Abstract
Lithium, a drug commonly used to treat mood disorders, and the psychostimulant methamphetamine are both capable of altering circadian rhythmicity. Although the actions of lithium on the circadian system are thought to occur through inhibition of glycogen synthase kinase-3β (GSK3β), the mechanism by which methamphetamine alters circadian rhythms is unknown. We tested the effects of concurrent methamphetamine and lithium treatment on the circadian wheel-running behavior of mice. Methamphetamine alone lengthened both the active duration and the free-running period of locomotor activity in animals housed in constant conditions. Administering lithium enhanced the period-lengthening effects of methamphetamine in animals housed in constant darkness. This effect was even more pronounced when animals were housed in constant light. Lithium increased both methamphetamine intake and serum levels of methamphetamine, possibly contributing to the effects on circadian behavior. We also tested the effect of methamphetamine in mutant mice possessing only one allele for Gsk3β. These animals, when treated with methamphetamine, responded like wild-type mice treated with a combination of methamphetamine and lithium, displaying long, free-running rhythms. These data, together with many others in the literature, point to a complicated interaction between the circadian system and the development and possible treatment of psychopathologies such as bipolar disorder and drug addiction.
Keywords: circadian rhythms, glycogen synthase kinase-3β, lithium, methamphetamine, mouse
Introduction
There is a wealth of evidence pointing to a link between abnormal circadian rhythms and mood disorders (Wehr and Wirz-Justice, 1982; Winget et al., 1984; Katz et al., 2001). Many depressed and bipolar patients suffer from alterations in their sleep–wake cycles and circadian rhythms. The connection between the circadian system and mood disorders is further strengthened with the recent establishment of circadian Clock mutant mice as a model for human mania (Roybal et al., 2007). The manic-like behaviors displayed by Clock mutant mice are reversed by administering lithium (Roybal et al., 2007). In fact, perhaps not surprisingly, lithium and other compounds commonly used to treat mood disorders have striking effects on circadian outputs (Klemfuss, 1992; Dokucu et al., 2005; O’Donnell and Gould, 2007).
The effect of lithium on circadian rhythms has been extensively studied in an effort to understand the relationship between psychopathology and circadian regulation. Lithium produces a lengthening of the free-running period of circadian locomotor activity in many species including drosophila (Padiath et al., 2004), rats (Hafen and Wollnik, 1994), hamsters (LeSauter and Silver, 1993), mice (Iwahana et al., 2004), and humans (Johnsson et al., 1983). In addition to altering locomotor activity, lithium lengthens the free-running period of the electrical firing rate in isolated mouse suprachiasmatic nucleus (SCN) neurons (Abe et al., 2000).
The action of lithium on the circadian clock is thought to occur through inhibition of glycogen synthase kinase-3 (GSK3) (Iwahana et al., 2004; Padiath et al., 2004; Dokucu et al., 2005; Iitaka et al., 2005; Yin et al., 2006). Lithium inhibits GSK3 directly (Ryves and Harwood, 2001) and increases GSK3 phosphorylation (phosphorylated GSK3 is the inactive form) (De Sarno et al., 2002). GSK3β has a role in the mammalian circadian clock, phosphorylating Rev-erbα, which in turn results in repression of Bmal1 transcription (Yin et al., 2006). The inhibition of GSK3β by lithium thus leads to suppression of Rev-erbα, activation of Bmal1 (Yin et al., 2006), and a slowing of the molecular circadian feedback loop (i.e. lengthening the period). GSK3β has also been shown to phosphorylate PER2, possibly promoting nuclear translocation (Iitaka et al., 2005). Thus, lithium may also ultimately lead to delayed transfer of PER2 to the nucleus.
In addition to lithium, circadian rhythmicity is sensitive to stimulants such as methamphetamine (MAP). Chronic administration of MAP in the drinking water of rats and mice produces a lengthening of the free-running period and an increase in the duration of locomotor activity (Honma et al., 1986; Tataroglu et al., 2006). Animals treated with MAP [both in constant conditions and in light : dark (LD) cycles] often show a second, long-period rhythmic component of locomotor activity, which exhibits relative coordination with the main component (Honma et al., 1986; Tataroglu et al., 2006). MAP also induces circadian rhythmicity in animals that are otherwise arrhythmic because of lesions of their SCN (Honma et al., 1987; Tataroglu et al., 2006) or clock gene mutations (Masubuchi et al., 2001). These data have led to the hypothesis that there is a methamphetamine-sensitive circadian oscillator (MASCO), which is separate and distinct from the circadian oscillators in the SCN. The mechanism by which MAP influences circadian rhythms is unknown, but, given its stimulatory effects on the dopaminergic system, it has been hypothesized that the circadian consequences of MAP administration could be mediated by reward pathways (Tataroglu et al., 2006).
Lithium can reverse or inhibit some of the effects of amphetamines. Lithium has an antagonistic action on some dopamine-dependent behavioral effects of amphetamine (Beaulieu et al., 2004). This occurs through lithium’s interference with the Akt/GSK3 signaling cascade (Beaulieu et al., 2004). Lithium protects against dopaminergic neurotoxicity (Youdim and Arraf, 2004), reducing apoptosis by inhibiting GSK3β (Li et al., 2002) and reduces the c-Fos expression typically induced by MAP (Lee et al., 1999; Namima et al., 1999; Yang et al., 2001). Nevertheless, other effects of MAP are potentiated by lithium. Lithium administration exacerbates MAP-induced stereotypy in mice (Ozawa and Miyauchi, 1977), an effect that may be because of the ability of lithium to prolong the halflife of MAP in the rodent brain (Miyauchi et al., 1981).
Given the synergistic effects of lithium and MAP on stereotyped behaviors, this study aimed to determine the impact of lithium on the circadian locomotor behavior of mice chronically treated with MAP. In addition, we investigated the effect of MAP treatment on mice lacking one allele for Gsk3β. Although Gsk3β null mice are not viable, Gsk3β heterozygote mice display behavioral changes similar to those of mice chronically treated with lithium (O’Brien et al., 2004). This model system was used to test the hypothesis that genetic inhibition of GSK3β would alter the effect of MAP on circadian locomotor activity in the same way as does the addition of lithium.
Methods
Animals and housing
Male C57BL/6 mice (10–15 weeks old) were purchased from Jackson laboratories (Bar Harbor, Maine, USA) and individually housed in Nalgene cages equipped with running wheels. Wild-type and heterozygous male and female Gsk3β mice were used, where noted; these animals were obtained from a breeding colony at the University of Virginia. Colony founders were a generous gift from Dr Peter Klein, University of Pennsylvania, with permission from Dr James Woodgett, Samuel Lunenfeld Research Institute, who originally generated the line. Running wheel activity data were recorded using the ClockLab software (Actimetrics, Evanston, Illinois, USA). All animals were kept on a 12 : 12 h LD cycle for at least 1 week before experimental manipulation. During the light phase and in constant light (LL), light intensity at the level of the animals’ cages was approximately 350 lux. The Animal Care and Use Committee at the University of Virginia approved all experimental procedures.
Methamphetamine and lithium chloride administration
Water containing MAP (Methamphetamine hydrochloride, Sigma, St Louis, Missouri, USA) at 0.005 and 0.010% was available ad libitum, where indicated. Lithium chloride (LiCl) was administered using 0.212% LiCl food pellets (Harlan Teklad, Madison, Wisconsin, USA). Similar concentrations of LiCl chow have been reported to produce a serum lithium concentration of approximately 0.76 mmol/l (O’Brien et al., 2004). LiCl food pellets were ground and moistened with peanut butter (10%) to prevent weight loss, which can occur when animals consume LiCl pellets alone. This dietary modification has no effect on the free-running period of animals on clean water or MAP (Mohawk et al., unpublished data). LiCl-fed animals also received supplemental saline (0.9%, administered in drinking water) to avoid lithium toxicity (Jensen et al., 1976). All food was available ad libitum. When in constant dark (DD), animal care and maintenance were performed using an infrared light and viewer.
Effect of methamphetamine and lithium administration
Lithium chloride treatment
One group of animals was fed with standard chow and water in LD (n=6), DD (n=15), or LL (n=12) for at least 2 weeks. Food was then replaced with rodent chow containing 0.212% LiCl and animals were maintained on the LiCl diet for at least 3 more weeks.
Methamphetamine treatment
A second group of animals was housed with standard chow and water in either LD (n=12), DD (n=20), or LL (n=22) for at least 2 weeks. After this 2-week control period, 0.005% MAP was administered chronically in the drinking water for an additional 2 weeks.
Methamphetamine + lithium chloride
A third group of animals [LD (n=12); DD (n=20); LL (n=14)] was fed standard chow and water for 2 weeks, and then given 0.005% MAP with standard chow. After 2 weeks on the standard chow + 0.005% MAP regimen, the chow was replaced with 0.212% LiCl food. After 3 weeks on the LiCl diet, the 0.212% LiCl chow was removed and mice continued consuming 0.005% MAP and standard chow for the duration of the experiment.
Increased methamphetamine concentration
To compare the effects of lithium to an increased dose of MAP, another group of animals received 0.005% MAP for 2 weeks, and then the concentration of drug was doubled. These animals received a solution of 0.010% MAP for at least 3 weeks [DD (n=9); LL (n=8)].
Effect of lithium chloride on serum methamphetamine concentrations
To determine whether LiCl administration was altering MAP metabolism, a separate group of mice were used to assay for serum MAP levels. Mice (n=24) were housed in running wheels and assigned randomly to one of four groups: control (no drug treatment), 0.005% MAP, 0.010% MAP, or 0.005% MAP + LiCl. Mice were released into DD and administered drug as described above. After 1 week of drug treatment, animals were sacrificed (at approximately circadian time 12) and trunk blood was collected. Serum was analyzed by the Center for Human Toxicology at the University of Utah using liquid chromatography and tandem mass spectrometry as described earlier (Slawson et al., 2002).
Effect of methamphetamine on Gsk3β+/− mice
Mice from GSK3β+/− × C57BL/6 crosses (n=20 animals) were released into constant darkness for 2 weeks, to establish their free-running period length. Animals were then given 0.005% MAP drinking water for 7 weeks and the effect on their circadian wheel-running behavior was observed. The long duration of MAP treatment was necessary to allow stabilization of the free-running period in some animals. After the 7 weeks of MAP treatment, all animals were given clean drinking water and monitored until they returned to a normal (pre-MAP treatment) free-running period length. Upon completion of data collection, animals were genotyped by PCR analysis of tail DNA. The experimental animals included six heterozygote and 14 wild-type mice. All mice originating from litters that did not contain a single heterozygote littermate were excluded from analyses, resulting in a final number of six heterozygotes and six wild-type mice.
Statistical analyses
Activity data were double plotted in actograms using a 10 min block size. Circadian parameters of period (τ) and activity duration (α) were evaluated using the ClockLab software (Actimetrics). Alpha was defined as the distance between the onset and the offset of activity, by fitting a week of data from a free-running rhythm into its dominant period and obtaining an average waveform. Arrhythmic animals and those that did not survive the appropriate duration of drug treatment were excluded from τ and α summary data and summary figures. Activity level was defined as the total number of running wheel revolutions during a week of each treatment. Activity levels during drug treatment were expressed as a percentage of control (no drug treatment) levels. Period values were obtained by χ2 periodogram on at least 1 week of continuous data. Groups were compared using one-way analysis of variance (with post-hoc Scheffé and t-test comparisons) and unpaired t-tests; a P value of less than 0.05 was considered significant.
Results
Methamphetamine, but not lithium, lengthens the free-running circadian rhythm of locomotor activity
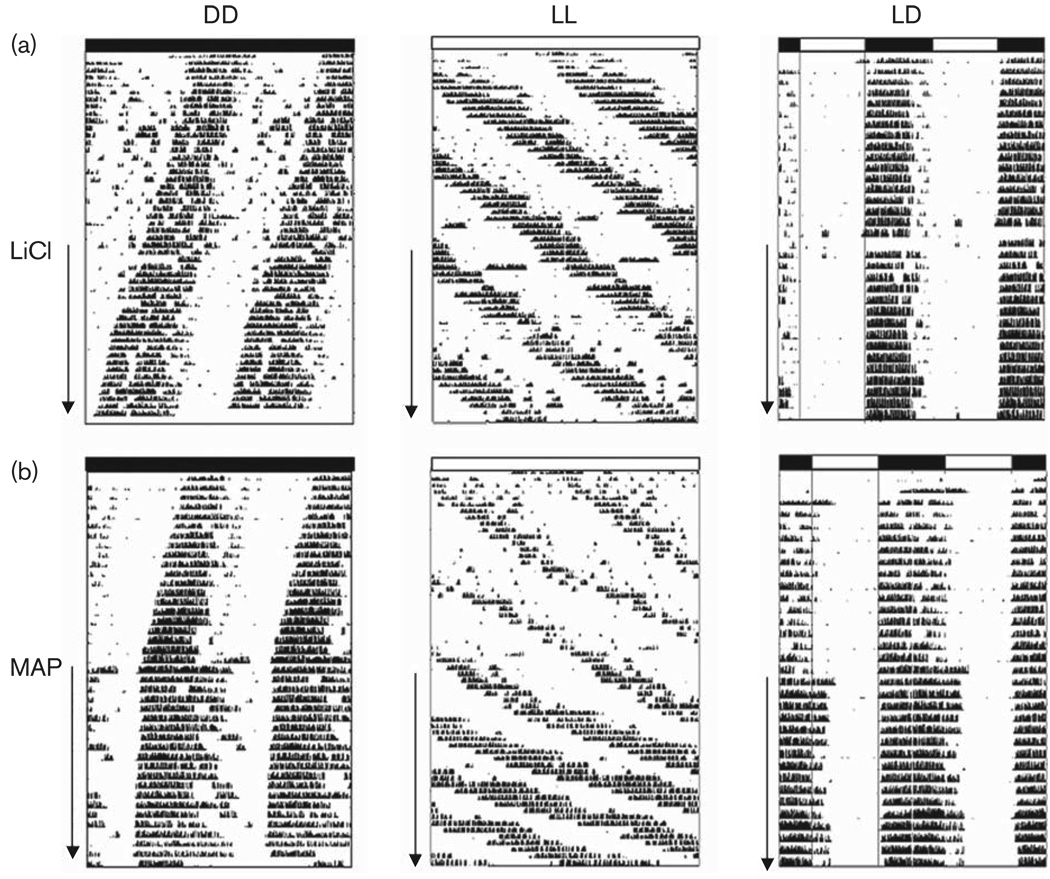
Figure 1 shows representative activity records from animals treated with either LiCl (Fig. 1a) or MAP (Fig. 1b) in DD, LL, and LD. As expected, animals treated with MAP alone lengthened period in DD and LL and increased activity level. In LD, they demonstrated a delayed, but stable, phase relationship between onset of activity and lights off (Ψ= − 0.8 ± 0.32 h), relative to control (untreated) conditions. There were no significant changes in circadian parameters during 2 weeks of LiCl treatment in LD.
Fig. 1.
Effect of 0.212% lithium chloride (LiCl) (a) or 0.005% methamphetamine (MAP) (b) on free-running period and entrainment of mice in constant darkness (DD), constant light (LL), and light: dark (LD). Running wheel activity data are double plotted, with the bars along the top representing light conditions (shaded=dark, open=light). Arrows along the side of each actogram indicate days of treatment with LiCl or MAP. Note the phase delay in activity onset after methamphetamine treatment in LD.
Table 1 shows the mean (±SEM) τ and α of mice housed in DD and LL. The group of mice consuming 0.005% MAP significantly lengthened both τ and α (P < 0.001). Mice on MAP consumed 8 ± 2.3 ml of water/day, which is an average of 0.4 mg of MAP/day. Water consumption levels did not differ between the control and MAP-treated conditions. After 2 weeks of consuming LiCl, a significant increase in α was observed in animals held in DD (P < 0.05). LiCl consumption did not significantly alter the free-running period length of animals in DD or LL. Average daily volume of (clean) water intake was 14 ± 1.2 ml in LiCl-treated mice and 8 ± 1.2 ml in untreated animals.
Table 1.
Mean±SEM free-running period(τ, in hours) and activity phase length (α, in hours) of animals in DD and LL after 2 weeks of either control conditions or drug treatment
| Control | 0.212% LiCl | Control | 0.005% MAP | ||
|---|---|---|---|---|---|
| DD | τ | 23.60 | 23.70 | 23.40 | 23.80 |
| (±0.23) | (±0.20) | (±0.08) | (±0.05)** | ||
| α | 13.55 | 14.95 | 14.87 | 18.51 | |
| (±0.47) | (±0.43)* | (±0.68) | (±0.63)** | ||
| LL | τ | 25.60 | 25.70 | 26.40 | 29.60 |
| (±0.47) | (±0.58) | (±0.12) | (±1.17)** | ||
| α | 11.10 | 10.40 | 12.80 | 14.32 | |
| (±0.87) | (±0.69) | (±0.66) | (±0.94) |
N in DD=15 LiCl-treated mice and 20 MAP-treated mice. N in LL = 12 LiCl-treated mice and 22 MAP-treated mice.
DD, constant darkness; LiCl, lithium chloride; LL, constant light; MAP, methamphetamine.
P< 0.05,
P< 0.001 as compared with control conditions.
Lithium and methamphetamine act synergistically to dramatically lengthen free-running period
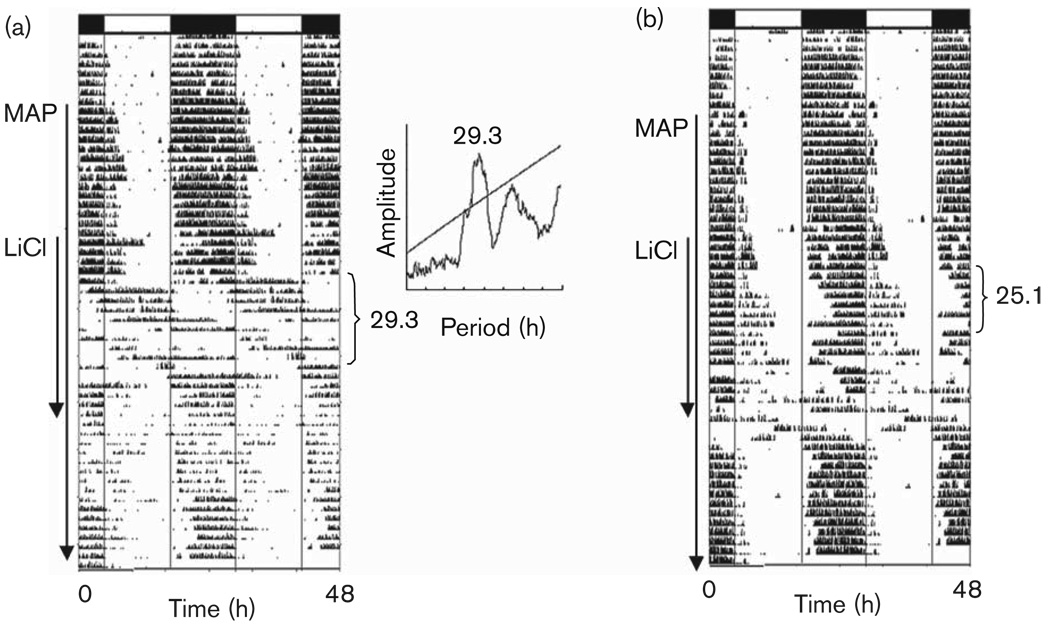
Combining LiCl and MAP treatment in animals housed in LD resulted in a delayed phase angle of activity onset and relative coordination, with episodes of free-running alternating with stable entrainment. Two weeks after withdrawing LiCl, phase was still unstable (Fig. 2).
Fig. 2.
Light entrainment and the effect of methamphetamine (MAP) and lithium chloride (LiCl). In (a), the animal begins to free-run after the addition of LiCl treatment. The periodogram appears in the inset. In (b), relative coordination to the light:dark cycle is observed. Arrows along the side of each actogram indicate days of treatment with MAP and LiCl. Periods (in hours) are noted to the right of the actograms.
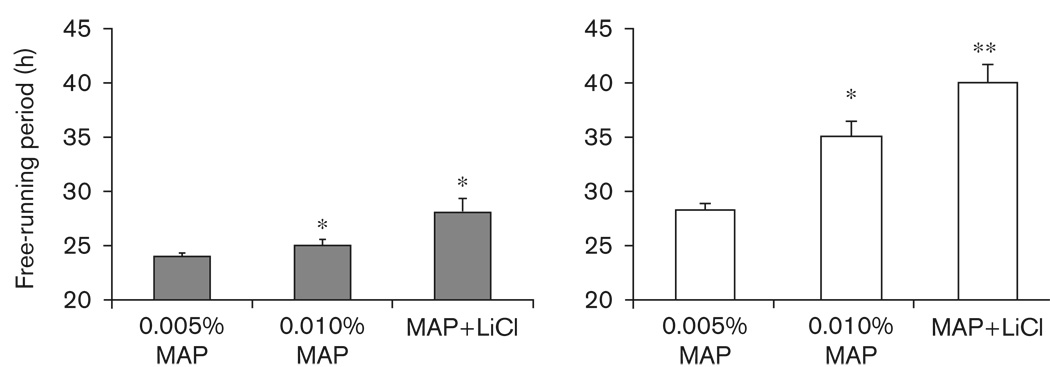
There was a significant effect of drug treatment (0.005% MAP, 0.010% MAP, or 0.005% MAP + LiCl) on period length in both DD [F(3,77) = 16.17, P < 0.001] and LL [F(3,53) = 74.84, P < 0.001] (Table 2 and Figs 2, 3, 4 and 5). Increasing the dose of MAP from 0.005% to 0.010% resulted in a lengthening of period in DD (P < 0.01) and LL (P < 0.001). When housed in DD, four out of nine mice on 0.010% MAP displayed a second component of activity, whereas in LL all animals tested (n=8) displayed a significant lengthening of τ when the MAP dose was increased from 0.005 to 0.010%.
Table 2.
Pooled mean±SEM (τ, in hours) of mice untreated (control), on 0.005% MAP, on 0.010% MAP, and on 0.005% MAP + LiCl
| τ in DD | n | τ in LL | n | |
|---|---|---|---|---|
| Control | 23.54 (±0.05)a | 29 | 26.36 (±0.08)a | 20 |
| 0.005% MAP | 24.05 (±0.13)b | 29 | 28.31 (±0.20)b | 21 |
| 0.010% MAP | 25.09 (±0.55)c | 9 | 35.09 (±1.53)c | 8 |
| 0.005% MAP + LiCl | 28.05 (±1.27)c | 14 | 40.16 (±1.74)d | 8 |
DD, constant darkness; LiCl, lithium chloride; LL, constant light; MAP, methamphetamine.
Different letters next to treatment groups represent significant differences from other free-running periods in that lighting condition (DD or LL; P< 0.05).
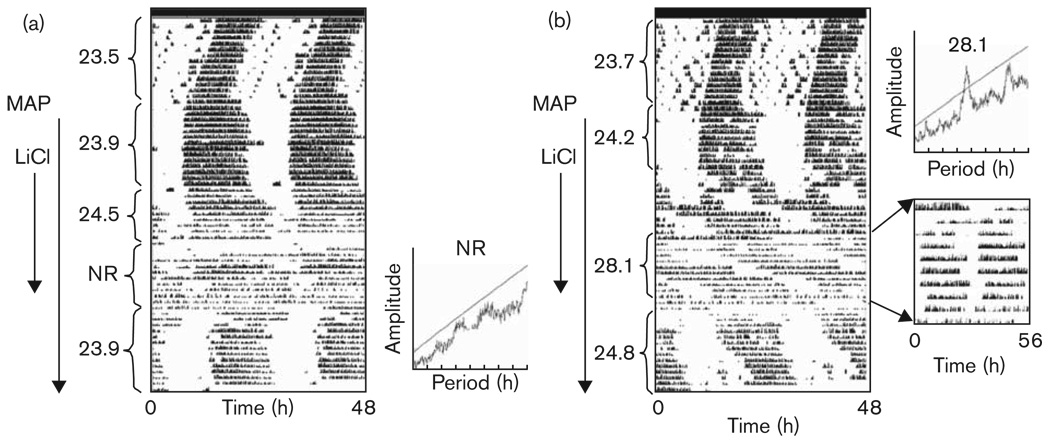
Fig. 3.
Effect of lithium chloride (LiCl) and methamphetamine (MAP) on the free-running rhythm of wheel-running activity in constant darkness. Arrows along the side of each actogram indicate days of treatment with MAP and LiCl. In (a), the animal experiences a lengthening of τ followed by arrhythmicity (no rhythm, NR). In (b), τ lengthens to 28.1 h (replotted in insert on a time base of 28h). Periods (in hours) are noted to the left of the actograms.
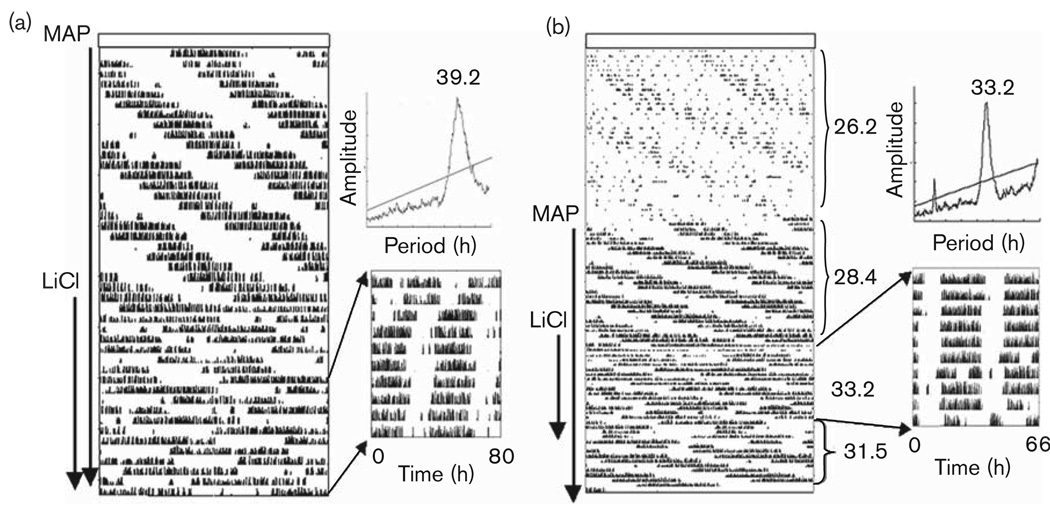
Fig. 4.
Effect of lithium chloride (LiCl) and methamphetamine (MAP) on the free-running rhythm of wheel-running activity in constant light. Arrows along the side of each actogram indicate days of treatment with MAP and LiCl. Periodograms along with the replotted activity data [with period of the time base set to match τ, i.e. 40h in (a) and 33 h in (b), double plotted] appear in the inset. In (b), periods (in hours) are noted to the right of the actogram.
Fig. 5.
Average±SEM τ observed when mice were treated with 0.005% methamphetamine (MAP), 0.010% MAP, or a combination of 0.005% MAP and lithium chloride (LiCl) (MAP + LiCl). All drug treatments resulted in longer τs compared with control conditions (data not shown). The gray bars are data from animals housed in constant darkness; open bars are data from animals housed in constant light. *Significantly longer than 0.005% MAP, **Significantly longer than 0.005 and 0.010% MAP.
Given LiCl, animals already consuming MAP further lengthened τ. In DD (Fig. 3), 0.005% MAP + LiCl resulted in a longer τ than that observed when animals were treated with 0.005% MAP alone (P < 0.001); 0.005% MAP + LiCl also tended toward a longer τ than was observed after 0.010% MAP treatment in DD, though this effect did not reach significance (P=0.08). After withdrawing LiCl in food, animals housed in DD continued to have low activity levels for at least 3 more days before reverting to a shorter, pre-LiCl exposure τ. Of the DD group, five mice (25%) on LiCl displayed arrhythmic behavior, seven mice (35%) had a free-running period longer than 27 h, and the remaining animals had an average τ lengthening of 0.4 h. In LL (Fig. 4), 0.005% MAP + LiCl resulted in a longer τ than that of animals on either 0.005% (P < 0.001) or 0.010% MAP (P < 0.05) alone. Of the animals housed in constant light, only two animals displayed arrhythmic behavior when treated with a combination of 0.005% MAP and LiCl, whereas six mice displayed a τ approximating 39 h or longer after the addition of LiCl. When LiCl was given in food, mice consumed an average of 13.4 ± 1.2 ml/day of 0.005% MAP solution, which is 0.67 mg of MAP/day; body weight loss was about 8% by the end of the third week.
Effect of lithium chloride treatment on methamphetamine-induced hyperactivity
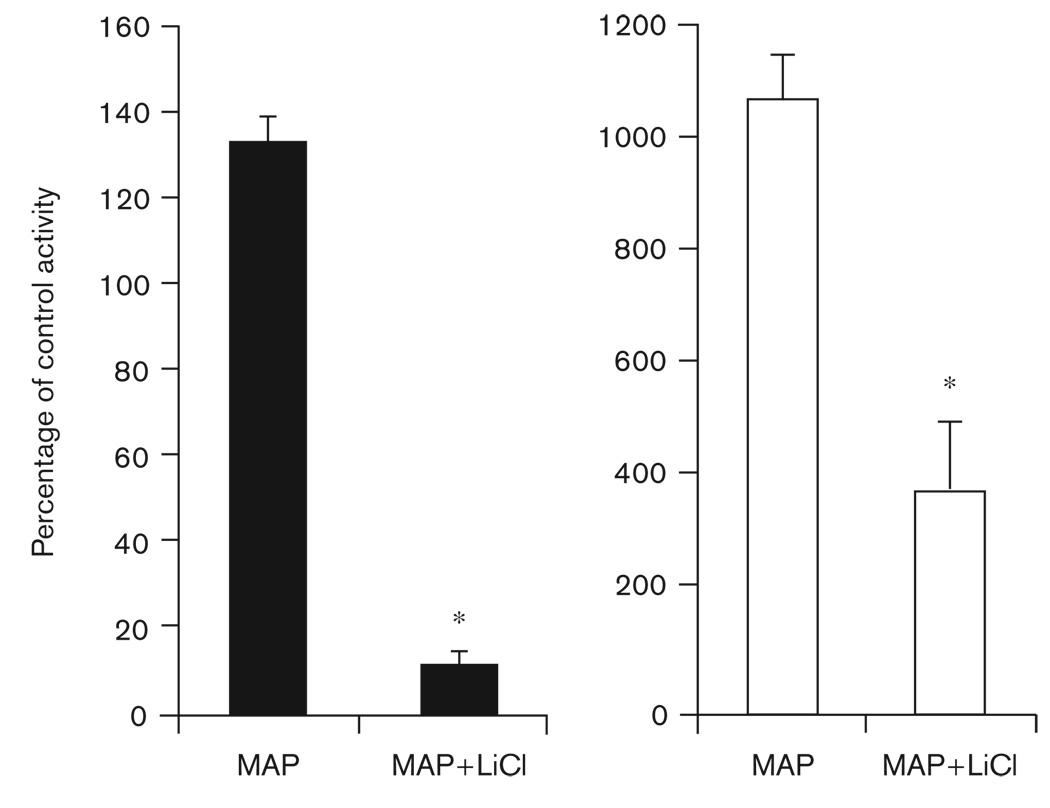
Lithium consumption reduced the hyperactivity resulting from MAP treatment (Fig. 6). In DD, MAP increased activity levels by approximately 35%, whereas adding LiCl in chow reduced overall wheel-running activity to only 13% of control levels (P < 0.01). In LL, untreated animals display low levels of activity (Fig. 4b). When consuming 0.005% MAP in LL, activity levels increased approximately 10 fold; when consuming lithium, the hyperactivity produced by MAP decreased significantly (P < 0.01; Fig. 6).
Fig. 6.
Average±SEM activity levels on 0.005% methamphetamine (MAP) alone and on 0.005% MAP with lithium chloride (LiCl) (MAP + LiCl). Data are presented as a percentage of control conditions (where control activity level=100%). The black bars represent data from mice housed in constant darkness (DD), the open bars are data from mice housed in constant light (LL). *Significant difference from 0.005% MAP (P<0.01). Note the difference in scale between the DD and LL charts.
Effect of lithium chloride on serum methamphetamine levels
There was a main effect of drug treatment on serum levels of MAP (F=41.685, P < 0.001; Table 3). Post-hoc comparisons revealed that animals consuming 0.005% MAP did not have serum MAP levels that were significantly increased over those of control animals. Mice consuming 0.010% MAP had serum levels that were significantly higher than controls (P < 0.05), but not significantly higher than those of 0.005% MAP-treated animals (P=0.10). Coadministration of LiCl and MAP resulted in increased levels of MAP in serum, with serum levels in these mice being higher than any other group (P < 0.05).
Table 3.
Mean±SEM serum methamphetamine for mice consuming clean water (control), 0.005% MAP, on 0.010% MAP, and on 0.005% MAP + LiCl
| Serum MAP (ng/ml) | n | |
|---|---|---|
| Control | 0 (±00) | 6 |
| 0.005% MAP | 41 (±26) | 6 |
| 0.010% MAP | 219 (±89)* | 4 |
| 0.005% MAP +LiCl | 593 (±50)** | 6 |
LiCl, lithium chloride; MAP, methamphetamine.
Significantly greater than control (P< 0.05).
Significantly greater than all other groups (P< 0.05).
Gsk3β+/− mice are hypersensitive to the circadian effects of methamphetamine in constant darkness
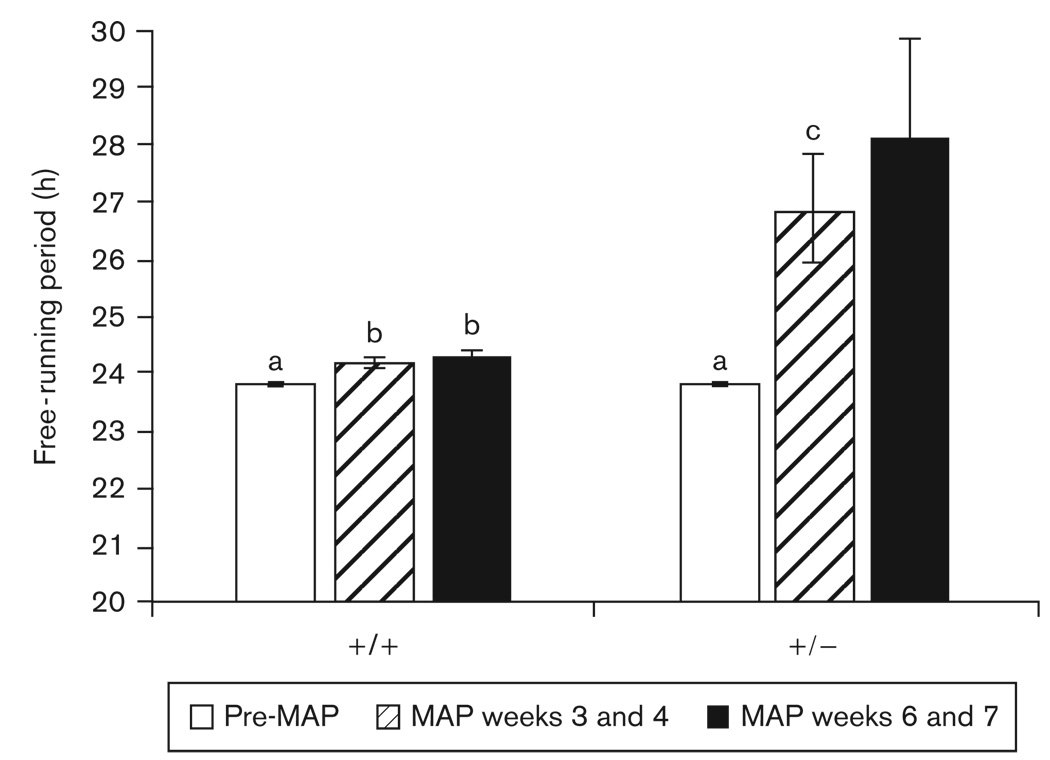
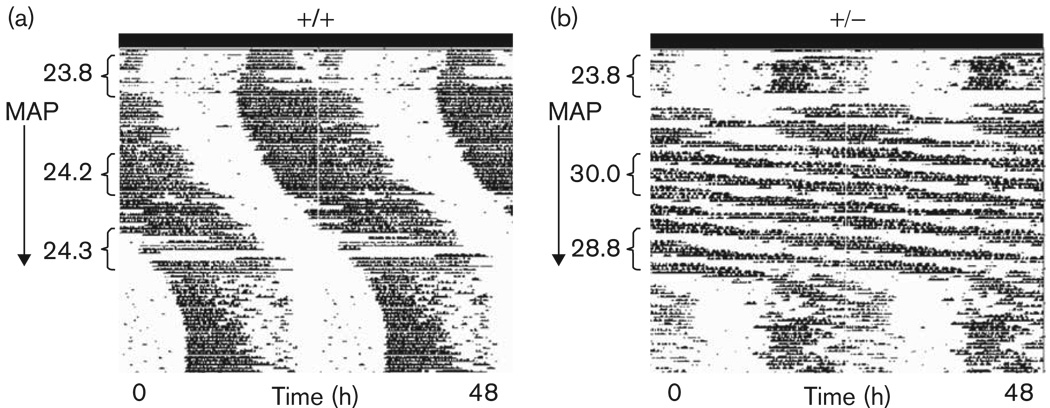
The τ of Gsk3β+/− mice did not differ from that of wild-type mice before drug treatment (t=0.47, P > 0.05). Some animals exhibited multiple free-running period lengths while on MAP; for these, the period with the larger amplitude (as determined by χ2 periodogram) was used in the statistical analyses. During weeks 3 and 4 of MAP treatment, τ was significantly lengthened in all mice [F(1,10)=13.35, P < 0.01], and there was a significant interaction between MAP-treatment and genotype [F(1,10)=8.03, P < 0.05]. MAP lengthened τ to a much greater extent in Gsk3β+/− mice than in their wild-type counterparts (P < 0.05). After continued MAP exposure, the difference between the τ of wild-type and Gsk3β+/− mice continued to increase, as did the variance in τ among Gsk3β+/− mice, resulting in unequal variances (P < 0.05). Despite the increased variability among Gsk3β+/− mice, there was still a trend towards a longer τ in the Gsk3β+/− mice as compared with wild-type mice during weeks 6 and 7 of MAP treatment (P=0.085 by t-test assuming unequal variances) (Figs 7 and 8).
Fig. 7.
Mean ±SEM free-running period lengths of wild-type (+/ +) and heterozygote (+ /−) Gsk3β knockout mice. Different letters above bars denote significant differences between groups (P<0.05). The τ of Gsk3β+/− mice during weeks 6 and 7 of methamphetamine (MAP) treatment were highly variable and the mean did not differ significantly from any other group.
Fig. 8.
Representative actograms from (a) wild-type (+/+) and (b) heterozygote (+/−) Gsk3β mice housed in DD. Activity data are double plotted. The arrow to the left of each actogram denotes the 7-week period of 0.005% methamphetamine (MAP) administration. Periods (in hours) are noted to the left of the actograms. MAP administration resulted in a lengthening of τ in both +/+ and +/− mice. The free-running periods of +/− animals, however, were affected to a much greater extent.
Discussion
Lithium treatment and Gsk3β haploinsufficiency further lengthened the circadian free-running periods of MAP-treated mice. The observation that Gsk3β+/− animals on 0.005% MAP had free-running period lengths approximating those of lithium-treated mice on the same dosage of MAP is consistent with the hypothesis that the synergistic effects of lithium and MAP on the circadian system may be accomplished through the inhibitory action of lithium on GSK3β. The increased serum levels of MAP in lithium-treated mice may also partly explain the lengthened free-running periods of these animals.
In this study, 0.212% LiCl alone did not produce noticeable changes in the free-running period length. However, a significant increase in a was observed in DD (Table 1). Lithium has previously been reported to alter period length in a number of animals including drosophila (Padiath et al., 2004; Dokucu et al., 2005), primates (Welsh and Moore-Ede, 1990), and mice (Iwahana et al., 2004). It is possible that administering LiCl to our mice for longer lengths of time, or at higher doses (Iwahana et al., 2004, administered 0.3% lithium), would have produced serum concentrations capable of altering τ. The method of lithium administration may also have played a role in the less dramatic effects documented in this study. It should be noted that feeding mice using LiCl in pellets within the cage top hopper while administering MAP in the drinking water resulted in high levels of mortality during a pilot experiment (data not shown). This problem was avoided by feeding the animals powdered food moistened with peanut butter in a dish at the bottom of the cage (De Groot and Rusak, 2004). This paradigm change also decreased weight loss of the animals during the experiment.
Chronic MAP treatment lengthened τ and α (Table 1), confirming earlier reports (Honma et al., 1986; Tataroglu et al., 2006). The mechanism by which MAP controls circadian rhythms is not yet known. MAP enhances monoamine neurotransmission (dopamine, norepinephrine, and serotonin), and the effects of MAP on circadian rhythms may be occurring through a monoaminergic pathway.
Not surprisingly, the overall activity levels of mice were increased by MAP. Although there are reports of increase in activity altering circadian free-running period (Mrosovsky, 1999), in this study, the effects on free-running period are unlikely to be secondary to changes in locomotor behavior. For one, increased activity levels typically lead to a shortening of τ (Yamada et al., 1988; Mrosovsky, 1999). Second, administering lithium to the animals attenuated the activity increase induced by MAP but continued to lengthen the free-running period. There does not seem to be a correlation between activity counts and τ in this situation.
Combining LiCl and 0.005% MAP altered circadian rhythms in a manner that varied depending on the photic environment in which the animals were housed. In LD, the response to LiCl and 0.005% MAP ranged from free-running activity rhythms (i.e. animals failed to remain entrained to the LD cycle) to relative coordination. Lithium is capable of reducing visual sensitivity in the mammalian retina (for review, see Klemfuss, 1992). Thus, the results obtained in LD could be the consequence of decreased salience of the photic zeitgeber (as a consequence of LiCl treatment) failing to entrain the SCN or the MASCO.
The combination of LiCl and 0.005% MAP treatment in constant conditions resulted in a consistent lengthening of τ beyond that observed with either drug singly. This effect was much larger in constant light, with many animals displaying free-running periods of 39 h or longer. The reason for the difference in effect size between DD and LL is not entirely clear. In nocturnal mammals, constant light lengthens the free-running period (Aschoff, 1960). It is possible that the lengthening effects of LL on τ further enhanced the lengthening effects of the drug treatment. Bright light, much like lithium, has been used extensively in the treatment of mood disorders (Golden et al., 2005; Wirz-Justice et al., 2005). Indeed, light may be acting as another drug affecting the circadian system. There is a dose-response curve to the phase-shifting and period-lengthening effects of light on the circadian system, with brighter light intensities resulting in larger phase-shifts and a longer τ in most species (Aschoff, 1960; Takahashi et al., 1984; Ruis et al., 1991; Boivin et al., 1996). It is possible that the parametric effects of constant light are enhanced in mice consuming both MAP and lithium, but the nonparametric, entraining effects of light are blunted resulting in the relative coordination seen when these mice are housed in LD cycles. Alternatively, the increased lengthening of τ during LiCl and MAP treatment in LL could be because of suppressed outputs from the SCN itself. Extended exposure to constant light can lead to behavioral arrhythmicity (Aschoff, 1960; Daan and Pittendrigh, 1976) and blunted rhythms of clock gene expression within the SCN (Sudo et al., 2003; Munoz et al., 2005). Suppressing signals from the SCN may allow MASCO more control over locomotor rhythmicity, resulting in the long free-running periods observed.
Animals fed with LiCl-containing chow had higher serum MAP levels than animals consuming 0.005 or 0.010% MAP alone. Serum samples were taken at only one circadian time, however, and it is possible that there is a circadian pattern of MAP clearance that is altered in LiCl-treated animals. This hypothesis has yet to be tested. When mice were consuming LiCl they increased consumption of 0.005% MAP water, which could explain the observed increase in serum MAP levels. Lithium may also alter the metabolism of MAP. An influence of lithium on psychostimulant metabolism has been implicated in the effect of lithium on amphetamine-induced stereotypy (Miyauchi et al., 1981).
Interestingly, despite the increased serum MAP levels, there were no health problems observed in mice treated with 0.005% MAP and LiCl. When animals were given 0.010% MAP directly (for the purposes of serum analysis, without having been treated with a lower dose first), two of the six mice died within a week of the onset of drug treatment. In contrast, all six of the 0.005% MAP + LiCl animals survived until blood collection despite their greatly increased serum levels of MAP. This leads us to speculate that LiCl may be protective against some of the toxic effects of MAP. Such a conclusion is supported in part by the established ability of lithium to reduce N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity (Youdim and Arraf, 2004).
Gsk3β heterozygote mice on 0.005% MAP exhibited circadian free-running periods in a range similar to 0.005% MAP-treated wild-type mice on lithium. This suggests a possible role for the inhibition of this enzyme in altering free-running period length. There is an emerging body of evidence supporting a role for GSK inhibition in the circadian effects of lithium. GSK3β phosphorylates the clock gene product Rev-erbα (Yin et al., 2006). Lithium treatment leads to degradation of Rev-erbα, and a mutated form of Rev-erbα insensitive to phosphorylation by GSK3β results in dampened circadian oscillation and an insensitivity of the circadian clock to lithium (Yin et al., 2006). Moreover, phase delays of the circadian clock in mouse embryonic fibroblasts have been observed after genetic or pharmacological inhibition of GSK activity (Kaladchibachi et al., 2007). It is possible that the action of GSK on the molecular clock is mediating the effects of MAP on the free-running periods of lithium-treated and Gsk3β heterozygote mice. Further experiments showing that lithium treatment of Gsk3β heterozygote mice does not further enhance the effect of MAP, or experiments rescuing GSK3β function in the deficient mice, would provide more definitive support for this hypothesis.
Studying the combined actions of drugs of abuse and those used to treat mood disorders is of great clinical importance. Studies have found that 55–60% of patients being treated for bipolar disorder also had a lifetime history of alcohol or substance abuse (Cassidy et al., 2001; Bizzarri et al., 2007). Lithium has light antidepressant effects and can potentiate the action of stronger antidepressants as a treatment for mood disorders (Bauer et al., 2003; Gould et al., 2004; Gould and Manji, 2005). The effects of combining lithium and similar pharmaceutical treatments for mood disorders with drugs of abuse, such as MAP, are not fully understood. Our data add to the current literature suggesting both additive and opposing effects of lithium and amphetamines on physiology and behavior. Investigating the impact of combined lithium and psychostimulant use on the circadian system may help us to understand the contribution of circadian malfunction to psychopathology and drug abuse.
Acknowledgements
The authors thank Naomi Ihara and Denise Holmes for their technical expertise and Luke Benvenuto for his assistance. They also thank the NIDA Drug Supply program for providing analytical services for the serum samples and Dr Rodger Foltz and the Center for Human Toxicology (University of Utah) for carrying out the subsequent analyses. This research was supported by Conte Center grant 1P50MH074924 and NSBRI NCC 9-58-HPF 00406 to M. Menaker. M. Miranda was supported by DGAPA, UNAM. J.M. was supported by NIH T32DK007646 and NIH F32DA024542.
References
- Abe M, Herzog ED, Block GD. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport. 2000;11:3261–3264. doi: 10.1097/00001756-200009280-00042. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Bauer M, Adli M, Baethge C, Berghofer A, Sasse J, Heinz A, et al. Lithium augmentation therapy in refractory depression: clinical evidence and neurobiological mechanisms. Can J Psychiatry. 2003;48:440–448. doi: 10.1177/070674370304800703. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarri JV, Sbrana A, Rucci P, Ravani L, Massei GJ, Gonnelli C, et al. The spectrum of substance abuse in bipolar disorder: reasons for use, sensation seeking and substance sensitivity. Bipolar Disord. 2007;9:213–220. doi: 10.1111/j.1399-5618.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Ahearn EP, Carroll BJ. Substance abuse in bipolar disorder. Bipolar Disord. 2001;3:181–188. [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. Functional-analysis of circadian pacemakers in nocturnal rodents. 3. heavy-water and constant light-homeostasis of frequency. J Comp Physiol. 1976;106:267–290. [Google Scholar]
- De Groot MH, Rusak B. Housing conditions influence the expression of food-anticipatory activity in mice. Physiol Behav. 2004;83:447–457. doi: 10.1016/j.physbeh.2004.08.037. [DOI] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Dokucu ME, Yu L, Taghert PH. Lithium- and valproate-induced alterations in circadian locomotor behavior in Drosophila. Neuropsychopharmacology. 2005;30:2216–2224. doi: 10.1038/sj.npp.1300764. [DOI] [PubMed] [Google Scholar]
- Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162:656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry. 2004;9:734–755. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- Hafen T, Wollnik F. Effect of lithium carbonate on activity level and circadian period in different strains of rats. Pharmacol Biochem Behav. 1994;49:975–983. doi: 10.1016/0091-3057(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Hiroshige T. Disorganization of the rat activity rhythm by chronic treatment with methamphetamine. Physiol Behav. 1986;38:687–695. doi: 10.1016/0031-9384(86)90265-9. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Hiroshige T. Activity rhythms in the circadian domain appear in suprachiasmatic nuclei lesioned rats given methamphetamine. Physiol Behav. 1987;40:767–774. doi: 10.1016/0031-9384(87)90281-2. [DOI] [PubMed] [Google Scholar]
- Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Akiyama M, Miyakawa K, Uchida A, Kasahara J, Fukunaga K, et al. Effect of lithium on the circadian rhythms of locomotor activity and glycogen synthase kinase-3 protein expression in the mouse suprachiasmatic nuclei. Eur J Neurosci. 2004;19:2281–2287. doi: 10.1111/j.0953-816X.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- Jensen J, Thomsen K, Olesen OV. Current determination of lithium-induced minimum sodium requirement in rats. Psychopharmacologia. 1976;45:295–299. doi: 10.1007/BF00421143. [DOI] [PubMed] [Google Scholar]
- Johnsson A, Engelmann W, Pflug B, Klemke W. Period lengthening of human circadian rhythms by lithium carbonate, a prophylactic for depressive disorders. Int J Chronobiol. 1983;8:129–147. [PubMed] [Google Scholar]
- Kaladchibachi SA, Doble B, Anthopoulos N, Woodgett JR, Manoukian AS. Glycogen synthase kinase 3, circadian rhythms, and bipolar disorder: a molecular link in the therapeutic action of lithium. J Circadian Rhythms. 2007;5:3. doi: 10.1186/1740-3391-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz G, Durst R, Zislin Y, Barel Y, Knobler HY. Psychiatric aspects of jet lag: review and hypothesis. Med Hypotheses. 2001;56:20–23. doi: 10.1054/mehy.2000.1094. [DOI] [PubMed] [Google Scholar]
- Klemfuss H. Rhythms and the pharmacology of lithium. Pharmacol Ther. 1992;56:53–78. doi: 10.1016/0163-7258(92)90037-z. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hamamura T, Ohashi K, Fujiwara Y, Kuroda S. The effect of lithium on methamphetamine-induced regional Fos protein expression in the rat brain. Neuroreport. 1999;10:895–900. doi: 10.1097/00001756-199904060-00001. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Silver R. Lithium lengthens the period of circadian rhythms in lesioned hamsters bearing SCN grafts. Biol Psychiatry. 1993;34:75–83. doi: 10.1016/0006-3223(93)90259-g. [DOI] [PubMed] [Google Scholar]
- Li X, Bijur GN, Jope RS. Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord. 2002;4:137–144. doi: 10.1034/j.1399-5618.2002.40201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi S, Honma S, Abe H, Nakamura W, Honma K. Circadian activity rhythm in methamphetamine-treated Clock mutant mice. Eur J Neurosci. 2001;14:1177–1180. doi: 10.1046/j.0953-816x.2001.01749.x. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Kikuchi K, Satoh S. Further studies on the potentiating effect of lithium chloride on methamphetamine-induced stereotypy in mice. Jpn J Pharmacol. 1981;31:61–68. doi: 10.1254/jjp.31.61. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Further experiments on the relationship between the period of circadian rhythms and locomotor activity levels in hamsters. Physiol Behav. 1999;66:797–801. doi: 10.1016/s0031-9384(99)00022-0. [DOI] [PubMed] [Google Scholar]
- Munoz M, Peirson SN, Hankins MW, Foster RG. Long-term constant light induces constitutive elevated expression of mPER2 protein in the murine SCN: a molecular basis for Aschoff’s rule? J Biol Rhythms. 2005;20:3–14. doi: 10.1177/0748730404272858. [DOI] [PubMed] [Google Scholar]
- Namima M, Sugihara K, Watanabe Y, Sasa H, Umekage T, Okamoto K. Quantitative analysis of the effects of lithium on the reverse tolerance and the c-Fos expression induced by methamphetamine in mice. Brain Res Brain Res Protoc. 1999;4:11–18. doi: 10.1016/s1385-299x(99)00002-1. [DOI] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa H, Miyauchi T. Potentiating effect of lithium chloride on methamphetamine-induced stereotypy in mice. Eur J Pharmacol. 1977;41:213–216. doi: 10.1016/0014-2999(77)90211-4. [DOI] [PubMed] [Google Scholar]
- Padiath QS, Paranjpe D, Jain S, Sharma VK. Glycogen synthase kinase 3beta as a likely target for the action of lithium on circadian clocks. Chronobiol Int. 2004;21:43–55. doi: 10.1081/cbi-120027981. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruis JF, Rietveld WJ, Buys JP. Properties of parametric photic entrainment of circadian rhythms in the rat. Physiol Behav. 1991;50:1233–1239. doi: 10.1016/0031-9384(91)90588-f. [DOI] [PubMed] [Google Scholar]
- Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- Slawson MH, Taccogno JL, Foltz RL, Moody DE. Quantitative analysis of selegiline and three metabolites (N-desmethylselegiline, methamphetamine, and amphetamine) in human plasma by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J Anal Toxicol. 2002;26:430–437. doi: 10.1093/jat/26.7.430. [DOI] [PubMed] [Google Scholar]
- Sudo M, Sasahara K, Moriya T, Akiyama M, Hamada T, Shibata S. Constant light housing attenuates circadian rhythms of mPer2 mRNA and mPER2 protein expression in the suprachiasmatic nucleus of mice. Neuroscience. 2003;121:493–499. doi: 10.1016/s0306-4522(03)00457-3. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. J Biol Rhythms. 2006;21:185–194. doi: 10.1177/0748730406287529. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A. Circadian rhythm mechanisms in affective illness and in antidepressant drug action. Pharmacopsychiatria. 1982;15:31–39. doi: 10.1055/s-2007-1019506. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Moore-Ede MC. Lithium lengthens circadian period in a diurnal primate, Saimiri sciureus. Biol Psychiatry. 1990;28:117–126. doi: 10.1016/0006-3223(90)90629-g. [DOI] [PubMed] [Google Scholar]
- Winget CM, DeRoshia CW, Markley CL, Holley DC. A review of human physiological and performance changes associated with desynchronosis of biological rhythms. Aviat Space Environ Med. 1984;55:1085–1096. [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Berger M, Lam RW, Martiny K, Terman M, Wu JC. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35:939–944. doi: 10.1017/s003329170500437x. [DOI] [PubMed] [Google Scholar]
- Yamada N, Shimoda K, Ohi K, Takahashi S, Takahashi K. Free-access to a running wheel shortens the period of free-running rhythm in blinded rats. Physiol Behav. 1988;42:87–91. doi: 10.1016/0031-9384(88)90265-x. [DOI] [PubMed] [Google Scholar]
- Yang P, Singhal N, Modi G, Swann A, Dafny N. Effects of lithium chloride on induction and expression of methylphenidate sensitization. Eur J Pharmacol. 2001;426:65–72. doi: 10.1016/s0014-2999(01)01213-4. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Arraf Z. Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax. Neuropharmacology. 2004;46:1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]