Abstract
Background and objectives: Achieving and maintaining dry-weight appears to be an effective but forgotten strategy in controlling and maintaining normotension among hypertensive patients on hemodialysis.
Methods: Qualitative review of literature to define dry-weight and its utility in achieving blood pressure control.
Results: The concept of dry-weight has evolved over time and its definition has changed. One such definition defines dry-weight as the lowest tolerated postdialysis weight achieved via gradual change in postdialysis weight at which there are minimal signs or symptoms of hypovolemia or hypervolemia. Although clinical examination does not perform well in detecting latent increase in dry-weight, several technologies such as relative plasma volume monitoring and body impedance analysis are emerging that may help in assessing dry-weight in the future. Sodium restriction is a modifiable risk factor that can lead to better blood pressure (BP) control. However, dietary sodium restriction requires lifestyle modifications that are difficult to implement and even harder to sustain over the long term. Restricting dialysate sodium is a simpler but underexplored strategy that can reduce thirst, limit interdialytic weight gain, and assist the achievement of dry-weight. Achievement of dry-weight can improve interdialytic BP, reduce pulse pressure, and limit hospitalizations.
Conclusions: Avoiding medication-directed control of BP may enhance the opportunity to probe dry-weight, facilitate removal of volume, and limit the risk for pressure-volume overload, which may be a significant concern leading to myocardial remodeling in the hemodialysis patient. Probing dry-weight among patients with ESRD has the potential to improve dismal cardiovascular outcomes.
Nearly 40 years ago, when dialysis was still in its infancy, John Merrill and his colleagues predicted that if dialysis patients lived long enough, they would die of cardiovascular disease (1). Their prophecy has not only proven to be true, but disappointingly, nearly all trials done in patients with ESRD have not managed to reduce the enormous burden of cardiovascular morbidity and mortality. One factor that has caused more controversy than the rest has been the issue of hypertension among patients with ESRD (2,3). Epidemiologic studies performed when blood pressure (BP) is measured before and after dialysis have failed to incriminate hypertension as a cardiovascular risk factor. In fact, these studies, whether done in incident or prevalent patients, find that low BP or BP that declines over time is associated with poor outcomes (4). Accordingly, this important cardiovascular risk factor in the general population has taken a backstage in the management of hemodialysis patients. However, two meta-analyses suggest that the use of antihypertensive drugs can improve cardiovascular outcomes by lowering BP (5,6). Even the studies that form the basis of these meta-analyses do not address the issue of nonpharmacologic management in the case of hypertensive hemodialysis patients. One study suggested that the use of more antihypertensive drugs in hemodialysis patients is paradoxically associated with even worse BP control (7). It is likely that too much medication may actually limit the opportunity to probe dry-weight and lead to BP resistance through expanded volume. Subsequent pressure/volume overload could lead to cardiac remodeling and increase the risk for development of congestive heart failure and arrhythmia (8,9). In managing hypertension among hemodialysis patients, this review discusses the definition and relevance of dry-weight and barriers to its achievement.
Dry-Weight
The concept of dry-weight is as old as dialysis itself and has been defined various ways. These definitions have evolved over time.
Definition
In 1967, dry-weight was initially defined by Thomson and colleagues as reduction of BP to hypotensive levels during ultrafiltration and unassociated with other obvious causes (7). Then, in 1980 dry weight was defined by Henderson as the weight obtained at the conclusion of a regular dialysis treatment below which the patient more often than not will become symptomatic and go into shock. In 1996, dry-weight was defined by Charra and colleagues as that body weight at the end of dialysis at which the patient can remain normotensive until the next dialysis despite the retention of saline and ideally without the use of antihypertensive medications (8). In 2008, Raimann et al. proposed a definition of dry-weight defined by continuous calf bioimpedance analysis during dialysis. They defined dry-weight as a flattening of the baseline/instantaneous impedance ratio curve for at least 20 minutes in the presence of ongoing ultrafiltration. Finally, in 2009, Sinha and Agarwal (9) proposed a definition that combines subjective and objective measurements. According to this definition, dry-weight is defined as the lowest tolerated postdialysis weight achieved via gradual change in postdialysis weight at which there are minimal signs or symptoms of hypovolemia or hypervolemia.
Because excess dietary or dialysate sodium may provoke excess interdialytic weight gain, clinicians often confuse that a strong link exists between salt and dry-weight. Notably, none of the definitions of dry-weight include dietary or dialysate sodium measurements. Although large interdialytic weight gains may impair achieving dry-weight, limiting interdialytic weight gain by restricting dialysate or dietary sodium intake does not guarantee the achievement of dry-weight. In fact, patients who gain limited amount of interdialytic weight may do so because they are above dry-weight. Although the reason for this is not immediately apparent, one possibility is visceral congestion due to subtle volume overload that may suppress appetite.
Assessment of Dry-Weight
Pedal edema does not correlate with dry-weight very well. In a case control study, Agarwal et al. (10) found that inferior vena cava diameter, blood volume monitoring, plasma volume markers, and inflammation markers were not determinants of edema. Pedal edema correlated with cardiovascular risk factors such as age, obesity, and left ventricular mass but not volume markers in hemodialysis patients. For most part, the assessment and achievement of dry-weight is an iterative process that often provokes uncomfortable intradialytic symptoms such as hypotension, dizziness, cramps, nausea, and vomiting. The symptoms lead to interventions such as cessation of ultrafiltration, administration of saline, the premature cessation of dialysis, or placing the patient in the head-down (Trendelenburg) position. Interestingly, placing the patient in the Trendelenburg position does little to protect the BP, and this practice is questionable (11); however, raising the leg passively without lowering the head can be effective for raising ventricular filling pressure (12) Often physicians will respond to these distressing symptoms by raising dry-weight, and then add more antihypertensive medication. Paradoxically, this may make subsequent achievement of dry-weight more difficult. However, if dry-weight is reduced gently by setting the ultrafiltration goal to just a little above the previous achieved postdialysis weight (say by 0.2 to 0.3 kg in an adult) without changing the dialysis time or better still by prolonging the dialysis time to allow for slower ultrafiltration with dialysis, then dry-weight can be successfully achieved.
Newer Developments in the Assessment of Dry-Weight
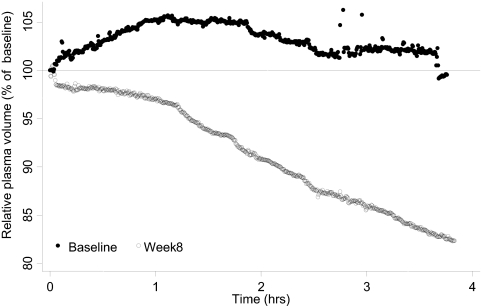
Relative plasma volume (RPV) monitoring utilizes photooptical technology to noninvasively measure absolute hematocrit through a transparent chamber affixed to the arterial end of the dialyzer. Accordingly, percent blood volume change during the dialysis procedure can be calculated in real time. RPV slope is a function of ultrafiltration rate and the plasma refill rate. Patients who are “wet” have large interstitial fluid volumes and therefore a high plasma refill rate; their RPV slope will be flat. Patients with a low plasma refill rate will have steeper slopes and are more likely to be at their “dry-weight” (Figure 1).
Figure 1.
Example of RPV monitoring as an indicator of dry-weight. A 42-year-old black man with ESRD on chronic hemodialysis for 8 years treated with four antihypertensive medications consented to participate in the DRIP trial after he was noted to be hypertensive. Interdialytic ambulatory BP monitoring revealed a BP value of 149/89 mmHg. At baseline, RPV monitoring demonstrated no change in RPV. Dry-weight was probed in the subsequent 8 weeks. He lost 2.0 kg of postdialysis weight from 62.0 to 60.0 kg. At 8 weeks, RPV monitoring revealed a 3.15% reduction in RPV per hour. Interdialytic ambulatory BP improved to 125/77 mmHg. RPV monitoring may be a useful tool to assess dry-weight.
In the Dry-weight Reduction In hypertensive hemodialysis Patients (DRIP) trial, RPV monitoring was performed in all patients at the beginning and end of the study (13). RPV slopes were defined as flat when they were less than the median (1.33% per hour) at the baseline visit. The study found that RPV slopes suggest a volume-overloaded state for four reasons: (1) probing dry-weight in these patients led to steeper slopes; (2) those with flatter slopes at baseline had greater weight loss; (3) baseline RPV slopes and the intensity of weight loss were found to be important for subsequent change in RPV slopes; and, most importantly, (4) RPV slopes predicted the subsequent reduction in interdialytic ambulatory systolic BP—those with the flat test slopes had the greatest decline in BP on probing dry-weight. Thus, RPV slope monitoring may be useful to assess dry-weight among hypertensive hemodialysis patients. RPV monitoring, combined with clinical assessment of intradialytic hypovolemia and postdialytic fatigue, can help assess patient dry-weight and optimize volume status while reducing dialysis-associated morbidity (14).
Wabel et al. (15) measured body composition through body impedance analysis and predialysis systolic BP among 500 patients from eight dialysis centers in Europe. One-third of the patients had normal BP and normal fluid status by the definitions used by the authors. Hypertension and volume expansion was found in 15% of the patients. Hypertension with no volume expansion was found in 13% of the patients, and BP was reasonable but patients were volume-expanded in 10% of the patients. The joint consideration of hydration state and BP provides a tool for classifying patients in terms of volume-sensitive and volume-resistant hypertension. This study represents an important conceptual advance when designing optimal treatment strategies.
Absolute measurements of total body water may become more feasible with the use of portable mass spectrometers. Chan et al. (16) reported about the use of a flowing afterglow mass spectrometer after ingestion of heavy water immediately after dialysis among 12 hemodialysis patients. Measurements of total body water immediately after hemodialysis and immediately preceding the following dialysis showed excellent agreement between the two measurements after accounting for insensible losses and urine output. The coefficient of variation in total body water between the two measurements was 2.6%. This proof-of-principle study demonstrated that absolute total body water can be determined among hemodialysis patients. Further work is required before this study can be used for day-to-day decision-making about volume management.
Benefits of Probing Dry-Weight
Dry-weight was probed without changing the dialysis time in a randomized controlled trial of hypertensive hemodialysis patients (15). Interdialytic ambulatory BP was reduced within 4 weeks by 11/6 mmHg (17). This level of BP reduction was achieved despite stable concurrent use of 2.7 antihypertensive drugs. The magnitude of reduction in BP is therefore much larger than what would be expected by adding an additional antihypertensive agent. Because the control group had a placebo effect, the placebo-corrected ambulatory BP reduction was 7/3 mmHg. This antihypertensive effect was sustained for 8 weeks of observation. Despite provoking occasional uncomfortable intradialytic symptoms, the quality of life was not impaired. Notably, in this study, patients with obvious volume overload were excluded. Thus, the study tested the hypothesis that hypertension among hemodialysis patients who do not manifest overt signs of volume overload is mediated by excess volume. The results of this study reject the null hypothesis. In fact, the presence or absence of edema, which is often taken to be as a reliable sign of volume overload, had no predictive value in separating the responders from nonresponders. Furthermore, 10% of the patients in the control group developed accelerated hypertension defined as BP ≥175/105 mmHg by interdialytic ambulatory monitoring. This study provides support to the notion that among hemodialysis patients, dry-weight reduction is an effective strategy for reducing BP.
Observational studies also support the practice of probing dry-weight. For example, in a report from Turkey, Kayikcioglu et al. (18) compared the benefit of nonpharmacologic to pharmacologic therapy for control of left ventricular mass among hemodialysis patients. In a cross-sectional study, patients who had been treated at one center with salt restriction and dry-weight reduction were compared with another center where antihypertensive-based therapy was the primary method for management of hypertension. The center using dry-weight and salt restriction as a strategy had the following benefits: lower antihypertensive drug use (7% versus 42%), lower interdialytic weight gain, lower left ventricular mass, better diastolic and systolic left ventricular function, and fewer episodes of intradialytic hypotension. These observations are important and of clinical relevance; they suggest that probing for dry-weight as opposed to adding more antihypertensive drugs perhaps diminishes the risk for cardiac remodeling. Although, a cross-sectional study cannot assert causation, the results of this study support the use of nonpharmacologic therapies in the management of patients with ESRD.
Dry-Weight and Outcomes
Studies among hemodialysis patients in adults and children suggest that managing intradialytic RPV may reduce the number of hospital admissions due to fluid overload (14,19), improve BP control, and decrease hypotension-associated dialysis symptoms (20). It is possible that the latter benefit is, in part, related to diminished use of antihypertensive medication. Accordingly, monthly monitoring of relative blood volume and home BP may offer an attractive way to assess the adequacy of volume control among hemodialysis patients.
To study the effect of volume status on mortality, Wizeman et al. (21) followed 269 prevalent hemodialysis patients for several years. They measured hydration state using a body composition analyzer. If there was >15% excess of extracellular water (2.5-L volume excess), they classified such patients as volume-overloaded. In a multivariate adjusted analysis, they found that excess hydration was associated with high mortality. The hazard ratio of mortality with excess fluid volume was 2.1 times greater (P = 0.003) compared with those without. All in all, 25% of the patients had excess extracellular fluid (ECF) volume. Although the study did not examine reduction in ECF volume in subsequent outcomes, it is quite likely that improvement in ECF volume will be associated with better mortality outcomes if such studies are performed in the future.
Inrig et al. (22) compared the change in pulse pressure during dialysis as a risk factor for hospitalization and mortality among prevalent hemodialysis patients participating in a randomized controlled trial. They found that patients who had the least change in pulse pressure from before to after dialysis had clinical characteristics indicating volume overload. Among these patients, lowering of the pulse pressure from before to after dialysis was associated with lower hospitalization and mortality outcomes. Because systolic BP largely drives pulse pressure, it is likely that lowering of pulse pressure with dialysis reflects more volume loss, a lesser hydration state, and may provide better cardiovascular outcomes, perhaps through less pressure/volume stress on the heart.
Potential Hazards of Probing Dry-Weight
There are potential hazards related to probing dry-weight, including (1) increased risk of clotted angioaccess, (2) increased rate of attrition in residual renal function, and (3) complications related to interdialytic hypotension. Intradialytic hypotension, in addition to requiring more nursing interventions, can be complicated by cerebral hypoperfusion, seizures, myocardial dysfunction, and mesenteric ischemia. The relative risks and benefits of probing dry-weight have not been qualified in long-term randomized trials.
Barriers to the Achievement of Dry-Weight
Nonadherence with Prescription
Patients often miss dialysis or want to reduce their time on dialysis. This may be a significant but often overlooked factor that limits the achievement of dry-weight. Missing dialysis or cutting time may not be captured by the measurement of Kt/V if the patients stay the full time on the day of the measurement. Compliance with dialysis therapy should be carefully assessed in those individuals with hypertension that is difficult to control.
Too Short Dialysis
Short-duration dialysis may limit the achievement of dry-weight. Long-duration dialysis with slow, continuous ultrafiltration has been reported to lower BP and facilitate withdrawal of antihypertensive medications. This is likely related to better achievement of dry-weight (23). In Tassin, France, Charra et al. (24) have maintained patients on long, slow hemodialysis with an overall excellent patient survival. They believe that achievement of BP control without use of antihypertensive medication should be used to judge adequacy. In a randomized trial, long-duration dialysis was found to regress left ventricular hypertrophy (25). Is this simply related to lower BP, or is it an effect on reducing cardiac pressure and volume? It is likely related to sustained achievement of dry-weight. Further randomized trials are awaited.
Excess Dietary Sodium
Because of the anephric state, patients on dialysis demonstrate a direct effect of salt intake with intravascular volume increase proportional to the level of salt intake. Limiting ECF expansion offers the potential of diminishing the adverse effects of pressure and volume overload on the function of the heart, lungs, liver, and other organs. Animal and human studies demonstrate a role for excess dietary salt intake as a cardiovascular risk factor (26). Despite these considerations, randomized, controlled studies suggest that an effect of controlled dietary salt intake on mortality in dialysis patients is absent. A meta-analysis of trials of sodium restriction in normotensive and hypertensive individuals concluded that a 50-mEq/d reduction in dietary sodium (that can simply be achieved by taking away table salt or healthier choices of nonprocessed food) would lead to a fall in systolic BP of 5 mmHg on average and 7 mmHg in those who are more hypertensive (27). Furthermore, at least 5 weeks of sodium restriction would be required to see such an effect.
Monitoring interdialytic weight gain serves as a convenient tool to monitor dietary salt intake. The management of patients with ESRD requires counseling to limit dietary salt intake when weight gain becomes excessive. Restricting fluid intake without restricting salt has no scientific basis for the management of hemodialysis patients. Sodium as an extracellular cation has an important effect on volume, whereas water distributes into cells (two-thirds of it) and thus has less of an effect on volume and BP. Fluid restriction should not be the focus of management among hemodialysis patients—sodium restriction should.
Patients with ESRD may have salt craving and may therefore consume excess salt. Kusaba et al. (28) compared 11 healthy volunteers to 29 patients with chronic kidney disease (CKD) using a taste test with sodium-impregnated test strips. They found that oral sodium intake was proportional to the taste threshold for sodium. The taste threshold for sodium was blunted in patients with CKD. Furthermore, zinc deficiency was associated with this latent taste dysfunction. These findings suggest that latent gustatory dysfunction and zinc deficiency may underlie excess sodium intake among patients with CKD. Although this study was limited to patients with earlier stages of CKD, similar mechanisms may mediate gustatory dysfunction among those with ESRD on hemodialysis.
There is no evidence that loop diuretics, even when given in high doses (as high as 250 mg furosemide intravenously), among anuric hemodialysis patients leads to changes in central cardiac hemodynamics using tissue Doppler echo imaging (29). Thus, loop diuretics appear to be of little value in the management of hypertension among those with ESRD.
Dialysate Sodium Excess
High dialysate sodium improves hemodynamic stability but may aggravate interdialytic hypertension. A simple strategy to limit sodium exposure is to reduce dialysate sodium (30). In a recent nonrandomized trial, reduction in sodium load was found to improve BP control even among peritoneal dialysis patients (31). In some patients, low sodium dialysate prescription may aggravate intradialytic hypotension. Reducing the dialysate temperature to 35°C may help sustain intradialytic BP in such patients.
Research from Titze's group has demonstrated in animal experiments that sodium can be rendered osmotically inactive (32). They postulate that sodium can be stored in the skin without net expansion of net plasma volume. Heer et al. (33) have demonstrated similar results among normal healthy volunteers. What role, if any, nonosmotic sodium regulation has in the control of volume among chronic hemodialysis patients remains to be demonstrated.
Conclusions
Achieving and maintaining dry-weight appears to be an effective but forgotten strategy in controlling and maintaining normotension among hypertensive patients on hemodialysis. Dietary or dialysate sodium intake is a modifiable risk factor that can lead to better BP control. However, dietary sodium restriction requires lifestyle modifications that are difficult to implement and even harder to sustain over the long term. Restricting dialysate sodium is a simpler but underexplored strategy that can reduce thirst, limit interdialytic weight gain, and assist the achievement of dry-weight. Dry-weight can be assessed inexpensively through RPV monitoring and body impedance analysis. Achievement of dry-weight can improve interdialytic BP, reduce pulse pressure, and limit hospitalizations. Probing dry-weight among patients with ESRD has the potential to improve dismal cardiovascular outcomes through reducing cardiac pressure/volume load and limit remodeling. Thus, medication-directed approaches for BP control should be a secondary consideration to manipulating the diet and dialysis prescription to achieve dry-weight.
Disclosures
None.
Acknowledgments
This work was supported by a research award to R.A. (2 RO1-DK062030-06).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Lazarus JM, Hampers C, Merrill JP: Hypertension in chronic renal failure. Treatment with hemodialysis and nephrectomy. Arch Intern Med 133: 1059–1066, 1974 [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD: Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63: 793–808, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Agarwal R: Hypertension is harmful to dialysis patients and should be controlled. Semin Dial 20: 518–522, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Lacson E, Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW: The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis 48: 606–615, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Heerspink HJ, Ninomiya T, Zoungas S, de ZD, Grobbee DE, Jardine MJ, Gallagher M, Roberts MA, Cass A, Neal B, Perkovic V: Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: A systematic review and meta-analysis of randomised controlled trials. Lancet 373: 1009–1015, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal R, Sinha AD: Cardiovascular protection with antihypertensive drugs in dialysis patients: Systematic review and meta-analysis. Hypertension 53: 860–866, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson GE, Waterhouse K, McDonald HP, Jr, Friedman EA: Hemodialysis for chronic renal failure. Clinical observations. Arch Intern Med 120: 153–167, 1967 [PubMed] [Google Scholar]
- 8.Charra B, Laurent G, Chazot C, Calemard E, Terrat JC, Vanel T, Jean G, Ruffet M: Clinical assessment of dry weight. Nephrol Dial Transplant 11[ Suppl 2]: 16–19, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Sinha AD, Agarwal R: Can chronic volume overload be recognized and prevented in hemodialysis patients? The pitfalls of the clinical examination in assessing volume status. Semin Dial 22: 480–482, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Andersen MJ, Pratt JH: On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol 3: 153–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridges N, Jarquin-Valdivia AA: Use of the Trendelenburg position as the resuscitation position: to T or not to T? Am J Crit Care 14: 364–368, 2005 [PubMed] [Google Scholar]
- 12.Monnet X, Teboul JL: Passive leg raising. Intensive Care Med 34: 659–663, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Sinha AD, Light RP, Agarwal R: Relative plasma volume monitoring during hemodialysis aids the assessment of dry weight. Hypertension 55: 305–311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez HJ, Domenici R, Diroll A, Goykhman I: Assessment of dry weight by monitoring changes in blood volume during hemodialysis using Crit-Line. Kidney Int 68: 854–861, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, Taborsky P, Tetta C, Velasco N, Vlasak J, Zaluska W, Wizemann V: Towards improved cardiovascular management: The necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant 23: 2965–2971, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Chan C, Smith D, Spanel P, McIntyre CW, Davies SJ: A non-invasive, on-line deuterium dilution technique for the measurement of total body water in haemodialysis patients. Nephrol Dial Transplant 23: 2064–2070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R, Alborzi P, Satyan S, Light RP: Dry-weight reduction in hypertensive hemodialysis patients (DRIP): A randomized, controlled trial. Hypertension 53: 500–507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayikcioglu M, Tumuklu M, Ozkahya M, Ozdogan O, Asci G, Duman S, Toz H, Can LH, Basci A, Ok E: The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant 24: 956–962, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein SL, Smith CM, Currier H: Noninvasive interventions to decrease hospitalization and associated costs for pediatric patients receiving hemodialysis. J Am Soc Nephrol 14: 2127–2131, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Patel HP, Goldstein SL, Mahan JD, Smith B, Fried CB, Currier H, Flynn JT: A standard, noninvasive monitoring of hematocrit algorithm improves blood pressure control in pediatric hemodialysis patients. Clin J Am Soc Nephrol 2: 252–257, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D: The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 24: 1574–1579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inrig JK, Patel UD, Toto RD, Reddan DN, Himmelfarb J, Lindsay RM, Stivelman J, Winchester JF, Szczech LA: Decreased pulse pressure during hemodialysis is associated with improved 6-month outcomes. Kidney Int 76: 1098–1107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagugli RM, Pasini P, Quintaliani G, Pasticci F, Ciao G, Cicconi B, Ricciardi D, Santirosi PV, Buoncristiani E, Timio F, Valente F, Buoncristiani U: Association between extracellular water, left ventricular mass and hypertension in haemodialysis patients. Nephrol Dial Transplant 18: 2332–2338, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Charra B, Calemard M, Laurent G: Importance of treatment time and blood pressure control in achieving long-term survival on dialysis. Am J Nephrol 16: 35–44, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ: Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA 298: 1291–1299, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Sanders PW: Assessment and treatment of hypertension in dialysis: The case for salt restriction. Semin Dial 20: 408–411, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Law MR, Frost CD, Wald NJ: By how much does dietary salt reduction lower blood pressure? III. Analysis of data from trials of salt reduction. BMJ 302: 819–824, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusaba T, Mori Y, Masami O, Hiroko N, Adachi T, Sugishita C, Sonomura K, Kimura T, Kishimoto N, Nakagawa H, Okigaki M, Hatta T, Matsubara H: Sodium restriction improves the gustatory threshold for salty taste in patients with chronic kidney disease. Kidney Int 76: 638–643, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Hayashi SY, Seeberger A, Lind B, Gunnes S, Alvestrand A, do Nascimento MM, Lindholm B, Brodin LA: Acute effects of low and high intravenous doses of furosemide on myocardial function in anuric haemodialysis patients: A tissue Doppler study. Nephrol Dial Transplant 23: 1355–1361, 2008 [DOI] [PubMed] [Google Scholar]
- 30.de Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJ, Santos SF: Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int 66: 1232–1238, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Davies S, Carlsson O, Simonsen O, Johansson AC, Venturoli D, Ledebo I, Wieslander A, Chan C, Rippe B: The effects of low-sodium peritoneal dialysis fluids on blood pressure, thirst and volume status. Nephrol Dial Transplant 24: 1609–1617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van RN, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J: Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15: 545–552, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C: High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol 278: F585–F595, 2000 [DOI] [PubMed] [Google Scholar]