Abstract
Background and objectives: Cardiovascular disease is highly prevalent in chronic kidney disease. Traditional risk factors are insufficient to explain the high cardiovascular disease prevalence. Free p-cresol serum concentrations, mainly circulating as its derivative p-cresyl sulfate, are associated with cardiovascular disease in hemodialysis patients. It is not known if p-cresol is associated with cardiovascular disease in patients with chronic kidney disease not yet on dialysis.
Design, setting, participants, & measurements: In a prospective observational study in 499 patients with mild-to-moderate kidney disease, we examined the multivariate association between p-cresol free serum concentrations and cardiovascular events.
Results: After a mean follow-up of 33 mo, 62 patients reached the primary end point of fatal or nonfatal cardiovascular events. Higher baseline concentrations of free p-cresol were directly associated with cardiovascular events (univariate hazard ratio [HR] 1.79, P < 0.0001). In multivariate analysis, p-cresol remained a predictor of cardiovascular events, independent of GFR and independent of Framingham risk factors (full model, HR 1.39, P = 0.04).
Conclusions: These findings suggest that p-cresol measurements may help to predict cardiovascular disease risk in renal patients over a wide range of residual renal function, beyond traditional markers of glomerular filtration. Whether p-cresol is a modifiable cardiovascular risk factor in CKD patients remains to be proven.
Chronic kidney disease reaches epidemic dimensions. In the recent National Health and Nutrition Examination Survey (NHANES), the overall prevalence of CKD (stages 1 to 4) increased from 10.0% in 1988 to 1994 to 13.1% in 1999 to 2004 (1). Mounting data point to the lethal synergy between chronic kidney disease (CKD) and cardiovascular disease (CVD) (2–4) and between CKD and overall mortality (5). Cardiovascular mortality in CKD patients treated with hemodialysis is more than fivefold higher than in patients in the general population, even after stratification for age, gender, race, and the presence of diabetes. The increased incidence of cardiovascular disease is not limited to patients with end-stage renal disease (6). The hazard ratio for cardiovascular events increases inversely with the estimated GFR (eGFR) (7). Individuals with earlier stages of CKD are more likely to die of CVD than to develop kidney failure.
Although kidney disease-associated CVD is potentially preventable and treatable, therapies targeting traditional risk factors prove less effective in CKD. For example, two large randomized trials failed to demonstrate a benefit of statin therapy to reduce cardiovascular disease risk in hemodialysis patients (8,9). As traditional cardiovascular risk factors are insufficient to predict and treat true cardiovascular disease risk in patients with CKD (10,11), it is assumed that CKD-specific pathways contribute substantially to the high cardiovascular disease risk.
Retention of organic waste solutes might be in the causal chain between CKD and cardiovascular disease. In hemodialysis patients, free serum concentrations of p-cresol (including its sulfate and glucuronide conjugates) were found to be associated with cardiovascular disease (12). More recently, a direct association between indoxyl sulfate and cardiovascular outcomes was observed (13). Intriguingly, whereas p-cresyl sulfate and indoxyl sulfate behave similarly during dialysis and share the same albumin binding site, total serum concentrations in hemodialysis patients are unrelated (14).
Whether p-cresol is associated with cardiovascular disease in patients with chronic kidney disease not yet on dialysis is not known. We therefore performed a prospective observational study in patients with chronic kidney disease not yet on dialysis (clinical trials protocol NCT00441623). The aims were, first, to explore the relationship between chronic kidney disease and p-cresol serum concentrations and, second, to explore the relationship between p-cresol and cardiovascular disease and to compare the strength of this relationship relative to traditional and novel cardiovascular risk factors.
Materials and Methods
Patients
Prevalent chronic kidney disease patients, followed at the CKD outpatient clinic of the University Hospital Gasthuisberg, 18 years or older and able to provide consent, were eligible for inclusion. Patients were screened between November 2005 and September 2006. The study was performed according to the Declaration of Helsinki and approved by the ethics committee of the University Hospital Leuven. Informed consent was obtained from all patients. The trial was prospectively registered at clinicaltrials.gov (NCT00441623).
Baseline Evaluation and Biochemical Measurements
Data on baseline demographics, smoking habit, presence of diabetes, prevalent cardiovascular disease, and cause of kidney disease were collected at time of informed consent. At inclusion, blood was taken by venous puncture for measurement of creatinine (mg/dl), hemoglobin (g/dl), biointact parathormone (PTH) (ng/L), calcium (mg/dl), phosphate (mg/dl), albumin (g/dl), C-reactive protein (CRP) (mg/L), cholesterol (mg/dl), and total and free p-cresol (mg/L).
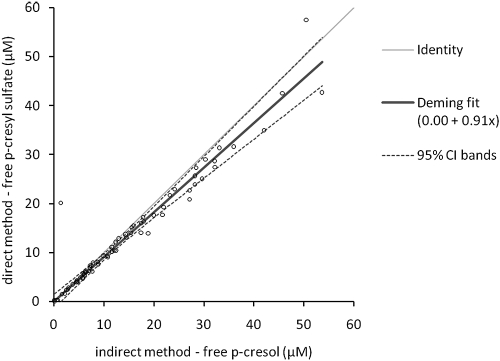
Creatinine, hemoglobin, PTH, calcium, phosphate, CRP, and cholesterol were all measured using standard laboratory techniques. The eGFR was calculated using the four-variable MDRD (Modification of Diet in Renal Disease study) equation (15). Albumin was measured using the bromcresol green method. Total and free (not bound to proteins) p-cresol serum concentrations were measured using a gas chromatography-mass spectrometry (GC-MS) method on serum stored at −80°C until batch analysis. After heat-acid denaturation of binding proteins, p-cresol was extracted in ethyl acetate and injected on the GC-MS (Trace GC-MS; Thermo Finnigan, San José, CA). p-Cresol-d8 was used as internal standard. Free p-cresol concentrations were measured in serum ultrafiltered at 37°C using 30,000 D molecular cutoff filters (Centifree UF devices; Amicon, Beverly, MA). We and others have recently demonstrated that in vivo p-cresol is almost entirely sulfated (16,17). Both p-cresol and its conjugated metabolites are measured by this analytical technique (17). We demonstrated good agreement between the GC-MS–based method for indirect quantification of p-cresyl sulfate after hydrolysis to p-cresol and a HPLC method for direct quantification of total serum concentrations (18). We performed additional method comparison for unbound p-cresol/p-cresyl sulfate (Figure 1). We chose to use the GC-MS–based method because of its lower limit of quantification. Values below the limit of quantification (0.02 mg/L; 0.18 μM) were treated as 0.09 μM for statistical analysis. Intra- and interassay coefficients of variation were 3.3% and 5.3%, respectively.
Figure 1.
Method comparison between indirect quantification of unbound p-cresyl sulfate concentrations after hydrolysis to p-cresol by gas chromatography-mass spectrometry and direct quantification of unbound p-cresyl sulfate concentrations by HPLC. Deming fit demonstrated excellent agreement. As expected, we observed a nonsignificant (P = 0.1) small proportional bias (y = 0.00 + 0.91 · x). Indeed, besides p-cresyl sulfate, small amounts of p-cresyl glucuronide and unconjugated p-cresol are quantified by the indirect GC-MS method.
End Point Evaluation
Patients were followed at the nephrology CKD outpatient clinic at 3- to 6-month intervals. Patients were prospectively followed until December 1, 2008. If the patient's last study visit was before October 2008 or a scheduled visit was missed, patients and/or their general practitioner were contacted by phone to ensure completeness of the study data. If information could not be obtained, the patient was assumed to be lost to follow-up starting from the date of the last actual visit. Cardiovascular end points were prospectively recorded and coded, blinded from clinical and biochemical data. The primary end point (first cardiovascular event) was a composite of death from cardiac causes, nonlethal myocardial infarction, myocardial ischemia, coronary intervention, ischemic stroke, or new peripheral vascular disease, whichever occurred first. Only one event per subject was included in the analysis.
After review of available information, cause of death was classified as either cardiovascular, infectious, malignancy, or other. Cardiovascular deaths included fatal myocardial infarction, sudden death, and death due to congestive heart failure. Cases of unobserved sudden death were considered cardiovascular death only when other potential causes could be excluded. Otherwise, they were classified as “other cause of death.” Out-of-hospital deaths were coded after consultation of the general practitioner. Nonlethal cardiovascular events included myocardial infarction, diagnosed based on elevated levels of cardiac enzymes and/or typical electrocardiography changes, myocardial ischemia with typical electrocardiography changes without elevated cardiac enzymes, and coronary intervention (thrombolysis, percutaneous coronary intervention, or coronary artery bypass grafting). Ischemic stroke was defined as a neurologic deficit lasting more than 24 hours. Hemorrhagic stroke was excluded from the primary end point. Peripheral vascular disease included new-onset ischemic pain in the lower limbs, with abnormal ankle brachial pressure index or radiologic evidence of peripheral vascular disease, new-onset ischemic necrotic lesions, or surgical arterial intervention.
Statistical Analysis
Continuous variables are expressed as mean (SD) for normally distributed variables or median (interquartile range) otherwise. Differences between baseline variables according to free p-cresol tertiles were analyzed by the Kruskal-Wallis test. Associations between p-cresol free serum concentrations and other variables were analyzed using a Spearman rank correlation matrix. The Kaplan-Meier method was used to estimate cumulative incidence of the primary end point. Time to first cardiovascular event analysis was performed using Cox proportional hazards analysis. The relative risk of a new cardiovascular event was expressed as a hazard ratio. A two-sided P < 0.05, unadjusted for multiple comparisons, was considered statistically significant. Several models were built, including different sets of covariates, to adjust for demographics (age and gender), medical history (coronary artery disease and peripheral artery disease), presence of diabetes, CKD markers [hemoglobin, calcium, phosphate, and PTH (Ln) concentrations], markers of inflammation [C-reactive protein (Ln) concentrations], markers of nutrition (body mass index and albumin concentrations), Framingham risk factors (age, gender, systolic BP, smoking, diabetes, and serum cholesterol concentrations), and medical therapy (use of converting enzyme inhibitors/Angiotensin receptor blockers, phosphate binders, statin intake, and 25-hydroxy vitamin D supplements). For each model, the proportionality assumption was tested against log(time). In case the proportionality assumption was violated, follow-up time-adjusted variables were entered as covariates. Owing to relatively low event number, candidate covariates for the final model were selected from these models (hierarchical elimination approach). Variables with P < 0.2 in individual models were retained. For sensitivity testing, additional best-fit models were constructed using backward elimination (Pexclude > 0.05) and stepwise selection (Pinclude < 0.20, Pexclude > 0.05). Two different approaches were used to select covariates. In a first approach we included all covariates associated at the P = 0.2 level with new cardiovascular events on univariate analysis. Using this approach, we included age, gender, history of coronary artery disease, history of peripheral vascular disease, diabetes, eGFR, hemoglobin, calcium, phosphate, CRP, albumin, systolic BP, and free p-cresol concentrations. As the number of covariates is sizeable, we performed backward elimination at P ≥ 0.2 followed by backward elimination at P ≥ 0.05 to reduce the risk of overfitting. In a second approach we included the most widely accepted cluster of covariates used to estimate cardiovascular disease risk. This approach yielded age, gender, diabetes, smoking habit, systolic BP, and serum cholesterol as covariates. To study the potential confounding effect of kidney function, eGFR was entered both as covariate and as stratifying variable. As eGFR is modeled from age and gender, creatinine was entered as a traditional marker of kidney function in multivariate models where age and gender were included. For statistics, SAS (version 9.1; the SAS Institute, Cary, NC) and SPSS (version 15.0; Chicago, IL) software packages were used. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree with the manuscript as written.
Results
Study Population
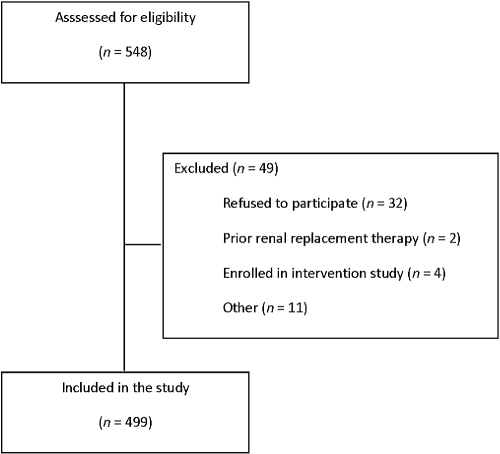
Between November 2005 and September 2006, 548 prevalent patients with chronic kidney disease Kidney Disease Outcomes Quality Initiative (KDOQI) stages 1 to 5, followed at the nephrology outpatient clinic of the University Hospital Gasthuisberg, Leuven, Belgium, were found eligible to be enrolled in the study. Four hundred ninety-nine patients providing informed consent were included in this prospective cohort study (Figure 2). Table 1 represents the demographic and baseline characteristics of the study population. Baseline characteristics of the 49 excluded individuals were similar to the 499 included patients.
Figure 2.
Patient inclusion. Flow chart demonstrating patient screening and inclusion.
Table 1.
Baseline demographic and laboratory data
| Variable | Overall |
p-Cresol |
P | ||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||
| Age (yr) | 64 (50–75) | 53 (39–63) | 69 (54–75) | 73 (61–78) | <0.0001 |
| Gender: male/female (%) | 274/225 (55/45) | 82/85 (49/51) | 101/65 (61/39) | 91/75 (55/45) | 0.10 |
| BMI (kg/m2) | 26.5 (5.2) | 26.5 (5.5) | 26.2 (4.8) | 26.7 (5.3) | 0.8 |
| Diabetes: yes/no (%) | 91/408 (18/82) | 19/148 (11/89) | 22/144 (13/87) | 50/116 (30/70) | <0.0001 |
| Prior CVD: yes/no (%) | 139/360 (28/72) | 22/145 (13/87) | 47/121 (28/72) | 70/96 (42/58) | <0.0001 |
| Current smoker: yes/no (%) | 90/409 (18/82) | 39/128 (23/77) | 24/142 (14/86) | 27/139 (16/84) | 0.09 |
| Therapy with ACEI/ARB:a yes/no (%) | 352/146 (71/29) | 112/55 (67/33) | 117/49 (70/30) | 123/42 (74/26) | 0.3 |
| Therapy with statins:a yes/no (%) | 241/257 (48/52) | 55/112 (33/67) | 93/73 (56/44) | 93/72 (56/44) | <0.0001 |
| Blood pressure: systolic/diastolic (mmHg) | 138 (22)/78 (12) | 133 (22)/78 (11) | 139 (21)/78 (12) | 142 (24)/77 (12) | 0.0008/0.3 |
| Serum creatinine (mg/dl) | 1.79 (1.28–2.47) | 1.18 (0.96–1.57) | 1.72 (1.43–2.02) | 2.58 (2.07–3.46) | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 35.7 (24.63–55.3) | 61.2 (41.9–75.6) | 38.1 (31.2–48.3) | 22.9 (16.0–28.3) | <0.0001 |
| Cholesterol (mg/dl) | 180.6 (43.2) | 185.0 (41.4) | 185.3 (45.8) | 171.8 (41.4) | 0.001 |
| LDL (mg/dl) | 90.1 (36.3) | 93.3 (35.6) | 92.7 (40.2) | 84.5 (32.3) | 0.02 |
| Albumin (g/L) | 44.3 (3.9) | 44.7 (3.9) | 44.8 (4.0) | 43.5 (3.8) | 0.001 |
| Calcium (mg/dl) | 9.55 (0.51) | 9.51 (0.46) | 9.64 (0.48) | 9.50 (0.58) | 0.06 |
| Phosphate (mg/dl) | 3.39 (0.74) | 3.19 (0.67) | 3.19 (0.57) | 3.79 (0.80) | <0.0001 |
| CRP (mg/L) | 1.0 (1.0–6.0) | 2.0 (1.0–6.0) | 2.0 (1.0–4.0) | 3.0 (1.0–7.0) | 0.02 |
| Hemoglobin (mg/dl) | 13.4 (1.8) | 14.0 (1.8) | 13.7 (1.7) | 12.4 (1.5) | <0.0001 |
| Total p-cresol (μM) | 43.6 (17.8–91.3) | 12.0 (4.5–18.9) | 44.1 (33.7–57.1) | 118.2 (87.9–156.6) | <0.0001 |
| Free p-cresol (μM) | 1.57 (0.60–3.56) | 0.41 (0.09–0.60) | 1.59 (1.15–1.97) | 4.74 (3.60–6.57) | <0.0001 |
Main demographic, clinical, and biochemical characteristics of study population. Data are expressed as mean (SD) or median (25–75th percentile), as appropriate. Differences between tertiles were tested using Kruskal-Wallis test or χ2 test as appropriate. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein.
Data on drug therapy are missing for one individual in p-cresol tertile 3.
Baseline Correlates of p-Cresol
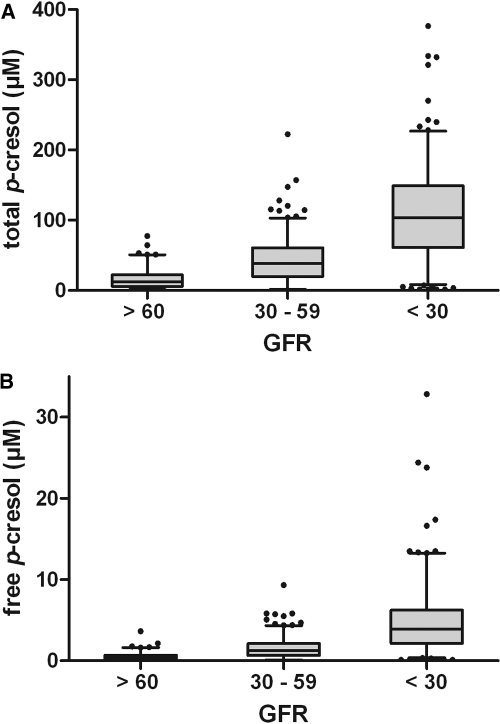
Total and free p-cresol serum concentrations were tightly correlated (r = 0.95, P < 0.0001). At baseline, free p-cresol serum concentrations were inversely associated with eGFR (Spearman rank r = −0.70, P < 0.0001) (Figure 3; Table 2). Other variables significantly associated with free p-cresol concentrations were markers of nutrition (albumin and cholesterol), serum phosphate, and hemoglobin (Table 2). Patients with diabetes had higher p-cresol concentrations than patients without diabetes (Mann-Whitney U P < 0.0001). eGFRs of patients with diabetes were significantly lower as compared with those of patients without diabetes (eGFR 31.3 (14.7) ml/min per 1.73 m2 versus 43.1 (22.7) ml/min per 1.73 m2). After adjustment for residual renal function diabetes was no longer associated with total p-cresol serum concentrations (P = 0.3). However, diabetes remained independently associated with higher free p-cresol serum concentrations (P = 0.03).
Figure 3.
p-Cresol and kidney function. Distribution of (A) total p-cresol serum concentrations and (B) free p-cresol serum concentrations as a function of estimated GFR (ml/min per 1.73 m2).
Table 2.
Spearman rank correlations of baseline characteristics with free p-cresol serum concentrations
| Free p-cresol |
||
|---|---|---|
| r | P | |
| Age | 0.43 | <0.0001 |
| Gender | −0.04 | 0.4 |
| BMI | 0.03 | 0.5 |
| Diabetes | 0.22 | <0.0001 |
| Previous CV history | 0.61 | <0.0001 |
| Current smoking | −0.09 | 0.06 |
| Systolic blood pressure | 0.18 | <0.0001 |
| Diastolic blood pressure | −0.05 | 0.3 |
| Pulse pressure | 0.21 | <0.0001 |
| Serum creatinine | 0.68 | <0.0001 |
| eGFR | −0.70 | <0.0001 |
| KDOQI stage | 0.67 | <0.0001 |
| Cholesterol | −0.14 | 0.002 |
| LDL | −0.10 | 0.02 |
| Albumin | −0.15 | 0.0008 |
| Calcium | −0.03 | 0.6 |
| Phosphate | 0.45 | <0.0001 |
| C-reactive protein | 0.09 | 0.06 |
| Hemoglobin | −0.37 | <0.0001 |
BMI, body mass index; CV history, cardiovascular disease history; eGFR, estimated glomerular filtration rate; KDOQI, Kidney Disease Outcomes Quality Initiative; LDL, low-density lipoprotein.
Cardiovascular Event Analysis
After a mean follow-up of 32.6 (SD 2.9) months, 62 patients reached the combined primary end point of a new nonfatal cardiovascular event (n = 50) or death caused by cardiovascular disease (n = 12) (Table 3). Patients were censored at start of renal replacement therapy (n = 94), death other than cardiovascular (n = 26), loss to follow-up (n = 43, 3.2%/year of follow-up), and at the end of the study observation period.
Table 3.
Cardiovascular events
| n (%) | |
|---|---|
| Nonfatal events | 50 (81) |
| Cardiac events | 24 (39) |
| new onset angina, conservative | 9 (15) |
| new onset angina, invasive therapy | 5 (8) |
| myocardial infarction | 8 (13) |
| ventricular arrhythmia | 2 (3) |
| Ischemic CVA | 6 (10) |
| Peripheral vascular disease | 20 (32) |
| thoracic aorta | 3 (5) |
| abdominal aorta | 8 (13) |
| carotid artery | 3 (5) |
| lower limbs | 6 (10) |
| Fatal cardiovascular events | 12 (19) |
| heart failure | 1 (2) |
| myocardial infarction | 2 (3) |
| sudden death, in hospital | 2 (3) |
| sudden death, out of hospital | 7 (11) |
Table 4.
Cox regression models of time to first cardiovascular events as a function of free p-cresol serum concentrations
| Free p-Cresol(Ln μM) |
||
|---|---|---|
| HR (95% CI) | P | |
| (A) Unadjusted | 1.79 (1.41–2.27) | <0.0001 |
| (B) Demographics: age, gender | 1.43 (1.10–1.87) | 0.008 |
| (C) Diabetes | 1.71 (1.34–2.17) | <0.0001 |
| (D) Past medical history: CAD, PAD | 1.59 (1.24–2.02) | 0.0002 |
| (E) eGFR | 1.49 (1.09–2.04) | 0.01 |
| (F) Markers of CKD: Hb, calcium, phosphorus, PTH (Ln) | 1.76 (1.33–2.32) | <0.0001 |
| (G) Markers of inflammation: CRP (Ln) | 1.74 (1.37–2.21) | <0.0001 |
| (H) Markers of nutrition: albumin, BMI | 1.70 (1.33–2.16) | <0.0001 |
| (I) Framingham risk factorsa | 1.40 (1.07–1.84) | 0.01 |
| (J) Current therapy: CEI/ARB, statins, PBT, 25-hydroxy vitamin D | 1.82 (1.40–2.37) | <0.0001 |
| Full model: age, gender, CAD, albumin, calcium, creatinine, 25-hydroxy vitamin D therapy | 1.39 (1.02–1.89) | 0.04 |
ARB, angiotensin receptor blockers; BMI, body mass index; CAD, coronary artery disease; CEI, converting enzyme inhibitors; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate (ml/min per 1.73 m2); Hb, haemoglobin; PAD, peripheral artery disease; PBT, phosphate binder therapy; PTH, parathormone.
Framingham risk factors include age, gender, systolic blood pressure, smoking, diabetes, and serum cholesterol concentrations. In the multivariate Framingham risk factor adjusted model, the proportionality assumption for cholesterol was violated. The results of a time-dependent model are given, in which follow-up time-adjusted cholesterol was entered as covariate.
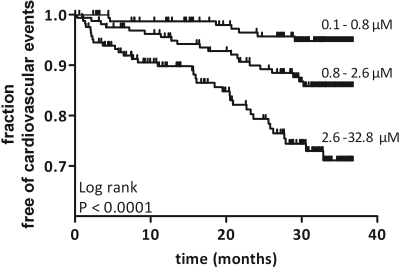
In univariate analysis, free p-cresol serum concentrations (hazard ratio [HR] 1.79, P < 0.0001) were directly associated with new-onset cardiovascular disease (Figure 4). Demographics (age, P < 0.0001; gender, P = 0.03), past medical history (coronary artery disease, P < 0.0001; peripheral artery disease, P < 0.0001), diabetic status (P = 0.003), traditional markers of kidney function (creatinine, P = 0.003; eGFR, P < 0.0001; KDOQI stage, P < 0.0001), anemia (hemoglobin, P = 0.01), bone-mineral disorders (calcium, P = 0.02; phosphate, P = 0.04), and markers of inflammation (CRP, P = 0.03; albumin, P < 0.0001) were all significantly associated with cardiovascular disease. In contrast, several Framingham risk factors (systolic BP, P = 0.08; total cholesterol, P = 0.4; LDL cholesterol, P = 0.9) and current smoking habits (P = 0.3) were not significantly associated with cardiovascular disease in this cohort.
Figure 4.
Kaplan-Meier curve of time to first lethal or nonlethal cardiovascular event. Tertiles of free p-cresol serum concentrations. p-Cresol tertiles 1 to 3: 7, 20, and 35 events, respectively. Log rank P < 0.0001.
Free p-cresol serum concentrations remained an independent predictor after adjustment (Table 3) for demographics (age and gender), diabetes, past medical history (coronary artery disease and peripheral artery disease), eGFR, markers of CKD (calcium, phosphate, PTH, and hemoglobin), markers of inflammation (CRP), nutritional markers (albumin and BMI), all Framingham risk factors (age, gender, current smoking habits, cholesterol, systolic BP, and presence of diabetes), and drug therapy (use of converting enzyme inhibitors/Angiotensin receptor blockers, phosphate binders, statin intake, and 25-hydroxy vitamin D supplements). In the final model (see Materials and Methods), free p-cresol serum concentrations were independently associated with cardiovascular disease (P = 0.04).
We performed additional sensitivity analyses to probe for residual confounding. Backward elimination and stepwise selection procedures were used to construct best-fit models. On the basis of this approach and including all variables univariately associated (P < 0.2) with cardiovascular events (see Materials and Methods), free p-cresol concentrations were independently associated with new cardiovascular events (HR 1.32, P = 0.03), together with age, gender, history of coronary artery disease, and serum albumin. Alternatively, we built a best-fit model to test the strength of the association between free p-cresol and cardiovascular disease relative to those variables broadly used to estimate future cardiovascular risk. Free p-cresol was added to age, gender, diabetes, smoking habit, systolic BP, and serum cholesterol, which constitute the Framingham risk equation and the Systematic Coronary Risk Evaluation (SCORE) (19). In this cohort, besides age and gender, only free p-cresol concentrations were independently associated with new cardiovascular disease (HR 1.41, P = 0.01). When traditional markers of kidney function were forced into these analyses, they lost their predictive power and were not retained in the final models.
Discussion
In this prospective study in chronic kidney disease patients not yet on dialysis, free p-cresol concentrations were associated with new cardiovascular disease, independent of demographics, Framingham risk factors, and traditional markers of kidney function.
Cardiovascular mortality in patients with end-stage renal disease is substantially higher than that in the general population. The high prevalence of cardiovascular disease in incident hemodialysis patients suggests that earlier stages of CKD promote the genesis and/or progression of cardiovascular disease (2,3). Indeed, large epidemiologic studies demonstrate a graded association between reduced estimated GFR and the risk of cardiovascular disease (7,20). Although part of this association can be explained by a higher prevalence and possibly larger impact of known risk factors, such as older age, hypertension, and diabetes (3), such “classical” cardiovascular risk factors are insufficient to accurately predict cardiovascular risk in patients with CKD (21). Several CKD-associated conditions are postulated to contribute to the high cardiovascular disease prevalence. Besides anemia, disturbances of bone and mineral metabolism, and fluid overload, retention of molecules that are cleared by the kidneys is thought to contribute to this increased risk (22).
One such uremic retention solute is p-cresol. We previously demonstrated that free p-cresol serum concentrations were independently associated with cardiovascular disease in maintenance hemodialysis patients (12). In the present study, we found a dose-response relationship between p-cresol serum concentrations and cardiovascular disease in CKD patients not yet on dialysis, confirming our initial findings and extending the relevance of p-cresol to the far larger population of patients with earlier stages of CKD (1).
In these patients, kidney function appears to be an important determinant of p-cresol serum concentrations. Nevertheless, p-cresol remained associated with the primary end point after adjustment for traditional markers of kidney function, including eGFR. In addition, p-cresol is associated with cardiovascular disease in HD patients in which residual renal function is minimal and does not contribute to variation in p-cresol serum concentrations (12). Together these observations suggest that, rather than innocent bystander, p-cresol is a mediator of CKD-associated cardiovascular disease.
The mechanisms underlying this association are only partly understood. The analytical technique used in the current study measures p-cresol, including its conjugates. While previous in vitro studies focused on the effects of unconjugated p-cresol, several groups recently demonstrated that the vast majority (>95%) of p-cresol circulates as its sulfate conjugate (16,17), shifting the focus of research toward the effects of p-cresyl sulfate.
In vitro, p-cresyl sulfate induced shedding of endothelial microparticles, which could be abrogated by blockage of the Rho-kinase pathway (23). In addition, p-cresyl sulfate disrupts nitric oxide signaling. Mechanistically, p-cresyl sulfate shifts the sGC redox equilibrium toward the NO-insensitive heme-oxidized/heme-free form, thereby reducing nitric oxide signaling. Although these in vitro studies provide additional evidence that p-cresyl sulfate contributes directly to the observed associations with cardiovascular disease, it is unclear whether this confers an additional therapeutic target.
Further studies are needed to clarify whether reduction of p-cresol/p-cresyl sulfate serum concentrations indeed lowers cardiovascular disease risk in patients with CKD. Given the wide range in p-cresol serum concentrations within different strata of eGFR (Figure 1), additional as yet unknown determinants of p-cresol serum concentrations should be present. Indeed, p-cresol concentrations are very low, even in some patients with eGFR < 30 ml/min per 1.73 m2. In contrast, p-cresol serum concentrations are significantly higher in patients with diabetes as compared with patients without diabetes (P < 0.0001), as was previously reported in diabetic versus nondiabetic HD patients (12).
A limitation of this study is that urinary indices, including microalbuminuria, were not systematically collected. Microalbuminuria is an independent cardiovascular risk predictor over a wide range of residual kidney function (24,25). Moreover, we used eGFR as a marker of kidney function. This may lead to residual confounding with respect to true kidney function. In addition, the study was conducted among prevalent patients followed at a university hospital in northwestern Europe. The hospital functions as a combined secondary/tertiary referral center, which might have affected case mix. Moreover, our findings may not be completely generalizable to unreferred patients or patients in other geographical regions. Nevertheless, the current findings confirm and expand the previously reported association between free p-cresol and cardiovascular disease in hemodialysis patients.
Conclusions
The results of the current study suggest that p-cresol measurements may help to stratify the cardiovascular risk in CKD patients beyond traditional and nontraditional risk factors including kidney function. Whether p-cresol is a modifiable cardiovascular risk factor in CKD patients remains to be proven.
Disclosures
None.
Acknowledgments
During this study, B.M. was the recipient of a doctoral fellowship (FWO-Vlaanderen, Grant 1.1.382.06N). The research support by FWO-Vlaanderen (Project G.0408.06) is highly appreciated. We thank all patients involved in this study. We also thank M. Dekens, E. Vanhalewyck, H. De Loor, and A. Van Esch for excellent technical assistance. S. Cecere and S. Fieuws (Leuven Biostatistics and Statistical Bio-informatics Center) are acknowledged for statistical counseling. Part of this work was presented at the World Congress of Nephrology, May 22 through 26, 2009, Milan, Italy; and at the American Society of Nephrology Renal Week, October 27 through November 1, 2009, San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1..Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van LF, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038– 2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2..Sarnak M, Levey A: Cardiovascular disease and chronic renal disease: A new paradigm. Am J Kidney Dis 35Suppl 1: s117–s131, 2000 [DOI] [PubMed] [Google Scholar]
- 3..Foley R, Parfrey P, Sarnak M: The clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 4..Foley RN, Wang C, Collins AJ: Cardiovascular risk factor profiles and kidney function stage in the US general population: The NHANES III study. Mayo Clin Proc 80: 1270–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 5..de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De MJ, Wetzels JF, Rosendaal FR, Dekker FW: Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 6..Schiffrin EL, Lipman ML, Mann JFE: Chronic kidney disease: Effects on the cardiovascular system. Circulation 116: 85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 7..Go AS, Chertow GM, Fan D, McCulloch CE, Hsu Cy: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 8..Wanner C, Krane V, Marz W, Olschewski M, Mann JFE, Ruf G, Ritz E, the German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 9..Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F, the AURORA Study Group : Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 10..Sarnak M, Coronado B, Greene T: Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol 57: 327–335, 2002 [DOI] [PubMed] [Google Scholar]
- 11..Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12..Meijers BKI, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1173–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 13..Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA: Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14..Meijers BK, Loor HD, Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P: p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin J Am Soc Nephrol 4: 1932–1938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15..Levey AS, Coresh J, Greene T, Stevens LA, Zhang Y, Hendriksen S, Kusek JW, Van Lente F, for the Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 16..Martinez AW, Recht NS, Hostetter TH, Meyer TW: Removal of p-cresol sulfate by hemodialysis. J Am Soc Nephrol 16: 3430–3436, 2005 [DOI] [PubMed] [Google Scholar]
- 17..de Loor H, Bammens B, Evenepoel P, De Preter V, Verbeke K: Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin Chem 51: 1535–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 18..de Loor H, Meijers BK, Meyer TW, Bammens B, Verbeke K, Dehaen W, Evenepoel P: Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography. J Chromatogr A 1216: 4684–4688, 2009 [DOI] [PubMed] [Google Scholar]
- 19..De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Manger Cats V, Orth-Gomer K, Perk J, Pyorala K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D: European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 24: 1601–1610, 2003 [DOI] [PubMed] [Google Scholar]
- 20..Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de ZD, Pocock S, van Veldhuisen DJ: Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 113: 671–678, 2006 [DOI] [PubMed] [Google Scholar]
- 21..Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 22..Meyer TW, Hostetter TH: Uremia. N Engl J Med 357: 1316–1325, 2007 [DOI] [PubMed] [Google Scholar]
- 23..Meijers BK, Van kerckhoven S, Verbeke k, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P: The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 54: 891–901, 2009 [DOI] [PubMed] [Google Scholar]
- 24..Hillege HL, Fidler V, Diercks GFH, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans ROB, Janssen WMT, Grobbee DE, de Jong PE, for the Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group : Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 25..Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D'Agostino RB, Vasan RS: Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 112: 969–975, 2005 [DOI] [PubMed] [Google Scholar]