Abstract
Background and objectives: Soluble TNF-like weak inducer of apoptosis (sTWEAK) and long pentraxin-3 (PTX3) concentrations have been associated with endothelial function in patients with chronic kidney disease (CKD). This study tested the hypothesis that the improvement in endothelial function after initiation of angiotensin II receptor blocker (valsartan), calcium channel blocker (amlodipine) therapy, or a combination of both is directly linked to the normalization of sTWEAK and PTX3.
Design, setting, participants, & measurements: One-hundred-eight diabetic CKD stage I patients with hypertension (56% men, 46.7 ± 5.3 years) were allocated to a 12-week intervention with amlodipine (10 mg/d), valsartan (160 mg/d), or their combination. Plasma levels of sTWEAK, PTX3, and flow-mediated dilation (FMD) were studied during the interventions.
Results: All treatment strategies effectively increased FMD and reduced proteinuria, confirming a more prone reduction with the combined therapy. These improvements were followed by significant PTX3 reductions. Valsartan alone and in combination with amlodipine achieved significant incremental raises in sTWEAK plasma levels. More importantly, the changes observed in sTWEAK (β = 0.25, P = 0.006) or PTX3 (β = −0.24, P = 0.007) plasma levels were independently associated with the improvement in ultrasonographically measured FMD.
Conclusions: This study shows that treatment with antihypertensive drugs improves FMD and normalizes proteinuria, PTX3, and sTWEAK in diabetic CKD stage I patients with hypertension. The improvement in FMD was independently associated with PTX3 and sTWEAK normalization. Two surrogate biomarkers of endothelial function are therefore identified with potential as therapeutic targets. The study was registered in clinicaltrials.gov as NCT00921570.
Chronic kidney disease (CKD) patients die at a markedly accelerated rate, principally from cardiovascular disease (CVD) (1). The mechanisms for the elevated CVD risk in CKD are complex and may involve changes in the heart and vasculature already at early stages. Endothelial dysfunction (ED) is commonly observed along the CKD spectrum, being an obligatory prodromal phase in the atherosclerosis process that likely precedes other cardiovascular complications (2). Hypertension closely associates with ED and is a negative feature in all progressive renal diseases, especially diabetic nephropathy. Patients with a history of hypertension and kidney disease or diabetes are recommended to have an annual check for albuminuria and, if elevated (>200 mg/d), strategies for blood pressure (BP) lowering therapy should be taken (3). Use of angiotensin converting enzyme (ACE) inhibitors and/or angiotensin receptor blockers (ARBs), strict glycemic control, and antilipidemic drugs may in this way show improvements in retarding diabetic nephropathy progression (4). However, combined therapy with angiotensin II (AII) receptor blocker and calcium channel blockers has been suggested to provide additional advantages (5,6).
Long pentraxin-3 (PTX3) is a multimeric mediator that shares structural homology with hepatic short pentraxins such as C-reactive protein (CRP) and serum amyloid P component, but it is expressed by many cell types in response to different stimuli (7). Although PTX3 levels are known to be increased in individuals with impaired renal function, it may share strong independent links with ED and albuminuria, perhaps contributing to increase the cardiovascular and mortality risk (8–10).
TNF-like weak inducer of apoptosis (TWEAK) is a type II transmembrane glycoprotein of the TNF superfamily that circulates in plasma as a soluble form (sTWEAK) with a molecular weight of 18 kD (11–13). TWEAK is widely expressed in many tissues including heart, skeletal muscle, and kidney (11,14). Binding of TWEAK to its receptor, Fn14 (13), mediates multiple biologic effects such as cellular growth, proliferation, and migration; osteoclastogenesis; angiogenesis; and apoptosis (15–17). sTWEAK plasma levels decrease with impaired renal function and associate with the aggravation of the endothelial function and the mortality risk (18,19). In the same line, diminished sTWEAK levels have been reported in patients with subclinical atherosclerosis (20).
A recent report was published by our group demonstrating that short-term ACE-inhibitor treatment significantly improved flow-mediated dilation (FMD) and normalized PTX3 and urinary protein excretion in type 2 diabetic patients with proteinuria (21). It is presently unknown whether sTWEAK levels can be normalized by short-term pharmacologic treatment. In the study presented here, we aimed to test the hypothesis that the previously demonstrated improvement in ED after initiation of AII receptor blocker (valsartan) (22), calcium channel blocker (amlodipine) (23) therapy, or a combination of both (6) is directly linked to the normalization of PTX3 and sTWEAK. We tested this in 108 type 2 diabetic hypertensive proteinuric patients.
Materials and Methods
Patients and Controls
A randomized prospective study was performed with patients referred to the outpatient clinics at the Department of Nephrology of the Gulhane School of Medicine in Ankara, Turkey, during the period of June 2008 to June 2009. From these referrals, after a 24-hour urine collection, we selected patients with CKD stage 1 (24-hour protein excretion 1 to 2 g/d) who were older than 18 years of age, were willing to participate, and characterized as having type 2 diabetes mellitus as the only cause of nephropathy (renal biopsy and medical history) and hypertension (systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg). A total of 375 patients fulfilled the above inclusion criteria, but from these we then excluded patients who were previously treated using ACE inhibitors or ARBs (medical history); who were obese [body mass index (BMI) >30 kg/m2]; or who had dyslipidemia (total cholesterol >200 mg/dl, fasting triglycerides (TG) >150 mg/dl), renal failure, (estimated GFR <90 ml/min), nephrotic syndrome, elevated liver enzymes, or a history of CVD [medical history, abnormal electrocardiogram (ECG), see below]. Of 174 patients who met these criteria, smokers and those taking statins or renin-angiotensin blockers were further excluded because of the effect of these factors on ED. A total of 118 patients met all study criteria and were included in this study. The duration of proteinuria and diabetic nephropathy after initial diagnosis was not known.
We also recruited 35 healthy subjects to serve as controls. They had no known diseases and were not currently taking any drugs. Also, they were subject to the same inclusion and exclusion criteria as the patients. The informed consent to participate in the study was obtained from patients and controls. The drug ethical committee of the Gulhane School of Medicine approved the study. The study was registered in clinicaltrials.gov with the reference number NCT00921570.
Baseline Characterization
Recruited patients were evaluated by standard physical examination; chest x-ray; baseline ECG; two-dimensional echocardiography; and routine clinical laboratory tests, including liver and kidney function tests and 24-hour urinary protein measurements. Arterial BP was measured in the right arm by a mercury sphygmomanometer 3 times in a resting condition in the morning, and mean values were calculated for diastolic and systolic pressures. The exclusion criteria were as follows: nephrotic syndrome (urinary protein excretion >3000 mg/d), coronary heart disease (patients with ischemic ST-T wave changes in ECG alterations, voltage criteria for left ventricular hypertrophy, and a history of revascularization or myocardial infarction), elevated liver enzymes (aspartate amino transferase or alanine amino transferase levels ≥40 U/L), and renal failure (serum creatinine levels >1.3 mg/dl). GFR was calculated according to the simplified version of the Modification of Diet in Renal Disease study prediction equation formula as defined by Levey (24).
Intervention and Follow-Up Measurements
Patients were randomized to one of the following intervention protocols that were given a (1) AII receptor blocker (valsartan, 160 mg once per day), (2) calcium channel blocker (amlodipine, 10 mg once per day), or (3) their combination (valsartan 160 mg + amlodipine 10 mg once per day) for 12 weeks immediately after baseline measurements. During the study period, serum creatinine and potassium concentrations were measured every 2 weeks and the dose of valsartan was titrated to achieve a serum potassium concentration <5.5 mEq/L. After this period, blood samples were again obtained for measurements as shown below. Urine samples were also collected over 24 hours to determine the degree of proteinuria after intervention.
Laboratory Measurements
After an overnight fasting, venous blood samples from patients and controls were obtained to calculate fasting plasma glucose, serum albumin, total serum cholesterol, TG, and HDL and LDL cholesterol. Total plasma cholesterol, TG, and HDL cholesterol were measured by enzymatic colorimetric method with Olympus AU 600 autoanalyzer using reagents from Olympus Diagnostics, GmbH (Hamburg, Germany). LDL cholesterol was calculated by Friedewald's formula (25). Plasma PTX3 concentration was measured from frozen samples using a commercially available ELISA kit (Perseus Proteomics, Inc., Japan). The same was true for plasma concentrations of sTWEAK, determined in duplicate with commercially available ELISA kits (Bender MedSystems, Vienna, Austria). Basal insulin level was measured by the coated tube method (DPC, United States). An insulin resistance score Homeostasis Model Assessment-Insulin resistance (HOMA-IR) was computed by the following formula (26): HOMA-IR = fasting plasma glucose (mg/dl) × immunoreactive insulin (μIU/ml)/405. Serum samples were diluted with a ratio of 1:101 with the diluents solution and high-sensitivity CRP (hsCRP) was measured as described previously (8).
Vascular Assessment
According to the method of Celermajer et al. (27) the endothelium-dependent vasodilation (FMD) and endothelium-independent vasodilation (NMD) of the brachial artery were assessed by using high-resolution ultrasound. Measurements were made by a single observer using an Advanced Technology Laboratories 5000 ultrasound system (Bothell, WA) with a 12-Mhz probe. All vasoactive medications were withheld for 24 hours before the procedure. The subjects remained at rest in the supine position for at least 15 minutes before the examination started. The subject's arm was comfortably immobilized in the extended position to allow consistent recording of the brachial artery 2 to 4 cm above the antecubital fossa. Three adjacent measurements of end-diastolic brachial artery diameter were made from single two-dimensional frames. All ultrasound images were recorded on S-VHS videotape for subsequent blinded analysis. A pneumatic tourniquet was inflated to 200 mmHg with obliteration of the radial pulse. After 5 minutes, the cuff was deflated. Flow measurements were made at 60 seconds postdeflation. After a further 15 minutes, measurements were repeated and again 3 minutes after administration of sublingual glyceryl trinitrate 400 μg per os. The maximum FMD and NMD dilation diameters were calculated as the average of the three consecutive maximum diameter measurements. The FMD and NMD were then calculated as the percent change in diameter compared with baseline resting diameters.
Statistical Analyses
Non-normally distributed variables were expressed as median (range) and normally distributed variables were expressed as mean ± SD as appropriate. P < 0.05 was considered to be statistically significant. Between-group comparisons were assessed for nominal variables with the χ2 test. Differences induced by treatment were analyzed by paired t test. Differences among the groups were analyzed by a one-way ANOVA test followed by a post hoc Tukey–Kramer test for multiple comparisons. Pearson rank correlation was used to determine correlations between two variables. Stepwise multivariate regression analysis was used to assess predictors of specific changes before and after intervention. All of the statistical analyses were performed using the SPSS 11.0 (SPSS, Inc., Chicago, IL) statistical package.
Results
Effects of Amlodipine, Valsartan, and Their Combination on FMD, sTWEAK, and PTX3 Concentrations
As compared with healthy individuals with similar age, BMI, and GFR, patients presented increased hsCRP protein and PTX3 levels and reduced FMD and sTWEAK values at the beginning of the study (Table 1). Patients were randomized to the three intervention groups, being well balanced with regards to age, sex distribution, BMI, estimated GFR, and 24-hour proteinuria. Baseline biochemistry, FMD values, and PTX3 or sTWEAK plasma concentrations were not different across these groups (Table 2). During the 12-week study period, ten patients were excluded because of the AII receptor blocker or calcium channel blocker treatment side effects (cough, n = 1; hyperkalemia, n = 2; edema, n = 3) or incompliance (n = 4).
Table 1.
General characteristics of patients included in the study and compared to healthy individuals
| Group | Healthy (n = 35) | Patients (n = 108) | P |
|---|---|---|---|
| Age, years | 46 ± 5 | 48 ± 7 | 0.3 |
| Gender, male/female | 17/18 | 51/57 | 0.8 |
| BMI, kg/m2 | 26 (22 to 30) | 27 (21.5 to 30) | 0.6 |
| Estimated GFR, ml/min/1.73 m2 | 112 ± 7.7 | 113 ± 8.9 | 0.4 |
| 24-hour proteinuria, mg/d | 40 (10 to 90) | 1590 (600 to 2800) | <0.001 |
| FMD, % | 8.8 (7.6 to 12.4) | 6.4 (5.5 to 8.3) | <0.001 |
| NMD, % | 13.4 (11.8 to 13.9) | 13 (11.8 to 13.8) | 0.01 |
| hsCRP, mg/L | 2.4 ± 0.8 | 12.9 ± 4.4 | <0.001 |
| PTX3, ng/ml | 1.3 (0.1 to 2.7) | 7.7 (1.8 to 32.9) | <0.001 |
| sTWEAK, pg/ml | 472 ± 81 | 411 ± 77 | <0.001 |
Data are presented as mean ± SD, median (25th to 75th percentile), or number of patients.
Table 2.
Effect of a 12-week administration of amlodipine, valsartan, or their combination on type 2 diabetic hypertensive patients with proteinuria
| Group | Amlodipine (n = 35) |
Valsartan (n = 37) |
Amlodipine + Valsartan (n = 36) |
|||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Age, years | 47 ± 5 | – | 47 ± 9 | – | 49 ± 6 | – |
| Gender, male/female | 16/19 | – | 17/20 | – | 18/18 | – |
| BMI, kg/m2 | 26.2 ± 2.1 | – | 26.5 ± 1.9 | – | 26.4 ± 2.2 | – |
| Estimated GFR, ml/min/1.73 m2 | 115 ± 8.6 | 105 ± 12c | 112 ± 8.4 | 104 ± 9.5c | 112 ± 9.6 | 105 ± 11b |
| 24-hour proteinuria, mg/d | 1560 (600 to 2800) | 1200 (200 to 2130)a | 1560 (780 to 2700) | 670 (220 to 2000)c | 1590 (600 to 2800) | 745 (110 to 2130)c |
| Serum albumin, g/dl | 3.8 ± 0.3 | 4 ± 0.3a | 4 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.4 | 4 ± 0.4 |
| Total cholesterol, mg/dl | 180 ± 23 | 157 ± 31b | 179 ± 36 | 151 ± 34b | 182 ± 33 | 164 ± 32a |
| TG, mg/dl | 135 ± 13 | 121 ± 23b | 135 ± 25 | 99 ± 31c | 134 ± 22 | 106 ± 32c |
| LDL cholesterol, mg/dl | 120 ± 27 | 97 ± 28b | 118 ± 24 | 113 ± 25 | 121 ± 22 | 106 ± 25b |
| HDL cholesterol, mg/dl | 37 ± 6 | 41 ± 5b | 39 ± 7 | 42 ± 7a | 37 ± 6 | 42 ± 4.6c |
| Plasma insulin, mIU/ml | 5.2 (3.8 to 7.3) | 4.2 (2.1 to 5.8)b | 5.2 (3.6 to 8) | 4.4 (1.3 to 7)c | 5.2 (3.8 to 8.7) | 3.7 (1.3 to 5.6)c |
| Plasma glucose, mg/dl | 162 (115 to 214) | 153 (115 to 213) | 169 (107 to 219) | 149 (114 to 214)c | 164 (129 to 211) | 157 (115 to 199)a |
| HOMA-IR | 2.1 ± 0.5 | 1.6 ± 0.4c | 2.3 ± 0.6 | 1.6 ± 0.6c | 2.2 ± 0.6 | 1.4 ± 0.5c |
| Systolic BP, mmHg | 150 (140 to 165) | 130 (110 to 137)c | 148 (133 to 160) | 125 (100 to 141)c | 152 (140 to 167) | 120 (110 to 140)c |
| Diastolic BP, mmHg | 90 (80 to 98) | 80 (70 to 91)c | 92 (82 to 100) | 80 (70 to 90)c | 91 (88 to 100) | 80 (70 to 92)c |
| hsCRP, mg/L | 11.8 ± 4.5 | 8.1 ± 3.2c | 13.2 ± 4.6 | 7.8 ± 3.9c | 13.7 ± 4 | 8 ± 3.4c |
| PTX3, ng/ml | 6.5 (1.8 to 33) | 2.5 (1.6 to 16)c | 8.5 (1.8 to 33) | 3.9 (1 to 11)c | 8.1 (2.2 to 33) | 2.2 (1.4 to 7.9)c |
| sTWEAK, pg/ml | 418 ± 71 | 418 ± 77 | 407 ± 77 | 452 ± 92a | 409 ± 84 | 524 ± 82d |
| FMD, % | 6.7 (5.5 to 8.3) | 7.5 (5 to 8.7)c | 6.3 (5.5 to 8) | 7.8 (6 to 9.3)c | 6.4 (5.5 to 7.3) | 8 (6.9 to 9.3)c |
| NMD, % | 13 (12.1 to 13.7) | 13 (12 to 13.8) | 13 (11.8 to 13.8) | 13 (11.6 to 13.8) | 13 (12 to 13.8) | 13 (12 to 13.8) |
Data are presented as mean ± SD and median (range). HOMA, homeostasis model assessment.
Paired t test, P < 0.05.
Paired t test, P < 0.01.
Paired t test, P < 0.001.
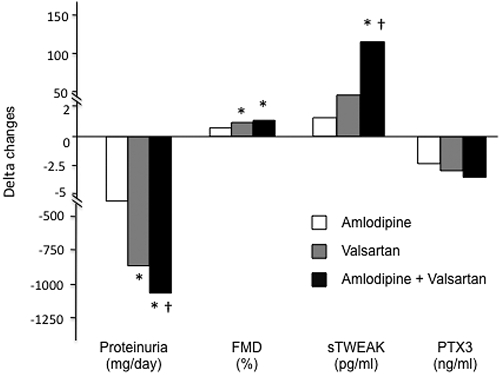
Changes induced in different arms during the 12-week study periods are summarized in Table 2 and in Figure 1. Briefly, proteinuria diminished after all interventions, observing the highest improvement in the patients that received the combined therapy. FMD was improved and PTX3 was reduced after all interventions, but the effect observed in the group treated with the combined therapy did not differ from that of valsartan alone. sTWEAK levels did not vary after amlopidine intervention. Nonetheless, treatment with valsartan alone or combined with amlodipine resulted in higher sTWEAK values.
Figure 1.
Changes in proteinuria, FMD, PTX3, and sTWEAK (delta values) after a 12-week treatment with different antihypertensive therapies. *P < 0.05 versus amlodipine; †P < 0.05 versus valsartan.
Univariate and Multivariate Correlations
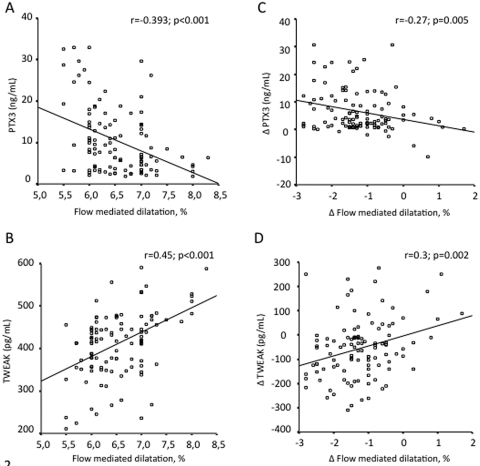
At the beginning of the study, and pooling all patients, FMD was negatively associated with hsCRP (rho = −0.21; P = 0.03) and PTX3 (rho = −0.39; P < 0.001) concentrations (Figure 2A) but positively correlated with sTWEAK (rho = 0.45; P < 0.001) (Figure 2B). These associations persisted after treatment and were also true for each treatment subgroup of patients (data not shown). Univariate and multivariate associations of the treatment-induced changes in diverse parameters are shown in Table 3. Briefly, the percent increase in FMD was negatively associated with the reductions in diastolic BP, hsCRP, and PTX3 levels (Figure 2C) but positively associated with the increase in sTWEAK concentrations (Figure 2D).
Figure 2.
Scatterplots showing the univariate associations between FMD and (A) PTX3 or (B) sTWEAK before treatment, as well as the association of 12-week changes in FMD with (C) the reduction in PTX3 and (D) the increase in sTWEAK.
Table 3.
Univariate and multivariate associations between intervention-induced changes (D) in FMD and relevant parameters
| ΔFMD |
||
|---|---|---|
| Univariate r (P) | Multivariate b (P) | |
| ΔeGFR, ml/min/1.73 m2 | 0.21 (0.03) | NS |
| ΔSBP, mmHg | −0.22 (0.02) | NS |
| ΔDBP, mmHg | −0.26 (0.006) | −0.22 (0.01) |
| ΔHOMA-IR | −0.22 (0.02) | NS |
| ΔhsCRP, mg/L | −0.27 (0.005) | −0.2 (0.04) |
| ΔFMD, % | – | – |
| ΔPTX3, ng/ml | −0.27 (0.005) | −0.24 (0.007) |
| ΔsTWEAK, pg/ml | 0.30 (0.002) | 0.25 (0.006) |
| Δ24-hour proteinuria | −0.27 (0.005) | NS |
SBP, systolic BP; DBP, diastolic BP.
We used a multiple linear regression model to analyze the independent contributors to the changes in FMD. This model included age and sex, as well as variables significantly associated with FMD in univariate analysis. After stepwise exclusion, the increase in FMD was multivariably explained by the improvement in diastolic BP, the reductions in hsCRP and PTX3, and the sTWEAK increase (r2 of the model = 0.17).
Discussion
In this study, we report the effects of an open-label, 12-week pharmacologic intervention trial analyzing the effect of initiation with amlodipine, valsartan, or their combination on diabetic proteinuric subjects with hypertension. We found that all treatment strategies effectively increased FMD and reduced proteinuria, confirming a more prone reduction with the combined therapy. These improvements were also followed by PTX3 reductions. Interestingly, valsartan alone and in combination with amlodipine achieved significant raises in sTWEAK plasma levels. More importantly, the changes observed in sTWEAK or PTX3 plasma levels were independently associated with the improvement in ultrasonographically measured FMD.
On one hand, our results evidence the possible role of PTX3 on local vascular health and proteinuria. PTX3 is a recently described multimeric inflammatory mediator structurally linked to CRP. As opposed to CRP, exclusively derived from hepatocytes, PTX3 is synthesized by various tissues and cells, including vascular endothelial cells (28) and macrophages (29), thus reflecting local tissue inflammatory activity. In the study presented here and in previous studies, we have shown that PTX3 levels are elevated across the CKD spectrum (8,9), and that in dialysis patients PTX3 predicts mortality independent of traditional risk factors and CRP (10,21). Strengthening our previous reports (8,21), results from the study presented here demonstrate that PTX3 is a strong and independent determinant of proteinuria and ED, as demonstrated here in a cross-sectional manner and longitudinally through pharmacologic intervention. Renin-angiotensin system blockers (21) and calcium channel blockers normalized PTX3 levels, but no additive reduction effect was observed with the combination of these drugs.
However, this is the first time in which the effect of a drug intervention on sTWEAK has been evaluated. TWEAK has been recently implicated in the development of vascular and renal damage (30–33), being expressed in endothelial and vascular smooth muscle cells, macrophages, and tubular and glomerular cells (31,34). The binding of TWEAK to its receptor Fn14 increases the inflammatory response associated with atherosclerotic plaque development and renal lesions (32,35). Unexpectedly, lower plasma sTWEAK has been reported in subjects with subclinical atherosclerosis, diabetic subjects, CKD patients (18–20,36), and in this study. In CKD patients, sTWEAK appeared to be robustly and negatively associated with ED (19), and sTWEAK plasma concentrations were good prognosticators of all-cause and CVD mortality in patients undergoing hemodialysis (18). Interestingly, in patients with chronic stable heart failure, reduced sTWEAK levels predicted an adverse prognosis independently of established risk markers such as the N-terminal prohormone of brain natriuretic peptide (37) and contributed to improve the prediction of coronary artery disease (38). These findings could be explained, at least in part, by the presence of a newly reported scavenger receptor of sTWEAK, CD163, which under proinflammatory conditions can sequester and degrade sTWEAK (39). In support of this, CD163 has been reported to be upregulated in CKD patients (40). Our results now show that treatment with valsartan alone, or in combination with amlodipine, normalized sTWEAK plasma concentrations after a 12-week intervention.
An important finding in the study presented here is the demonstration in multivariate analysis that the improvement in FMD was independently associated with sTWEAK and PTX3 changes, with these associations being somewhat stronger for sTWEAK. Several studies implicate both molecules in the worsening of endothelial function: PTX3 has been detected in atherosclerotic lesions and implicated on neointimal thickening (41,42). However, the recent observation that double knockout mice lacking ApoE and PTX3 displayed an increment in aortic lesion size and a higher inflammatory response compared with ApoE knockout mice expressing PTX3 (43) has lead to the hypothesis that PTX3 may be a failed compensatory mechanism to endothelial damage. On the other hand, systemic injection of human recombinant sTWEAK increased lesion development in a model of atheroclerosis in mice (35), and treatment with Fn14-Fc diminished atherosclerotic lesion size in ApoE knockout mice (44). However, it is important to note that our study was not designed to explain the mechanisms behind these associations and we cannot exclude the possibility of a reverse causality, whereby lower BP leads to lower levels of systemic inflammation. AII is an important inductor of vascular injury by inducing ED, promoting vascular remodeling, and accelerating atherosclerotic plaque development (45). At the same time, restoration of calcium homeostasis in the vasculature may improve vascular resistance and structural arterial wall reorganization (46). It is thus possible that an increase of AII concentrations and/or inhibition of calcium channels linked to hypertension could be responsible for reduced local inflammation, thereby explaining the observed changes in PTX3 and sTWEAK.
Some limitations should be considered in this study. First, the number of subjects included in each group of treatment was small. Second, factors that were not controlled for (e.g., glycemic control) could reasonably also affect endothelial function and proteinuria. Third, because we evaluated a specifically selected group of type 2 diabetic CKD stage 1 patients who do not represent the heterogeneous CKD patient population at large, these results need confirmation in other studies. Finally, because of the nature of the study design, it is unclear if the normalization observed in sTWEAK and PTX3 levels are because of changes in systolic BP or because of specific cellular properties from specific effects of the pharmacologic agents used.
In summary, we observe that a 12-week treatment with antihypertensive drugs significantly reduced PTX3 and increased sTWEAK concentrations. These changes were associated with an increase in FMD and a reduction in proteinuria. In addition to adding to the wealth of evidence linking treatment with ACE inhibitors and calcium channel blockers to beneficial vascular and anti-inflammatory effects in diabetic kidney disease, our study may also indicate that, at least in theory, these drugs could mediate their actions through PTX3 and sTWEAK modulation. This may encourage further mechanistic research regarding the potential of these molecules as therapeutic targets in inflammation- or atherosclerosis-related diseases.
Disclosures
None.
Acknowledgments
This study was supported by the Gulhane School of Medicine Research Center. The authors were supported by grants from the Center of Gender Medicine at Karolinska Institutet, the Loo and Hans Osterman Foundation, the Swedish Kidney Association, Ministerio de Ciencia y Tecnología (SAF 2007/60896, SAF2007/63648), Comunidad de Madrid (S2006/GEN-0247), Fondo de Investigaciones Sanitarias (Programa Miguel Servet to L.M.B.-C.), Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo, (RETICS RD06/0014/0035), and the Sociedad Española de Arteriosclerosis and Fundación Ramón Areces. M.I.Y and J.J.C contributed equally to this work.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, Wetzels JF, Rosendaal FR, Dekker FW: Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Endemann DH, Schiffrin EL: Endothelial dysfunction. J Am Soc Nephrol 15: 1983–1992, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kalaitzidis RG, Bakris GL: Should proteinuria reduction be the criterion for antihypertensive drug selection for patients with kidney disease? Curr Opin Nephrol Hypertens 18: 386–391, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Staessen JA, Li Y, Thijs L, Wang JG: Blood pressure reduction and cardiovascular prevention: An update including the 2003–2004 secondary prevention trials. Hypertens Res 28: 385–407, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Locatelli F, Del Vecchio L, Andrulli S, Colzani S: Role of combination therapy with ACE inhibitors and calcium channel blockers in renal protection. Kidney Int Suppl 82: S53–S60, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A; VALUE trial group Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet 363: 2022–2031, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, Spreafico F, Pausa M, D'Ettorre C, Gianazza E, Tagliabue A, Salmona M, Tedesco F, Introna M, Mantovani A: Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem 272: 32817–32823, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Suliman ME, Yilmaz MI, Carrero JJ, Qureshi AR, Saglam M, Ipcioglu OM, Yenicesu M, Tong M, Heimbürger O, Barany P, Alvestrand A, Lindholm B, Stenvinkel P: Novel links between the long pentraxin 3, endothelial dysfunction, and albuminuria in early and advanced chronic kidney disease. Clin J Am Soc Nephrol 3: 976–985, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong M, Carrero JJ, Qureshi AR, Anderstam B, Heimbürger O, Bárány P, Axelsson J, Alvestrand A, Stenvinkel P, Lindholm B, Suliman ME: Plasma pentraxin 3 in patients with chronic kidney disease: Associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol 2: 889–897, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Suliman ME, Qureshi AR, Carrero JJ, Bárány P, Yilmaz MI, Snaedal-Jonsdottir S, Alvestrand A, Heimbürger O, Lindholm B, Stenvinkel P: The long pentraxin PTX-3 in prevalent hemodialysis patients: Associations with comorbidities and mortality. QJM 101: 397–405, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL: TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272: 32401–32410, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Winkles JA: The TWEAK-Fn14 cytokine-receptor axis: Discovery, biology and therapeutic targeting. Nat Rev Drug Discov 7: 411–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiley SR, Winkles JA: TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev 14: 241–249, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Desplat-Jego S, Varriale S, Creidy R, Terra R, Bernard D, Khrestchatisky M, Izui S, Chicheportiche Y, Boucraut J: TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol 133: 116–123, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Lynch CN, Wang YC, Lund JK, Chen YW, Leal JA, Wiley SR: TWEAK induces angiogenesis and proliferation of endothelial cells. J Biol Chem 274: 8455–8459, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Polek TC, Talpaz M, Darnay BG, Spivak-Kroizman T: TWEAK mediates signal transduction and differentiation of RAW264.7 cells in the absence of Fn14/TweakR. Evidence for a second TWEAK receptor. J Biol Chem 278: 32317–32323, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Ho DH, Vu H, Brown SA, Donohue PJ, Hanscom HN, Winkles JA: Soluble tumor necrosis factor-like weak inducer of apoptosis overexpression in HEK293 cells promotes tumor growth and angiogenesis in athymic nude mice. Cancer Res 64: 8968–8972, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Carrero JJ, Ortiz A, Qureshi AR, Martín-Ventura JL, Bárány P, Heimbürger O, Marrón B, Metry G, Snaedal S, Lindholm B, Egido J, Stenvinkel P, Blanco-Colio LM: Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin J Am Soc Nephrol 4: 110–118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yilmaz MI, Carrero JJ, Ortiz A, Martín-Ventura JL, Sonmez A, Saglam M, Yaman H, Yenicesu M, Egido J, Blanco-Colio LM: Soluble TWEAK plasma levels as a novel biomarker of endothelial function in patients with chronic kidney disease. Clin J Am Soc Nephrol 4: 1716–1723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanco-Colio LM, Martín-Ventura JL, Muñóz-García B, Orbe J, Páramo JA, Michel JB, Ortiz A, Meilhac O, Egido J: Identification of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) as a possible biomarker of subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 27: 916–922, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Yilmaz MI, Axelson J, Sonmez A, Carrero JJ, Saglam M, Eyileten T, Caglar K, Kirkpantur A, Celik T, Oguz Y, Vural A, Yenicescu M, Lindholm B, Stenvinkel P: Effect of renin angiotensin system blockade on pentraxin 3 levels in type-2 diabetic patients with proteinuria. Clin J Am Soc Nephrol 4: 535–541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klingbeil AU, John S, Schneider MP, Jacobi J, Handrock R, Schmieder RE: Effect of AT1 receptor blockade on endothelial function in essential hypertension. Am J Hypertens 16: 123–128, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Koh KK, Han SH, Ahn JY, Chung WJ, Lee Y, Shin EK: Amlodipine improves endothelial function and metabolic parameters in patients with hypertension. Int J Cardiol 133: 23–31, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Berg RL, Gassman JJ, Hall PM, Walker WG: Creatinine filtration, secretion and excretion during progressive renal disease. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney Int Suppl 27: S73–S80, 1989 [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 27.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID: Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Introna M, Breviario F, d'Aniello EM, Golay J, Dejana E, Mantovani A: IL-1 inducible genes in human umbilical vein endothelial cells. Eur Heart J 14[ Suppl K]: K78–K81, 1993 [PubMed] [Google Scholar]
- 29.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A: Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood 84: 3483–3493, 1994 [PubMed] [Google Scholar]
- 30.Muñoz-García B, Martín-Ventura JL, Martínez E, Sánchez S, Hernández G, Ortega L, Ortiz A, Egido J, Blanco-Colio LM: Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: Modulation by atorvastatin. Stroke 37: 2044–2053, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Sanz AB, Sanchez-Niño MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Egido J, Ortiz A: TWEAK induces proliferation in renal tubular epithelium: A role in uninephrectomy-induced renal hyperplasia. J Cell Mol Med 13: 3329–3342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanz AB, Justo P, Sanchez-Niño MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A: The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 19: 695–703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Justo P, Sanz AB, Sanchez-Niño MD, Winkles JA, Lorz C, Egido J, Ortiz A: Cytokine cooperation in renal tubular cell injury: The role of TWEAK. Kidney Int 70: 1750–1758, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Ortiz A, Sanz AB, Muñoz García B, Moreno JA, Sánchez Niño MD, Martín-Ventura JL, Egido J, Blanco-Colio LM: Considering TWEAK as a target for therapy in renal and vascular injury. Cytokine Growth Factor Rev 20: 251–258, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Muñoz-García B, Moreno JA, López-Franco O, Sanz AB, Martín-Ventura JL, Blanco J, Jakubowski A, Burkly LC, Ortiz A, Egido J, Blanco-Colio LM: Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) enhances vascular and renal damage induced by hyperlipidemic diet in ApoE-knockout mice. Arterioscler Thromb Vasc Biol 29: 2061–2068, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Kralisch S, Ziegelmeier M, Bachmann A, Seeger J, Lössner U, Blüher M, Stumvoll M, Fasshauer M: Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis, 199: 440–444, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Chorianopoulos E, Rosenberg M, Zugck C, Wolf J, Katus HA, Frey N: Decreased soluble TWEAK levels predict an adverse prognosis in patients with chronic stable heart failure. Eur J Heart Fail 11: 1050–1056, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Jelić-Ivanović Z, Bujisić N, Spasić S, Bogavac-Stanojević N, Spasojević-Kalimanovska V, Kotur-Stevuljević J: Circulating sTWEAK improves the prediction of coronary artery disease. Clin Biochem 42: 1381–1386, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Moreno JA, Muñoz-García B, Martín-Ventura JL, Madrigal-Matute J, Orbe J, Páramo JA, Ortega L, Egido J, Blanco-Colio LM: The CD163-expressing macrophages recognize and internalize TWEAK: Potential consequences in atherosclerosis. Atherosclerosis 207: 103–110, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Axelsson J, Møller HJ, Witasp A, Qureshi AR, Carrero JJ, Heimbürger O, Bárány P, Alvestrand A, Lindholm B, Moestrup SK, Stenvinkel P: Changes in fat mass correlate with changes in soluble sCD163, a marker of mature macrophages, in patients with CKD. Am J Kidney Dis 48: 916–925, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Savchenko A, Imamura M, Ohashi R, Jiang S, Kawasaki T, Hasegawa G, Emura I, Iwanari H, Sagara M, Tanaka T, Hamakubo T, Kodama T, Naito M: Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol 215: 48–55, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Kotooka N, Inoue T, Fujimatsu D, Morooka T, Hashimoto S, Hikichi Y, Uchida T, Sugiyama A, Node K: Pentraxin3 is a novel marker for stent-induced inflammation and neointimal thickening. Atherosclerosis 197: 368–374, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F, Pizzitola I, Garlanda C, Mantovani A, Catapano AL: Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation 120: 699–708, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Schapira K, Burkly LC, Zheng TS, Wu P, Groeneweg M, Rousch M, Kockx MM, Daemen MJ, Heeneman S: Fn14-Fc fusion protein regulates atherosclerosis in ApoE−/− mice and inhibits macrophage lipid uptake in vitro. Arterioscler Thromb Vasc Biol 29: 2021–2027, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Ortega M, Esteban V, Rupérez M, Sánchez-López E, Rodríguez-Vita J, Carvajal G, Egido J: Renal and vascular hypertension-induced inflammation: Role of angiotensin II. Curr Opin Nephrol Hypertens 15: 159–166, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Inoue R, Hai L, Honda A: Pathophysiological implications of transient receptor potential channels in vascular function. Curr Opin Nephrol Hypertens 17: 193–198, 2008 [DOI] [PubMed] [Google Scholar]