Abstract
Introduction
Adipose tissue-derived stem cells (ADSC) could potentially restore the endothelial function in vasculogenic erectile dysfunction (ED). The mechanism for ADSC endothelial differentiation remained unidentified.
Aim
To test whether ADSC could differentiate into endothelial cells in the penis and to identify the underlying mechanism of ADSC endothelial differentiation.
Methods
For in vivo endothelial differentiation, ADSC were labeled with bromodeoxyuridine (BrdU), injected into rat corpus cavernosa, and localized by Immunofluorescence and phase-contrast microscopy. For in vitro endothelial differentiation, ADSC were grown in endothelial growth medium EGM2, stained for endothelial markers CD31, vWF, and eNOS, and assessed for ability to form tube-like structures in Matrigel and to endocytose acetylated low-density lipoprotein (Ac-LDL). To identify factors that promote ADSC endothelial differentiation, ADSC were grown in various media, each of which contained a specific combination of EGM2 supplemental factors, and assessed for LDL-uptake. PD173074, a selective inhibitor of FGF2 receptor, was used to confirm the importance of FGF2 signaling for ADSC endothelial differentiation.
Main Outcome Measures
In vivo endothelial differentiation was assessed by immunofluorescence microscopy. In vitro endothelial differentiation was assessed by immunofluorescence, Matrigel tube formation, and Ac-LDL uptake.
Results
Injected ADSC were localized to the sinusoid endothelium, some of which stained positive for both BrdU and endothelial antigen RECA. ADSC proliferated at a faster rate in EGM2 than in standard DMEM, expressed endothelial markers CD31, vWF, and eNOS, formed tube-like structures in Matrigel, and endocytosed Ac-LDL. These properties were greatly diminished when ADSC were grown in the absence of FGF2 but were unaffected when grown in the absence of VEGF, IGF, or EGF. Furthermore, ADSC grown FGF2-supplemented basic medium displayed similar endothelial properties as in EGM2. Finally, blockade of FGF2 signaling with PD173074 abrogated ADSC endothelial differentiation.
Conclusions
ADSC could differentiate into endothelial cells in the penis. FGF2 signaling mediates ADSC endothelial differentiation.
Keywords: adopose tissue-derived stem cells, penis, endothelial differentiation, FGF2
INTRODUCTION
Endothelial dysfunction/injury is a prominent feature of a wide variety of diseases, including coronary artery disease [1], diabetes mellitus [2], stroke [3], and erectile dysfunction (ED) [4]. One of the strategies to restore endothelial function is “therapeutic angiogenesis”, in which proangiogenic agents are employed to promote revascularization of ischemic tissues [5]. Initial efforts of this therapeutic strategy largely focused on single agent therapy by direct or indirect delivery (via viral or plasmid vector) of a proangiogenic protein such as VEGF or FGF. These efforts were fruitful in preclinical studies but encountered obstacles in clinical trials [6,7]. Growth factor therapy for ED has also been successfully demonstrated in animal models but has not reached clinical trials [8].
Recent discovery that bone marrow-derived circulating endothelial progenitor cells (EPC) are involved in postnatal neovascularization raised hope that a cell-based alternative for therapeutic angiogenesis might be feasible [9]. However, clinical application of EPC is difficult to implement due to their scarcity, particularly in patients who could benefit most from therapeutic angiogenesis [10]. As an alternative, stem cells, either embryonic or adult, have been shown to possess endothelial properties. For example, bone marrow stem cells (BMSC) have been shown to express a wide spectrum of angiogenic growth factors and may stimulate collateral vessel formation by paracrine mechanisms [11]. Transplanted BMSC have also been shown to incorporate into new blood vessels [12]. In addition, when cultured in the presence of endothelial growth factors, BMSC showed a strong increase of expression of endothelial-specific markers such as VEGF receptors (VEGFR-1 and VEGFR-2) and von Willebrand factor (vWF) [13].
Adipose tissue-derived stem cells (ADSC) isolated from the stromal vascular fraction (SVF) of adipose tissue are an attractive alternative stem cell source to BMSC. Several head-on comparison studies have shown that ADSC and BMSC are similar in cell-surface expression profile, transcriptome, differentiation potential, and therapeutic efficacy [14-18]. In murine models of hindlimb ischemia, transplanted ADSC and BMSC both differentiated into endothelial cells, incorporated into blood vessels, and promoted neovascularization [19-23]. Thus, it appears that both ADSC and BMSC are ideal cell source for therapeutic angiogenesis. However, because adipose tissue is much more abundant and expendable than bone marrow, it can be reasonably expected that ADSC will be a better choice than BMSC for clinical application.
We have previously shown that ADSC were multipotent and were localized within the vasculature of adipose tissue [24,25]. Studies by others have also shown that ADSC could differentiate into endothelial cells [19-21,23]. However, in spite of the in vivo and in vitro evidence for ADSC endothelial differentiation, little is known about its underlying mechanism. Published experimental procedures for ADSC endothelial differentiation generally employed culture media that contained VEGF as the principal inducing factor or in combination with IGF or FGF2 [19-21,23]. Indeed it has been assumed that VEGF was the responsible inducing factor for ADSC endothelial differentiation [26]. To identify the responsible factors, we first demonstrated that ADSC could indeed differentiate into endothelial cells when injected into rat penis. We then grew ADSC in a widely used endothelial growth medium EGM2 to see if ADSC could differentiate into endothelial cells in culture. As this was confirmed, we used a “subtraction-addition” strategy to screen the supplemental factors of EGMS. This resulted in the identification of FGF2 as the critical inducing factor and the dismissal of VEGF, IGF, or EGF as unnecessary. Surprisingly, vitamin C was also found to be essential for ADSC endothelial differentiation. Its role is most likely the maintenance of a healthy growth environment for endothelial cells by protecting them from oxidative stress.
MATERIALS AND METHODS
ADSC isolation
Rat adipose tissue was excised from the inguinal region of male Sprague-Dawley rats, as approved by the Institutional Animal Care and Use Committee. These tissue samples were processed for ADSC isolation as described previously [25]. Briefly, the tissue was rinsed with phosphate-buffered saline (PBS) containing 1% penicillin and streptomycin, minced into small pieces, and then incubated in a solution containing 0.075% collagenase type IA (Sigma-Aldrich, St. Louis, MO) for 1 h at 37 °C with vigorous shake. The top lipid layer was removed and the remaining liquid portion was centrifuged at 220 g for 10 minutes at room temperature. The pellet was treated with 160 mM NH4Cl for 10 min to lyse red blood cells. The remaining cells were suspended in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), filtered through a 40-mm cell strainer (BD Biosciences, Bedford, MA), and plated at a density of 1 × 106 cells in a 10-cm dish. After reaching 80% confluence, the cells were harvested and stored in liquid nitrogen at a density of 5 × 105 cells per ml of freezing media (DMEM, 20% FBS, and 10% DMSO). Cells were thawed and re-cultured as needed.
Cell culture
Human umbilical vein endothelial cell strain, HUVEC, was purchased from Lonza Biologics Inc. (Portsmouth, NH) and cultured in EGM2 medium supplemented with growth factors and cytokines (Lonza Biologics Inc.). Unless otherwise indicated (see below and Results), all human and rat ADSC cell lines were cultured in DMEM supplemented with 10% FBS, 1% non-essential amino acid, 1% PSF (10,000 units/ml penicillin, 10,000 mcg/ml streptomycin SO4, and 0.025 mg/ml fungizone) and 110 μg/ml sodium pyruvate. Culture incubator was set at 37 °C with 5% CO2.
In vivo endothelial differentiation
For the purpose of cell tracking, ADSC were labeled with 10 μM BrdU for 12 hr. A total 1×106 BrdU-labeled ADSC in 200 ml PBS were injected into each of the corpus cavernosa of Sprague-Dawley rats. Control animals were similarly injected, but without cells. Four weeks later, cavernous tissue samples were fixed in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1 M phosphate buffer, pH 8.0, for 4 h followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (Sakura Finetic USA, Torrance, CA) and stored at −70 °C until use. Fixed frozen tissue specimens were cut at 5 microns, mounted onto SuperFrost-Plus charged slides (Fisher Scientific, Pittsburgh, PA) and air dried for 5 min. The slides were then placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining this solution from the tissue section, the slides were pre-treated with 2N HCI for 10 min, followed by incubation overnight at 4°C with rabbit anti-BrdU antibodies and mouse anti-rat endothelial cell antigen (RECA-1)( Santa Cruz Biotechnlogy, Santa Cruz, CA). Control tissue sections were similarly prepared except no primary antibody was added. The tissue was then incubated with secondary antibody conjugated with FITC or Texas Red (Vector Labs, Burlingame, CA). Stained tissues were examined by fluorescence microscopy. All animal experiments complied with National Institute of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
In vitro endothelial differentiation
ADSC were seeded into 100-mm dishes or 6-well plate at 40-60% confluence in DMEM. The next day, the culture media were discarded by aspiration and the cells washed with PBS, followed with the addition of fully or partially supplemented EGM2. Fully supplemented EGM2 contained 0.02 ml/ml FBS, 1 μg/ml vitamin C, 22.5 μg/ml heparin, 0.2 μg/ml hydrocortisone, 5 ng/ml EGF, 10 ng/ml FGF2, 20 ng/ml IGF-I, and 0.5 ng/ml VEGF. Partially supplemented EGM2 contained factors as indicated in the relevant figures in the Results section. Induction proceeded for 6-10 days, with the medium replenished every three days.
Reversibility test
To test whether ADSC endothelial induction is reversible, cells were first induced in fully supplemented EGM2 as above. The cells were then detached from the dish by trypsinization. Half of the detached cells were subjected to tests for endothelial markers (immunocytochemistry, capillary tube formation and LDL uptake, see below). The other half of the detached cells were seeded into 100-mm dishes or 6-well plates in regular DMEM medium and cultured for another 6 days, with one medium change on the third day. After that, the cells were split again and half of the cells were used to do immunostaining, capillary formation assay or LDL uptake assay and half of the cells were cultured in EGM2 medium again for 6 days. The medium was changed every three days. After induction, the cells were projected to immunostaining, capillary formation or LDL-uptake assay.
Inhibition of FGF2 signaling
ADSC were seeded in 6-well plate at 40–60% confluence in DMEM. The next day, cells were washed with PBS and then incubated in EBM2, in the presence or absence of 100 nM of PD173074 (#341607, Calbiochem, San Diego, CA) for 3 h. The medium was then changed to EGM2 or FGF2/vitamin C-supplemented EBM2, with or without 100 nM of PD173074. The cells were incubated in these media for 6-10 days, with fresh medium replenishment once every three days. In each replenishment, the cells were first incubated in EBM2 with or without 100 nM of PD173074 for 3 h and then maintained in EGM2 or FGF2/vitamin C-supplemented EBM2, with or without 100 nM of PD173074, for 3 days. At the conclusion of the treatment period, cells were harvested and re-seeded into 6-well plates for LDL-uptake assay. Note that because PD173074 was dissolved in DMSO, the above-mentioned terms “absence” and “without” in effect refer to media that were added with this solvent.
Immunocytochemistry and fluorescence microscopy
Cells were seeded onto a coverslip inside each well of a 6-well plate at 40–60% confluence in DMEM. The next day, the cells were rinsed with PBS and fixed with ice-cold methanol for 5 min. The cells were rinsed with PBS again and permeabilized with 0.05% triton X-100 for 8 min. After another PBS rinse, the cells were incubated with 5% horse serum for 1 h and then with anti-CD31 antibody CD31 (sc-1506, Santa Cruz Biotechnology, Santa Cruz, CA), anti-vWF antibody (ab6494-100, Abcam Inc., Cambridge, MA), or anti-eNOS antibody (#610296, BD Biosciences, San Diego, CA) for 1 h. After 3 rinses with PBS, the cells were incubated with FITC- or Texas red-conjugated goat anti-rabbit IgG or rabbit anti-goat IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h. After 3 rinses with PBS, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 mg/ml, Sigma-Aldrich, St. Louis, MO) for 5 min. The stained cells were examined with Nikon Eclipse E600 fluorescence microscope and the images recorded with Retiga 1300 Q-imaging camera.
Low-density lipoprotein (LDL) uptake
Cells were seeded into 6-well plates at of 5×104 cells per well in DMEM or EGM2 medium and incubated at 37 °C. The next day, 10 mg/ml of acetylated low-density lipoprotein DiI complex (DiI AcLDL, Invitrogen corporation, Carlsbad, CA) was added to the culture medium. The next day, after the medium was removed, the cells were washed 3 times, examined by phase-contrast and fluorescence microscopy, and photographed.
Matrigel-based capillary-like tube formation assay
Cells were cultured in EGM2 for 6 days prior to the assay. HUVEC was always maintained in EGM2. The assay was initiated by coating a 4-well CultureSlide (BD Biosciences, San Jose, CA) with 150 μl of growth factor-reduced Matrigel (BD Biosciences) per well. Approximately 5 × 104 cells of in 500 μl of EGM2 were then seeded into each well and incubated at 37 °C. Sixteen hours later, development of capillary-like networks was examined by phase-contrast microscopy and photographed.
RESULTS
In vivo endothelial differentiation
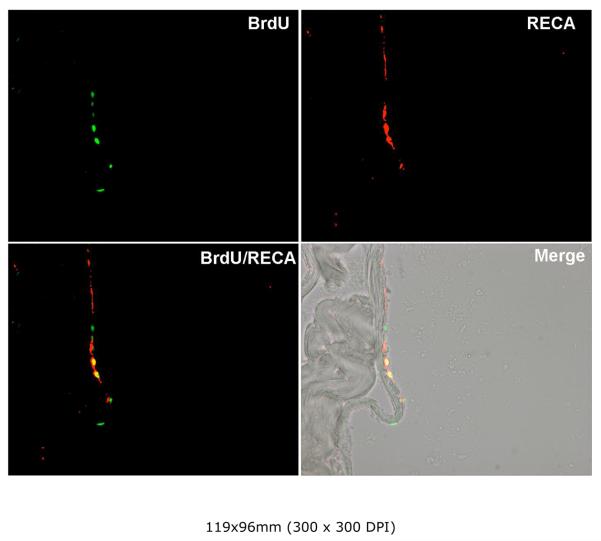
To test whether ADSC could differentiate into endothelial cells, we injected ADSC into the penis of rats and examined the tissue 4 weeks later. As shown in Fig. 1, ADSC were identified by BrdU staining, and some of them were also stained positive for rat endothelial cell antigen (RECA-1). These cells were localized to the sinusoid endothelium as revealed by the superimposed images of fluorescence and phase-contrast microscopy.
Figure 1. Endothelial differentiation of ADSC in the penis.
ADSC were labeled with BrdU and injected into the corpus cavernosa of rats. Four weeks later the tissues were examined by immunofluoresce microscopy. Anti-BrdU and RECA-1antibodies identified the injected ADSC (green) and endothelial cells (red), respectively. Superimposed image (BrdU/RECA) shows that some ADSC (yellow) also stained positive for RECA-1. Another superimposed image (Merge) with the phase-contrast image shows the localization of ADSC to the sinusoid endothelium.
Morphology and growth characteristics of cells grown in EGM2 medium
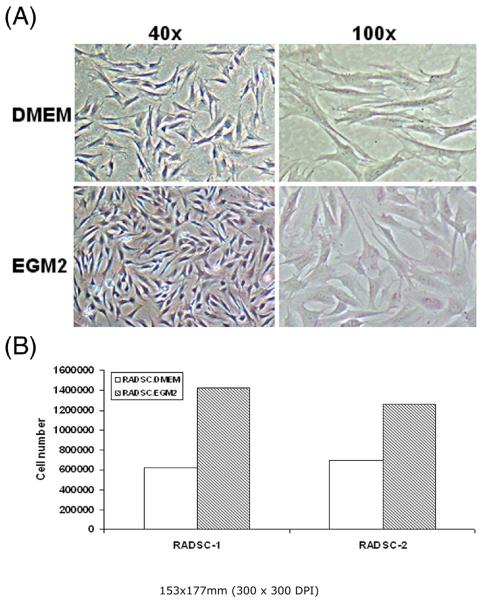
We routinely cultured ADSC in DMEM as the majority of published ADSC studies do. We also routinely cultured endothelial cells in EGM2, which is a commercially available endothelial growth medium. When DMEM in ADSC cultures was replaced with EGM2, the cells reached confluence faster and appeared more compact (larger nuclei) than cells that remained in DMEM (Fig. 2A). Proliferation assay confirmed that ADSC grew much more rapidly in EGM2 than in DMEM (Fig. 2B).
Figure 2. Comparison of cell morphology and growth rate in DMEM and EGM2.
Two rat ADSC lines, RADSC-1 and RADSC-2, were seeded into 100-mm dishes at identical density (300,000 cells/dish) and grown for 3 days in DMEM or EGM2. The cell morphology of RADSC-1 is shown in panel A. The growth rate of both cell lines is shown in panel B.
Endothelial characteristics
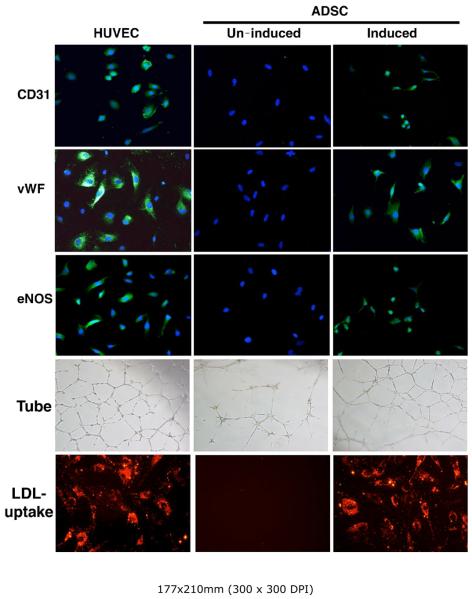
Immunocytochemistry showed that ADSC grown in EGM2 expressed endothelial specific markers CD31, vWF, and eNOS (Fig. 3). Matrigel tube formation assay also showed that ADSC grown in EGM2 were able to form endothelial-like tube structures (Fig. 3). Additionally, LDL uptake assay showed that ADSC grown in EGM2 were capable of LDL uptake (Fig. 3). The endothelial specificity of these three assays was supported by positive results with HUVEC cells (Fig. 3) and negative results with either ADSC grown in DMEM (Fig. 3) or urethral smooth muscle cells grown in EGM2 (data not shown).
Figure 3. Expression of endothelial markers in cells grown in EGM2.
RADSC-1 and RADSC-2 were grown in DMEM (un-induced) or EGM2 (induced). They were then stained for endothelial markers CD31, vWF, and eNOS. Green color indicates expression of CD31, vWF, or eNOS; blue color indicates cell nuclei. Original magnification was 200x. The cells were also assayed for Matrigel tube formation (Tube) and LDL-uptake. Red color indicates the presence of LDL, which was in a conjugated form with the red fluorescence dye DiI. The results were similar for both cell lines; only those of RADSC-1 are shown. Human umbilical vein endothelial cells (HUVEC) served as positive control.
Reversibility and re-inducibility of endothelial differentiation
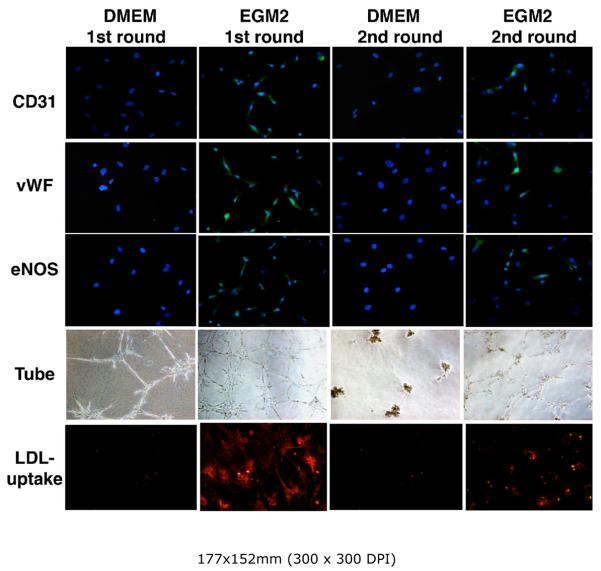
Whether ADSC differentiation is reversible was tested by replacing EGM2 medium with DMEM for the. This resulted in the disappearance of all endothelial characteristics (Fig. 4). Whether endothelial differentiation can be re-induced was tested by reintroducing EGM2 to the cells, and this resulted in the reappearance of all endothelial characteristics (Fig. 4), albeit at reduced levels. These tests established that the EGM2 medium contains specific factors capable of inducing ADSC endothelial differentiation.
Figure 4. Reversibility and re-inducibility of endothelial differentiation.
RADSC-1 and RADSC-2 were grown in DMEM for 6-10 days (DMEM 1st round); half of the cells were assayed for endothelial markers. The other half of the cells were switched to EGM2, grown for 6 days (first round EGM2), and half of the cells were assayed for endothelial markers. The other half of the cells were switched to DMEM, grown for 10 days (2nd round DMEM), and half of the cells were assayed for endothelial markers. Finally, the other half of the cells were switched to EGM2, grown for 6 days (2nd round EGM2), and assayed for endothelial markers. The results were similar for both cell lines; only those of RADSC-1 are shown. Green color indicates expression of CD31, vWF, or eNOS; blue color indicates cell nuclei. Original magnification was 200x. Red color indicates the presence of LDL, which was in a conjugated form with the red fluorescence dye DiI.
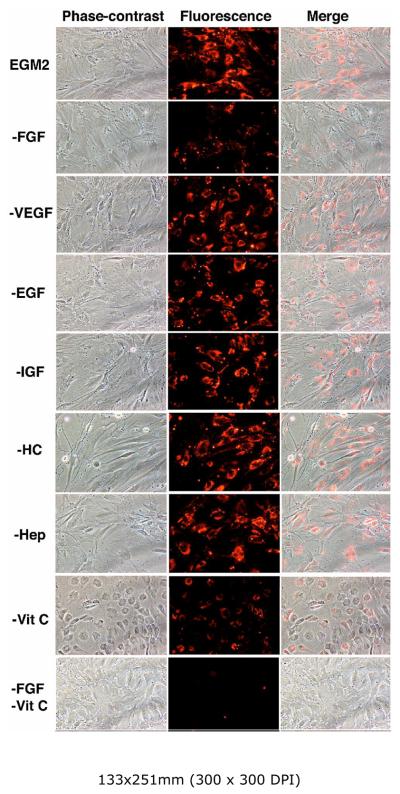
Identification of endothelial-inducing factors
EGM2 medium is supplied by the manufacturer in the form of a basal medium (EBM2) and individual vials of supplemental factors. This packaging format allowed us to test the importance of each supplemental factor as related to ADSC endothelial differentiation. Specifically, we prepared “subtraction” EGM2 media by omitting one supplemental factor at a time. We then maintained ADSC in each subtracted EGM2 medium for one week and then assayed for their LDL-uptake ability. The results show that, among growth factors, the omission of VEGF, EGF, or IGF had essentially no effect, whereas the omission of FGF2 greatly diminished ADSC's LDL-uptake ability (Fig. 5). Among non-growth factors, the omission of hydrocortisone or heparin had essentially no effect, whereas the omission of vitamin C greatly diminished ADSC's LDL-uptake ability (Fig. 5). When both FGF2 and vitamin C were omitted, ADSC exhibited essentially no LDL-uptake ability (Fig. 5). To further confirm the importance of FGF2 and vitamin C, we prepared “addition” media by adding FGF2 and/or vitamin C to EBM2, maintained ADSC in these media for one week, and then assayed ADSC's LDL-uptake ability.
Figure 5. Identification of endothelial inducing factor by “subtraction”.
RADSC-1 cells were grown in fully or partially supplemented EGM2 and then assayed for LDLuptake. Each partially supplemented EGM2 is indicated by the omitted factor; for example, “FGF” denotes EGM2 without FGF2.
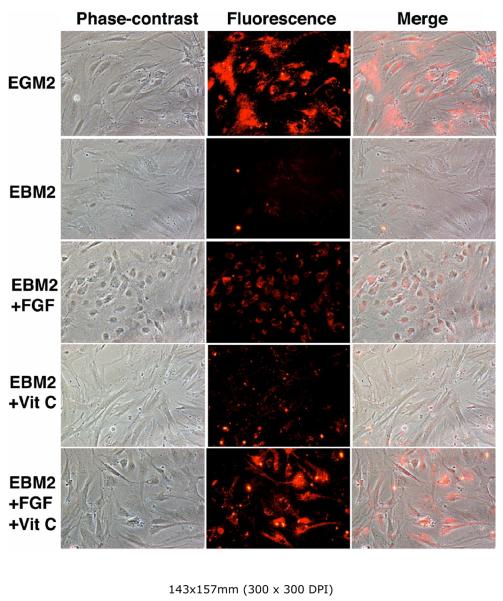
The results show that (1) EBM2 supplemented with FGF2 and vitamin C was nearly as effective as EGM2, (2) EBM2 supplemented with FGF2 was still effective, albeit at a reduced level, and (3) EBM2 supplemented with vitamin C was still somewhat effective, but at a much reduced level (Fig. 6). It is also noteworthy that cells grown in EGM2 without vitamin C (Fig. 5) or in FGF2-supplemented EBM2 (Fig. 6) assumed a round-shape morphology, which may be indicative of oxidative stress (see Discussion).
Figure 6. Identification of endothelial inducing factor by “addition”.
RADSC-1 cells were grown in EGM2, EBM2, or EBM2 supplemented with the indicated factor, and then assayed for LDL-uptake.
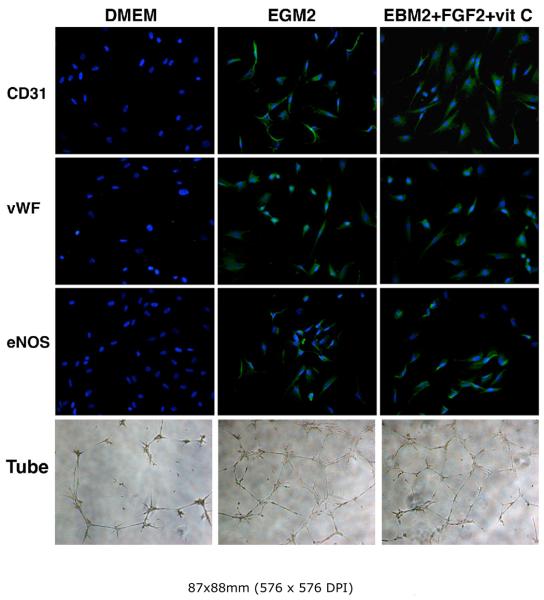
Induction of other endothelial characteristics by FGF2
The above experiments identified FGF2 as the only growth factor required for the induction of ADSC's LDL-uptake ability. We then tested whether FGF2 was able to induce the expression of additional endothelial characteristics. The results show that indeed this was the case; that is, cells grown in FGF2/vitamin C-supplemented EBM2 acquired all of the tested endothelial markers, as did cells grown in the completely supplemented EGM2 (Fig. 7).
Figure 7. Induction of endothelial markers by FGF2.
RADSC-1 cells were grown in DMEM, EGM2, or EBM2 supplemented with FGF2 and vitamin C. They were then stained for endothelial markers CD31, vWF, and eNOS. Green color indicates expression of CD31, vWF, or eNOS; blue color indicates cell nuclei. Original magnification was 200x. The cells were also assayed for Matrigel tube formation (Tube).
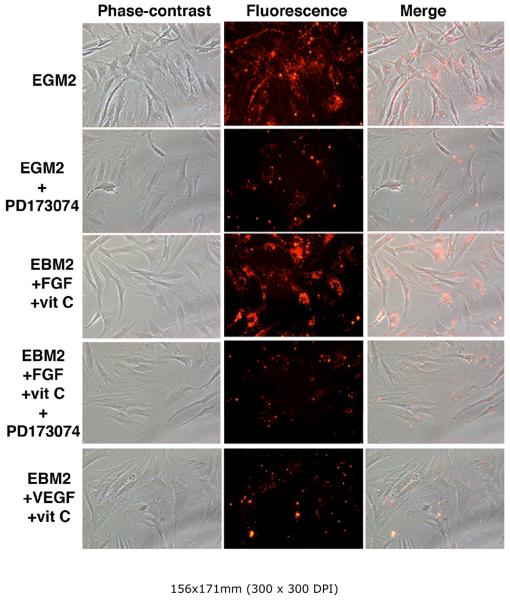
FGFR1 inhibitor blocks ADSC endothelial differentiation
To further confirm the critical role of FGF2 in ADSC endothelial differentiation, we conducted ADSC differentiation experiments in the presence or absence of PD173074, a selective inhibitor for FGF receptor (FGFR1) [27]. In the absence of PD173074 (with the addition of solvent only), cells grown in either the completely supplemented EGM2 or the FGF2/vitamin C-supplemented EBM2 acquired the LDL-uptake ability (Fig. 8). On the other hand, in the presence of PD173074, cells grown in either medium were unable to do so (Fig. 8). To ensure that VEGF signaling did not interfere with this test, as PD173074 is known to have a weaker inhibitory action on VEGF receptor (VEGFR2) [27], we showed that ADSC grown in VDGF/vitamin C-supplemented EBM2 did not acquire the LDL-uptake ability (Fig. 8).
Figure 8. Effect of FGFR inhibitor on endothelial differentiation.
RADSC-1 cells were grown in EGM2 or EBM2 supplemented with FGF2 and vitamin C in the presence or absence of FGFR inhibitor PD173074. The cells were then assayed for LDL-uptake. Red color indicates the presence of LDL, which was in a conjugated form with the red fluorescence dye DiI.
DISCUSSION
Endothelial dysfunction is a prominent feature of a wide variety of diseases including ED, and therapeutic angiogenesis is one of the strategies that have been considered for the restoration of endothelial function. Earlier efforts of this therapeutic strategy largely focused on injection of proangiogenic proteins or vectors expressing such proteins. On the other hand, recent studies have shifted to cell-based therapies, such as using progenitor and stem cells. We have previously shown that ADSC were multipotent and were localized within the vasculature of adipose tissue [24,25]. Studies by others have also shown that ADSC could differentiate into endothelial cells [19-21,23]. We thus hypothesized that ADSC could be used to restore the endothelial function in vasculogenic ED. As a first step, we demonstrated in the present study that indeed ADSC differentiated into endothelial cells in the penile sinusoids. We then sought to identify the mechanism underlying ADSC endothelial differentiation.
Using cell cultures we first tested whether ADSC could differentiate into endothelial cells when grown in EGM2, a commonly used endothelial growth medium. We observed that switching ADSC from DMEM to EGM2 for approximately 7 days was sufficient to induce expression of endothelial markers, LDL-uptake, and endothelial tube formation. We also observed that this endothelial differentiation was reversible because switching ADSC back to DMEM resulted in the disappearance of all of the above-mentioned endothelial characteristics. Upon returning to EGM2, the endothelial characteristic reappeared, albeit at reduced levels. Thus, EGM2 did contain factors necessary and sufficient to induce ADSC endothelial differentiation. Subsequent experiments led to the identification of FGF2 and vitamin C as the only two plausible factors.
The importance of vitamin C for endothelial cell culture has been reported previously [28]. Human aortic endothelial cells cultured without vitamin C supplementation were essentially scorbutic. Addition of 100 μM of vitamin C to the culture media significantly lowered oxidative stress and increased eNOS activity by 600%. In the present study we observed that ADSC cultured in EGM2 without vitamin C supplementation displayed a morphology that was indicative of oxidative stress (Fig. 4). Therefore, the reduced LDL-uptake ability of ADSC grown in such medium was likely due to oxidative stress, and based on this consideration, vitamin C was added to the culture media to maintain a healthy environment for endothelial cells in all subsequent experiments.
In the presence of vitamin C, the omission of EGF, IGF, or VEGF from EGM2 had no or little effect on ADSC's LDL-uptake ability. In contrast, the omission of FGF2 greatly impeded ADSC's LDL-uptake ability. Furthermore, addition of FGF2 alone in the vitamin C-supplemented EBM2, without EGF, IGF, or VEGF, was sufficient to induce expression of endothelial markers and endothelial tube formation. Finally, addition of FGFR1 inhibitor PD173074 to EGM2 or FGF2-supplemented EBM2 blocked the induction of ADSC's LDL-uptake ability. Together, these results point to FGF2 signaling as a critical determinant of ADSC endothelial differentiation.
Autocrine FGF2 signaling has been shown to be critical for self-renewal of human ADSC [29]. In this case, endogenously expressed FGF2 is exported to the cell surface without being released into the culture medium. As ADSC are propagated in culture, they gradually express less FGF2 and become less able to differentiate, which can be circumvented by chronic treatment with exogenous FGF2. Furthermore, treatment of FGF2-expressing ADSC with PD173074 dramatically decreases their differentiation potential. Together, these data are consistent with our finding that exogenously applied FGF2 induces endothelial differentiation and PD173074 treatment inhibits endothelial differentiation.
CONCLUSIONS
ADSC can differentiate into endothelial cells in tissues such as the penis. FGF2 signaling mediates ADSC endothelial differentiation. Further experiments are required to test whether ADSC, possibly with enhanced FGF2 siganling, can treat vasculogenic ED.
ACKNOWLEDGMENTS
This work was supported by grants from the California Urology Foundation, Mr. Arthur Rock and the Rock Foundation, and the National Institutes of Health.
REFERENCES
- 1.Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart. 2006;92:441–444. doi: 10.1136/hrt.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diab Vasc Dis Res. 2007;4:84–88. doi: 10.3132/dvdr.2007.025. [DOI] [PubMed] [Google Scholar]
- 3.Slevin M, Kumar P, Gaffney J, Kumar S, Krupinski J. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci (Lond) 2006;111:171–183. doi: 10.1042/CS20060049. [DOI] [PubMed] [Google Scholar]
- 4.Gholami SS, Rogers R, Chang J, Ho HC, Grazziottin T, Lin CS, Lue TF. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J Urol. 2003;169:1577–1581. doi: 10.1097/01.ju.0000055120.73261.76. [DOI] [PubMed] [Google Scholar]
- 5.Vartanian SM, Sarkar R. Therapeutic angiogenesis. Vasc Endovascular Surg. 2007;41:173–185. doi: 10.1177/1538574407302849. [DOI] [PubMed] [Google Scholar]
- 6.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 7.Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, Botker HE, Dudek D, Drvota V, Hesse B, Thuesen L, Blomberg P, Gyongyosi M, Sylven C. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 8.Lin CS, Lue TF. Growth factor therapy and neuronal nitric oxide synthase. Int J Impot Res. 2004;16(Suppl 1):S38–39. doi: 10.1038/sj.ijir.3901214. [DOI] [PubMed] [Google Scholar]
- 9.Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 11.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 12.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 13.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 14.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 16.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 17.Dicker A, Le Blanc K, Astrom G, van Harmelen V, Gotherstrom C, Blomqvist L, Arner P, Ryden M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283–290. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 19.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 20.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 22.Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279–290. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- 23.Sumi M, Sata M, Toya N, Yanaga K, Ohki T, Nagai R. Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci. 2007;80:559–565. doi: 10.1016/j.lfs.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Lin G, Garcia M, Ning H, Banie L, Gio YL, Lue TF, Lin CS. Defining Stem and Progenitor Cells within Adipose Tissue. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–518. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 26.DiMuzio P, Tulenko T. Tissue engineering applications to vascular bypass graft development: the use of adipose-derived stem cells. J Vasc Surg. 2007;45(Suppl A):A99–103. doi: 10.1016/j.jvs.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, Eliseenkova AV, Green D, Schlessinger J, Hubbard SR. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. Embo J. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith AR, Visioli F, Hagen TM. Vitamin C matters: increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. Faseb J. 2002;16:1102–1104. doi: 10.1096/fj.01-0825fje. [DOI] [PubMed] [Google Scholar]
- 29.Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006;24:2412–2419. doi: 10.1634/stemcells.2006-0006. [DOI] [PubMed] [Google Scholar]