Abstract
Background and objectives: Acute kidney injury is an independent predictor of short- and long-term survival; however, data on the relationship between reversible transitory decline of kidney function and chronic kidney disease (CKD) are lacking. We assessed the prognostic value of temporary renal function decline on the development of long-term CKD.
Design, setting, participants, & measurements: The study included 1308 patients who were undergoing major vascular surgery (aortic aneurysm repair, lower extremity revascularization, or carotid surgery), divided into three groups on the basis of changes in Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) estimated GFR (eGFR) on days 1, 2, and 3 after surgery, compared with baseline: Group 1, improved or unchanged (change in CKD-EPI eGFR ±10%); group 2, temporary decline (decline >10% at day 1 or 2, followed by complete recovery within 10% to baseline at day 3); and group 3, persistent decline (>10% decrease). Primary end point was the development of incident CKD during a median follow-up of 5 years.
Results: Perioperative renal function was classified as unchanged, temporary decline, and persistent decline in 739 (57%), 294 (22%), and 275 (21%) patients, respectively. During follow-up, 272 (21%) patients developed CKD. In multivariate logistic regression analyses, temporary and persistent declines in renal function both were independent predictors of long-term CKD, compared with unchanged renal function.
Conclusion: Vascular surgery patients have a high incidence of temporary and persistent perioperative renal function declines, both of which were independent predictors for development of long-term incident CKD.
Acute kidney injury (AKI) is a common and serious complication in hospitalized patients and is associated with a high rate of in-hospital morbidity and mortality and prolonged length of stay (1). The incidence of AKI ranges between 2 and 45% and depends on the type of population, underlying comorbidities, and the definition used to define AKI (2–4). During the past decade, the incidence of AKI has increased to approximately 500 events per 100,000 in the general population (1).
Although episodes of AKI seem to be reversible, there is a silent, ongoing inflammatory and fibrotic process that leads to progressive structural kidney damage (5,6). This process predisposes to worsening BP control, proteinuria, and more rapid decreases in GFR, which are widely known risk factors for incident chronic kidney disease (CKD) and cardiovascular disease (CVD) (7). Meta-analyses have demonstrated an increased risk for short- and long-term mortality after an episode of AKI (6,8). In these analyses, several definitions of AKI were used, including small temporary kidney function decline. In fact, a meta-analysis by Coca et al. (8) demonstrated that even a 10 to 24% increase in serum creatinine (Scr) levels was strongly associated with an in increased mortality risk; therefore, small changes in Scr levels seem to provide a sensitive definition of AKI and could promote early prevention or treatment strategies. Although numerous studies (4,6,9,10) have investigated the predictive value of AKI for long-term CVD and mortality, data regarding the relationship between a temporary decline of renal function during the perioperative period with incident CKD are lacking; therefore, we performed this study to assess the relationship between temporary decline of renal function and the development of CKD during long-term follow-up.
Materials and Methods
Study Design and Population
This single-center, retrospective study comprised a source population of 2933 consecutive patients who had peripheral arterial disease and were referred for elective major vascular surgery. All patients underwent a major vascular surgery procedure between 1990 and 2008 and included lower extremity revascularization (n = 1031 [35%]), aneurysmal abdominal aorta surgery (n = 1170 [40%]), or carotid surgery (n = 732 [25%]). Patients with known CKD at baseline or unavailable Scr levels before surgery were excluded (n = 481 [16%]). Scr levels during the perioperative period were available for all patients (n = 2452). Patients with an estimated GFR (eGFR) <60 ml/min per 1.73 m2 at day 3 after surgery were excluded for the analyses that examined the primary end point (n = 234). Of the remaining 2218 patients, 1308 (59%) had a Scr measurement at least 1 year after surgery. These 1308 patients composed the final analyzed study population. Baseline characteristics between the source population and the final study population were compared to rule out the possibility of selection bias. No significant differences with respect to all baseline characteristics, cardiac risk factors, and medication use at discharge between the two populations were observed. The study complies with the Declaration of Helsinki, and enrollment was performed after approval of the hospital’s ethics committee and after informed consent from all patients (or their guardians).
Renal Function Assessment
Scr was assessed by means of a nonkinetic alkaline picrate (Jaffé) method (11). eGFR was calculated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula: eGFR = 141 × min (Scr/κ, 1)a × max (Scr/κ, 1)−1.209 × 0.993age × 1.018 [if female] × 1.159 [if black] (12). Scr is micromoles per liter, κ is 0.7 for women and 0.9 for men, a is −0.329 for women and −0.411 for men, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1.
Renal Function Groups
Before surgery (1 to 3 days), Scr level was measured at baseline in all patients. Scr level was measured at day 1 or 2 and day 3 after surgery. Patients were categorized into three groups on the basis of changes in CKD-EPI eGFR from baseline to day 1 or 2 and from day 1 or 2 to day 3: Group 1, unchanged or improved renal function (change in CKD-EPI GFR function −10 to 10% compared with baseline); group 2, temporary decline of renal function (temporary decline >10% at day 1 or 2, then complete recovery within 10% of baseline value at day 3); and group 3, persistent decline of renal function (>10% decrease compared with baseline). Baseline CKD-EPI eGFR was defined as the value recorded within 3 days before surgery. The purpose of this study was to investigate the prognostic value for the risk for development of CKD after small decrements in renal function. On the basis of our previous studies, we used a cutoff value of ±10% in eGFR (8,10). To confirm the validity of our initial definition, we performed additional sensitivity analyses by repeating all analyses using the Risk, Injury, Failure, Loss, and ESRD (RIFLE) classification for AKI (13).
Follow-up and End Points
During the follow-up period, long-term Scr level was used to determine the primary end point of the development of incident CKD. Long-term creatinine measurements had to be obtained at least 1 year after surgery. The median follow-up period between date of surgery and last Scr level recorded was 5.0 years (interquartile range [IQR] 2.6 to 8.5). The primary outcome of interest, incident CKD, was defined using the National Kidney Foundation Disease Outcome Quality Initiative (NKF/DOQI) definition of eGFR <60 ml/min per 1.73 m2, calculated using the CKD-EPI equation (11). In addition, for prevention of the occurrence of information bias as a result of misclassification of CKD, patients were classified as having CKD only when the CKD-EPI eGFR was decreased by at least 25% from the baseline GFR. In 916 (70%) patients, more than one Scr measurement was used to define the presence of CKD.
Statistical Analysis
Continuous data were described as mean ± SD or median (IQR). Categorical data were presented as percentage frequencies and compared using χ2 tests. Differences in baseline characteristics between the perioperative renal function groups were analyzed with χ2 test for trend and ANOVA for trend, when appropriate. We performed multivariate logistic regression analyses to investigate the independent value of perioperative temporary or persistent decline of renal function and the development of CKD. Adjustments were made for demographics (age, gender), cerebrocardiovascular history, cardiovascular risk factors (body mass index, smoking status, hypertension [BP ≥140/90 mmHg in patients who did not have diabetes and ≥130/90 mmHg in patients who had diabetes or required antihypertensive medication), diabetes (fasting blood glucose ≥6.1 mmol/L or need for insulin and/or oral antidiabetic medication), hypercholesterolemia (LDL cholesterol >135 mg/dl and/or the need for lipid-lowering medication), chronic obstructive pulmonary disease (14), baseline CKD-EPI eGFR, and BP-lowering drugs (diuretics, angiotensin-converting enzyme inhibitors, calcium antagonists, and angiotensin receptor blockers). Furthermore, because the Scr levels were not measured at a prespecified time after surgery but at least 1 year after surgery, we also adjusted for time between surgery and last Scr level recorded and used for prediction of CKD-EPI eGFR. To evaluate further the strength of the 10% change in CKD-EPI eGFR, we performed additional sensitivity analyses using a 20, 30, 40, and 50% cutoff value for renal function decline. Because the incidence of the outcome of interest (CKD) in the study population was >10%, we calculated relative risks (RRs) along with their 95% confidence intervals (CIs) from the adjusted odds ratios (ORs). Zhang et al. proposed the following formula to calculate RR from OR, because this better represents the true relative risk: RR = OR/[(1 − P0) + (P0 × OR)]. P0 indicates the incidence of the outcome of interest in the nonexposed group. Statistical analyses were performed using SPSS 15.0 (SPSS, Inc., Chicago, IL). P < 0.05 (two-sided) was considered statistically significant.
Results
Description of the Study Population
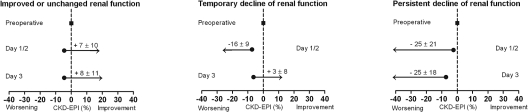
The study population consisted of 1308 consecutive patients who had peripheral arterial disease and were referred for elective major vascular surgery. Lower extremity revascularization was performed in 527 (40%) patients, abdominal aortic surgery in 514 (39%) patients, and carotid surgery in 267 (21%) patients. In the source population (n = 2452), 1569 (64%) patients were classified as having normal or improved renal function, whereas 441 (18%) and 436 (18%) had temporary and persistent declines in renal function, respectively. Of the patients in the final study population, 815 (62%) had no change or had improvement in CKD-EPI eGFR compared with baseline during the first 3 postoperative days. Temporary and persistent declines of renal function were observed in 261 (20%) and 232 (18%) patients, respectively. At baseline, patients with temporary renal function decline had a significantly lower CKD-EPI eGFR compared with patients with normal renal function (P = 0.01). Mean changes in CKD-EPI eGFR on day 1 or 2 and day 3 for the groups are shown in Figure 1. Patients with temporary or persistent renal function decline more often had vascular disease, resulting in a higher incidence of polyvascular disease (P = 0.03; Table 1). Risk factors for CKD, including hypertension and chronic heart failure, were more often present in patients with temporary or persistent renal function decline (P = 0.05). There was a significant trend for increased use of statins, diuretics, and angiotensin-converting enzyme inhibitors in patients with temporary or persistent renal function decline (P < 0.05).
Figure 1.
Subdivision of renal function groups on the basis of CKD-EPI eGFR, with changes in CKD-EPI eGFR (mean ± SD). (Left) Improved or unchanged renal function; ΔCKD-EPI eGFR −10 to 10% function compared with baseline. (Middle) Temporary decline of renal function; temporary decline >10% at day 1 or 2, then complete recovery within 10% of baseline at day 3. (Right) Persistent decline of renal function of >10% compared with baseline.
Table 1.
Baseline characteristics of the study population
| Characteristic | No Change/Improved (n = 815) | Temporary Decline (n = 261) | Persistent Decline (n = 232) | P for Trend |
|---|---|---|---|---|
| Demographics | ||||
| age (years; mean ± SD) | 64 ± 11 | 67 ± 10 | 64 ± 11 | 0.01 |
| male (n [%]) | 605 (74) | 201 (77) | 167 (72) | 0.73 |
| baseline eGFR (mean ± SD) | 77 ± 20 | 74 ± 19 | 76 ± 22 | 0.01 |
| Cardiac history (n [%]) | ||||
| ischemic heart disease | 358 (44) | 148 (57) | 125 (54) | 0.01 |
| CVA/TIA | 276 (34) | 71 (27) | 54 (23) | 0.01 |
| lower extremity arterial disease | 197 (24) | 70 (27) | 59 (25) | 0.55 |
| aortic aneurysm disease | 20 (3) | 8 (3) | 6 (3) | 0.80 |
| polyvascular disease | 440 (54) | 168 (64) | 145 (63) | 0.03 |
| Cardiovascular risk factors (n [%]) | ||||
| smoking | 0.87 | |||
| no | 304 (37) | 92 (35) | 83 (36) | |
| current | 321 (39) | 101 (39) | 89 (38) | |
| history | 190 (23) | 68 (26) | 60 (26) | |
| hypertension | 377 (46) | 142 (54) | 124 (53) | 0.02 |
| diabetes | 115 (14) | 51 (20) | 36 (16) | 0.28 |
| hypercholesterolemia | 223 (27) | 64 (25) | 69 (30) | 0.71 |
| chronic heart failure | 40 (5) | 20 (8) | 18 (8) | 0.05 |
| COPD | 140 (17) | 52 (20) | 47 (20) | 0.21 |
| Medication at discharge (n [%]) | ||||
| aspirin | 382 (47) | 121 (46) | 1108 (47) | 0.91 |
| statin | 278 (34) | 92 (35) | 99 (43) | 0.03 |
| β-blocking agents | 306 (38) | 108 (41) | 91 (41) | 0.29 |
| diuretics | 158 (19) | 65 (25) | 56 (24) | 0.05 |
| ACEIs | 181 (22) | 72 (28) | 69 (30) | 0.01 |
| calcium antagonists | 196 (24) | 77 (30) | 59 (25) | 0.37 |
| angiotensin receptor blockers | 36 (4) | 13 (5) | 12 (5) | 0.59 |
ACEI, angiotensin-converting enzyme inhibitor; COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack.
Long-Term Incident CKD
Median follow-up between surgery and the last Scr level recorded was 5.0 years (IQR 2.6 to 8.5 years). Median CKD-EPI eGFR during long-term follow-up in patients with normal or improved renal function, temporary decline of renal function, and persistent decline of renal function was 81 ml/min per 1.73 m2 (IQR 63 to 95 ml/min per 1.73 m2), 61 ml/min per 1.73 m2 (IQR 40 to 82 ml/min per 1.73 m2), and 59 ml/min per 1.73 m2 (IQR 36 to 76 ml/min per 1.73 m2). During the observation period, 272 (21%) patients developed CKD. Of the patients without a change or with an improvement in perioperative renal function, 87 (11%) developed CKD. In contrast, 94 (32%) and 84 (36%) patients with temporary and persistent renal function declines, respectively, developed CKD. There was a significant association between the presence of perioperative renal function decline and the development of CKD in unadjusted analyses (P < 0.001). In multivariate regression analyses, adjusted for demographics, cerebrovascular history, cardiovascular risk factors, BP-lowering agents, and time of Scr measurement, the presence of temporary renal function decline was independently associated with an increased risk for CKD (RR 3.4; 95% CI 2.7 to 4.1) compared with no change or improvement in renal function. Persistent renal function decline in the perioperative period was associated with a 3.6-fold increased risk for CKD (RR 3.6; 95% CI 2.8 to 4.4). Other covariates that were significantly associated with the outcome of interest were age (RR 1.04; 95% CI 1.02 to 1.06), diabetes (RR 1.9; 95% CI 1.4 to 2.5), and smoking (RR 1.2; 95% CI 1.0 to 1.4).
Sensitivity Analysis
To evaluate the strength of the 10% cutoff value for temporary changes in CKD-EPI eGFR, we performed additional analyses using 20, 30, 40, and 50% cutoff values, respectively (Table 2). Using a 20% change in CKD-EPI eGFR compared with baseline, temporary renal function decline was independently associated with a 3.5-fold increased risk (RR 3.5; 95% CI 2.8 to 4.2). Furthermore, for a 50% change in CKD-EPI eGFR compared with baseline, a comparable independent association was observed (RR 3.8; 95% CI 2.9 to 4.3). Importantly, as the change in CKD-EPI eGFR cutoff values increased, the independent risk remained stable for patients with a temporary decline of renal function. In contrast, performing the same analyses for patients with a persistent decline of perioperative renal function, there was a graded relation between the cutoff value and the estimated risk for CKD up to a cutoff value of 30%. Using a 30% change in CKD-EPI eGFR, there was a 4.3-fold increased risk (RR 4.3; 95% CI 3.3 to 5.4); however, when a cutoff value of 40 or 50% was used, the risk estimates stabilized at a fourfold increased risk (RR 3.9; 95% CI 2.8 to 5.1).
Table 2.
Predictive value of temporary renal dysfunction on long-term CKD, using various (small) changes in CKD-EPI eGFR
| Cutoff Values | No Change | Temporary Decline | Persistent Decline |
|---|---|---|---|
| 10% | |||
| patients (n [%]) | 815 (62) | 261 (20) | 232 (18) |
| RR (95% CI) | Reference | 3.4 (2.7 to 4.1) | 3.6 (2.8 to 4.4) |
| 20% | |||
| patients (n [%]) | 856 (65) | 313 (24) | 139 (11) |
| RR (95% CI) | Reference | 3.5 (2.8 to 4.2) | 4.2 (3.2 to 5.2) |
| 30% | |||
| patients (n [%]) | 863 (66) | 337 (26) | 108 (8) |
| RR (95% CI) | Reference | 3.5 (2.8 to 4.2) | 4.3 (3.3 to 5.4) |
| 40% | |||
| patients (n [%]) | 866 (66) | 355 (27) | 87 (7) |
| RR (95% CI) | Reference | 3.6 (2.9 to 4.3) | 3.9 (2.8 to 5.1) |
| 50% | |||
| patients (n [%]) | 866 (66) | 365 (28) | 77 (6) |
| RR (95% CI) | Reference | 3.8 (2.9 to 4.3) | 3.9 (2.8 to 5.1) |
Adjustment for demographics (age, gender), cerebrovascular history, cardiovascular risk factors (polyvascular disease, smoking, hypertension, diabetes, hypercholesterolemia, heart failure, chronic obstructive pulmonary disease), baseline CKD-EPI eGFR, and BP-lowering drugs (diuretics, angiotensin-converting enzyme inhibitors, calcium antagonists, and angiotensin receptor blockers).
We performed additional sensitivity analyses to test more specific definitions of CKD, using eGFR <45 and <30 ml/min, respectively. In total, 252 (19%) patients had an eGFR <45 ml/min and 124 (9%) patients had eGFR <30 ml/min. For both definitions, temporary renal function decline remained an independent predictor of long-term CKD (eGFR <45: RR 2.2 [95% CI 1.7 to 2.8]; eGFR <30: RR 3.1 [95% CI 2.1 to 4.5]).
To confirm the validity of the results, we repeated all analyses using the RIFLE classification for AKI. Logistic regression analyses demonstrated that Risk (RR 3.6; 95% CI 2.6 to 4.8), Injury (RR 3.3; 95% CI 1.8 to 5.1), and Failure (RR 4.6; 95% CI 2.6 to 6.6) were independent predictors of CKD. Finally, to address that death is a competing end point of CKD, we studied the combined end point of death and CKD. Using the combined end point, temporary and persistent declines remained independent predictors of the primary end point incident CKD and death (temporary: RR 2.1 [95% CI 1.6 to 3.0]; persistent: RR 2.2 [95% CI 1.6 to 3.1]); however, the magnitude of the ORs decreased.
Discussion
To our knowledge, this study is the first to show that temporary perioperative renal function decline is an independent predictor for the development of incident CKD during long-term follow-up. During the perioperative period, >40% of the patients developed temporary or persistent renal function decline, which was invariably associated with the development of CKD independent of other important confounders that are known to be associated with kidney disease progression.
Ischemia-reperfusion injury as a result of hypotension or sepsis is one of the major causes of AKI (15). Ischemia and/or reperfusion initiates changes in vascular endothelial cells, tubular epithelial cells, and leukocytes that result in the loss of immune system homeostasis in the kidney with a consequent inflammatory response (16). Animal studies have demonstrated that AKI causes permanent damage to the microvasculature with subsequent abnormalities in kidney structure and function (17). Basile et al. (18) demonstrated that recovery from ischemia-reperfusion injury in rats is not complete and that it compromises sodium hemostasis and predisposes to hypertension and secondary renal disease. In addition, this same group of investigators reported that in rats that were recovering from acute renal failure, genes with known inflammatory, remodeling, and vasoactive activities were identified, some of which may play a role in altering long-term renal function (19–21). In human studies, it has been demonstrated that after an episode of AKI, residual kidney injury promotes the release of inflammatory markers such as C-reactive protein, IL-6, and D-dimer (22). In combination with fibrotic signaling pathways, these inflammatory pathways can lead to progressive structural kidney damage (6). This silent, ongoing process predisposes to worsening hypertension, proteinuria, and more rapid decreases in renal function, all of which are widely known risk factors for kidney disease progression and establishment of CVD (5,7).
The incidence of AKI varies between 2 and 45%, depending on the study population and the definition used for AKI (2–4). Especially, patients who undergo surgical procedures and have a high risk for ischemia-reperfusion injury as a consequence of hemodynamic instabilities are at increased risk for AKI (2,4). The association between the development of AKI and increased rates of in-hospital mortality has been widely known for decades and has been reported in several studies (4,23,24); however, the association of AKI and long-term outcome, especially incident CKD, has been less studied. This could be a result of the seeming reversibility of the clinical episode as observed by improvements in Scr levels. Better understanding of the impact of AKI on long-term outcomes may identify a segment of patients who may need extended follow-up after discharge. A meta-analysis by Coca et al. (6) demonstrated that in hospitalized patients, AKI is common and is an independent predictor of long-term myocardial infarction and all-cause mortality (RRs 1.6 to 2.6 and 1.6 to 3.9, respectively); however, in this analysis, the RR for incident CKD after AKI was unattainable because of lack of follow-up studies with appropriate control subjects who did not have AKI. In this study, 11% of the patients with normal or improved renal function during the perioperative period developed CKD during long-term follow-up. These findings are in line with several (general) population studies in which the incidence of CKD ranged between 10 and 17% (7,25,26). CKD was defined according the NKF/DOQI definition, using a cutoff value of eGFR <60 ml/min per 1.73 m2. Stevens et al. (27,28) demonstrated that eGFR near 60 ml/min per 1.73 m2 should be interpreted with caution; therefore, we performed additional sensitivity analyses using more specific definitions of CKD (eGFR <45 and <30 ml/min per 1.73 m2), which demonstrated a change in the strength of the predictive value. Both temporary and persistent renal function decline remained independent predictors of long-term CKD, however. Importantly, because the number of patients with more advance CKD decreased significantly, the results for these cutoffs need to be interpreted with some caution.
In this study we are the first to describe the value of a small, temporary impairment in renal function as an independent predictor of long-term CKD. The prognostic importance of small, acute decrements in renal function has been studied in several surgical and intensive care unit populations. Coca et al. (8) summarized these results in a meta-analysis and demonstrated that patients with a 10 to 24% increase in Scr levels had a RR for death of 1.8 (95% CI 1.3 to 2.5). A graded relation between the increase in Scr levels and adverse outcome in a variety of clinical settings and patient types was observed. Welten et al. (10) demonstrated that although renal function may recover completely after aortic surgery, temporary decline of renal function was still associated with increased mortality during long-term follow-up. It has been noted by other investigators that the use of smaller changes in Scr levels to define AKI are more likely to be only a reflection of hemodynamic changes than real kidney injury (29) and that small or very temporary increases in Scr are simply markers for comorbid conditions. In this study, we used a cutoff value of 10% change in CKD-EPI eGFR compared with baseline preoperative CKD-EPI eGFR. To evaluate the predictive strength of this small, temporary change in CKD-EPI eGFR, we performed additional sensitivity analyses using a stepwise increased cutoff value of 20, 30, 40, and 50% change in CKD-EPI GFR compared with baseline. It is interesting that there seemed to be a dosage-dependent relationship between the magnitude of exposure and the risk for outcome in patients with a temporary decline of renal function. In patients with a persistent decline of renal function after the perioperative period, the dosage-dependent relationship was observed up to a cutoff of 30%. Although the graded association was not completely observed for both groups, these findings strongly suggest that even small, temporary deteriorations in renal function are already an independent predictor for development of long-term CKD. Importantly, in additional sensitivity analyses using the RIFLE criteria for AKI, both temporary and persistent declines in renal function became even stronger predictors for development of long-term CKD; however, because the focus of this study was on small changes of renal function in relation to outcome, the 10% definition was used for the study outcomes.
This study supports the use of small changes in renal function in daily clinical practice, because both temporary and persistent declines in renal function were independent predictors of development of CKD. These small changes in Scr are a reflection of the onset of AKI; therefore, early management of AKI should be initiated. Despite the seeming reversible nature of clinical AKI, more attention should be given to perioperative impairment in renal function. Episodes of AKI confer increased risk for CKD and CVD; therefore, follow-up and risk factor should be monitored more closely. In addition, medical therapies that are known to prevent these outcomes could be applied earlier and more aggressively.
Potential limitations of this study merit consideration. This study has the disadvantage of a retrospective design. As a consequence, data on long-term Scr levels were not available for all patients in the source population but for 59% of the patients. To study the possibility of selection bias, we compared the source population and the study population regarding baseline characteristics, and no significant differences were observed. Furthermore, patients with established CKD before surgery were excluded from the study. As a consequence of the study design, we were not able to study the influence of AKI on kidney disease progression in patients with established CKD. During the follow-up period, Scr levels were not measured at prespecified time points. Multivariate analyses were adjusted for the time between surgery and the last Scr level recorded, and additional sensitivity analyses were performed; however, the presence of potential survival bias in patients with multiple Scr measurements during long-term follow-up has to be acknowledged.
There is a possibility of ascertainment bias because patients with increased risk for CKD were likely to have more frequent Scr measurements. In addition, informative censoring could not be completely ruled out regarding the influence of CKD and death on the occurrence of the end point; however, sensitivity analyses using a combined end point of CKD and death demonstrated that temporary and persistent renal function declines remained independent predictors of the primary end point.
There are several definitions for AKI; however, none of them includes small changes in renal function. In addition, we evaluated the presence of AKI only during the first 3 postoperative days and did not examine specific causes of AKI. Furthermore, Scr levels at day of hospital discharge were not available. In the literature, there is a growing consensus to use the RIFLE criteria, but these do not include small changes in Scr levels; therefore, in this study, we used the cutoff value of 10% in CKD-EPI eGFR on the basis of previous studies and a meta-analysis (8,10). It has to be noted that, independent of which prediction equation is used to estimate GFR, the changes in GFR in the acute setting are always difficult to interpret and a limitation of this study. Finally, although a minor dosage-dependent relationship between the magnitude of exposure (e.g., higher cutoff values) and the risk for outcome was observed, these data should be viewed as hypothesis-generating for future studies.
Conclusions
Temporary or persistent perioperative decline of renal function has a high incidence in the vascular surgery population. Temporary decline of renal function was an independent prognostic predictor for the development of CKD during long-term follow-up.
Disclosures
None.
Acknowledgments
J.-P.v.K., W.-J.F., and T.A.W. are supported by an unrestricted grant from “lijf en leven” Foundation, Rotterdam, Netherlands.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellenberger C, Schweizer A, Diaper J, Kalangos A, Murith N, Katchatourian G, Panos A, Licker M: Incidence, risk factors and prognosis of changes in serum creatinine early after aortic abdominal surgery. Intensive Care Med 32: 1808–1816, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kheterpal S, Tremper KK, Englesbe MJ, O’Reilly M, Shanks AM, Fetterman DM, Rosenberg AL, Swartz RD: Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 107: 892–902, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Basile DP: The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int 72: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR: The prognostic importance of a small acute decrement in kidney function in hospitalized patients: A systematic review and meta-analysis. Am J Kidney Dis 50: 712–720, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA: Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 16: 195–200, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Welten GM, Schouten O, Chonchol M, Hoeks SE, Feringa HH, Bax JJ, Dunkelgrun M, van Gestel YR, van Domburg RT, Poldermans D: Temporary worsening of renal function after aortic surgery is associated with higher long-term mortality. Am J Kidney Dis 50: 219–228, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Perrone RD, Madias NE, Levey AS: Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem 38: 1933–1953, 1992 [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176: 532–555, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ: Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kinsey GR, Li L, Okusa MD: Inflammation in acute kidney injury. Nephron Exp Nephrol 109: e102–e107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burne-Taney MJ, Liu M, Ascon D, Molls RR, Racusen L, Rabb H: Transfer of lymphocytes from mice with renal ischemia can induce albuminuria in naive mice: A possible mechanism linking early injury and progressive renal disease? Am J Physiol Renal Physiol 291: F981–F986, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP: Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Basile DP, Fredrich K, Alausa M, Vio CP, Liang M, Rieder MR, Greene AS, Cowley AW, Jr: Identification of persistently altered gene expression in the kidney after functional recovery from ischemic acute renal failure. Am J Physiol Renal Physiol 288: F953–F963, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Spurgeon KR, Donohoe DL, Basile DP: Transforming growth factor-beta in acute renal failure: Receptor expression, effects on proliferation, cellularity, and vascularization after recovery from injury. Am J Physiol Renal Physiol 288: F568–F577, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL: Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 297: R1358–F1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 107: 87–92, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA: Acute renal failure in patients with sepsis in a surgical ICU: Predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol 14: 1022–1030, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT: Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 128: 194–203, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Hsu CY, Vittinghoff E, Lin F, Shlipak MG: The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med 141: 95–101, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, Rahman M, Deysher AE, Zhang YL, Schmid CH, Levey AS: Evaluation of the Modification of Diet in Renal Disease study equation in a large diverse population. J Am Soc Nephrol 18: 2749–2757, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Stevens LA, Greene T, Levey AS: Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol 1: 874–884, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lo L, Liu KD, Hsu CY: Long-term outcomes after acute kidney injury: Where we stand and how we can move forward. Am J Kidney Dis 53: 928–931, 2009 [DOI] [PubMed] [Google Scholar]