Abstract
Objective
To describe the safety and efficacy of highly active antiretroviral therapy (HAART) in pregnant women treated in an integrated antenatal antiretroviral programme (ANC ARV).
Methods
A retrospective analysis was performed on patients attending the ANC ARV from August 2004 through February 2007.
Results
Data was collected on 689 treatment-naïve pregnant women initiated on HAART. The mean age was 29.2 years. The mean baseline CD4+ count was 154 cells/uL and mean baseline HIV viral load was 101,561 copies/ml. Tuberculosis was the most prevalent presenting opportunistic infection (7.7%). Stavudine, lamivudine, and nevirapine were initiated in 82% of women with the most frequent adverse drug reaction being nevirapine-associated skin rash (3.5%). Mean gestational age at HAART initiation was 27 weeks. Among women with follow-up data, 80% gained 50 or more CD4 cells/uL, and 80.5% achieved viral suppression to <1000 copies/ml. Of 302 mother/infant pairs who completed postnatal follow-up, the HIV transmission rate was 5%. In women who received more than seven weeks of HAART during pregnancy, transmission was 0.3%.
Conclusions
Within the ANC ARV programme, initiating pregnant women on HAART was feasible, safe, and effective. Advanced gestational age at treatment initiation and loss to follow-up emerge as important challenges in this population.
Keywords: HIV/AIDS, pregnancy, high active antiretroviral therapy (HAART), prevention of mother-to-child transmission (PMTCT)
INTRODUCTION
There are an estimated five and a half million people infected with HIV in South Africa, many of whom are unaware of their status. The majority of HIV infections occur in individuals of reproductive age, and more than half of these are women. Surveys place antenatal prevalence in the range of 15-39%, with a national average of 29.1%.1 The South African HIV prevention of mother-to-child (PMTCT) programme has utilized the HIVNET 012 strategy of single dose nevirapine (sdNVP) to mother and infant from 2001 until March 2008.2 Uptake of this programme has been poor with less than 20% of HIV infected women receiving any form of antiretroviral therapy prior to 2005.3 Rates of HIV transmission in the developing world range from 5%-40%4-6 depending on access to PMTCT programmes.
The South African antiretroviral treatment programme, in the period under review, provided highly active antiretroviral therapy (HAART) to patients with CD4 counts below 200 cells/uL or with World Health Organisation (WHO) Stage 4 classification. HIV treatment programmes and antenatal care are currently provided as separate health services, and due to long waiting lists, many pregnant women present for antenatal services in the third trimester. Pregnant women who test positive for HIV-infection and qualify for HAART rely on referrals from antenatal clinics to HIV treatment programmes in order to access this care, with resultant delays in initiating treatment, often until after delivery. To address this problem with health services, an integrated antiretroviral antenatal clinic (ANC ARV clinic) was established within an existing antenatal service at Johannesburg Hospital. The goals of the integrated ANC ARV are to expedite initiation of HAART in pregnant women qualifying for this service, both to improve maternal health and to prevent mother-to-child HIV transmission. HIV/AIDS accounts for more than one-third of non-obstetric maternal and neonatal deaths, and South Africa is one of only twelve countries in the world where infant and child mortality rates increased in the period from 1990-2004, largely due to HIV/AIDS. 7 The ANC ARV is an intervention designed to improve maternal and infant health in South Africa. This paper describes the programme experience from August 2004 to February 2007.
BACKGROUND
Johannesburg Hospital is an academic public institution offering both ANC and HAART services. Pregnant women are offered voluntary counselling and testing (VCT) for HIV at their first antenatal visit. Those who test HIV-positive have a CD4 cell count specimen taken on the same day. Those with CD4+ counts less than 200 cells/uL are referred to the ANC ARV clinic. As the programme has become established, and the clinic's capacity expanded, a higher CD4 cell count cut off (250 cells/uL) has been employed for referral into the clinic. HIV-infected women with WHO Stage 4 classification, poor obstetric history, and conditions requiring amniocentesis are also referred to the ANC ARV. Women who do not qualify for HAART are seen in routine antenatal clinics and receive sdNVP for PMTCT as per National Guidelines.
All women with CD4+ counts below 200 cells/uL are offered prophylactic co-trimoxazole, and all pregnant women receive folate and iron supplementation. Upon entering care at the ANC ARV clinic, women have baseline clinical assessment, laboratory testing, and adherence counselling. At the follow-up visit one-week later a second adherence session is conducted and, if no mitigating factors exist, HAART is initiated. During the period of data collection, the first-line treatment regimen consisted of stavudine 30mg or 40mg tablets according to weight, lamivudine 150mg tablets, and nevirapine 200mg tablets. Nevirapine toxicity is monitored clinically and with alanine transaminase (ALT) measurements. For cases of toxicity or resistance, alternative drugs include zidovudine, didanosine, ritonavir boosted lopinavir (kaletra capsule), and efavirenz. Efavirenz is used in women with TB co-infection who are beyond the first trimester of pregnancy.
Women are followed at the ANC ARV clinic weekly until stable on HAART. Stability is defined as a minimum of eight weeks of treatment with absence of side effects and adequate adherence, defined as no missed doses over two consecutive visits (determined by pill count and self-report). Women who have missed doses by self-report and/or pill count remain in the ANC ARV and receive intensive follow-up adherence counselling. Those with no complications or ongoing opportunistic infections are then referred to community-based antenatal clinics for management, to ensure the ANC ARV clinic can continue to accommodate new patients.
No routine postnatal follow-up existed when the ANC ARV was initiated. Pregnant women were referred to adult HAART clinics, with high rates of loss to follow up during the referral process. In October 2005 a postnatal clinic (PNC) was established to follow postpartum women initiated on HAART during pregnancy and to provide postpartum HIV care for women identified with infection during the peripartum period. In addition, at six weeks postpartum the PNC offers HIV testing for exposed infants (HIV-1 DNA Roche Amplicor). At ten weeks postpartum, women are referred to a local HAART clinic for further care.
METHODS
A retrospective analysis was performed on data from the ANC ARV cohort from August 2004 through February 2007. Data were collected by the ANC ARV care team, which consists of a physician, nurse, and counsellor. Data were recorded for all treatment-naïve women (with the exception of prior sdNVP) referred to clinic and initiated on HAART, regardless of the duration of follow-up. Women who conceived on HAART were excluded for this analysis. Data recorded included patient demographics, baseline clinical status, laboratory values, HAART regimens, treatment complications, medical complications, and delivery characteristics. Data from clinical charts were entered into Excel spreadsheets, coded, and transferred into STATA (STATA version 9, StataCorp, College Station, Texas). All summary statistics were obtained utilizing standard STATA commands. Missing data points were excluded from the analysis and all summary statistics are reported in relation to the total number of observations available. Analysis of Variance (ANOVA) was performed in STATA to determine differences in baseline maternal characteristics for HIV-infected, HIV-uninfected, and HIV status unknown infants.
Women presenting to the obstetric clinic at Johannesburg Hospital are provided voluntary rapid HIV testing with pre and post-test counselling per South African National Guidelines. Those women referred to the ANC ARV for HAART give verbal consent to have their medical information entered into a database. Ethical approval for data collection was obtained from the University of the Witwatersrand (certificate number M050445), and exemption was given by the University of California, Los Angeles Internal Review Board.
RESULTS
Data were collected on all treatment-naïve women referred to the clinic who were subsequently initiated on HAART during pregnancy (N=689). The mean age of the cohort was 29.2 years (standard deviation 5.0, range 18-48 years). The timing of HIV diagnosis was obtained in 388 women of whom 77.3% were diagnosed during the current pregnancy. Of 410 women with prior pregnancy data available, 11.5% (N=47) were primaparous. The mean number of pregnancies per woman was 2.7 (standard deviaton 1.1 and range 1-8). Only 1.6% (N=11) of multiparous women reported taking single dose nevirapine for PMCTC during a prior pregnancy. The mean baseline CD4+ cell count performed at first clinic visit for the majority of women was 154 cells/uL (n=639, SD 92, range 6 to 784 cells/uL), with 81.3% of women having CD4 counts below 200 cells/uL. The mean baseline HIV-1 viral load was 101,561 copies/ml and standard deviation 177,585 copies/ml. The distribution of viral loads was asymmetric, with a skew towards lower ranges, as demonstrated by the median baseline HIV-1 viral load (n=452) of 29,000 copies/ml.
The most common opportunistic infection (OI) at presentation was tuberculosis (7.7%). Serious OIs identified at baseline included cryptococcal meningitis (0.4%) and Kaposi's sarcoma (0.3%). Syphilis was the only sexually transmitted infection routinely screened in this cohort. Among 457 women with rapid plasma reagin (RPR) test results, 4.8% (N= 22) were positive. The most common non-infectious medical co-morbidities were hypertension (pre-existing/non-gestational hypertension 1.0% (N=7) and gestational hypertension (hypertension developing after 20 weeks of gestation without proteinuria) 3.0% (N=21). There were also six cases of preeclampsia (0.9%) and 2 cases of eclampsia (0.3%), with no maternal deaths or adverse fetal outcomes. No cases of gestational diabetes were reported (monitoring performed with urine dipsticks); however, three (0.4%) women in the cohort had pre-existing diabetes and there were no adverse outcomes among this group.
The mean gestational age at HAART initiation was 27 weeks (data available on 437 women) and the mean number of weeks on HAART during pregnancy was 10.4 (data available on 250 women). The most commonly utilized HAART regimen in the cohort was stavudine, lamivudine, and nevirapine (82% of 620 women for whom data were available) (Table 1). Follow-up CD4+ cell counts were done in most women who remained in the program for at least 24 weeks (N=244). Of these women, 80% gained at least 50 or more cells/uL a mean of 24 weeks after baseline, and half of this group (41.4%) gained 100-250 cells/uL. Approximately 8.2% of women experienced a drop in CD4 count.
Table 1.
Antiretroviral Regimens Utilized in the Antenatal Clinic at Johannesburg General Hospital
| Regimen | Frequency (N) | Percent |
|---|---|---|
| stavudine, lamivudine, efavirenz | 47 | 7.6 |
| stavudine, lamivudine, nevirapine | 509 | 82 |
| zidovudine, didanosine, ritonavir boosted lopinavir | 1 | 0.2 |
| Other: mixed nucleosides* and NNRTI | 29 | 4.7 |
| Other: mixed nucleosides* and PI | 34 | 5.5 |
| Total | 620 | 100 |
Includes the following: stavudine, lamivudine, zidovudine, didanosine.
NNRTI: non-nucleoside reverse transcriptase inhibitor (nevirapine or efavirenz)
PI: protease inhibitor (ritonavir/lopinavir in kaletra capsule formulation)
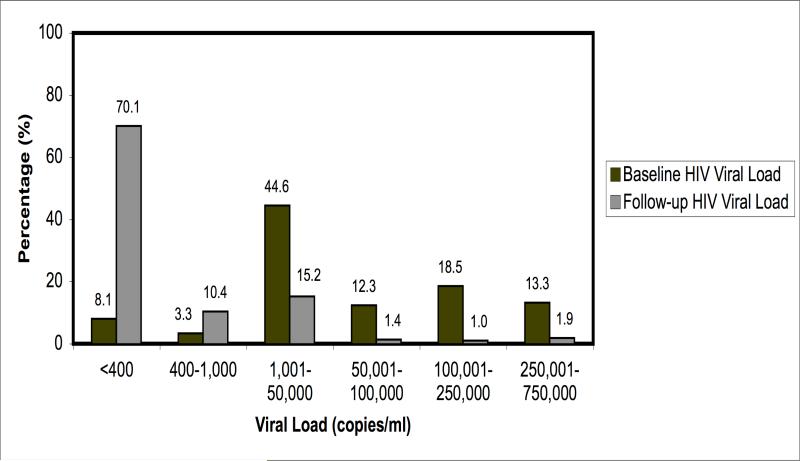
Baseline and follow-up HIV-1 viral load data were available in a subset of 211 women. At baseline 44% of these women had viral loads greater than 50,000 copies/ml. A mean of 14.4 weeks later, 80.5% were suppressed to <1000 copies/ml and only six women continued to have viremia at a level of greater than 100,000 copies/ml (Figure 1). The most common adverse reaction among women in the cohort was nevirapine associated skin rash (18 episodes, 3.5%) including 2 women with Stevens-Johnson syndrome. Clinically significant nevirapine-associated hepatitis occurred in 1% of the cohort, with no deaths. Mitochondrial toxicities were rare with neuropathy reported in two women (0.3%), although the mean duration of exposure in the cohort was brief (ten weeks). Only 35 women received protease inhibitors at any point during pregnancy and no adverse reactions were reported. Regimen changes during pregnancy were uncommon, with only 2.6% (N=16, data available on 620 women) switching to a different regimen for serious toxicity.
Figure 1.
Comparison of Baseline and Follow-up HIV-1 Viral Load Measurements on a Subset of Pregnant Women Started on HAART during Pregnancy (N=211).
(Mean of 14.4 weeks between baseline and follow-up viral load)
Among 455 women in whom delivery information was available 56.9% (N=259) had normal vaginal deliveries, 25.7% (N=117) had non-emergent Caesarean sections for reasons unrelated to HIV infection (fetal position, failure to progress, postdates, prior Caesarean section), and 17.4% (N=79) had emergent Caesarean sections for pre-eclampsia/eclampsia, fetal distress, and placental abruption or placenta previa. Among the women on whom postpartum data was available (N=355), complications included postpartum haemorrhage 2.0 % (N =7), retained products of conception 0.3 % (N=1), postpartum psychosis 0.3% (N=1), and prolapsed uterus 0.3% (N=1).
Gestational age at delivery was available for 266 infants in the cohort. Among this group, 19.5% (N=52) were premature (delivered prior to 37 weeks). Adjusted for gestational age, three premature infants were below the third percentile for weight. There were no neonatal deaths among this group. Excluding premature infants, the mean birth weight was 3023 grams (standard deviation 479 grams). Forty-eight infants (13.5%) were low birth weight (<2500 grams), and none met criteria for very low birth weight (<1500 grams). One each of the following congenital abnormalities was noted in the newborns: enlarged kidneys, bilateral clubbed feet, extra digit, and absent left hand (secondary to amniotic band).
For 302 mother/infant pairs who completed six weeks of postnatal follow-up, the HIV-1 transmission rate was 5% (N=15) as determined by HIV-1 DNA testing. Sixty-seven women (9.7%) delivered infants prior to inception of the PNC in October 2005, and 116 (16.8%) had not reached six weeks post-partum by the time the dataset was closed in February 2007, and therefore infant HIV status was unavailable. There were eleven women with fetal or infant loss, including three reports of intrauterine fetal demise, three stillbirths, and five neonatal deaths. One neonatal death was in an HIV negative infant (cause unknown), the remaining four infant deaths had unknown HIV status. Two deaths were from unknown causes and two were attributed to infection (gastroenteritis and pneumonia). The remainder of women did not return for infant HIV testing at six weeks postpartum (N=191, 27.7%), and therefore were classified as lost to follow-up. ANOVA did not reveal significant differences in the ages or baseline viral loads among women with HIV-infected versus uninfected versus status-unknown infants. For baseline CD4 cell counts, the two sample t-tests did not reach significance after Bonferroni correction, although the uncorrected p-value for the comparison of the three groups was 0.0243, with the mothers of HIV-infected infants showing lower baseline cell counts (Table 2).
Table 2.
ANOVA Comparison of Characteristics of Women in Antenatal Clinic for women with (1) HIV-uninfected babies at 6 weeks follow-up (2) HIV-infected babies at 6 weeks follow-up (3) HIV-status unknown at 6 weeks (lost to follow-up)
| Infant Cohort (defined by HIV result at 6 week follow-up) N=689 | Mean age of mother (years) | Baseline CD4 cell count (cells/uL) of mother | Mean and median initial viral load of mother (copies/ml) |
|---|---|---|---|
| Infant HIV-uninfected (N=287) | 29.3 years (N=283) | 148 cells/uL (N=275) | mean 114,253 copies/ml and median 35,950 copies/ml (N=182) |
| Infant HIV-infected (N=15) | 30.3 years (N=15) | 106 cells/uL (N=15) | mean 132,629 copies/ml and median 56,100 copies/ml (N=14) |
| HIV Status Unknown (N=387) | 29.1 years (N=376) | 161 cells/uL (N=345) | mean 91,276 copies.ml and median 25,000 copies/ml (N=257) |
| ANOVA p-value | 0.581 | 0.0278* | 0.345** |
Two sample t-tests for HIV-infected versus HIV-uninfected (Bonferonni adjusted) p-value 0.264 and for HIV-infected versus HIV-unknown (Bonferonni adjusted) p-value=0.073.
Based on mean viral load
Among those who completed follow-up infant HIV testing, the transmission rate for women who received more than seven weeks of HAART during pregnancy was 0.3% (N=1). Due to national policies limiting viral loads to twice-yearly, the cohort lacks viral load data at the time of delivery. Therefore no statistical analysis could be performed evaluating the relationship between viral load at or near the time of delivery and risk of infant HIV infection. All but two neonates received single dose nevirapine. Both of these infants had follow-up HIV testing at six weeks, with one testing positive. The majority of women (99%) chose to formula feed.
DISCUSSION
Safety and Efficacy of HAART in Pregnant Women with Advanced HIV Infection
Data from the ANC ARV clinic suggests that HAART is well tolerated and effective in pregnancy. Experience with stavudine in pregnancy has been largely limited to animal studies, with one small clinical trial in which women receiving stavudine as part of a PMTCT regimen experienced no significant toxicity.8, 9 Extensive use of stavudine in non-pregnant women in Africa has revealed high rates of mitochondrial toxicity, with overweight women particularly at risk for symptomatic and life-threatening lactic acidosis.10-12 The few human pregnancy studies evaluating NRTI-mitochondrial toxicity are limited to case reports of maternal deaths due to severe lactic acidosis, especially with the combination of stavudine and didanosine.13-16 In this Johannesburg cohort of treatment-naïve women initiating stavudine-containing regimens during pregnancy, adverse reactions were extremely rare. The low rates of observed toxicity may be due to the limited duration of NRTI exposure. The absence of reported complications during the initial period of drug exposure is reassuring, but further information is needed in regard to rates of toxicity in these women following prolonged antiretroviral exposure.
The efficacy of HAART in non-pregnant women from developed countries has been reported in a number of studies, but data in pregnant women with advanced infection is sparse.17, 18 Most women in this cohort entered clinical care late in pregnancy. In the subset of women in whom baseline and follow-up CD4+ cell counts were performed, excellent immune recovery was observed, with over 80% of this subset gaining 50 or more CD4+ cells/uL during the brief time they were on HAART. Reasons for CD4+ cells loss in 8% of women are unclear, but may be secondary to the presence of opportunistic infections causing transient suppression of CD4+ cells, haemodilution of pregnancy19, 20, or long delays between baseline CD4 cell count and the initiation of HAART.
Virologic response was difficult to assess in this cohort due to a national policy limiting viral load testing to twice yearly; however, in the group of women who underwent baseline and repeat HIV-1 RNA quantitation, more than 80% had virologic suppression to less than 1,000 copies/ml, the cut off value for predictable HIV-1 transmission, and 75.6% had an undetectable viral load. Resistance testing was not available for the subset of women who failed to suppress their viral load on HAART. Resistance from previous sdNVP exposure is unlikely to have played a role in treatment failure since only 1.6% of the cohort reported having received PMTCT during a prior pregnancy.
Successful immunologic and virologic response in this subset of women may be due to availability of intensive adherence counselling, which occurs at entry to the clinic and at each of the clinic appointments, and high motivation of women for prevention of infant HIV infection. Efficacy data may be biased by both missing observations among those who completed follow-up and by high rates of loss to follow-up. Individuals lost from the program may have been most at risk for poor adherence and less favourable outcomes.
At entry to the ANC ARV programme, evaluation for OIs was performed. Overall, OIs were uncommon, with tuberculosis most commonly identified. Pregnant women may represent a healthier category of women with AIDS, since a certain degree of body weight, reproductive health, and immune robustness must exist to conceive and support gestation. Recent data suggest that pregnancy is associated with a lower risk of HIV disease progression and experience with the ANC ARV cohort supports this finding.21
The mother-to-child transmission rate for this group of women with advanced HIV disease is low (5%) compared to women who do not access HAART, and is much lower than the rate of 11.1% reported from a recent study of healthier women (CD4 cell counts > 200/uL) who received sdNVP for PMTCT in Soweto, Johannesburg.22 However, the number of infected infants in the ANC ARV cohort is high relative to developed countries where HAART is used for PMTCT and transmission rates are less than 2%.23, 24 The high rate of HIV transmission relative to developed country settings is multifactorial, with this data reinforcing the importance of duration of HAART on rates of transmission. With one exception, all HIV-infected infants were born to women who received seven or fewer weeks of HAART.
Maximizing Safety and Efficacy of HAART in the ANC ARV: The Challenge of Late Presentation and Closing the Treatment Gap
Many HIV-infected individuals in South Africa are unaware of their status, do not perceive their risk, and only present for HIV testing late in the course of disease when symptomatic with opportunistic infections. Pregnant women are an exception to this paradigm, since most are healthy and undergo HIV testing in the setting of existing antenatal services. Despite the opportunity that antenatal care presents for diagnosing HIV in women of reproductive age, many of these HIV diagnoses occur late in gestation. Additionally, 23% of women in this cohort knew their HIV status prior to conception yet did not present for medical care until the third trimester. HIV-infected patients who do not qualify for HAART are referred to wellness centres for follow-up and care, but retention in these programmes is poor.
Delayed diagnosis and late presentation to medical care have implications for optimal management of HIV during pregnancy, particularly with regard to the timing of HAART initiation. Ideally, women should be empowered to undergo HIV testing prior to conception, and if positive, encouraged to access services for health optimization. For women who qualify for HAART, goals include early initiation of antiretroviral regimens safe in pregnancy and complete viral suppression prior to delivery. The success of this approach is illustrated by extremely low transmission rates in countries that use HAART for PMTCT and within this cohort for women who received more than seven weeks of HAART.
Loss to Follow-up and Other Limitations of Data Analysis
In this cohort 27.7% of women did not return to the PNC for infant HIV testing. The issue of missing data as well as loss to follow-up of mother/infant pairs is significant. Missing data points are the result of referral to routine clinics after stabilization, as well as return of women to rural homes around the time of delivery. Although ANOVA did not detect differences in mean age, baseline CD4 cell count, or baseline viral load for individuals who remained in the program as compared to those lost to follow-up, women who stayed in the ANC ARV programme through the postnatal follow-up period may represent a distinctly different group based on socio-economic factors, clinical status, and HIV infection characteristics. These differences are likely to bias the data, particularly in regard to HIV transmission rates.
CONCLUSIONS
Within the ANC ARV programme, initiating women on HAART during pregnancy was feasible, safe, and effective. HAART-related complications were low, and in the subset of women on whom follow-up CD4+ cell counts and HIV viral loads were available, most had an excellent response to therapy with good short-term immune reconstitution and successful viral suppression. Advanced immunosuppression, late gestational age at presentation, and high rates of loss to follow-up emerge as important challenges in this population.
In South Africa, antenatal care, HIV treatment programmes, and HIV prevention services are often delivered separately, and this division of health services creates barriers to comprehensive care. The ANC ARV programme combines antenatal care, delivery of HAART to pregnant women with advanced HIV, and interventions for the prevention of mother-to-child HIV transmission in a coordinated and expedited fashion. As a result of the success of the ANC ARV at Johannesburg Hospital, four additional sites have been opened in the surrounding area, including two programmes managed by midwives within primary health care clinics. It is anticipated that with ongoing support from public health leadership, sustainability will be attainable. This type of integrated programme may be a crucial step towards closing the HIV treatment gap in South Africa.
Acknowledgements
The authors thank Dr. Thomas Coates and the UCLA Program in Global Health for their support, PEPFAR and UNAIDS who assist with funding the ANC and PNC, the South African Department of Health, and the staff and patients of the ANC ARV and PNC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Department of Health RoSA [May 8, 2008];National HIV and Syphilis Prevalence Survey South Africa 2005. at http://www.hivan.org.za/admin/documents/anchivsummary.pdf.
- 2.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999 Sep 4;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 3.Barron P, Day C, Monticelli F. District Health Barometer 2005-2006. http://www.hst.org.za/uploads/files/secB_gp.pdf.
- 4.Wiktor SZ, Ekpini E, Karon JM, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet. 1999 Mar 6;353(9155):781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 5.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004 Jul 15;351(3):217–228. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 6.HIV WGoM-t-CTo Rates of mother-to-child transmission of HIV-1 in Africa, America and Europe: results from 13 perinatal studies. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:506–510. doi: 10.1097/00042560-199504120-00011. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw D, Chopra M, Kerber K, et al. Every death counts: use of mortality audit data for decision making to save the lives of mothers, babies, and children in South Africa. Lancet. 2008 Apr 12;371(9620):1294–1304. doi: 10.1016/S0140-6736(08)60564-4. [DOI] [PubMed] [Google Scholar]
- 8.Barreto RL, Soares JM, Jr., Simoes RS, Maciel GA, Simoes Mde J, Kulay L., Jr Effects of chronic stavudine exposure on liver, pancreas and kidneys of pregnant rats and their fetuses: morphological and biochemical aspects. Eur J Obstet Gynecol Reprod Biol. 2006 Sep-Oct;128(1-2):50–53. doi: 10.1016/j.ejogrb.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Wade NA, Unadkat JD, Huang S, et al. Pharmacokinetics and safety of stavudine in HIV-infected pregnant women and their infants: Pediatric AIDS Clinical Trials Group protocol 332. J Infect Dis. 2004 Dec 15;190(12):2167–2174. doi: 10.1086/425903. [DOI] [PubMed] [Google Scholar]
- 10.Geddes R, Knight S, Moosa MY, Reddi A, Uebel K, Sunpath H. A high incidence of nucleoside reverse transcriptase inhibitor (NRTI)-induced lactic acidosis in HIV-infected patients in a South African context. S Afr Med J. 2006 Aug;96(8):722–724. [PubMed] [Google Scholar]
- 11.Currier JS. Sex Differences in Antiretroviral Therapy Toxicity: Lactic Acidosis, Stavudine, and Women. Clin Infect Dis. 2007 July 15;(45) doi: 10.1086/518977. [DOI] [PubMed] [Google Scholar]
- 12.Bolhaar MG, Karstaedt AS. A high incidence of lactic acidosis and symptomatic hyperlactatemia in women receiving highly active antiretroviral therapy in Soweto, South Africa. Clin Infect Dis. 2007 Jul 15;45(2):254–260. doi: 10.1086/518976. [DOI] [PubMed] [Google Scholar]
- 13.Luzzati R, Del Bravo P, Di Perri G, Luzzani A, Concia E. Riboflavine and severe lactic acidosis. Lancet. 1999 Mar 13;353(9156):901–902. doi: 10.1016/S0140-6736(99)00523-1. [DOI] [PubMed] [Google Scholar]
- 14.Sarner L, Fakoya A. Acute onset lactic acidosis and pancreatitis in the third trimester of pregnancy in HIV-1 positive women taking antiretroviral medication. Sex Transm Infect. 2002 Feb;78(1):58–59. doi: 10.1136/sti.78.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandelbrot L, Kermarrec N, Marcollet A, et al. Case report: nucleoside analogue-induced lactic acidosis in the third trimester of pregnancy. Aids. 2003 Jan 24;17(2):272–273. doi: 10.1097/01.aids.0000050791.28043.19. [DOI] [PubMed] [Google Scholar]
- 16.(BMS) B-MSC Important Change in Sustiva (efavirenz) Package Insert---Change from Pregnancy Category C to D. [May 8, 2008];FDA Medwatch. at http://www.fda.gov/medwaTCH/safety/2005/Sustiva_DHCPletter-061005.pdf.
- 17.Cassol E, Page T, Mosam A, et al. Therapeutic response of HIV-1 subtype C in African patients coinfected with either Mycobacterium tuberculosis or human herpesvirus-8. J Infect Dis. 2005 Feb 1;191(3):324–332. doi: 10.1086/427337. [DOI] [PubMed] [Google Scholar]
- 18.Akileswaran C, Lurie MN, Flanigan TP, Mayer KH. Lessons learned from use of highly active antiretroviral therapy in Africa. Clin Infect Dis. 2005 Aug 1;41(3):376–385. doi: 10.1086/431482. [DOI] [PubMed] [Google Scholar]
- 19.Ureland K. Cardiorespiratory physiology of pregnancy. Vol. 3. Harper and Row; Baltimore: 1979. [Google Scholar]
- 20.Assali N. Pathophysiology of Gestation, Vol 1: Maternal Disorders. Academic Press; New York: 1972. [Google Scholar]
- 21.Tai JH, Udoji MA, Barkanic G, et al. Pregnancy and HIV Disease Progression during the Era of Highly Active Antiretroviral Therapy. J Infect Dis. 2007 Oct 1;196(7):1044–1052. doi: 10.1086/520814. [DOI] [PubMed] [Google Scholar]
- 22.Martinson NA, Ekouevi DK, Dabis F, et al. Transmission rates in consecutive pregnancies exposed to single-dose nevirapine in Soweto, South Africa and Abidjan, Cote d'Ivoire. J Acquir Immune Defic Syndr. 2007 Jun 1;45(2):206–209. doi: 10.1097/QAI.0b013e318050d652. [DOI] [PubMed] [Google Scholar]
- 23.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002 Apr 15;29(5):484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 24.Achievements in public health Reduction in perinatal transmission of HIV infection--United States, 1985-2005. MMWR Morb Mortal Wkly Rep. 2006 Jun 2;55(21):592–597. [PubMed] [Google Scholar]