyme forms covalent bonds between active site tyrosyl residues and the 5′-DNA termini created by scission of the double helix (54–56). These covalent topoisomerase II-cleaved DNA intermediates are known as cleavage complexes. Under normal conditions, they are present at low equilibrium levels and are tolerated by the cell. However, conditions that significantly increase the concentration of cleavage complexes generate permanent DNA strand breaks that trigger illegitimate recombination, chromosomal aberrations, sister chromatid exchange, and cell death pathways (37, 40, 57–63).
Agents that increase the concentration of topoisomerase II-DNA cleavage complexes are called topoisomerase II poisons (37, 63–66). A variety of important anticancer drugs, such as etoposide and doxorubicin, kill cells by acting as topoisomerase II poisons (37, 63–67). Despite the importance of these compounds in cancer chemotherapy, ~2–3% of patients that are treated with regimens that include topoisomerase II-targeted agents eventually develop secondary leukemias (58, 61, 66, 68–71). Like the infant leukemias, these drug-related malignancies are characterized by rearrangements in the MLL gene (58, 61, 68–71). Agents such as etoposide display potent activity against both topoisomerase IIα and IIβ in vitro and in human cells (72–74), but the relative contributions of the two enzyme isoforms to either the therapeutic or leukemogenic properties of these drugs are not known.
Although bioflavonoids impact human health by a variety of processes, many of their chemopreventative, cytotoxic, and genotoxic properties are consistent with their activity as topoisomerase II poisons. Therefore, the present study more fully defined the activity and mechanism of action of three major classes of bioflavonoids, flavones, flavonols, and isoflavones, against human topoisomerase IIα and IIβ. Results provide novel insight into the mechanistic basis for the actions of these compounds.
EXPERIMENTAL PROCEDURES
Enzymes and Materials
Recombinant wild-type human topoisomerase IIα, IIβ, and htop2αG474A were expressed in Saccharomyces cerevisiae and purified as described previously (75–77). Negatively supercoiled pBR322 DNA was prepared from Escherichia coli using a Plasmid Mega Kit (Qiagen) as described by the manufacturer. Genistein was purchased from ICN. Chrysin, fisetin, galangin, and etoposide were purchased from Sigma. Luteolin, apigenin, diosmetin, myricetin, quercetin, kaempferol, isorhamnetin, daidzein, and biochanin A were obtained from LKT Laboratories. [γ-32P]ATP (~6000 Ci/mmol) and [14C]genistein (~16 mCi/mmol) were purchased from ICN and Moravek Biochemicals, respectively. All bioflavonoids and drugs were prepared as 20 mM stocks in 100% DMSO. Bioflavonoid stocks were stored at −20 °C, and etoposide was stored at 4 °C.
Generation of the G474A Mutant of Human Topoisomerase IIα
The G474A mutant of human topoisomerase IIα (htop2αG474A) was generated by cloning a SalI-KpnI fragment of YEpWob6 (78) that encoded the N-terminus of the human enzyme into pUC18. Site-directed mutagenesis was performed using the QuikChange II PCR site-directed mutagenesis kit (Stratagene). The sequence of the forward and reverse primers used to generate the G474A mutation were GGCTGTTTCAGGCCTTGCAGTGGTTGGGAGAGACAAATATGGGG and CCCATATTTGTCTCTCCCAACCACTGCAAGGCCTGAAACAGC, respectively. The mutagenized sequence is underlined. Mutations were verified by sequencing and the SalI-KpnI fragment was cloned back into YEpWob6. htop2αG474A was purified as described above.
Cleavage of Plasmid DNA
DNA cleavage reactions were carried out using the procedure of Fortune and Osheroff (79). Assay mixtures contained 220 nM topoisomerase IIα or IIβ, 10 nM negatively supercoiled pBR322 DNA, and 0–200 μM bioflavonoid or etoposide in 20 μL of DNA cleavage buffer [10 mM Tris-HCl, pH 7.9, 5 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, and 2.5% (v/v) glycerol]. DNA cleavage mixtures were incubated for 6 min at 37 °C, and enzyme-DNA cleavage intermediates were trapped by adding 2 μL of 5% SDS and 1 μL of 375 mM EDTA, pH 8.0. Proteinase K was added (2 μL of a 0.8 mg/mL solution) and reaction mixtures were incubated for 30 min at 45 °C to digest topoisomerase II. Samples were mixed with 2 μL of 60% sucrose in 10 mM Tris-HCl, pH 7.9, 0.5% bromophenol blue, and 0.5% xylene cyanol FF, heated for 2 min at 45 °C, and subjected to electrophoresis in 1% agarose gels in 40 mM Tris-acetate, pH 8.3, and 2 mM EDTA containing 0.5 μg/mL ethidium bromide. DNA cleavage was monitored by the conversion of negatively supercoiled plasmid DNA to linear molecules. DNA bands were visualized by ultraviolet light and quantified using an Alpha Innotech digital imaging system.
In reactions that determined whether DNA cleavage by human topoisomerase IIα or IIβ was reversible, EDTA (final concentration of 18 mM) was added prior to treatment with SDS. To determine whether cleaved DNA was protein-linked, proteinase K treatment was omitted. To examine the effects of a reducing agent on the actions of genistein against topoisomerase IIα and IIβ, 0.5 mM DTT was incubated with 50 μM genistein for ~5 min prior to initiation of the cleavage reaction.
To assess the effects of genistein on human topoisomerase IIα and IIβ in the absence of DNA, 50 μM genistein was incubated with the enzyme for ~5 min at 37 °C in 15 μL of DNA cleavage buffer. Cleavage was initiated by adding 10 nM negatively supercoiled pBR322 DNA to the reaction mixture. The final concentrations of topoisomerase II and plasmid molecules were 220 nM and 10 nM, respectively.
To determine the ability of daidzein, biochanin A, and chrysin to compete with genistein, DNA cleavage reactions with human topoisomerase IIα or IIβ were performed in the presence of 50 μM genistein and 0–500 μM of bioflavonoid. Competition was quantified by the loss of genistein-induced linear DNA molecules.
DNA Cleavage Site Utilization
DNA cleavage sites were mapped using a modification of the procedure of O’Reilly and Kreuzer (80). A linear 4330 bp fragment (HindIII/EcoRI) of pBR322 plasmid DNA singly labeled with 32P on the 5′-terminus of the HindIII site was used as the cleavage substrate. The pBR322 DNA substrate was linearized by treatment with HindIII. Terminal 5′-phosphates were removed by treatment with calf intestinal alkaline phosphatase and replaced with [32P]phosphate using T4 polynucleotide kinase and [γ-32P]ATP. The DNA was treated with EcoRI, and the 4330 bp singly end labeled fragment was purified from the small EcoRI-HindIII fragment by passage through a CHROMA SPIN+TE-100 column (Clontech). Reaction mixtures contained 0.7 nM labeled pBR322 DNA substrate and 90 nM human topoisomerase IIα or IIβ in 50 μL of DNA cleavage buffer. Assays were carried out in the absence of compound, or in the presence of 25 μM etoposide or 50 μM bioflavonoid. Reactions were initiated by the addition of the enzyme and were incubated for 6 min (topoisomerase IIα) or 0.5 min (topoisomerase IIβ) at 37 °C. Cleavage intermediates were trapped by adding 5 μL of 5% SDS followed by 3.75 μL of 250 mM EDTA, pH 8.0. Topoisomerase II was digested with proteinase K (5 μL of a 0.8 mg/mL solution) for 30 min at 45 °C. DNA products were precipitated twice in 100% ethanol, washed in 70% ethanol, dried, and resuspended in 6 μL of 40% formamide, 10 mM NaOH, 0.02% xylene cyanol FF, and 0.02% bromophenol blue. Samples were subjected to electrophoresis in a denaturing 6% polyacrylamide sequencing gel 100 mM Tris-borate, pH 8.3, and 2 mM EDTA. The gel was fixed in a 10% methanol/10% acetic acid mixture for 2 min and dried. DNA cleavage products were analyzed on a Bio-Rad Molecular Imager FX.
Ligation of Cleaved Plasmid DNA by Human Topoisomerase II
DNA ligation mediated by human topoisomerase IIα or IIβ was monitored according to the procedure of Byl et al. (81). DNA cleavage-ligation equilibria were established for 6 min at 37 °C as described above in the presence of 50 μM bioflavonoid or 50 μM etoposide. Ligation was initiated by shifting samples from 37 to 0 °C. Reactions were stopped at time points up to 20 s by the addition of 2 μL of 5% SDS followed by 1 μL of 375 mM EDTA, pH 8.0. Samples were processed and analyzed as above. Ligation was monitored by the loss of linear DNA.
Nitrocellulose Filter Binding
Topoisomerase II-bioflavonoid competition binding studies were performed using the procedure of Kingma and Osheroff (82). Nitrocellulose membranes (0.45 μm HA; Millipore) were soaked in binding buffer [10 mM Tris-HCl, pH 7.9, 0.1 mM EDTA, and 2.5% (v/v) glycerol] for 10 min. Reaction mixtures contained 25 μM [14C]genistein, 1.6 μM enzyme, and 0–250 μM daidzein, biochanin A, or chrysin in a total of 60 μL of binding buffer. Samples were incubated for 6 min at 37 °C and applied to the nitrocellulose membranes in vacuo. Filters were immediately washed three times with 1 mL of ice-cold binding buffer, dried, and submerged in 8 mL of scintillation fluid (Econo-Safe; Research Products International). Radioactivity remaining on the membranes was quantified using a Beckman LS 5000 TD scintillation counter. The amount of radioactive genistein remaining on the filter in the absence of enzyme was subtracted prior to binding calculations.
Formation of Topoisomerase II-DNA Cleavage Complexes in Cultured Human Cells
Human CEM leukemia cells were cultured under 5% CO2 at 37 °C in RPMI 1640 medium (Cellgro by Mediatch, Inc.), containing 10% heat-inactivated fetal calf serum (Hyclone) and 2 mM glutamine (Cellgro by Mediatech, Inc.). The in vivo complex of enzyme (ICE) bioassay (as modified on the TopoGen, Inc. website) (83, 84) was utilized to determine the ability of selected bioflavonoids to increase levels of topoisomerase II-DNA cleavage complexes in treated cells. Exponentially growing cultures were treated with 50 μM bioflavonoid or etoposide for 1 h. Cells (~ 5 × 106) were harvested by centrifugation and lysed by the immediate addition of 3 mL of 1% sarkosyl. Following gentle homogenization in a dounce homogenizer, cell lysates were layered onto a 2 mL cushion of CsCl (1.5 g/mL) and centrifuged at 45000 rpm for 15 h at 20 °C. DNA pellets were isolated, resuspended in 5 mM Tris-HCl, pH 8.0, and 0.5 mM EDTA, normalized for the amount of DNA present, and blotted onto nitrocellulose membranes using a Schleicher and Schuell slot blot apparatus. Covalent complexes formed between human topoisomerase IIα or IIβ and DNA were detected using a polyclonal antibody directed against either human topoisomerase IIα or IIβ (Abcam), respectively, at a 1:2000 dilution.
ICE bioassays were used to assess the effects of biochanin A and daizein on the ability of genistein to increase levels of topoisomerase II-DNA cleavage complexes in human CEM cells. Cultures were treated with 25 or 50 μM genistein in the presence of 250 or 500 μM biochanin A or daidzein, respectively. Competition was quantified by the reduction of genistein-induced topoisomerase II-DNA cleavage complexes.
RESULTS AND DISCUSSION
Bioflavonoids Enhance DNA Cleavage Mediated by Human Topoisomerase IIα and IIβ
Bioflavonoids increase levels of DNA cleavage mediated by purified calf thymus and Drosophila topoisomerase II, and by human nuclear extracts supplemented with human topoisomerase II. (16, 32, 85–87) [The calf thymus and human topoisomerase II used for these studies were not isoform specific. These enzymes were isolated from natural sources, presumably as a mixture of the α and β isoforms. Drosophila encodes only a single type II topoisomerase (40).] Furthermore, treatment of cultured human cells with flavones, flavonols, or isoflavones has been shown to generate DNA strand breaks and induce cleavage within the breakpoint cluster region of the MLL gene (16, 88, 89).
It should be noted that the sensitivity of topoisomerase II to bioflavonoids is species specific. Although genistein is the most active bioflavonoid against the type II enzymes listed above, yeast topoisomerase II is refractory to the compound.
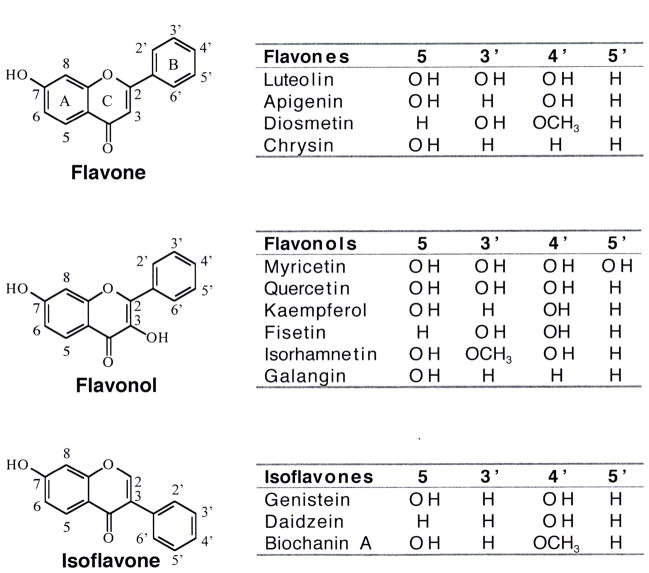
Despite the impact of bioflavonoids on human health, the effects of these compounds on the individual isoforms of human topoisomerase II and the mechanistic basis for their actions have not been characterized. As a first step toward this end, the ability of several flavones, flavonols, and isoflavones to enhance DNA cleavage mediated by human topoisomerase IIα and IIβ was assessed. The bioflavonoids utilized for these studies are shown in Figure 1.
Figure 1.
Structures of selected bioflavonoids. Flavones, flavonols, and isoflavones are shown.
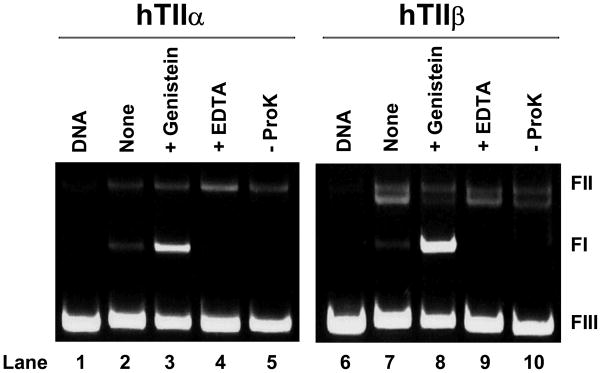
As seen in Figure 2, genistein enhanced DNA cleavage mediated by human topoisomerase IIα and IIβ. Scission was reversed when EDTA was added to reaction mixtures before cleavage complexes were trapped by SDS. This reversibility is inconsistent with a non-enzymatic reaction. In addition, the electrophoretic mobility of the cleaved DNA (i.e., the linear band) was dramatically reduced in the absence of proteinase K treatment, indicating that all of the cleaved plasmid molecules were covalently attached to either topoisomerase IIα or IIβ. Taken together, these findings provide strong evidence that bioflavonoids increase DNA cleavage through an enzyme-mediated reaction.
Figure 2.
Genistein-induced DNA cleavage is mediated by human topoisomerase IIα (hTIIα) and IIβ (hTIIβ). Ethidium bromide-stained agarose gels are shown. The reversibility of genistein-induced DNA cleavage complexes was determined by adding EDTA to reaction mixtures before these complexes were trapped by SDS (+EDTA; lanes 4 and 9). To determine whether DNA cleavage induced by genistein was protein-linked, proteinase K treatment was omitted (−ProK; lanes 5 and 10). Control reactions contained DNA alone (DNA; lanes 1 and 6), DNA and enzyme in the absence of genistein (none; lanes 2 and 7), or reaction mixtures treated with SDS prior to EDTA (Genistein; lanes 3 and 8). The mobility of negatively supercoiled DNA (form I, FI), nicked circular plasmid (form II, FII), and linear molecules (form III, FIII) are indicated. Data are representative of at five least independent experiments.
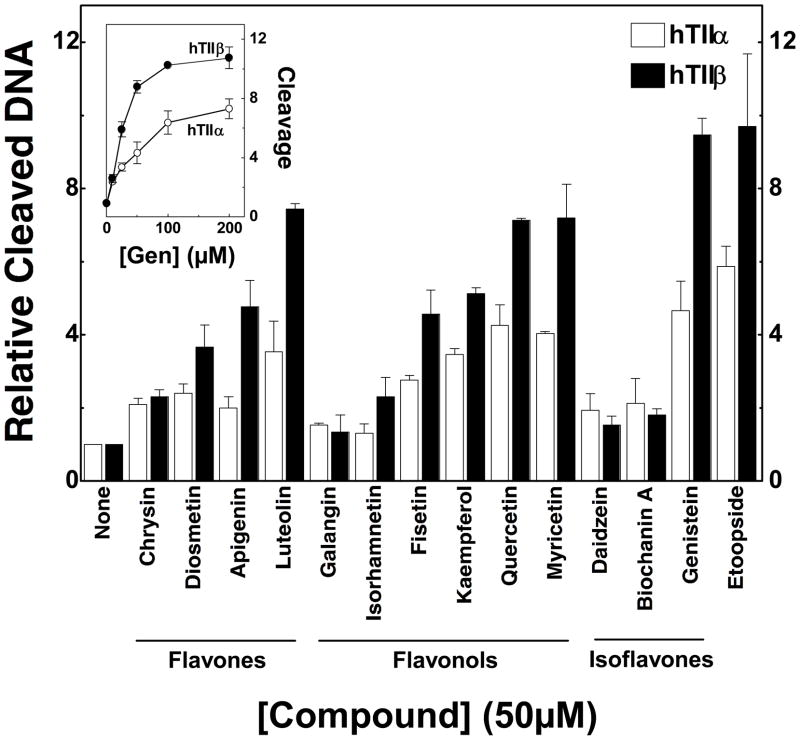
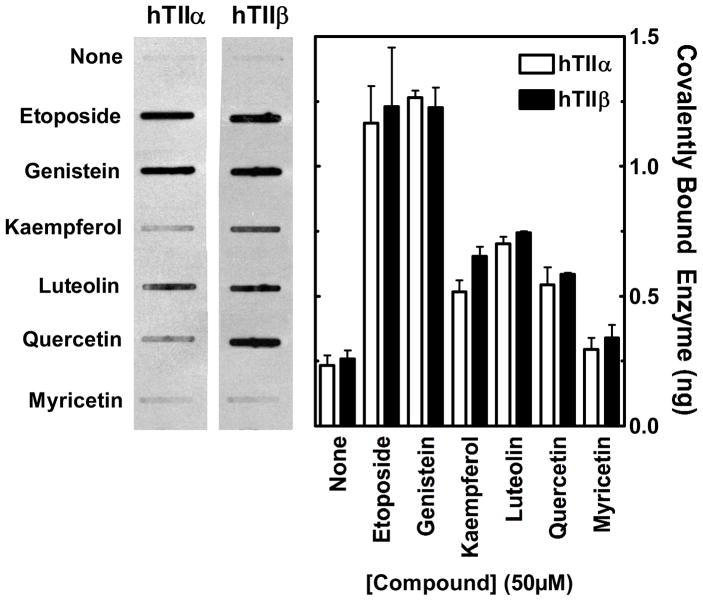
Several of the bioflavonoids tested enhanced DNA scission mediated by both human topoisomerase II isoforms (Figure 3). None of the bioflavonoids cleaved DNA in the absence of enzyme (not shown). The compounds utilized generated a wide range of topoisomerase II-mediated DNA cleavage. Complete titrations were carried out with each compound (see inset for a titration with genistein). A summary of data obtained with 50 μM bioflavonoid is shown. This concentration represented the maximum level of cleavage for most of the compounds tested. The only major exception was genistein, which plateaued at ~100–200 μM.
Figure 3.
Effects of bioflavonoids on double-stranded DNA breaks generated by human topoisomerase IIα and IIβ. Data for topoisomerase IIα– (hTIIα; open bars) and IIβ–(hTIIβ; closed bars) mediated DNA cleavage in the presence of 50 μM flavones, flavonols, and isoflavones are shown in the bar graph. The inset shows a titration for DNA cleavage meditated by topoisomerase IIα (open circles) and IIβ (closed circles) in the presence of 0–200 μM genistein. Error bars represent standard deviations for three independent experiments.
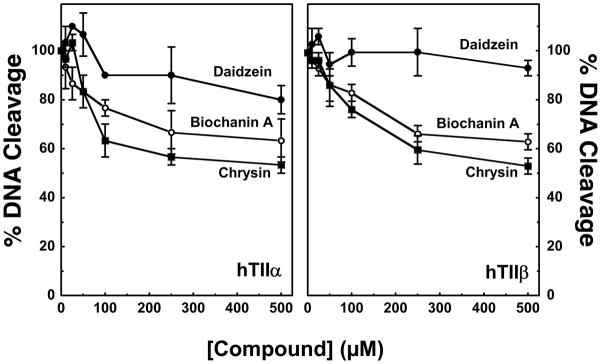
Whereas some compounds such as galangin had virtually no effect on DNA scission, others such as luteolin, kaempferol, quercetin, myricetin, and genistein enhanced cleavage several–fold. Three conclusions can be drawn from the data shown in Figure 3. First, bioflavonoids in all three classes that enhanced DNA cleavage generally had a substantially larger effect on topoisomerase IIβ than they did on topoisomerase IIα. In these cases, enhancement of cleavage was ~1.5– to 2–fold higher with the β isoform. Second, as proposed previously with mixed populations of mammalian type II topoisomerases (16, 32, 87), the presence of a hydroxyl moiety at the 5- or 4′-position greatly contributes to the enhancement of enzyme-mediated DNA cleavage. For example, substitution of the 5-OH with a hydrogen (daidzen) or the 4′-OH with a methoxy group (biochanin A) abrogates the activity of genistein. It is notable, however, that the requirement for these hydroxyl groups is not absolute. Fisetin (which lacks the 5-OH) and diosmetin (which lacks the 5-OH and contains a methoxy group in the 4′-position) both induce moderate levels of DNA cleavage. Third, the presence of additional hydroxyl moieties on the pendant ring (B-ring) at the 3′- and/or 5′-positions enhances bioflavonoid activity, especially against topoisomerase IIβ.
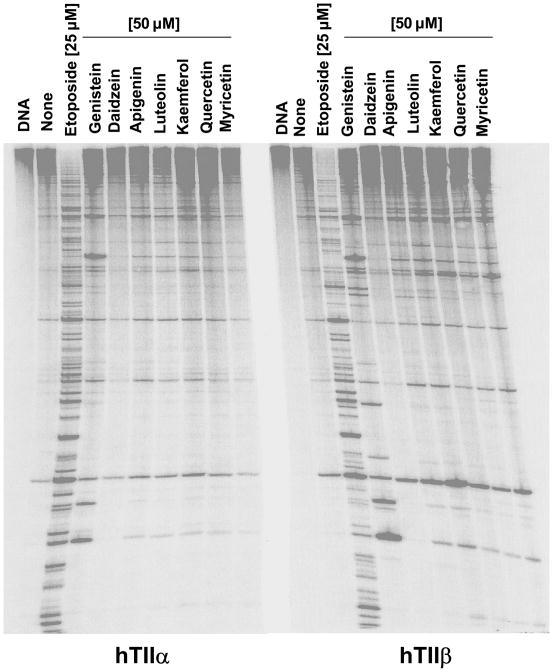
The ability of selected bioflavonoids to enhance topoisomerase II-mediated DNA scission also was examined using end-labeled linear plasmid molecules (Figure 4). This allows sites of DNA cleavage to be monitored. High levels of DNA cleavage were observed for those bioflavonoids that displayed activity with negatively supercoiled plasmid (compare with Figure 3). In contrast, no appreciable cleavage was seen with daidzein, which displayed little activity with negatively supercoiled molecules. Equivalent cleavage maps were observed for the flavones and flavonols with both topoisomerase IIα and IIβ, suggesting that these compounds interact in a similar fashion within the ternary enzyme-DNA-bioflavonoid complex.
Figure 4.
Effects of bioflavonoids on DNA cleavage site utilization by human topoisomerase IIα (hTIIα) and IIβ (hTIIβ). Autoradiograms of polyacrylamide gels are shown. DNA cleavage reactions contained no compound (none), 25 μM etoposide, or 50 μM bioflavonoid. A DNA control is shown in the far left lane of each autoradiogram (DNA). Data are representative of two independent experiments.
Sites of DNA cleavage observed in the presence of flavones and flavonols differed significantly from those seen for etoposide and were predominantly those generated by the enzyme isoforms in the absence of drugs.1 This further suggests that these bioflavonoids do not significantly alter the specificity of either topoisomerase IIα or IIβ. Slightly different results were seen with genistein, whose DNA cleavage pattern included several strong sites of action in addition to those observed with the flavones and flavonols. This finding implies that there may be subtle, but important differences in the spatial geometry of isoflavones within the ternary complex as compared to the other classes of bioflavonoids.
Role of the 5-OH and 4′-OH Groups in Mediating Isoflavone Activity
As discussed above, earlier studies (as well as Figure 3) point to the importance of the 5-OH and 4′-OH groups (16, 32, 87). However, their roles in mediating the activity of bioflavonoids have not been determined. Since genistein displayed the greatest activity against human topoisomerase II, competition studies were carried out to determine whether these groups contribute primarily to genistein binding or function.
In a first set of experiments, the ability of daidzein (which lacks the 5-OH) and biochanin A (which contains a 4′-methoxyl in place of the hydroxyl) to inhibit DNA cleavage induced by 50 μM genistein was assessed. Similar results were found for both human topoisomerase II isoforms (Figure 5). Even at a concentration of 500 μM, daidzein showed no significant ability to inhibit the actions of genistein. This finding indicates that the 5-OH moiety plays an important role in isoflavone binding within the ternary complex.
Figure 5.
Contributions of the 5-OH and 4′-OH moieties to the activity of genistein. Effects of daidzein (lacking 5-OH; closed circles), biochanin A (containing a 4′-methoxy group in place of the 4′-OH; open circles), and chrysin (a flavone that lacks the 4′-OH; closed squares) on the ability of genistein to enhance DNA cleavage mediated by human topoisomerase IIα (hTIIα) and IIβ (hTIIβ) are shown. DNA cleavage reactions were carried out in the presence of 50 μM genistein and 0–500 μM competing bioflavonoid. Competition was quantified by the loss of genistein-induced linear DNA molecules. DNA cleavage was set to 100% in the absence of competitor. Error bars represent standard deviations for three independent experiments.
The ability of biochanin A to complete with genistein was somewhat improved as compared to daidzein. Substitution of a methoxyl group for the 4′-OH was not as debilitating as the absence of the 5-OH. Unfortunately, it was not possible to use an isoflavone lacking the 4′-OH for this study, as none was available. However, to explore the role of the 4′-OH more fully, a parallel competition study was performed using chrysin. This compound is the flavone equivalent of genistein, but lacks the 4′-OH moiety.
Chrysin inhibited genistein-induced DNA cleavage slightly better than did either isoflavone (Figure 5). The fact that alterations at the 4′-OH decreased bioflavonoid-induced DNA cleavage to a similar extent as the loss of the 5-OH (see Figure 3) but allowed greater competition with genistein suggests that the 4′-OH plays a role in mediating bioflavonoid function beyond enzyme binding.
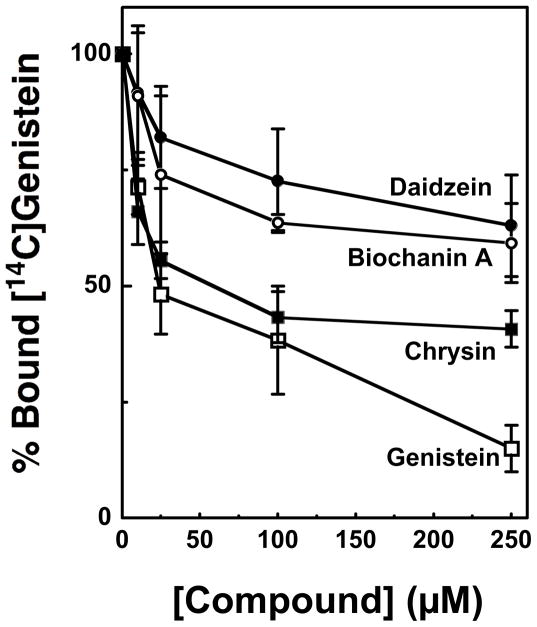
To further define the contributions of the 5-OH and 4′-OH moieties to isoflavone binding and function, the ability of daidzein, biochanin A, and chrysin to compete for [14C]genistein binding to human topoisomerase IIα was determined (Figure 6). Consistent with the DNA cleavage competition studies, chrysin competed the best followed by biochanin A then daidzein. These findings indicate that the 5-OH plays a more important role than the 4′-OH in mediating genistein binding to topoisomerase II and implies that the 4′-OH plays a significant functional role beyond any contribution to genistein-enzyme binding.
Figure 6.
Contributions of the 5-OH and 4′-OH moieties to the binding of genistein to topoisomerase IIα. Effects of daidzein (lacking 5-OH; closed circles), biochanin A (containing a 4′-methoxy group in place of the 4′-OH; open circles), chrysin (a flavone that lacks the 4′-OH; closed squares) and unlabeled genistein (open squares) on the ability of [14C]genistein to bind to human topoisomerase IIα are shown. Nitrocellulose filter binding assays were carried out in the presence of 25 μM [14C]genistein and 0–250 μM competing bioflavonoid. Competition was quantified by the loss of enzyme bound [14C]genistein. Enzyme binding was set to 100% in the absence of competitor. Error bars represent standard deviations for three independent experiments.
It is notable that two other potent topoisomerase II poisons, etoposide and the quinolone CP-115,953, both contain pendant rings that feature 4′-OH moieties. Furthermore, in both cases, the 4′-OH groups are essential for drug action (91, 92). Thus, this group may play equivalent roles across a spectrum of topoisomerase II poisons.
Mechanistic Basis for Bioflavonoid-Induced Enhancement of DNA Cleavage Mediated by Topoisomerase II
Topoisomerase II poisons act by two non-mutually exclusive mechanisms. Agents such as etoposide act primarily by inhibiting the ability of the enzyme to ligate cleaved nucleic acids (37, 63, 66, 93). Conversely, topoisomerase II poisons such as quinolones and a basic sites have little or no effect on rates of ligation. Thus, they are presumed to act primarily by stimulating the forward rate of enzyme-mediated DNA scission (37, 40, 63, 66, 94–98).
A previous study using Drosophila topoisomerase II suggested that genistein acted primarily by increasing rates of DNA cleavage (99). However, as discussed above, the effects of bioflavonoids on topoisomerase II are species specific. Therefore, DNA ligation assays were carried out with topoisomerase IIα and IIβ to characterize the mechanism by which bioflavonoids poison the human type II enzyme (Figure 7).
Figure 7.
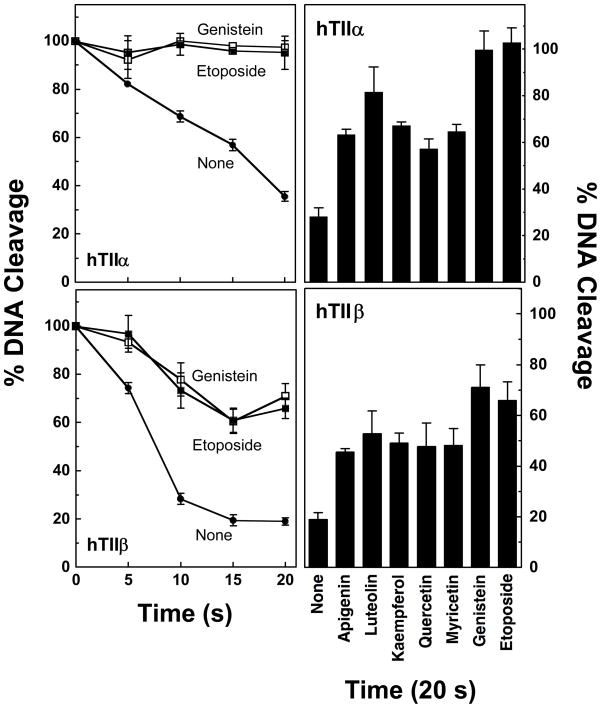
Effects of bioflavonoids on DNA ligation mediated by human topoisomerase IIα (hTIIα, top panels) and IIβ (hTIIβ, bottom panels). Left panels: DNA ligation was examined in the absence of compounds (closed circles), or in the presence of 50 μM genistein (open squares) or etoposide (closed squares). Samples were incubated at 37 °C to establish DNA cleavage/ligation equilibria and were shifted to 0 °C to initiate the ligation reaction. The amount of DNA cleavage observed at equilibrium for was set to 100% at time zero. Ligation was quantified by the loss of linear cleaved molecules. Right panels: Representative DNA ligation data for reactions containing no compound, 50 μM bioflavonoid, or 50 μM etoposide at 20 s after shifting samples to 0 °C are shown. Error bars represent standard deviations for three independent experiments.
In contrast to results with the Drosophila enzyme (99), genistein strongly inhibited the ability of human topoisomerase IIα and IIβ to ligate cleaved plasmid molecules. Results were comparable to those seen with etoposide. Other bioflavonoids that enhanced topoisomerase II-mediated DNA cleavage also decreased rates of ligation. In general, there was a strong correlation between the ability of the compounds to increase levels of cleavage complexes and to inhibit ligation. These data suggest that bioflavonoids poison human topoisomerase IIα and IIβ primarily by impairing the ability of these enzymes to ligate cleaved DNA molecules.
There is precedence for topoisomerase II poisons having differential effects on enzymes from different species. For example, the quinolone CP-115,953 strongly inhibits the ability of bacterial topoisomerase IV to ligate DNA, but has only a modest effect on the ligation activity of eukaryotic type II enzymes (94, 100).
Bioflavonoids contain multiple hydroxyl moieties and are capable of undergoing redox cycling (1, 3, 4, 7, 8). Recent studies demonstrated that a number of quinones, including N-acetyl-p-benzoquinone imine (the toxic metabolite of acetaminophen), 1,4-benzoquinone (a reactive metabolite of benzene), and several polychlorinated biphenyl (PCB) quinone metabolites, poison human topoisomerase IIα by a mechanism that involves protein adduction (101–104). Thus, it is possible that bioflavonoids poison topoisomerase II via an adduction-based mechanism.
Two sets of experiments were carried out to determine whether redox cycling plays a role in the actions of bioflavonoids as topoisomerase II poisons. The first set took advantage of the finding that reduction of quinones to hydroquinones dramatically reduces the activity of these compounds against human type II topoisomerases (103, 104). In contrast, reducing agents do not alter the efficacy of “traditional” topoisomerase II poisons such as etoposide (103, 104). Therefore, the effect of dithiothreitol on the activity of genistein was determined. As seen in Figure 8, treatment of the isoflavone with dithiothreitol prior to the addition to DNA cleavage reactions had no effect on genistein-induced enhancement of DNA scission by topoisomerase IIα or IIβ.
Figure 8.
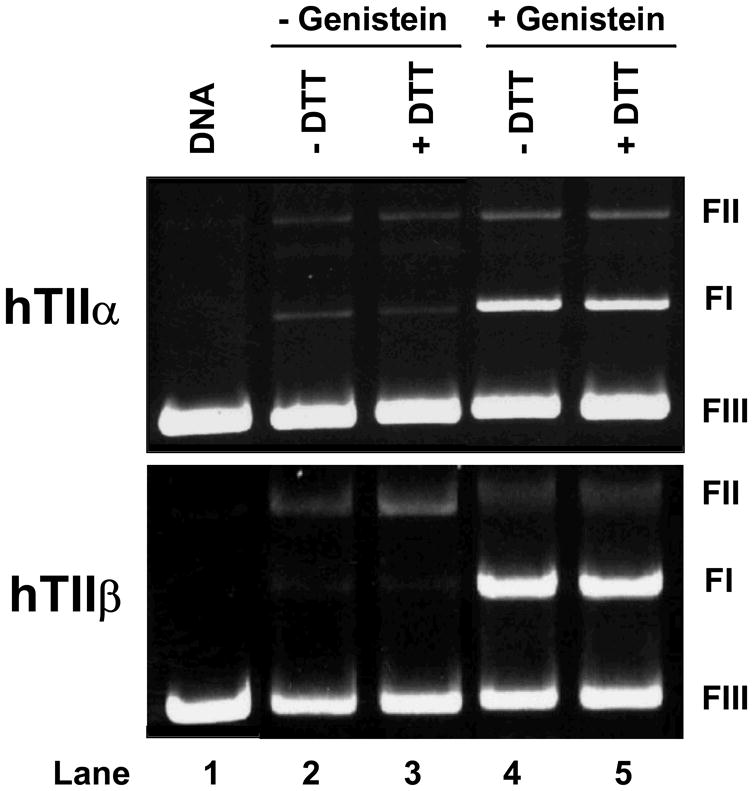
Dithiothreitol does not impair the ability of genistein to enhance DNA cleavage mediated by human topoisomerase IIα (hTIIα) or IIβ (hTIIβ). Ethidium bromide-stained agarose gels are shown. The positions of supercoiled (FI), nicked circular (FII), and linear (FIII) DNA molecules are labeled as in Figure 2. The gels show reactions in which 50 μM genistein was incubated without dithiothreitol (−DTT; lane 4) or with 500 μM dithiothreitol prior to its addition to cleavage reactions (+DTT; lane 5). Parallel control reactions mediated by topoisomerase IIα or IIβ in the absence of genistein are shown in lanes 2 and 3. A DNA standard is shown in lane 1. Results are representative of three independent experiments.
The second set of experiments took advantage of the finding that incubation of human topoisomerase IIα with quinones prior to the addition of DNA inactivates the enzyme (101, 103, 104). This inactivation results at least in part from a crosslinking of the N-terminal gate of the protein by the quinone (104). Once again, this enzyme inactivation is not observed with etoposide or other “traditional” topoisomerase II poisons. As seen in Figure 9, levels of DNA cleavage generated by topoisomerase IIα or IIβ were identical when genistein was added to reaction mixtures before or after the inclusion of DNA.
Figure 9.
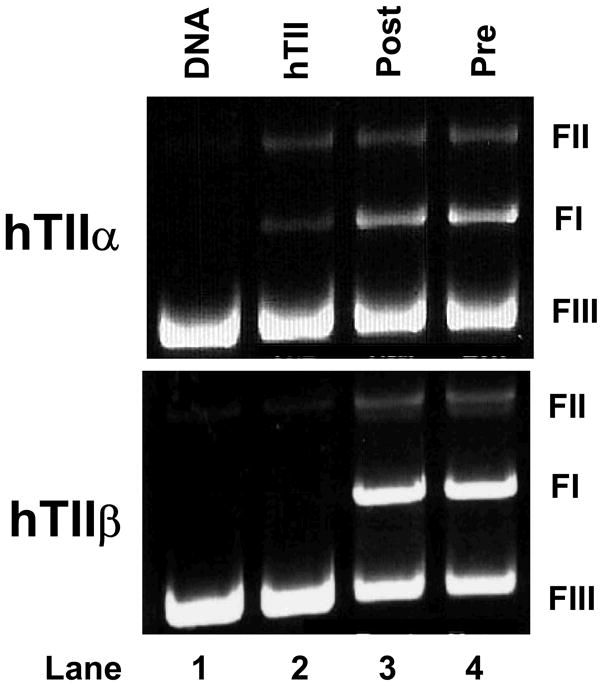
Genistein does not inactivate human topoisomerase IIα (hTIIα) or IIβ (hTIIβ) in the absence of DNA. Ethidium bromide-stained agarose gels of DNA cleavage reactions are shown. Enzymes were incubated simultaneously with plasmid DNA and 50 μM genistein (Same; lane 3) or with 50 μM genistein for 5 min prior to the addition of DNA (Pre; lane 4). A DNA standard (DNA; lane 1) and reactions mediated by topoisomerase IIα or IIβ in the absence of genistein (hTII; lane 2) are shown. The positions of supercoiled (FI), nicked circular (FII), and linear (FIII) DNA are labeled as in Figure 2. Results are representative of three independent experiments.
Taken together, these data demonstrate that the potential to undergo redox cycling does not contribute to the ability of genistein to increase topoisomerase II-mediated DNA cleavage. Thus, we conclude that bioflavonoids act as traditional topoisomerase II poisons rather than quinone-based compounds.
Mutation of Gly474 to Alanine in Human Topoisomerase IIα Reduces Sensitivity to Genistein
Many eukaryotic type II topoisomerases contain two Walker consensus ATP bindng motifs (105), Walker A and B (106–108). The Walker A site spans residues 161-166 (numbering is from human topoisomerase IIα) and is comprised of the G-X-X-G-X-G sequence (106–109). The Walker B site, at residues 472-477, contains the G-X-G-X-X-G consensus (Table 1) (106–109). The sequence at residues 161-166 is the site that actually is utilized for ATP binding in the eukaryotic type II enzyme (110). The second motif has no known function in topoisomerase II.
Table 1.
Walker B Consensus ATP Binding Site in Topoisomerase II
| Species | A.A. Sequence Position | Sequence |
|---|---|---|
| Consensus | ––– | G-X-G-X-X-G |
| Human Topoisomerase IIα | 472-477 | G-L-G-V-V-G |
| Human Topoisomerase IIβ | 488-493 | G-L-G-I-V-G |
| Drosophila Topoisomerase II | 452-457 | G-L-G-V-I-G |
| S. cerevisiae Topoiomerase II | 456-461 | G-L-A-V-V-G |
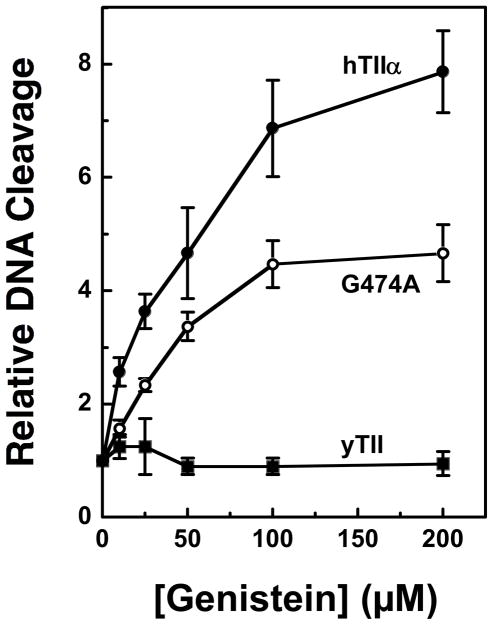
Genistein and other bioflavonoids are competitive inhibitors of ATP in a number of tyrosine kinases that utilize the G-X-G-X-X-G consensus for ATP binding (18–23, 111). This raises the intriguing possibility that bioflavonoids interact with the non-functional consensus (residues 472-477) on topoisomerase II. Two lines of circumstantial evidence support this possibility. First, this sequence is located in the TOPRIM region of the enzyme, which is in close proximity to the site of DNA cleavage and ligation (52). Second, genistein is a potent poison of human topoisomerase IIα and β and Drosophila topoisomerase II, each of which contains the consensus G-X-G-X-X-G motif. In contrast, yeast topoisomerase II, which carries the sequence G-X-A-X-X-G at this site (and thus lacks the ATP binding motif) (52), is refractory to genistein (see Figure 10). As seen in Table 1, the positions denoted by the “X”s are identical or highly conserved among these species.
Figure 10.
htop2αG474A displays decreased sensitivity to genistein. Levels of DNA cleavage for the wild-type human enzyme (hTIIα, closed circles) and hTop2αG474A (G474A, open circles) were quantified and expressed as the relative increase in linear molecules in the presence of 0–200 μM genistein. DNA cleavage for yeast topoisomerase II(yTII, closed squares) is shown for comparison. Error bars represent standard deviations for three independent experiments.
To determine if the ATP binding motif at residues 472-477 contributes to the actions of genistein against topoisomerase II, Gly474 in human topoisomerase IIα was mutated to Ala (hTop2αG474A). This substitution converts the consensus sequence of the human enzyme to the non-consensus sequence of yeast topoisomerase II. Although the intrinsic DNA cleavage activity of hTop2αG474A was similar to that of wild-type topoisomerase IIα (not shown), the sensitivity to genistein was reduced (Figure 10). Both the potency and efficacy of hTop2αG474A were ~2–fold lower than that of the wild-type enzyme. While this finding does not completely explain the lack of sensitivity of yeast topoisomerase II to genistein, it strongly suggests that the nonfunctional ATP binding motif at residues 472-477 contributes to the actions of the isoflavone against human topoisomerase II.
Bioflavonoids Increase Levels of DNA Cleavage Complexes Generated by Topoisomerase IIα and IIβ in Cultured Human Cells
Since bioflavonoids act as topoisomerase II poisons in vitro, it has been assumed that the DNA breaks generated by these compounds in cells are mediated by the type II enzyme(s) (16, 88, 89). However, the physiological effects of these compounds on topoisomerase II have never been confirmed. Therefore, the ICE bioassay was employed to determine whether bioflavonoids actually increase DNA cleavage mediated by topoisomerase IIα and/or IIβ in human cells. In this assay, cultured CEM leukemia cells were lysed with an ionic detergent, and proteins that were covalently attached to genomic DNA were separated from free proteins by sedimentation through a CsCl cushion.
As seen in Figure 11, bioflavonoids enhance DNA cleavage mediated by topoisomerase IIα and IIβ in cultured human cells. Results reflect those obtained in vitro, but differences were noted. First, while all of the compounds were more efficacious against purified topoisomerase IIβ in vitro (see Figure 3), similar levels of cleavage were observed with both enzyme isoforms in treated human cells. Since an identical result was seen with etoposide, this decreased cellular effect on topoisomerase IIβ does not appear to represent a bioflavonoid-specific property. Rather, it may reflect the different physiological functions or locations of topoisomerase IIα and IIβ (112, 113). Second, myricetin appeared to be less effective in cultured human cells than in purified systems. It is not known whether this is due to the uptake or metabolism of this flavonol. These differences notwithstanding, bioflavonoids clearly act as topoisomerase II poisons in cultured human cells.
Figure 11.
Bioflavonoids enhance DNA cleavage mediated by topoisomerase IIα and IIβ in cultured human CEM cells. The ICE bioassay was used to monitor levels of cleavage complexes in cells treated with selected bioflavonoids. DNA (3 μg) from cell cultures treated for 1 h in the absence of compound (none) or in the presence of 50 μM bioflavonoid or 50 μM etoposide was blotted onto a nitrocellulose membrane. Immunoblots were probed with a polyclonal antibody directed against either human topoisomerase IIα or IIβ, respectively. A representative immunoblot is shown. The bar graph shows quantified data for topoisomerase IIα (hTIIα; open bars) or IIβ (hTIIβ; closed bars). Levels of covalently bound topoisomerase II (expressed in ng) are based on standards of purified human type II topoisomerases. Error bars represent the standard deviation for three independent experiments.
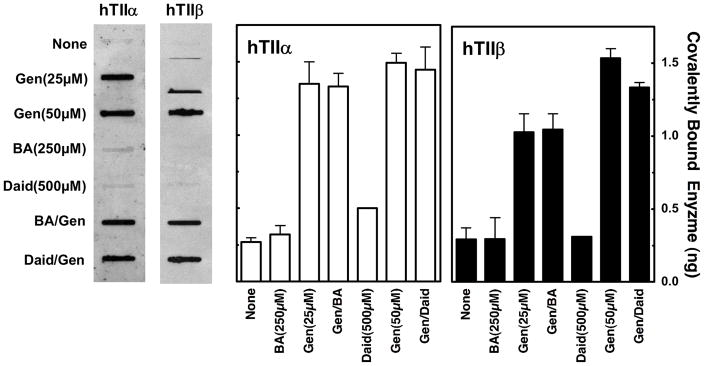
As described earlier, the 5-OH and 4′-OH moieties contribute to genistein binding and function. Neither daidzein nor biochanin A were able to compete effectively with genistein in DNA cleavage assays in vitro (see Figure 5). To confirm and extend these findings, competition studies were carried out in cultured CEM cells (Figure 12). As determined by levels of DNA cleavage complexes formed with either topoisomerase IIα and IIβ, daidzein and biochanin A displayed no ability to compete with genistein in human cells.
Figure 12.
Contribution of the 5-OH and 4′-OH moieties to the ability of genistein to enhance DNA cleavage mediated by human topoisomerase IIα or IIβ in cultured human CEM cells. The ICE bioassay was used to monitor the level of cleavage complexes in CEM cells treated with 25 or 50 μM genistein (Gen) in the absence or presence of 250 μM biochanin A (BA) or 500 μM daidzein (Daid), respectively. DNA (3 μg) from cells treated for 1 h were blotted onto a nitrocellulose membrane. Immunoblots were probed with a polyclonal antibody directed against either human topoisomerase IIα or IIβ, respectively. A representative immunoblot is shown. The bar graph shows quantified data for topoisomerase IIα (hTIIα; open bars) or IIβ (hTIIβ; closed bars). Levels of covalently bound topoisomerase II (expressed in ng) are based on standards of purified human type II topoisomerases. Error bars represent the standard deviation of three independent experiments.
Conclusions
Bioflavonoids are human dietary components that have been linked to the prevention of cancer in adults and the generation of specific types of leukemia in infants. While these compounds have a broad range of cellular activities, many of their genotoxic effects have been attributed to their actions as topoisomerase II poisons. The present study provides mechanistic details for the actions of flavones, flavonols, and isoflavones against human topoisomerase IIα and IIβ. It also supports roles for both topoisomerase II isoforms in mediating at least some of the cellular activities of bioflovanoids and sets the stage for future physiological studies.
Acknowledgments
We are grateful to Ryan Bender and Joseph Deweese for critical reading of the manuscript.
Footnotes
Products of DNA cleavage reactions shown in Figure 4 were analyzed on denaturing polyacrylamide gels. Therefore, both single- and double-stranded breaks were monitored by this assay. Etoposide generates high levels of single-stranded DNA breaks in addition to double-stranded breaks (90). As a result, the total level of cleavage products generated in the presence of etoposide is greater than that seen in reactions that contained genistein, despite the fact that both agents induce similar levels of double-stranded DNA breaks (see Figure 3).
This work was supported by National Institutes of Health research grant GM33944. OJB was a trainee under grant 5 T32 CA09582 from the National Institutes of Health and was supported in part by Ruth L. Kirschstein National Research Service Award Predoctoral Fellowship F31 GM78744 from the National Institutes of Health.
References
- 1.Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 2.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 3.Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Yao LH, Jiang YM, Shi J, Tomas-Barberan FA, Datta N, Singanusong R, Chen SS. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59:113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 5.Kanadaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 6.Siddiqui IA, Adhami VM, Saleem M, Mukhtar H. Beneficial effects of tea and its polyphenols against prostate cancer. Mol Nutr Food Res. 2006;50:130–143. doi: 10.1002/mnfr.200500113. [DOI] [PubMed] [Google Scholar]
- 7.Dragsted LO. Antioxidant actions of polyphenols in humans. Int J Vitam Nutr Res. 2003;73:112–119. doi: 10.1024/0300-9831.73.2.112. [DOI] [PubMed] [Google Scholar]
- 8.Sang S, Hou Z, Lambert JD, Yang CS. Redox properties of tea polyphenols and related biological activities. Antioxid Redox Signal. 2005;7:1704–1714. doi: 10.1089/ars.2005.7.1704. [DOI] [PubMed] [Google Scholar]
- 9.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 10.Lamartiniere CA. Protection against breast cancer with genistein: a component of soy. Am J Clin Nutr. 2000;71:1705S–1709S. doi: 10.1093/ajcn/71.6.1705S. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar FH, Adsule S, Padhye S, Kulkarni S, Li Y. The role of genistein and synthetic derivatives of isoflavone in cancer prevention and therapy. Mini Rev Med Chem. 2006;6:401–407. doi: 10.2174/138955706776361439. [DOI] [PubMed] [Google Scholar]
- 12.Usui T. Pharmaceutical prospects of phytoestrogens. Endocr J. 2006;53:7–20. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- 13.Ross JA, Potter JD, Robison LL. Infant leukemia, topoisomerase II inhibitors, and the MLL gene. J Natl Cancer Inst. 1994;86:1678–1680. doi: 10.1093/jnci/86.22.1678. [DOI] [PubMed] [Google Scholar]
- 14.Ross JA, Potter JD, Reaman GH, Pendergrass TW, Robison LL. Maternal exposure to potential inhibitors of DNA topoisomerase II and infant leukemia (United States): a report from the Children’s Cancer Group. Cancer Causes Control. 1996;7:581–590. doi: 10.1007/BF00051700. [DOI] [PubMed] [Google Scholar]
- 15.Ross JA. Maternal diet and infant leukemia: a role for DNA topoisomerase II inhibitors? Int J Cancer Suppl. 1998;11:26–28. [PubMed] [Google Scholar]
- 16.Strick R, Strissel PL, Borgers S, Smith SL, Rowley JD. Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc Natl Acad Sci U S A. 2000;97:4790–4795. doi: 10.1073/pnas.070061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spector LG, Xie Y, Robison LL, Heerema NA, Hilden JM, Lange B, Felix CA, Davies SM, Slavin J, Potter JD, Blair CK, Reaman GH, Ross JA. Maternal diet and infant leukemia: the DNA topoisomerase II inhibitor hypothesis: a report from the children’s oncology group. Cancer Epidemiol Biomarkers Prev. 2005;14:651–655. doi: 10.1158/1055-9965.EPI-04-0602. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama T, Ishida K, Nakagawa S, Ogaara H, Watanabe S, Itoh NM, Shibuya M, Fukami Y. Genistein, a Specific Inhibitor of Tyrosine-Specific Protein Kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 19.Hagiwara M, Inoue S, Tanaka T, Nunoki K, Ito M, Hidaka H. Differential effects of flavonoids as inhibitors of tyrosine protein kinases and serine/threonine protein kinases. Biochem Pharmacol. 1988;37:2987–2992. doi: 10.1016/0006-2952(88)90286-9. [DOI] [PubMed] [Google Scholar]
- 20.Geahlen RL, Koonchanok NM, McLaughlin JL, Pratt DE. Inhibition of protein-tyrosine kinase activity by flavanoids and related compounds. J Nat Prod. 1989;52:982–986. doi: 10.1021/np50065a011. [DOI] [PubMed] [Google Scholar]
- 21.Cushman M, Nagarathnam D, Burg DL, Geahlen RL. Synthesis and protein-tyrosine kinase inhibitory activities of flavonoid analogues. J Med Chem. 1991;34:798–806. doi: 10.1021/jm00106a047. [DOI] [PubMed] [Google Scholar]
- 22.Yang EB, Guo YJ, Zhang K, Chen YZ, Mack P. Inhibition of epidermal growth factor receptor tyrosine kinase by chalcone derivatives. Biochim Biophys Acta. 2001;1550:144–152. doi: 10.1016/s0167-4838(01)00276-x. [DOI] [PubMed] [Google Scholar]
- 23.Hollosy F, Keri G. Plant-derived protein tyrosine kinase inhibitors as anticancer agents. Curr Med Chem Anticancer Agents. 2004;4:173–197. doi: 10.2174/1568011043482124. [DOI] [PubMed] [Google Scholar]
- 24.Baker ME. Evolution of regulation of steroid-mediated intercellular communication in vertebrates: insights from flavonoids, signals that mediate plant-rhizobia symbiosis. J Steroid Biochem Mol Biol. 1992;41:301–308. doi: 10.1016/0960-0760(92)90355-m. [DOI] [PubMed] [Google Scholar]
- 25.Beck V, Rohr U, Jungbauer A. Phytoestrogens derived from red clover: an alternative to estrogen replacement therapy? J Steroid Biochem Mol Biol. 2005;94:499–518. doi: 10.1016/j.jsbmb.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 26.Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alpha- or Beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood) 2005;230:558–568. doi: 10.1177/153537020523000807. [DOI] [PubMed] [Google Scholar]
- 27.Oh SM, Kim YP, Chung KH. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch Pharm Res. 2006;29:354–362. doi: 10.1007/BF02968584. [DOI] [PubMed] [Google Scholar]
- 28.Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 29.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Fresco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26:747–766. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 31.Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Le Pecq JB, Larsen AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- 32.Austin CA, Patel S, Ono K, Nakane H, Fisher LM. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem J. 1992;282:883–889. doi: 10.1042/bj2820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markovits J, Junqua S, Goldwasser F, Venuat AM, Luccioni C, Beaumatin J, Saucier JM, Bernheim A, Jacquemin-Sablon A. Genistein resistance in human leukaemic CCRF-CEM cells: selection of a diploid cell line with reduced DNA topoisomerase II beta isoform. Biochemical Pharmacology. 1995;50:177–186. doi: 10.1016/0006-2952(95)00131-i. [DOI] [PubMed] [Google Scholar]
- 34.Wang JC. DNA Topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 35.Austin CA, Marsh KL. Eukaryotic DNA topoisomerase IIbeta. BioEssays. 1998;20:215–226. doi: 10.1002/(SICI)1521-1878(199803)20:3<215::AID-BIES5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 36.Nitiss JL. Investigating the biological functions of DNA topoisomerases in eukaryotic cells. Biochim Biophys Acta. 1998;1400:63–81. doi: 10.1016/s0167-4781(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 37.Fortune JM, Osheroff N. Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice. Prog Nucleic Acid Res Mol Biol. 2000;64:221–253. doi: 10.1016/s0079-6603(00)64006-0. [DOI] [PubMed] [Google Scholar]
- 38.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 39.Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 40.Velez-Cruz R, Osheroff N. DNA Topoisomerases: Type II. In: Lennarz W, Lane MD, editors. Encyclopedia of Molecular Biology. Elsevier Science; San Diego: 2004. pp. 806–811. [Google Scholar]
- 41.Drake FH, Zimmerman JP, McCabe FL, Bartus HF, Per SR, Sullivan DM, Ross WE, Mattern MR, Johnson RK, Crooke ST, Mirabelli CK. Purification of topoisomerase II from amsacrine-resistant P388 leukemia cells. Evidence for two forms of the enzyme. J Biol Chem. 1987;262:16739–16747. [PubMed] [Google Scholar]
- 42.Drake FH, Hofmann GA, Bartus HF, Mattern MR, Crooke ST, Mirabelli CK. Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry. 1989;28:8154–8160. doi: 10.1021/bi00446a029. [DOI] [PubMed] [Google Scholar]
- 43.Heck MM, Earnshaw WC. Topoisomerase II: A specific marker for cell proliferation. J Cell Biol. 1986;103:2569–2581. doi: 10.1083/jcb.103.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsiang YH, Wu HY, Liu LF. Proliferation-dependent regulation of DNA topoisomerase II in cultured human cells. Cancer Res. 1988;48:3230–3235. [PubMed] [Google Scholar]
- 45.Woessner RD, Mattern MR, Mirabelli CK, Johnson RK, Drake FH. Proliferation- and cell cycle-dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 1991;2:209–214. [PubMed] [Google Scholar]
- 46.Bauman ME, Holden JA, Brown KA, Harker WG, Perkins SL. Differential immunohistochemical staining for DNA topoisomerase II alpha and beta in human tissues and for DNA topoisomerase II beta in non-Hodgkin’s lymphomas. Mod Pathol. 1997;10:168–175. [PubMed] [Google Scholar]
- 47.Grue P, Grasser A, Sehested M, Jensen PB, Uhse A, Straub T, Ness W, Boege F. Essential mitotic functions of DNA topoisomerase IIα are not adopted by topoisomerase IIβ in human H69 cells. J Biol Chem. 1998;273:33660–33666. doi: 10.1074/jbc.273.50.33660. [DOI] [PubMed] [Google Scholar]
- 48.Isaacs RJ, Davies SL, Sandri MI, Redwood C, Wells NJ, Hickson ID. Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta. 1998;1400:121–137. doi: 10.1016/s0167-4781(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 49.Chen M, Beck WT. DNA topoisomerase II expression, stability, and phosphorylation in two VM-26-resistant human leukemic CEM sublines. Oncol Res. 1995;7:103–111. [PubMed] [Google Scholar]
- 50.Dereuddre S, Delaporte C, Jacquemin-Sablon A. Role of topoisomerase II beta in the resistance of 9-OH-ellipticine-resistant Chinese hamster fibroblasts to topoisomerase II inhibitors. Cancer Res. 1997;57:4301–4308. [PubMed] [Google Scholar]
- 51.Yang X, Li W, Prescott ED, Burden SJ, Wang JC. DNA topoisomerase IIbeta and neural development. Science. 2000;287:131–134. doi: 10.1126/science.287.5450.131. [DOI] [PubMed] [Google Scholar]
- 52.Berger JM, Gamblin SJ, Harrison SC, Wang JC. Structure and mechanism of DNA topoisomerase II. Nature. 1996;379:225–232. doi: 10.1038/379225a0. [DOI] [PubMed] [Google Scholar]
- 53.Wang JC. Moving one DNA double helix through another by a type II DNA topoisomerase: the story of a simple molecular machine. Quart Rev Biophys. 1998;31:107–144. doi: 10.1017/s0033583598003424. [DOI] [PubMed] [Google Scholar]
- 54.Sander M, Hsieh T. Double strand DNA cleavage by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983;258:8421–8428. [PubMed] [Google Scholar]
- 55.Liu LF. DNA topoisomerases--enzymes that catalyse the breaking and rejoining of DNA. CRC Crit Rev Biochem. 1983;15:1–24. doi: 10.3109/10409238309102799. [DOI] [PubMed] [Google Scholar]
- 56.Zechiedrich EL, Christiansen K, Andersen AH, Westergaard O, Osheroff N. Double-stranded DNA cleavage/religation reaction of eukaryotic topoisomerase II: evidence for a nicked DNA intermediate. Biochemistry. 1989;28:6229–6236. doi: 10.1021/bi00441a014. [DOI] [PubMed] [Google Scholar]
- 57.Baguley BC, Ferguson LR. Mutagenic properties of topoisomerase-targeted drugs. Biochim Biophys Acta. 1998;1400:213–222. doi: 10.1016/s0167-4781(98)00137-7. [DOI] [PubMed] [Google Scholar]
- 58.Felix CA. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim Biophys Acta. 1998;1400:233–255. doi: 10.1016/s0167-4781(98)00139-0. [DOI] [PubMed] [Google Scholar]
- 59.Kaufmann SH. Cell death induced by topoisomerase-targeted drugs: more questions than answers. Biochim Biophys Acta. 1998;1400:195–211. doi: 10.1016/s0167-4781(98)00136-5. [DOI] [PubMed] [Google Scholar]
- 60.Kaufmann SH, Gore SD, Miller CB, Jones RJ, Zwelling LA, Schneider E, Burke PJ, Karp JE. Topoisomerase II and the response to antileukemic therapy. Leukemia Lymph. 1998;29:217–237. doi: 10.3109/10428199809068560. [DOI] [PubMed] [Google Scholar]
- 61.Rowley JD. Seminars from the University of Minnesota. Chromosome translocations: dangerous liaisons. J Lab Clin Med. 1998;132:244–250. doi: 10.1016/s0022-2143(98)90036-1. [DOI] [PubMed] [Google Scholar]
- 62.Sordet O, Khan QA, Kohn KW, Pommier Y. Apoptosis induced by topoisomerase inhibitors. Curr Med Chem Anticancer Agents. 2003;3:271–290. doi: 10.2174/1568011033482378. [DOI] [PubMed] [Google Scholar]
- 63.Wilstermann AM, Osheroff N. Stabilization of eukaryotic topoisomerase II-DNA cleavage complexes. Curr Top Med Chem. 2003;3:321–338. doi: 10.2174/1568026033452519. [DOI] [PubMed] [Google Scholar]
- 64.Li TK, Liu LF. Tumor cell death induced by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol. 2001;41:53–77. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- 65.Walker JV, Nitiss JL. DNA topoisomerase II as a target for cancer chemotherapy. Cancer Invest. 2002;20:570–589. doi: 10.1081/cnv-120002156. [DOI] [PubMed] [Google Scholar]
- 66.Baldwin EL, Osheroff N. Etoposide, topoisomerase II and cancer. Curr Med Chem Anticancer Agents. 2005;5:363–372. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- 67.Hande KR. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer. 1998;34:1514–1521. doi: 10.1016/s0959-8049(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 68.Pui CH, Relling MV. Topoisomerase II inhibitor-related acute myeloid leukaemia. Br J Haematol. 2000;109:13–23. doi: 10.1046/j.1365-2141.2000.01843.x. [DOI] [PubMed] [Google Scholar]
- 69.Felix CA. Leukemias related to treatment with DNA topoisomerase II inhibitors. Med Pediatr Oncol. 2001;36:525–535. doi: 10.1002/mpo.1125. [DOI] [PubMed] [Google Scholar]
- 70.Leone G, Voso MT, Sica S, Morosetti R, Pagano L. Therapy related leukemias: susceptibility, prevention and treatment. Leuk Lymphoma. 2001;41:255–276. doi: 10.3109/10428190109057981. [DOI] [PubMed] [Google Scholar]
- 71.Felix CA, Kolaris CP, Osheroff N. Topoisomerase II and the etiology of chromosomal translocations. DNA Repair (Amst) 2006;5:1093–1108. doi: 10.1016/j.dnarep.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 72.Cornarotti M, Tinelli S, Willmore E, Zunio F, Fisher LM, Austin CA, Capranico G. Drug sensitivity and sequence specificity of human recombinant DNA topoisomerase IIalpha and IIbeta. Mol Pharm. 1996;50:1463–1471. [PubMed] [Google Scholar]
- 73.Willmore E, Frank AJ, Padget K, Tilby MJ, Austin CA. Etoposide targets topoisomerase IIalpha and IIbeta in leukemic cells: Isoform-specific cleavable comlexes visualized and quantified in situ by a novel immunofluorescence technique. Mol Pharmacol. 1998;53:78–85. doi: 10.1124/mol.54.1.78. [DOI] [PubMed] [Google Scholar]
- 74.Gatto B, Leo E. Drugs acting on the beta isoform of human topoisomerase II (p180) Curr Med Chem Anticancer Agents. 2003;3:173–185. doi: 10.2174/1568011033482486. [DOI] [PubMed] [Google Scholar]
- 75.Worland ST, Wang JC. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J Biol Chem. 1989;264:4412–4416. [PubMed] [Google Scholar]
- 76.Elsea SH, Hsiung Y, Nitiss JL, Osheroff N. A yeast type II topoisomerase selected for resistance to quinolones. Mutation of histidine 1012 to tyrosine confers resistance to nonintercalative drugs but hypersensitivity to ellipticine. J Biol Chem. 1995;270:1913–1920. doi: 10.1074/jbc.270.4.1913. [DOI] [PubMed] [Google Scholar]
- 77.Kingma PS, Greider CA, Osheroff N. Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry. 1997;36:5934–5939. doi: 10.1021/bi970507v. [DOI] [PubMed] [Google Scholar]
- 78.Wasserman RA, Austin CA, Fisher LM, Wang JC. Use of yeast in the study of anticancer drugs targeting DNA topoisomerases: expression of a functional recombinant human DNA topoisomerase II alpha in yeast. Cancer Res. 1993;53:3591–3596. [PubMed] [Google Scholar]
- 79.Fortune JM, Osheroff N. Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J Biol Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- 80.O’Reilly EK, Kreuzer KN. A unique type II topoisomerase mutant that is hypersensitive to a broad range of cleavage-inducing antitumor agents. Biochemistry. 2002;41:7989–7997. doi: 10.1021/bi025897m. [DOI] [PubMed] [Google Scholar]
- 81.Byl JA, Fortune JM, Burden DA, Nitiss JL, Utsugi T, Yamada Y, Osheroff N. DNA topoisomerases as targets for the anticancer drug TAS-103: primary cellular target and DNA cleavage enhancement. Biochemistry. 1999;38:15573–15579. doi: 10.1021/bi991791o. [DOI] [PubMed] [Google Scholar]
- 82.Kingma PS, Burden DA, Osheroff N. Binding of etoposide to topoisomerase II in the absence of DNA: decreased affinity as a mechanism of drug resistance. Biochemistry. 1999;38:3457–3461. doi: 10.1021/bi982855i. [DOI] [PubMed] [Google Scholar]
- 83.Shaw JL, Blanco J, Mueller GC. Simple procedure for isolation of DNA, RNA and protein fractions from cultured animal cells. Anal Biochem. 1975;65:125–131. doi: 10.1016/0003-2697(75)90498-4. [DOI] [PubMed] [Google Scholar]
- 84.Subramanian D, Kraut E, Staubus A, Young DC, Muller MT. Analysis of topoisomerase I/DNA complexes in patients administered topotecan. Cancer Res. 1995;55:2097–2103. [PubMed] [Google Scholar]
- 85.Robinson MJ, Corbett AH, Osheroff N. Effects of topoisomerase II-targeted drugs on enzyme-mediated DNA cleavage and ATP hydrolysis: evidence for distinct drug interaction domains on topoisomerase II. Biochemistry. 1993;32:3638–3643. doi: 10.1021/bi00065a016. [DOI] [PubMed] [Google Scholar]
- 86.Elsea SH, Westergaard M, Burden DA, Lommenick JP, Osheroff N. Quinolones share a common interaction domain on topoisomerase II with other DNA cleavage-enhancing antineoplastic drugs. Biochemistry. 1997;36:2919–2924. doi: 10.1021/bi962488f. [DOI] [PubMed] [Google Scholar]
- 87.Constantinou A, Mehta R, Runyan C, Rao K, Vaughan A, Moon R. Flavonoids as DNA topoisomerase antagonists and poisons: structure-activity relationships. J Nat Prod. 1995;58:217–225. doi: 10.1021/np50116a009. [DOI] [PubMed] [Google Scholar]
- 88.Salti GI, Grewal S, Mehta RR, Das Gupta TK, Boddie AW, Jr, Constantinou AI. Genistein induces apoptosis and topoisomerase II-mediated DNA breakage in colon cancer cells. Eur J Cancer. 2000;36:796–802. doi: 10.1016/s0959-8049(00)00017-4. [DOI] [PubMed] [Google Scholar]
- 89.Lynch A, Harvey J, Aylott M, Nicholas E, Burman M, Siddiqui A, Walker S, Rees R. Investigations into the concept of a threshold for topoisomerase inhibitor-induced clastogenicity. Mutagenesis. 2003;18:345–353. doi: 10.1093/mutage/geg003. [DOI] [PubMed] [Google Scholar]
- 90.Bromberg KD, Burgin AB, Osheroff N. A two drug model for etoposide action against human topoisomerase IIα. J Biol Chem. 2003;278:7406–7412. doi: 10.1074/jbc.M212056200. [DOI] [PubMed] [Google Scholar]
- 91.Long BH, Musial ST, Brattain MG. Comparison of cytotoxicity and DNA breakage activity of congeners of podophyllotoxin including VP16–213 and VM26: a quantitative structure-activity relationship. Biochemistry. 1984;23:1183–1188. doi: 10.1021/bi00301a024. [DOI] [PubMed] [Google Scholar]
- 92.Elsea SH, McGuirk PR, Gootz TD, Moynihan M, Osheroff N. Drug features that contribute to the activity of quinolones against mammalian topoisomerase II and cultured cells: correlation between enhancement of enzyme-mediated DNA cleavage in vitro and cytotoxic potential. Antimicrob Agents Chemother. 1993;37:2179–2186. doi: 10.1128/aac.37.10.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Osheroff N. Effect of antineoplastic agents on the DNA cleavage/religation reaction of eukaryotic topoisomerase II: inhibition of DNA religation by etoposide. Biochemistry. 1989;28:6157–6160. doi: 10.1021/bi00441a005. [DOI] [PubMed] [Google Scholar]
- 94.Robinson MJ, Martin BA, Gootz TD, McGuirk PR, Moynihan M, Sutcliffe JA, Osheroff N. Effects of quinolone derivatives on eukaryotic topoisomerase II. A novel mechanism for enhancement of enzyme-mediated DNA cleavage. J Biol Chem. 1991;266:14585–14592. [PubMed] [Google Scholar]
- 95.Kingma PS, Corbett AH, Burcham PC, Marnett LJ, Osheroff N. Abasic sites stimulate double-stranded DNA cleavage mediated by topoisomerase II. DNA lesions as endogenous topoisomerase II poisons. J Biol Chem. 1995;270:21441–21444. doi: 10.1074/jbc.270.37.21441. [DOI] [PubMed] [Google Scholar]
- 96.Kingma PS, Osheroff N. Spontaneous DNA damage stimulates topoisomerase II-mediated DNA cleavage. J Biol Chem. 1997;272:7488–7493. doi: 10.1074/jbc.272.11.7488. [DOI] [PubMed] [Google Scholar]
- 97.Cline SD, Jones WR, Stone MP, Osheroff N. DNA abasic lesions in a different light: solution structure of an endogenous topoisomerase II poison. Biochemistry. 1999;38:15500–15507. doi: 10.1021/bi991750s. [DOI] [PubMed] [Google Scholar]
- 98.Sabourin M, Osheroff N. Sensitivity of human type II topoisomerases to DNA damage: stimulation of enzyme-mediated DNA cleavage by abasic, oxidized and alkylated lesions. Nucleic Acids Res. 2000;28:1947–1954. doi: 10.1093/nar/28.9.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corbett AH, Osheroff N. When good enzymes go bad: conversion of topoisomerase II to a cellular toxin by antineoplastic drugs. Chemical Research in Toxicology. 1993;6:585–597. doi: 10.1021/tx00035a001. [DOI] [PubMed] [Google Scholar]
- 100.Anderson VE, Zaniewski RP, Kaczmarek FS, Gootz TD, Osheroff N. Quinolones inhibit DNA religation mediated by Staphylococcus aureus topoisomerase IV. Changes in drug mechanism across evolutionary boundaries. J Biol Chem. 1999;274:35927–35932. doi: 10.1074/jbc.274.50.35927. [DOI] [PubMed] [Google Scholar]
- 101.Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry. 2001;40:3316–3323. doi: 10.1021/bi002786j. [DOI] [PubMed] [Google Scholar]
- 102.Bender RP, Lindsey RH, Jr, Burden DA, Osheroff N. N-acetyl-p-benzoquinone imine, the toxic metabolite of acetaminophen, is a topoisomerase II poison. Biochemistry. 2004;43:3731–3739. doi: 10.1021/bi036107r. [DOI] [PubMed] [Google Scholar]
- 103.Lindsey RH, Jr, Bromberg KD, Felix CA, Osheroff N. 1,4-Benzoquinone is a topoisomerase II poison. Biochemistry. 2004;43:7563–7574. doi: 10.1021/bi049756r. [DOI] [PubMed] [Google Scholar]
- 104.Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Polychlorinated Biphenyl Quinone Metabolites Poison Human Topoisomerase IIalpha: Altering Enzyme Function by Blocking the N-Terminal Protein Gate. Biochemistry. 2006;45:10140–10152. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- 105.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. Embo J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bugg BY, Danks MK, Beck WT, Suttle DP. Expression of a mutant DNA topoisomerase II in CCRF-CEM human leukemic cells selected for resistance to teniposide. Proc Natl Acad Sci U S A. 1991;88:7654–7658. doi: 10.1073/pnas.88.17.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wessel I, Jensen LH, Jensen PB, Falck J, Rose A, Roerth M, Nitiss JL, Sehested M. Human small cell lung cancer NYH cells selected for resistance to the bisdioxopiperazine topoisomerase II catalytic inhibitor ICRF-187 demonstrate a functional R162Q mutation in the Walker A consensus ATP binding domain of the alpha isoform. Cancer Res. 1999;59:3442–3450. [PubMed] [Google Scholar]
- 108.Wessel I, Jensen LH, Renodon-Corniere A, Sorensen TK, Nitiss JL, Jensen PB, Sehested M. Human small cell lung cancer NYH cells resistant to the bisdioxopiperazine ICRF-187 exhibit a functional dominant Tyr165Ser mutation in the Walker A ATP binding site of topoisomerase II alpha. FEBS Lett. 2002;520:161–166. doi: 10.1016/s0014-5793(02)02805-3. [DOI] [PubMed] [Google Scholar]
- 109.Tsai-Pflugfelder M, Liu LF, Liu AA, Tewey KM, Whang-Peng J, Knutsen T, Huebner K, Croce CM, Wang JC. Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21–22. Proc Natl Acad Sci U S A. 1988;85:7177–7181. doi: 10.1073/pnas.85.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wigley DB, Davies GJ, Dodson EJ, Maxwell A, Dodson G. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature. 1991;351:624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- 111.Denis L, Morton MS, Griffiths K. Diet and its preventive role in prostatic disease. Eur Urol. 1999;35:377–387. doi: 10.1159/000019912. [DOI] [PubMed] [Google Scholar]
- 112.Zini N, Santi S, Ognibene A, Bavelloni A, Neri LM, Valmori A, Mariani E, Negri C, Astaldi-Ricotti GC, Maraldi NM. Discrete localization of different DNA topoisomerases in HeLa and K562 cell nuclei and subnuclear fractions. Experimental Cell Research. 1994;210:336–348. doi: 10.1006/excr.1994.1046. [DOI] [PubMed] [Google Scholar]
- 113.Cowell IG, Willmore E, Chalton D, Marsh KL, Jazrawi E, Fisher LM, Austin CA. Nuclear distribution of human DNA topoisomerase IIbeta: a nuclear targeting signal resides in the 116-residue C-terminal tail. Exp Cell Res. 1998;243:232–240. doi: 10.1006/excr.1998.4150. [DOI] [PubMed] [Google Scholar]