Abstract
One of the fundamental goals in proteomics and cell biology is to identify the functions of proteins in various cellular organelles and pathways. Information of subcellular locations of proteins can provide useful insights for revealing their functions and understanding how they interact with each other in cellular network systems. Most of the existing methods in predicting plant protein subcellular localization can only cover three or four location sites, and none of them can be used to deal with multiplex plant proteins that can simultaneously exist at two, or move between, two or more different location sites. Actually, such multiplex proteins might have special biological functions worthy of particular notice. The present study was devoted to improve the existing plant protein subcellular location predictors from the aforementioned two aspects. A new predictor called “Plant-mPLoc” is developed by integrating the gene ontology information, functional domain information, and sequential evolutionary information through three different modes of pseudo amino acid composition. It can be used to identify plant proteins among the following 12 location sites: (1) cell membrane, (2) cell wall, (3) chloroplast, (4) cytoplasm, (5) endoplasmic reticulum, (6) extracellular, (7) Golgi apparatus, (8) mitochondrion, (9) nucleus, (10) peroxisome, (11) plastid, and (12) vacuole. Compared with the existing methods for predicting plant protein subcellular localization, the new predictor is much more powerful and flexible. Particularly, it also has the capacity to deal with multiple-location proteins, which is beyond the reach of any existing predictors specialized for identifying plant protein subcellular localization. As a user-friendly web-server, Plant-mPLoc is freely accessible at http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/. Moreover, for the convenience of the vast majority of experimental scientists, a step-by-step guide is provided on how to use the web-server to get the desired results. It is anticipated that the Plant-mPLoc predictor as presented in this paper will become a very useful tool in plant science as well as all the relevant areas.
Introduction
Information of the subcellular localization of proteins is important because it can (1) indicate how and under what kind of cellular environments they interact with each other and with other molecules, (2) provide useful clues for revealing their functions, and (3) help understand the intricate pathways that regulate biological processes at the cellular level [1], [2]. Although this kind of information can be acquired by conducting various biochemical experiments, it is both time consuming and expensive to determine the subcellular localization of uncharacterized proteins one by one with experiments alone. With the avalanche of protein sequences generated in the Post-Genomic Age, it is highly desired to develop computational methods that can be used to identify the subcellular location site(s) of a newly found protein based on its sequence information alone.
During the past 17 years or so, numerous efforts have been made in this regard (see, e.g., [3], [4], [5], [6], [7], [8], [9], [10] as well as a long list of references cited in two comprehensive review articles [11], [12]). However, relatively much fewer predictors were developed specialized for predicting the subcellular localization of plant proteins. To the best of our knowledge, of the aforementioned methods only the one called “TargetP” [6] and the one called “Predotar” [8] are specialized for plant proteins. Ever since the two predictors were proposed, they have been widely used for studying various plant protein systems and related areas. However, TargetP and Predotar can discriminate plant proteins among only three or four location sites. For instance, TargetP [6] only covers the following sites: (1) mitochondria, (2) chloroplast, (3) secretory pathway, and (4) other. And Predotar [8] only covers the following sites: (1) endoplasmic reticulum, (2) mitochondrion, (3) plastid, and (4) other. After removing the ambiguous location of “other”, TargetP or Predotar actually covers only three subcellular location sites. If a user tried to use TargetP and Predotar to predict a query protein located outside the aforementioned sites, such as cell wall, peroxisome, Golgi apparatus, or vacuole, the two predictors would either fail to work or generate meaningless outcomes.
To improve the situation, the predictor called “Plant-PLoc” [13] was developed to extend the coverage scope for plant proteins from the three locations covered by TargetP or Predotar to the following eleven: (1) cell wall, (2) chloroplast, (3) cytoplasm, (4) endoplasmic reticulum, (5) extracellular, (6) mitochondrion, (7) nucleus, (8) peroxisome, (9) plasma membrane, (10) plastid, and (11) vacuole. The Plant-PLoc predictor was established by integrating the “higher-level” GO (gene ontology) [14] approach and PseAAC (pseudo amino acid composition) [15] approach. GO is a controlled vocabulary used to describe the biology of a gene product in any organism [16], [17]. The GO database was established based on the molecular function, biological process and cellular component [14], and hence proteins formulated in the GO database space would be clustered in a way much better reflecting their subcellular locations, as elucidated in [18]. For those proteins that cannot be meaningfully defined in the GO space, the PseAAC descriptor [15] would play a better complementary role than the classical AAC (amino acid composition) descriptor.
However, the existing Plant-PLoc [13] predictor has the following problems. (1) The accession number of a query protein is required as an input in order to utilize the advantage of GO approach. Many proteins, such as synthetic or hypothetical proteins, and newly discovered sequences without being deposited into databanks yet, do not have accession numbers, and hence cannot be treated with the GO approach. (2) Even with the accession numbers available, many proteins can still not be meaningfully formulated in a GO space because the current GO database is far from complete yet. (3) Although the PseAAC approach, a complementary approach to the GO approach in Plant-PLoc [13], can take into account some partial sequence order effects, the original PseAAC [15] did not contain the functional domain and sequential evolution informations, which have been proved to play an important role in enhancing the prediction quality of other protein attributes (see, e.g., [19], [20]). (4) Plant-PLoc [13] cannot be used to deal with multiplex proteins that may simultaneously exist at, or move between, two or more different subcellular locations. Proteins with multiple locations or dynamic feature of this kind are particularly interesting because they may have some very special biological functions intriguing to investigators in both basic research and drug discovery [2], [21]. Particularly, as pointed out by Millar et al. [22], recent evidence indicates that an increasing number of proteins have multiple locations in the cell.
The present study was initiated in an attempt to develop a new and more powerful predictor for predicting plant protein subcellular localization by addressing the above four problems.
Materials and Methods
Protein sequences were collected from the Swiss-Prot database at http://www.ebi.ac.uk/swissprot/. The detailed procedures are basically the same as those elaborated in [13]; the only differences are as follows. (1) To get the updated benchmark dataset, instead of version 49.3 of the Swiss-Prot database, the version 55.3 released on 29-Apr-2008 was adopted. (2) In order to make the new predictor also able to deal with proteins having two or more location sites, the multiplex proteins are no longer excluded in this study. Actually, according to a statistical analysis on the current database, about 8% of plant proteins were found located in more than one location.
After strictly following the aforementioned procedures, we finally obtained a benchmark
dataset 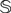 containing 978 different protein sequences, which are distributed
among 12 subcellular locations (
Fig. 1
); i.e.,
containing 978 different protein sequences, which are distributed
among 12 subcellular locations (
Fig. 1
); i.e.,
| (1) |
where  represents the subset for the subcellular location of cell membrane,
represents the subset for the subcellular location of cell membrane, 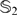 for cell wall,
for cell wall,  for chloroplast, and so forth; while
for chloroplast, and so forth; while  represents the symbol for “union” in the set
theory. A breakdown of the 978 plant proteins in the benchmark dataset
represents the symbol for “union” in the set
theory. A breakdown of the 978 plant proteins in the benchmark dataset  according to their 12 location sites is given in
Table 1
. To avoid redundancy and homology bias, none of the proteins in
according to their 12 location sites is given in
Table 1
. To avoid redundancy and homology bias, none of the proteins in 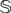 has
has 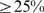 pairwise sequence identity to any other in a same subset. The
corresponding accession numbers and protein sequences are given in Table S1.
pairwise sequence identity to any other in a same subset. The
corresponding accession numbers and protein sequences are given in Table S1.
Figure 1. Schematic illustration to show the 12 subcellular locations of plant proteins.
The 12 location sites are: (1) cell membrane, (2) cell wall, (3) chloroplast, (4) cytoplasm, (5) endoplasmic reticulum, (6) extracellular, (7) Golgi apparatus, (8) mitochondrion, (9) nucleus, (10) peroxisome, (11) plastid, and (12) vacuole.
Table 1. Breakdown of the plant protein benchmark dataset 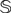 derived from Swiss-Prot database (release 55.3) according to the
procedures described in the Materials section.
derived from Swiss-Prot database (release 55.3) according to the
procedures described in the Materials section.
| Subset | Subcellular locationa | Number of proteins |
|
Cell membrane | 56 |

|
Cell wall | 32 |
|
Chloroplast | 286 |
|
Cytoplasm | 182 |
|
Endoplasmic reticulum | 42 |
|
Extracellular | 22 |
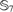
|
Golgi apparatus | 21 |
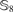
|
Mitochondrion | 150 |

|
Nucleus | 152 |

|
Peroxisome | 21 |

|
Plastid | 39 |

|
Vacuole | 52 |
Total number of locative proteins 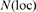
|
1,055b | |
Total number of different proteins 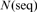
|
978c | |
None of proteins included here has 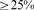 sequence identity to any other in a same subcellular
location.
sequence identity to any other in a same subcellular
location.
The benchmark dataset  here covers 12 plant subcellular locations and the
“Golgi apparatus” is newly added in comparison with the
dataset in [13] that covered 11 location sites.
here covers 12 plant subcellular locations and the
“Golgi apparatus” is newly added in comparison with the
dataset in [13] that covered 11 location sites.
See Eqs.2–3 for the definition about the number of locative proteins, and its relation with the number of different proteins.
Of the 978 different proteins, 904 have one subcellular location, 71 have two locations, 3 have three locations, and none have four or more locations.
Since some proteins in 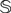 may occur in two or more locations, it is instructive to introduce the
concept of “locative protein” [23], as briefed as follows. A
protein coexisting at two different location sites will be counted as 2 locative
proteins even though the two are with completely the same sequence; if
coexisting at three sites, 3 locative proteins; and so forth. Thus, it follows
may occur in two or more locations, it is instructive to introduce the
concept of “locative protein” [23], as briefed as follows. A
protein coexisting at two different location sites will be counted as 2 locative
proteins even though the two are with completely the same sequence; if
coexisting at three sites, 3 locative proteins; and so forth. Thus, it follows
| (2) |
where 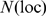 is the number of total locative proteins,
is the number of total locative proteins,  the number of total different protein sequences,
the number of total different protein sequences, 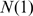 the number of proteins with one location,
the number of proteins with one location,  the number of proteins with two locations, and so forth;
while
the number of proteins with two locations, and so forth;
while  is the number of total subcellular location sites concerned (for the
current case,
is the number of total subcellular location sites concerned (for the
current case,  as shown in
Fig. 1
).
as shown in
Fig. 1
).
For the current 978 different protein sequences, 904 occur in one subcellular location, 71 in two locations, 3 in three locations, and none in four or more locations. Substituting these data into Eq.2, we have
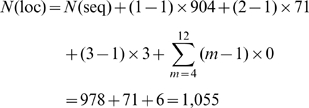 |
(3) |
which is fully consistent with the figures in Table 1 and the data in Table S1.
To develop a powerful method for predicting protein subcellular localization, it is very important to formulate the sample of a protein in terms of the core features that are intrinsically correlated with its localization in a cell. To realize this, the strategy by integrating the GO representation and PseAAC representation was adopted in the original Plant-PLoc [13]. In this study, the essence of such a strategy will be still kept. However, in order to overcome the four shortcomings as mentioned in Introduction for Plant-PLoc [13], a completely different combination approach has been developed, as described below.
1. Gene Ontology Descriptor
The gene ontology (GO) representation for a protein sample in the original Plant-PLoc [13] was derived through its accession number from the GO database [16]. Therefore, in using Plant-PLoc to conduct prediction, the accession number of a query protein would be indispensable as a part of input. To avoid such a requirement, the following different procedures are proposed to derive the GO representation.
Step 1
Use BLAST [24] to search the homologous proteins of the query
protein  from the Swiss-Prot database (version 55.3), with the BLAST
parameter of expect value
from the Swiss-Prot database (version 55.3), with the BLAST
parameter of expect value  .
.
Step 2
Those proteins that have  pairwise sequence identity with the query protein
pairwise sequence identity with the query protein 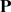 are collected into a set,
are collected into a set,  , called the “homology set” of
, called the “homology set” of 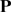 . All the elements in
. All the elements in  can be deemed as the representative proteins of
can be deemed as the representative proteins of  . Because these representative proteins were retrieved from the
Swiss-Prot database, they must each have their own accession numbers.
. Because these representative proteins were retrieved from the
Swiss-Prot database, they must each have their own accession numbers.
Step 3
Search each of these accession numbers collected in Step 2 against the GO database at http://www.ebi.ac.uk/GOA/ to find the corresponding GO numbers [16].
Step 4
The current GO database (version 70.0 released 10 March 2008) contains 60,020 GO
numbers, thus the query protein  can be formulated through its representative proteins in
can be formulated through its representative proteins in  by the following equation
by the following equation
| (4) |
where 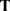 is the transposing operator, and
is the transposing operator, and
 |
(5) |
Through the above steps, we can use Eq.4 derived from the representative proteins
in  to investigate the query protein
to investigate the query protein  . The rationale of such a practice is based on the fact that
homology proteins generally share similar attributes, such as folding patterns
[25]
and biological functions [26], [27], [28]. Thus,
the accession number is no longer needed for the input of the query protein even
when using the high-level GO approach to predict its subcellular localization as
required in the old Plant-PLoc [13].
. The rationale of such a practice is based on the fact that
homology proteins generally share similar attributes, such as folding patterns
[25]
and biological functions [26], [27], [28]. Thus,
the accession number is no longer needed for the input of the query protein even
when using the high-level GO approach to predict its subcellular localization as
required in the old Plant-PLoc [13].
The above homology-based GO extraction method is particularly useful for studying
those proteins which do not have UniProt accession numbers. However, it would
still fail to work under any of the following situations: (1) the
query protein does not have significant homology to any protein in the Swiss-Prot
database, i.e.,  meaning the homology set is an empty one;
(2) its representative proteins do not contain any useful
information for statistical prediction based on a given training dataset.
meaning the homology set is an empty one;
(2) its representative proteins do not contain any useful
information for statistical prediction based on a given training dataset.
Therefore, it is necessary to consider the following representations for those proteins that fail to be meaningfully defined in the GO space.
2. Functional Domain Descriptor
The functional domain (FunD) is the core of a protein. Therefore, in determining the 3-D (dimensional) structure of a protein by experiments (see, e.g., [29], [30]) or by computational modeling (see, e.g., [28], [31]), the first priority was always focused on its FunD. Using FunD to formulate protein samples was originally proposed in [32], [33] based on the 2005 FunDs in the SBASE-A database [34]. Since then, a series of new protein FunD databases were established, such as COG [35], KOG [35], SMART [36], Pfam [37], and CDD [38]. Of these databases, CDD contains the domains imported from COG, Pfam, and SMART, and hence is relatively much more complete [38] and will be adopted in this study. The version 2.11 of CDD contains 17,402 characteristic domains. Thus, using each of these domains as a base vector, a given protein sample can be defined as a vector in the 17402-D (dimensional) FunD space according to the following procedures:
Step 1
Use RPS-BLAST (Reverse PSI-BLAST) program [24] to conduct sequence
alignment of the sequence of the query protein  with each of the 17,402 domain sequences in the CDD
database.
with each of the 17,402 domain sequences in the CDD
database.
Step 2
If the significance threshold value (expect value) is 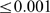 for the
for the 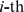 domain meaning that a “hit” is found, then
the
domain meaning that a “hit” is found, then
the  component of the protein
component of the protein 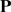 in the 17402-D space is assigned 1; otherwise, 0.
in the 17402-D space is assigned 1; otherwise, 0.
Step 3
The protein sample 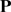 in the FunD space can thus be formulated as
in the FunD space can thus be formulated as
| (6) |
where 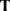 has the same meaning as in Eq.4, and
has the same meaning as in Eq.4, and
| (7) |
3. SeqEvo (Sequential Evolution) Descriptor
Biology is a natural science with historic dimension. All biological species have
developed continuously starting out from a very limited number of ancestral species.
The evolution in protein sequences involves changes of single residues, insertions
and deletions of several residues [39], gene doubling, and gene fusion. In the course of
time such changes accumulate, so that many similarities between initial and resultant
amino acid sequences are eliminated, but the corresponding proteins may still share
many common attributes, such as belonging to a same subcellular location and
possessing basically the same function. To incorporate this kind of evolutionary
effects, let us use the “Position-Specific Scoring Matrix” or
“PSSM” [24] to express the protein sample  , as formulated by
, as formulated by
 |
(8) |
where 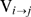 represents the score of the amino acid residue in the
represents the score of the amino acid residue in the 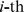 position of the protein sequence being changed to amino acid type
position of the protein sequence being changed to amino acid type  during the evolutionary process, and
during the evolutionary process, and 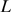 the sequence length of protein
the sequence length of protein 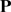 . Here, the numerical codes 1, 2, …, 20 are used to denote
the 20 native amino acid types according to the alphabetical order of their single
character codes. The
. Here, the numerical codes 1, 2, …, 20 are used to denote
the 20 native amino acid types according to the alphabetical order of their single
character codes. The 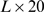 scores in Eq.8 were generated by using PSI-BLAST [24] to
search the Swiss-Prot database (version 55.3 released on 29-Apr-2008) through three
iterations with 0.001 as the
scores in Eq.8 were generated by using PSI-BLAST [24] to
search the Swiss-Prot database (version 55.3 released on 29-Apr-2008) through three
iterations with 0.001 as the 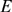 -value cutoff for multiple sequence alignment against the sequence
of the protein
-value cutoff for multiple sequence alignment against the sequence
of the protein 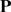 , followed by a standard conversion given below:
, followed by a standard conversion given below:
| (9) |
where 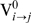 represent the original scores directly created by PSI-BLAST [24] that
are generally shown as positive or negative integers (the positive score means that
the corresponding mutation occurs more frequently than expected by chance, while the
negative means just the opposite); the symbol
represent the original scores directly created by PSI-BLAST [24] that
are generally shown as positive or negative integers (the positive score means that
the corresponding mutation occurs more frequently than expected by chance, while the
negative means just the opposite); the symbol  means taking the average of the quantity therein over 20 native
amino acids, and
means taking the average of the quantity therein over 20 native
amino acids, and 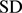 means the corresponding standard deviation. The converted values
obtained by Eq.9 will have a zero mean value over the 20 amino acids and will remain
unchanged if going through the same conversion procedure again. However, according to
the descriptor of Eq.8, proteins with different lengths will correspond to
row-different matrices causing difficulty for developing a predictor able to
uniformly cover proteins of any length. To make the descriptor become a size-uniform
matrix, one possible avenue is to represent a protein sample
means the corresponding standard deviation. The converted values
obtained by Eq.9 will have a zero mean value over the 20 amino acids and will remain
unchanged if going through the same conversion procedure again. However, according to
the descriptor of Eq.8, proteins with different lengths will correspond to
row-different matrices causing difficulty for developing a predictor able to
uniformly cover proteins of any length. To make the descriptor become a size-uniform
matrix, one possible avenue is to represent a protein sample  by
by
| (10) |
where
| (11) |
where 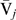 represents the average score of the amino acid residues in the
protein
represents the average score of the amino acid residues in the
protein 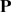 being changed to amino acid type
being changed to amino acid type  during the evolutionary process. However, if
during the evolutionary process. However, if 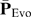 of Eq.10 was used to represent the protein
of Eq.10 was used to represent the protein 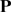 , all the sequence-order information during the evolutionary process
would be missed. To avoid complete loss of the sequence-order information, the
concept of the pseudo amino acid composition (PseAAC) as originally proposed in [15] was
utilized; i.e., instead of Eq.10, let us use the pseudo position-specific
scoring matrix as given by
, all the sequence-order information during the evolutionary process
would be missed. To avoid complete loss of the sequence-order information, the
concept of the pseudo amino acid composition (PseAAC) as originally proposed in [15] was
utilized; i.e., instead of Eq.10, let us use the pseudo position-specific
scoring matrix as given by
| (12) |
to represent the protein  , where
, where
| (13) |
meaning that 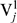 is the correlation factor by coupling the most contiguous
position-specific scoring matrix scores along the protein chain for the amino acid
type
is the correlation factor by coupling the most contiguous
position-specific scoring matrix scores along the protein chain for the amino acid
type  ;
; 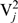 that by coupling the second-most contiguous position-specific
scoring matrix scores; and so forth. Note that, as mentioned in the Material
section of [13], the length of the shortest protein sequence in the
benchmark dataset is
that by coupling the second-most contiguous position-specific
scoring matrix scores; and so forth. Note that, as mentioned in the Material
section of [13], the length of the shortest protein sequence in the
benchmark dataset is  , and hence the value allowed for
, and hence the value allowed for 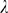 in Eq.13 must be smaller than 50. When
in Eq.13 must be smaller than 50. When 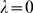 ,
,  becomes a naught element and Eq.12 is degenerated to Eq.10.
becomes a naught element and Eq.12 is degenerated to Eq.10.
It is instructive to point out that the above three protein descriptors, i.e., 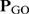 of Eq.4,
of Eq.4, 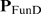 of Eq.6, and
of Eq.6, and 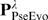 of Eq.12, can be actually deemed as three different kinds of PseAAC
as well [40]. This is because, according to its original
definition, the PseAAC is actually a set of discrete numbers [15] as long as it is different
from the classical amino acid composition and it is derived from a protein sequence
that is able to harbor some sort of sequence order or pattern information. The
concept of PseAAC has also been widely used to deal with many other protein-related
problems and sequence-related systems (see, e.g., [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]).
of Eq.12, can be actually deemed as three different kinds of PseAAC
as well [40]. This is because, according to its original
definition, the PseAAC is actually a set of discrete numbers [15] as long as it is different
from the classical amino acid composition and it is derived from a protein sequence
that is able to harbor some sort of sequence order or pattern information. The
concept of PseAAC has also been widely used to deal with many other protein-related
problems and sequence-related systems (see, e.g., [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]).
4. Prediction Engine and Process
The prediction engine used in this study is the ensemble classifier 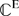 [12] formed
by fusing many basic individual classifiers operated according to the OET-KNN
(Optimized Evidence-Theoretic K Nearest Neighbor) rule [57], [58]. OET-KNN is a very powerful
classifier as demonstrated in identifying membrane protein types [58]. For
reader's convenience, a brief introduction about OET-KNN is given below.
[12] formed
by fusing many basic individual classifiers operated according to the OET-KNN
(Optimized Evidence-Theoretic K Nearest Neighbor) rule [57], [58]. OET-KNN is a very powerful
classifier as demonstrated in identifying membrane protein types [58]. For
reader's convenience, a brief introduction about OET-KNN is given below.
Let us consider a problem of classifying  plant protein entities into 12 categories (subcellular location
sites). The problem can be formulated as
plant protein entities into 12 categories (subcellular location
sites). The problem can be formulated as
| (14) |
The available information is assumed to consist in a training dataset
| (15) |
where the 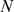 plant proteins
plant proteins 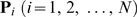 and their corresponding location labels
and their corresponding location labels 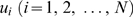 take the values in
take the values in  of Eq.14. According to the KNN (K-Nearest
Neighbors) rule [59], an unclassified protein
of Eq.14. According to the KNN (K-Nearest
Neighbors) rule [59], an unclassified protein  is assigned to the class (or location) represented by the majority
of its K nearest neighbors of
is assigned to the class (or location) represented by the majority
of its K nearest neighbors of  . Owing to its good performance and simple-to-use feature, the KNN
rule, also named as “voting KNN rule”, is quite popular in
pattern recognition community.
. Owing to its good performance and simple-to-use feature, the KNN
rule, also named as “voting KNN rule”, is quite popular in
pattern recognition community.
The ET-KNN (Evidence Theoretic K-nearest Neighbors) rule is a pattern classification method based on the Dempster-Shafer theory of belief functions [57]. In the classification process, each neighbor of a protein to be classified is considered as a piece of evidence supporting certain hypotheses concerning the class (or location) membership of that protein. Based on this kind of evidence, the basic belief masses are assigned to each subset concerned. Such masses are obtained for each of the K nearest neighbors of the protein under consideration and aggregated using the Dempster's rule of combination [60]. A decision is made by assigning the query protein to the class (or location) with the maximum credibility.
Suppose 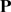 is a query protein to be classified, and
is a query protein to be classified, and 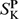 is the set of its K-nearest neighbors in the
training dataset
is the set of its K-nearest neighbors in the
training dataset 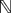 of Eq.15. Thus, for any
of Eq.15. Thus, for any  , the knowledge that
, the knowledge that 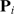 belongs to class (or location)
belongs to class (or location) 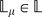 can be considered as a piece of evidence that increases our belief
that
can be considered as a piece of evidence that increases our belief
that 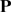 also belongs to
also belongs to  . According to the basic belief assignment mapping theory [60], this
piece of evidence can be formulated by
. According to the basic belief assignment mapping theory [60], this
piece of evidence can be formulated by
| (16) |
where 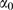 is a fixed parameter,
is a fixed parameter, 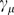 is a parameter associated with class (or location)
is a parameter associated with class (or location) 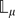 , and
, and 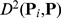 is the square distance between
is the square distance between 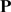 and
and 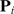 . In this study, when the proteins are represented by the GO
descriptor mode (cf. Eq.4) or the FunD mode (cf. Eq.6), then
. In this study, when the proteins are represented by the GO
descriptor mode (cf. Eq.4) or the FunD mode (cf. Eq.6), then 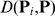 is defined as
is defined as 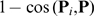 , i.e.
, i.e.
| (17) |
where  and
and 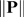 are the moduluses of
are the moduluses of  and P, respectively. When the proteins are represented
by the PseEvo model (cf. Eq.12), then
and P, respectively. When the proteins are represented
by the PseEvo model (cf. Eq.12), then  is defined as the Euclidean distance between
is defined as the Euclidean distance between 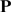 and
and  .
.
In the ET-KNN rule, it was not addressed how to optimally select the parameters. In
1998 an optimization procedure to determine the optimal or near-optimal parameter
values of  and
and  was proposed from the data by minimizing an error function [61]. It was
observed that the OET-KNN rule obtained thru such an optimization treatment would
lead to a substantial improvement in classification accuracy.
was proposed from the data by minimizing an error function [61]. It was
observed that the OET-KNN rule obtained thru such an optimization treatment would
lead to a substantial improvement in classification accuracy.
The belief function of  belonging to class (or location)
belonging to class (or location)  is a combination of its K-Nearest Neighbors, and
can be formulated as
is a combination of its K-Nearest Neighbors, and
can be formulated as
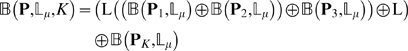 |
(18) |
where  is called the orthogonal sum, which is commutative and associative.
According to Dempster's rule [60], the belief function of
Eq.18 can be expressed as
is called the orthogonal sum, which is commutative and associative.
According to Dempster's rule [60], the belief function of
Eq.18 can be expressed as
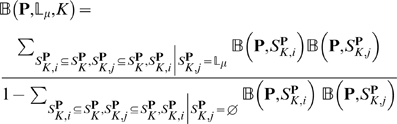 |
(19) |
where 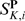 is the i-th possible subset of
is the i-th possible subset of 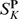 , and
, and  ,
,  , and
, and 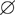 are the symbols in set theory, representing “contained
in”, “intersection”, and the empty set,
respectively.
are the symbols in set theory, representing “contained
in”, “intersection”, and the empty set,
respectively.
A decision is made by assigning the query protein 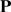 to the
to the 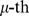
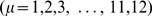 class (or location) with which the belief function of Eq.19 has the
maximum value; i.e.,
class (or location) with which the belief function of Eq.19 has the
maximum value; i.e.,
| (20) |
where 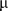 is the argument of
is the argument of  that maximizes the belief function
that maximizes the belief function 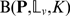 . If there are two and more arguments leading to a same maximum
value for
. If there are two and more arguments leading to a same maximum
value for 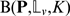 , the query protein will be randomly assigned to one of the
subcellular locations associated with these arguments although this kind of tie case
rarely happens.
, the query protein will be randomly assigned to one of the
subcellular locations associated with these arguments although this kind of tie case
rarely happens.
The power of the ensemble classifier  is also reflected by the fact that a statistical predictor
established by fusing many basic individual predictors will significantly improve its
performance as demonstrated by the recent studies on protein folding rate predictions
[62],
[63]. For
the detailed procedures of how to fuse many individual OET-KNN classifiers to form
the ensemble classifier
is also reflected by the fact that a statistical predictor
established by fusing many basic individual predictors will significantly improve its
performance as demonstrated by the recent studies on protein folding rate predictions
[62],
[63]. For
the detailed procedures of how to fuse many individual OET-KNN classifiers to form
the ensemble classifier 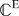 , see Eqs.30–35 in [12]. For the procedures of how
to make
, see Eqs.30–35 in [12]. For the procedures of how
to make 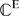 able to deal with both single-location and multiple-location
proteins, see Eqs.36–48 of [12].
able to deal with both single-location and multiple-location
proteins, see Eqs.36–48 of [12].
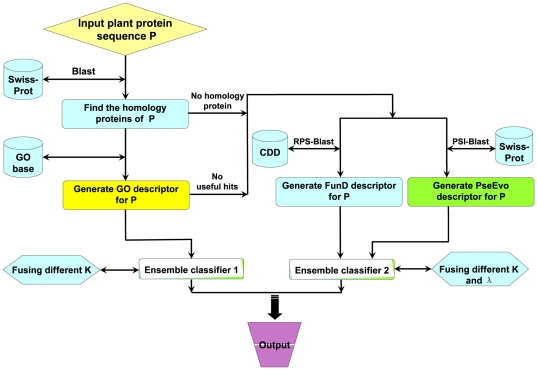
The prediction is processed according to the following order.
(1) If the query protein can be expressed as a meaningful or productive
descriptor in the GO database via its representative proteins in  , then
, then  of Eq.4 should be input into the prediction engine for identifying
its subcellular location site(s). And the output will be determined by fusing many
basic OET-KNN predictors [12] with different numbers of
of Eq.4 should be input into the prediction engine for identifying
its subcellular location site(s). And the output will be determined by fusing many
basic OET-KNN predictors [12] with different numbers of  (cf. Eq.18–20), the parameter of the nearest neighbor
rule [57].
(cf. Eq.18–20), the parameter of the nearest neighbor
rule [57].
(2) If the query protein does not have significant homology to any
protein in the Swiss-Prot database, i.e., 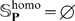 , or its representative proteins in
, or its representative proteins in 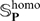 do not contain any useful GO information, then both the FunD
representation
do not contain any useful GO information, then both the FunD
representation 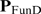 of Eq.6 and the pseudo position-specific scoring matrix
representation
of Eq.6 and the pseudo position-specific scoring matrix
representation  of Eq.12 should be input into the prediction engine. The output
will be determined by fusing many basic OET-KNN predictors [12] with different numbers of
of Eq.12 should be input into the prediction engine. The output
will be determined by fusing many basic OET-KNN predictors [12] with different numbers of  (cf. Eq.20) and
(cf. Eq.20) and 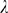 (cf. Eq.13).
(cf. Eq.13).
The whole process can be formulated as
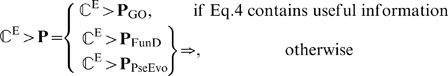 |
(21) |
where 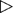 represents the identification operator, and
represents the identification operator, and 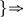 means fusing the results generated from its left side.
means fusing the results generated from its left side.
The entire ensemble classifier thus established is called “Plant-mPLoc”, where “m” stands for the first character of “multiple”, meaning that Plant-mPLoc is able to deal with proteins having both single and multiple subcellular locations. To provide an intuitive picture, a flowchart is given in Fig. 2 to illustrate the prediction process of Plant-mPLoc.
Figure 2. A flowchart to show the prediction process of Plant-mPLoc.
Protocol Guide
For the convenience of experimental scientists, a user-friendly web-server for Plant-mPLoc was established. Here let us provide a step-by-step guide on how to use the web-server to get the desired results.
Step 1
Open the web server at http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/ and you will see the top page of the predictor on your computer screen, as shown in Fig. 3a . Click on the Read Me button to see a brief introduction about Plant-mPLoc predictor and the caveat in using it.
Figure 3. Semi-screenshot to show the prediction steps.
(a) the top page of the Plant-mPLoc web server at http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/, (b) the input of a query protein in FASTA format, (c) the output predicted by Plant-mPLoc for the query protein 1 in the Example window, and (d) the output for the query protein 2 in the Example window.
Step 2
Either type or copy and paste the query protein sequence into the input box at the center of Fig. 3a . The input sequence should be in the FASTA format. A sequence in FASTA format consists of a single-line description, followed by lines of sequence data. The first character of the description line is a greater-than symbol (“>”) in the first column. All lines should be shorter than 80 characters. Examples to show the input sequences format can be seen by clicking on the Example button right above the input box. For more information about FASTA format, visit http://en.wikipedia.org/wiki/Fasta_format.
Step 3
Click on the Submit button to see the predicted result. For example, if you use the sequence of query protein 1 in the Example window, the input screen should look like the illustration in Fig. 3b ; after clicking the Submit button, you will see “Cytoplasm. Nucleus” shown on the predicted result window ( Fig. 3c ), meaning that the protein is a multiplex one, which can simultaneously occur in “cytoplasm” organelle and “nucleus” organelle, fully consistent with experimental observations. However, if using the sequence of query protein 2 in the Example window as an input, you will see “Cytoplasm” shown on the predicted result window ( Fig. 3d ), meaning that the protein is a single-location one occurring in “cytoplasm” compartment only, also consistent with experimental observations. It takes less than 15 seconds for a protein sequence of 300 amino acids before the predicted result appears on your computer screen. Generally speaking, the longer the sequence is, the more time it is needed.
Step 4
Click on the Citation button to find the relevant papers that document the detailed development and algorithm of Plant-mPLoc.
Step 5
Click on the Data button to download the benchmark datasets used to train and test the Plant-mPLoc predictor.
Caveat
To obtain the predicted result with the expected success rate, the entire sequence of the query protein rather than its fragment should be used as an input. A sequence with less than 50 amino acid residues is generally deemed as a fragment
Results and Discussion
In statistical prediction, the following three methods are often used to examine the
quality of a predictor: independent dataset test, subsampling test, and jackknife test
[64]. Since
independent dataset can be treated as a special case of sub-sampling test, one benchmark
dataset is sufficient to serve all the three kinds of cross-validation. However, as
elucidated in [18] and demonstrated by Eq.50 of [12], among the three
cross-validation methods, the jackknife test is deemed the most objective that can
always yield a unique result for a given benchmark dataset and hence has been
increasingly and widely adopted to examine the power of various predictors (see, e.g.,
[42], [46], [51], [53], [55], [65], [66], [67], [68], [69]). Particularly
for a benchmark dataset in which none of proteins included has 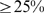 pairwise sequence identity to any other in a same subset (subcellular
location), such as the one used in the current study (cf. Table S1), it would be highly unlikely to get an over-estimated success rate by the
jackknife test. Quite the contrary, the success rate derived by the jackknife test on
such kind of stringent dataset would actually be under-estimated in comparison with the
success rates of practical applications in most cases, as will be seen later.
pairwise sequence identity to any other in a same subset (subcellular
location), such as the one used in the current study (cf. Table S1), it would be highly unlikely to get an over-estimated success rate by the
jackknife test. Quite the contrary, the success rate derived by the jackknife test on
such kind of stringent dataset would actually be under-estimated in comparison with the
success rates of practical applications in most cases, as will be seen later.
For the details of how to calculate the overall success rate for a statistical system with both single-location and multiple-location proteins, see Eqs.43–48 and Fig. 4 of [12], where the details of how to count the false positives (over-predictions) and false negatives (under-predictions) were also elaborated.
Let us first compare the current predictor Plant-mPLoc with the old Plant-PLoc [13]. Listed in Table 2 are the results obtained with Plant-PLoc [13] and Plant-mPLoc, respectively, on the benchmark dataset (cf. Table S1) by the jackknife cross-validation test. During the testing process, only the sequences of proteins in Table S1 but not their accession numbers were used as inputs in order to make the comparison between the two predictors under exactly the same condition. As we can see from Table 2 , for such a stringent benchmark dataset, the overall success rate achieved by the new predictor is 63.7%, which is more than 25% higher than that by Plant-PLoc [13].
Table 2. A comparison of the jackknife success rates by Plant-PLoc [13] and the
current Plant-mPLoc on the benchmark dataset (cf. Table S1) that covers 12 location sites of plant proteins in which none of the
proteins included has  25% pairwise sequence identity to any other in a same
location.
25% pairwise sequence identity to any other in a same
location.
| Subcellular location | Success ratea | |
| Plant-PLoc | Plant-mPLoc | |
| Cell membrane | 15/56 = 26.8% | 24/56 = 42.9% |
| Cell wall | 7/32 = 21.9% | 8/32 = 25.0% |
| Chloroplast | 184/286 = 64.3% | 248/286 = 86.7% |
| Cytoplasm | 51/182 = 28.0% | 72/182 = 39.6% |
| Endoplasmic reticulum | 1/42 = 2.4% | 17/42 = 40.5% |
| Extracellular | 4/22 = 18.2% | 3/22 = 13.6% |
| Golgi apparatus | 6/21 = 28.6% | 6/21 = 28.6% |
| Mitochondrion | 26/150 = 17.3% | 114/150 = 76.0% |
| Nucleus | 92/152 = 60.5% | 136/152 = 89.5% |
| Peroxisome | 2/21 = 9.5% | 14/21 = 66.7% |
| Plastid | 9/39 = 23.1% | 4/39 = 10.3% |
| Vacuole | 4/52 = 7.7% | 26/52 = 50.0% |
| Total | 401/1055 = 38.0% | 672/1055 = 63.7% |
Note that in order to make the comparison under exactly the same condition, only the sequences of proteins in the Table S1 but not their accession numbers were used as inputs during the prediction.
Now, let us compare the current predictor with TargetP [6] and Predotar [8], two popular predictors widely used for predicting the subcellular locations of plant proteins. As mentioned in Introduction, the two predictors only cover three or four location sites. Therefore, it can be easily conceived that they would yield even much lower success rates when tested by the current benchmark dataset that covers twelve location sites.
Actually, even if tested by a benchmark dataset within the scope that can be covered by TargetP [6] or Predotar [8], the success rate by the current Plant-mPLoc predictor is also much higher than those by the two predictors, as demonstrated below.
Let us compare Plant-mPLoc with TargetP [6] first. The TargetP predictor also has a web-server at http://www.cbs.dtu.dk/services/TargetP/, with a built-in training dataset covering the following four items: “mitochondria”, “chloroplast”, “secretory pathway”, and “other”. Since the “secretory pathway” is not a final destination of subcellular location as annotated in Swiss-Prot databank, and hence was removed from the comparison. Also, the location of “other” is not a clear site for comparison, and should be removed as well. Thus, in order to compare TargetP with the new predictor Plant-mPLoc, let us construct an independent testing dataset by randomly picking testing proteins according to the following criteria: (i) they must belong to plant proteins, as clearly annotated in Swiss-Prot database; (ii) they must neither occur in the training dataset of TargetP nor occur in the training dataset of Plant-mPLoc in order to avoid the memory bias; (iii) their experimentally observed subcellular locations are known as clearly annotated in Swiss-Prot database, and also these locations must be within the scope covered by TargetP as a compromise for rationally utilizing its web-server. By following the above procedures, we obtained a degenerate independent testing dataset consisting of 1,775 plant proteins, of which 1,500 belong to chloroplast and 275 belong to mitochondrion. The accession numbers and sequences of these 1,775 proteins are given in Table S2.
The predicted results by TargetP [6] and the current Plant-mPLoc for each of the 1,775 independent testing proteins are listed in Table S3, where for facilitating comparison, the corresponding experimental results are also given. By examining Table S3, we can see the following. (1) Many proteins whose subcellular locations were misidentified by TargetP have been corrected by Plant-mPLoc. (2) Many proteins, which were identified by TargetP as belonging to the location of “other”, have been identified as “chloroplast” or “mitochondrion”, fully consistent with experimental observations. (3) There are quite a few proteins whose subcellular locations were incorrectly predicted by Plant-mPLoc, or the results yielded by Plant-mPLoc contain some false positives. Even though, the overall success rate by Plant-mPLoc on the 1,755 independent proteins is over 86%, which is at least more than 40% higher than that by TargetP [6].
Now, let us compare Plant-mPLoc with Predotar [8]. The web-server of Predotar is at: http://urgi.versailles.inra.fr/predotar/predotar.html, with a built-in training dataset covering the following four items: “endoplasmic reticulum”, “mitochondrion”, “plastid”, and “other”. Since the term “other” is not a clear description for subcellular location, and was removed from comparison. Thus, by following the aforementioned similar criteria as in constructing the independent dataset for comparing TargetP with Plant-mPLoc, we also constructed a degenerate independent dataset to compare Predotar [8] with Plant-mPLoc. The dataset consists of 381 plant proteins, of which 48 belong to endoplasmic reticulum, 253 belong to mitochondrion, and 70 belong to plastid. The accession numbers and sequences of these 381 proteins are given in Table S4. The predicted results by Predotar [8] and the current Plant-mPLoc for the 381 independent testing proteins and their corresponding experimental results are listed in Table S5, from which we can see the following. (1) Many proteins whose subcellular locations were correctly identified by Plant-mPLoc were unable to identify by Predotar [8] although all these location sites are within its coverage scope. (2) Many proteins whose subcellular locations were misidentified by Predotar [8] have been corrected by Plant-mPLoc. (3) Although Plant-mPLoc also had quite a few incorrect and false positive predicted results, its overall success rate for the 381 independent proteins could still be over 70%, which is at least more than 30% higher than that by Predotar [8].
Furthermore, it is interesting to see from Table S3 and Table S5 that some proteins with multiple locations have been correctly predicted by Plant-mPLoc. For example, according to the annotations of Swiss-Prot databank, the proteins with codes Q5YLB5, Q9FV51, and Q9LJL3 can coexist in both “chloroplast” and “mitochondrion” while the protein with code Q42560 can coexist in both “cytoplasm” and “mitochondrion”, and the predicted results by Plant-mPLoc are exactly so. This is beyond the reach of TargetP [6] and Predotar [8].
From the above three comparisons, we can now make the following points more clear.
The more stringent a benchmark dataset is in excluding homologous and high similarity
sequences, or the more subcellular location sites it covers, the more difficult for a
predictor to achieve a high overall success rate, as can be easily understood by
considering the following cases. For a benchmark dataset only covering three subcellular
locations each containing same number of proteins, the overall success rate by random
assignments would generally be  ; while for a benchmark dataset covering 12 subcellular
locations, the overall success rate by random assignments would be only
; while for a benchmark dataset covering 12 subcellular
locations, the overall success rate by random assignments would be only 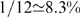 . This means that the former is more than four times the latter.
. This means that the former is more than four times the latter.
Also, a predictor tested by jackknife cross-validation is very difficult to yield a high
success rate when performed on a stringent benchmark dataset in which none of proteins
included has  pairwise sequence identity to any other in a same subset (subcellular
location). That is why the overall success rate achieved by Plant-mPLoc was only
63.7% when tested by the jackknife cross-validation on the benchmark dataset
of Table S1 but was over 86% and 70% when tested by the independent
datasets of Table S2 and Table S4, respectively. However, regardless of using what test methods or test datasets,
one thing is crystal clear, i.e., the overall success rates achieved by the current
Plant-mPLoc are significantly higher than those by its counterparts.
pairwise sequence identity to any other in a same subset (subcellular
location). That is why the overall success rate achieved by Plant-mPLoc was only
63.7% when tested by the jackknife cross-validation on the benchmark dataset
of Table S1 but was over 86% and 70% when tested by the independent
datasets of Table S2 and Table S4, respectively. However, regardless of using what test methods or test datasets,
one thing is crystal clear, i.e., the overall success rates achieved by the current
Plant-mPLoc are significantly higher than those by its counterparts.
Meanwhile, it has also become understandable why the success rates as originally
reported for TargetP [6] and Predotar [8] were over-estimated. This is
because the benchmark datasets adopted by the two predictors only cover less than
one-third of the location sites that are covered by the current Pant-mPLoc. Besides, the
benchmark datasets used by TargetP and Predotar to estimate their success rates contain
many homologous sequences. For the benchmark dataset used by Predotar [8], the cutoff
threshold was set at 80%, meaning that only those sequences which have 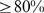 pairwise sequence identity to any other in a same subset were excluded
[8]; while for the benchmark dataset used in TargetP
[6],
even no such a cutoff percentage was indicated. Compared with the current benchmark
dataset (cf. Table S1) in which none of proteins included has
pairwise sequence identity to any other in a same subset were excluded
[8]; while for the benchmark dataset used in TargetP
[6],
even no such a cutoff percentage was indicated. Compared with the current benchmark
dataset (cf. Table S1) in which none of proteins included has 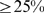 pairwise sequence identity to any other in a same subset, the
benchmark datasets adopted in Predotar and TargetP are much less stringent and hence
cannot avoid homologous bias and over estimation.
pairwise sequence identity to any other in a same subset, the
benchmark datasets adopted in Predotar and TargetP are much less stringent and hence
cannot avoid homologous bias and over estimation.
Plant-mPLoc was evolved from Plant-PLoc [13] through a top-down approach improvement. The new predictor distinguishes itself from the old one by the following remarkable features. (1) The ability of prediction is extended to cover both single-location and multiple-location proteins. (2) The input of accession number for using the higher-level GO approach [18] to perform the prediction is no longer needed; this is particularly useful when dealing with protein sequences without accession numbers available. (3) For those plant proteins without useful GO information to conduct the higher-level prediction, a sophisticated combination approach by fusing the FunD information and SeqEvo information is developed to replace the simple PseAAC approach [15].
It is instructive to point out that in a broader sense the protein descriptors,  ,
, 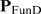 , and
, and  as introduced in the current study, are actually three different forms
of PseAAC [40].
Accordingly, it is essentially through the concept of PseAAC [15] that the GO information, FunD
information, and SeqEvo information have been effectively incorporated into the
predictor Plant-mPLoc. Plant-mPLoc is available as a web-server at http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/.
as introduced in the current study, are actually three different forms
of PseAAC [40].
Accordingly, it is essentially through the concept of PseAAC [15] that the GO information, FunD
information, and SeqEvo information have been effectively incorporated into the
predictor Plant-mPLoc. Plant-mPLoc is available as a web-server at http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/.
Finally, let us consider the following hypothetical case: a single amino acid mutation in the signal part of a protein sequence might lead it to a completely different subcellular location site. Can Plant-mPLoc be used to deal with such a subtle case? Like all existing predictors in this area, Plant-mPLoc is a statistical predictor. As a statistical predictor, it would generally not be so sensitive to reflect the change of only one amino acid. Nevertheless, since Plant-mPLoc is an ensemble classifier formed by fusing many basic individual classifiers as well as by incorporating functional domain and evolution informations, it would be relatively more competent in dealing with the cases of mutated sequences than those predictors based on single classifier alone. Of course, it remains a challenging problem how to incorporate into a statistical predictor with the subtle effect of a single amino acid mutation at the signal peptide of a protein.
Supporting Information
This benchmark dataset S for Plant-mPLoc includes 1,055 plant protein sequences (978 different proteins), classified into 12 plant subcellular locations. Among the 978 different proteins, 904 belong to one subcellular location, 71 to two locations, and 3 to three locations. Both the accession numbers and sequences are given. None of the proteins has ≥25% sequence identity to any other in the same subset (subcellular location). See the text of the paper for further explanation.
(0.78 MB PDF)
The degenerate testing dataset used for comparing the performance between TargetP (Emanuelsson, et al. J. of Mol. Biol. 2000, 300: 1005–1016) and Plant-mPLoc of this paper. The dataset contains 1,775 plant proteins classified into 2 subcellular locations: (1) chloroplast, and (2) mitochondrion. To avoid bias, none of the proteins included here occurs in the training dataset of TargetP, nor in the training dataset of Plant-mPLoc. See the text of the paper for further explanation.
(0.91 MB PDF)
List of the results predicted by TargetP (Emanuelsson et al. J. Mol. Biol. 2000, 300: 1005–1016) and Plant-mPLoc on the 1,775 independent proteins in the Table S2, and their experimental subcellular locations as annotated in Swiss-Prot databank (version 55.3 released on 29-Apr-2008). Note for TargetP outputs, “C” means “Chloroplast”, “M” means “Mitochondrion”, “S” means “Secretory pathway”, and “_” means “Any other location”.
(0.41 MB PDF)
The degenerate testing dataset used for comparing the performance between Predotar (Small et al., Proteomics 2004, 4: 1581–1590) and Plant-mPLoc of this paper. The dataset contains 381 plant proteins classified into 3 subcellular locations: (1) endoplasmic reticulum, (2) mitochondrion, and (3) plastid. To avoid bias, none of the proteins included here occurs in the training dataset of TargetP, nor in the training dataset of Plant-mPLoc. See the text of the paper for further explanation.
(0.25 MB PDF)
List of the results predicted by Predotar (Small et al., Proteomics 2004, 4:1581–90) and Plant-mPLoc on the 381 independent proteins in the Table S4, and their experimental subcellular locations as annotated in Swiss-Prot databank (version 55.3 released on 29-Apr-2008). Note for the Predotar output, “ER” means “Endoplasmic reticulum”.
(0.16 MB PDF)
Acknowledgments
The authors wish to thank the reviewers for the valuable suggestions and comments, which are very helpful for strengthening the presentation of this paper.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 60704047), Science and Technology Commission of Shanghai Municipality (Grant No. 08ZR1410600, 08JC1410600) and sponsored by the Shanghai Pujiang Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ehrlich JS, Hansen MD, Nelson WJ. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev Cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glory E, Murphy RF. Automated subcellular location determination and high-throughput microscopy. Dev Cell. 2007;12:7–16. doi: 10.1016/j.devcel.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima H, Nishikawa K. Discrimination of intracellular and extracellular proteins using amino acid composition and residue-pair frequencies. J Mol Biol. 1994;238:54–61. doi: 10.1006/jmbi.1994.1267. [DOI] [PubMed] [Google Scholar]
- 4.Cedano J, Aloy P, P'erez-Pons JA, Querol E. Relation between amino acid composition and cellular location of proteins. J Mol Biol. 1997;266:594–600. doi: 10.1006/jmbi.1996.0804. [DOI] [PubMed] [Google Scholar]
- 5.Chou KC, Elrod DW. Protein subcellular location prediction. Protein Engineering. 1999;12:107–118. doi: 10.1093/protein/12.2.107. [DOI] [PubMed] [Google Scholar]
- 6.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 7.Zhou GP, Doctor K. Subcellular location prediction of apoptosis proteins. PROTEINS: Structure, Function, and Genetics. 2003;50:44–48. doi: 10.1002/prot.10251. [DOI] [PubMed] [Google Scholar]
- 8.Small I, Peeters N, Legeai F, Lurin C. Predotar: A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics. 2004;4:1581–1590. doi: 10.1002/pmic.200300776. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda S, Vert JP, Saigo H, Ueda N, Toh H, et al. A novel representation of protein sequences for prediction of subcellular location using support vector machines. Protein Sci. 2005;14:2804–2813. doi: 10.1110/ps.051597405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierleoni A, Martelli PL, Fariselli P, Casadio R. BaCelLo: a balanced subcellular localization predictor. Bioinformatics. 2006;22:e408–416. doi: 10.1093/bioinformatics/btl222. [DOI] [PubMed] [Google Scholar]
- 11.Nakai K. Protein sorting signals and prediction of subcellular localization. Advances in Protein Chemistry. 2000;54:277–344. doi: 10.1016/s0065-3233(00)54009-1. [DOI] [PubMed] [Google Scholar]
- 12.Chou KC, Shen HB. Review: Recent progresses in protein subcellular location prediction. Analytical Biochemistry. 2007;370:1–16. doi: 10.1016/j.ab.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Chou KC, Shen HB. Large-scale plant protein subcellular location prediction. Journal of Cellular Biochemistry. 2007;100:665–678. doi: 10.1002/jcb.21096. [DOI] [PubMed] [Google Scholar]
- 14.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, et al. Gene ontology: tool for the unification of biology. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou KC. Prediction of protein cellular attributes using pseudo amino acid composition. PROTEINS: Structure, Function, and Genetics (Erratum: ibid, 2001, Vol44, 60) 2001;43:246–255. doi: 10.1002/prot.1035. [DOI] [PubMed] [Google Scholar]
- 16.Camon E, Magrane M, Barrell D, Binns D, Fleischmann W, et al. The Gene Ontology Annotation (GOA) project: implementation of GO in SWISS-PROT, TrEMBL, and InterPro. Genome Res. 2003;13:662–672. doi: 10.1101/gr.461403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrell D, Dimmer E, Huntley RP, Binns D, O'Donovan C, et al. The GOA database in 2009–an integrated Gene Ontology Annotation resource. Nucleic Acids Res. 2009;37:D396–403. doi: 10.1093/nar/gkn803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou KC, Shen HB. Cell-PLoc: A package of web-servers for predicting subcellular localization of proteins in various organisms. Nature Protocols. 2008;3:153–162. doi: 10.1038/nprot.2007.494. [DOI] [PubMed] [Google Scholar]
- 19.Chou KC, Shen HB. MemType-2L: A Web server for predicting membrane proteins and their types by incorporating evolution information through Pse-PSSM. Biochem Biophys Res Comm. 2007;360:339–345. doi: 10.1016/j.bbrc.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Chou KC, Shen HB. ProtIdent: A web server for identifying proteases and their types by fusing functional domain and sequential evolution information. Biochem Biophys Res Comm. 2008;376:321–325. doi: 10.1016/j.bbrc.2008.08.125. [DOI] [PubMed] [Google Scholar]
- 21.Smith C. Subcellular targeting of proteins and drugs. 2008. http://wwwbiocomparecom/Articles/TechnologySpotlight/976/Subcellular-Targeting-Of-Proteins-And-Drugshtml.
- 22.Millar AH, Carrie C, Pogson B, Whelan J. Exploring the function-location nexus: using multiple lines of evidence in defining the subcellular location of plant proteins. Plant Cell. 2009;21:1625–1631. doi: 10.1105/tpc.109.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou KC, Shen HB. Euk-mPLoc: a fusion classifier for large-scale eukaryotic protein subcellular location prediction by incorporating multiple sites. Journal of Proteome Research. 2007;6:1728–1734. doi: 10.1021/pr060635i. [DOI] [PubMed] [Google Scholar]
- 24.Schaffer AA, Aravind L, Madden TL, Shavirin S, Spouge JL, et al. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 2001;29:2994–3005. doi: 10.1093/nar/29.14.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou KC, Zhang CT. Predicting protein folding types by distance functions that make allowances for amino acid interactions. Journal of Biological Chemistry. 1994;269:22014–22020. [PubMed] [Google Scholar]
- 26.Loewenstein Y, Raimondo D, Redfern OC, Watson J, Frishman D, et al. Protein function annotation by homology-based inference. Genome Biol. 2009;10:207. doi: 10.1186/gb-2009-10-2-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerstein M, Thornton JM. Sequences and topology. Curr Opin Struct Biol. 2003;13:341–343. doi: 10.1016/s0959-440x(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 28.Chou KC. Review: Structural bioinformatics and its impact to biomedical science. Current Medicinal Chemistry. 2004;11:2105–2134. doi: 10.2174/0929867043364667. [DOI] [PubMed] [Google Scholar]
- 29.Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451:591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Pielak RM, McClintock MA, Chou JJ. Solution structure and functional analysis of the influenza B proton channel. Nat Struct Mol Biol. 2009;16:1267–1271. doi: 10.1038/nsmb.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou KC. Modelling extracellular domains of GABA-A receptors: subtypes 1, 2, 3, and 5. Biochemical and Biophysical Research Communications. 2004;316:636–642. doi: 10.1016/j.bbrc.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 32.Chou KC, Cai YD. Using functional domain composition and support vector machines for prediction of protein subcellular location. Journal of Biological Chemistry. 2002;277:45765–45769. doi: 10.1074/jbc.M204161200. [DOI] [PubMed] [Google Scholar]
- 33.Cai YD, Zhou GP, Chou KC. Support vector machines for predicting membrane protein types by using functional domain composition. Biophysical Journal. 2003;84:3257–3263. doi: 10.1016/S0006-3495(03)70050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murvai J, Vlahovicek K, Barta E, Pongor S. The SBASE protein domain library, release 8.0: a collection of annotated protein sequence segments. Nucleic Acids Research. 2001;29:58–60. doi: 10.1093/nar/29.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letunic I, Copley RR, Pils B, Pinkert S, Schultz J, et al. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 2006;34:D257–260. doi: 10.1093/nar/gkj079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finn RD, Mistry J, Schuster-Bockler B, Griffiths-Jones S, Hollich V, et al. Pfam: clans, web tools and services. Nucleic Acids Res. 2006;34:D247–251. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchler-Bauer A, Anderson JB, Derbyshire MK, DeWeese-Scott C, Gonzales NR, et al. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007;35:D237–240. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou KC. The convergence-divergence duality in lectin domains of the selectin family and its implications. FEBS Letters. 1995;363:123–126. doi: 10.1016/0014-5793(95)00240-a. [DOI] [PubMed] [Google Scholar]
- 40.Chou KC. Pseudo amino acid composition and its applications in bioinformatics, proteomics and system biology. Current Proteomics. 2009;6:262–274. [Google Scholar]
- 41.Esmaeili M, Mohabatkar H, Mohsenzadeh S. Using the concept of Chou's pseudo amino acid composition for risk type prediction of human papillomaviruses. Journal of Theoretical Biology. 2010;263:203–209. doi: 10.1016/j.jtbi.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Zhang GY, Fang BS. Predicting the cofactors of oxidoreductases based on amino acid composition distribution and Chou's amphiphilic pseudo amino acid composition. Journal of Theoretical Biology. 2008;253:310–315. doi: 10.1016/j.jtbi.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Lin H, Wang H, Ding H, Chen YL, Li QZ. Prediction of Subcellular Localization of Apoptosis Protein Using Chou's Pseudo Amino Acid Composition. Acta Biotheoretica. 2009;57:321–330. doi: 10.1007/s10441-008-9067-4. [DOI] [PubMed] [Google Scholar]
- 44.Ding YS, Zhang TL. Using Chou's pseudo amino acid composition to predict subcellular localization of apoptosis proteins: an approach with immune genetic algorithm-based ensemble classifier. Pattern Recognition Letters. 2008;29:1887–1892. [Google Scholar]
- 45.Lin H, Ding H, Feng-Biao Guo FB, Zhang AY, Huang J. Predicting subcellular localization of mycobacterial proteins by using Chou's pseudo amino acid composition. Protein & Peptide Letters. 2008;15:739–744. doi: 10.2174/092986608785133681. [DOI] [PubMed] [Google Scholar]
- 46.Lin H. The modified Mahalanobis discriminant for predicting outer membrane proteins by using Chou's pseudo amino acid composition. Journal of Theoretical Biology. 2008;252:350–356. doi: 10.1016/j.jtbi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Qiu JD, Huang JH, Liang RP, Lu XQ. Prediction of G-protein-coupled receptor classes based on the concept of Chou's pseudo amino acid composition: an approach from discrete wavelet transform. Analytical Biochemistry. 2009;390:68–73. doi: 10.1016/j.ab.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Georgiou DN, Karakasidis TE, Nieto JJ, Torres A. Use of fuzzy clustering technique and matrices to classify amino acids and its impact to Chou's pseudo amino acid composition. Journal of Theoretical Biology. 2009;257:17–26. doi: 10.1016/j.jtbi.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Gu Q, Ding YS, Zhang TL. Prediction of G-Protein-Coupled Receptor Classes in Low Homology Using Chou's Pseudo Amino Acid Composition with Approximate Entropy and Hydrophobicity Patterns. Protein Pept Lett. 2010;17:559–567. doi: 10.2174/092986610791112693. [DOI] [PubMed] [Google Scholar]
- 50.Zeng YH, Guo YZ, Xiao RQ, Yang L, Yu LZ, et al. Using the augmented Chou's pseudo amino acid composition for predicting protein submitochondria locations based on auto covariance approach. Journal of Theoretical Biology. 2009;259:366–372. doi: 10.1016/j.jtbi.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 51.Jiang X, Wei R, Zhang TL, Gu Q. Using the concept of Chou's pseudo amino acid composition to predict apoptosis proteins subcellular location: an approach by approximate entropy. Protein & Peptide Letters. 2008;15:392–396. doi: 10.2174/092986608784246443. [DOI] [PubMed] [Google Scholar]
- 52.Li FM, Li QZ. Predicting protein subcellular location using Chou's pseudo amino acid composition and improved hybrid approach. Protein & Peptide Letters. 2008;15:612–616. doi: 10.2174/092986608784966930. [DOI] [PubMed] [Google Scholar]
- 53.Ding H, Luo L, Lin H. Prediction of cell wall lytic enzymes using Chou's amphiphilic pseudo amino acid composition. Protein & Peptide Letters. 2009;16:351–355. doi: 10.2174/092986609787848045. [DOI] [PubMed] [Google Scholar]
- 54.Zhou XB, Chen C, Li ZC, Zou XY. Using Chou's amphiphilic pseudo-amino acid composition and support vector machine for prediction of enzyme subfamily classes. Journal of Theoretical Biology. 2007;248:546–551. doi: 10.1016/j.jtbi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Chen C, Chen L, Zou X, Cai P. Prediction of protein secondary structure content by using the concept of Chou's pseudo amino acid composition and support vector machine. Protein & Peptide Letters. 2009;16:27–31. doi: 10.2174/092986609787049420. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Diaz H, Gonzalez-Diaz Y, Santana L, Ubeira FM, Uriarte E. Proteomics, networks, and connectivity indices. Proteomics. 2008;8:750–778. doi: 10.1002/pmic.200700638. [DOI] [PubMed] [Google Scholar]
- 57.Denoeux T. A k-nearest neighbor classification rule based on Dempster-Shafer theory. IEEE Transactions on Systems, Man and Cybernetics. 1995;25:804–813. [Google Scholar]
- 58.Shen HB, Chou KC. Using optimized evidence-theoretic K-nearest neighbor classifier and pseudo amino acid composition to predict membrane protein types. Biochemical & Biophysical Research Communications. 2005;334:288–292. doi: 10.1016/j.bbrc.2005.06.087. [DOI] [PubMed] [Google Scholar]
- 59.Cover TM, Hart PE. Nearest neighbour pattern classification. IEEE Transaction on Information Theory. 1967;IT-13:21–27. [Google Scholar]
- 60.Shafer G. A mathematical theory of evidence. Princeton N.J.: Princeton University Press; 1976. [Google Scholar]
- 61.Zouhal LM, Denoeux T. An evidence-theoretic K-NN rule with parameter optimization. IEEE Transactions on Systems, Man and Cybernetics. 1998;28:263–271. [Google Scholar]
- 62.Shen HB, Song JN, Chou KC. Prediction of protein folding rates from primary sequence by fusing multiple sequential features. Journal of Biomedical Science and Engineering (JBiSE) 2009;2:136–143. (openly accessible at http://www.srpublishing.org/journal/jbise/) [Google Scholar]
- 63.Chou KC, Shen HB. FoldRate: A web-server for predicting protein folding rates from primary sequence. The Open Bioinformatics Journal. 2009;3:31–50. (openly accessible at http://www.bentham.org/open/tobioij/) [Google Scholar]
- 64.Chou KC, Zhang CT. Review: Prediction of protein structural classes. Critical Reviews in Biochemistry and Molecular Biology. 1995;30:275–349. doi: 10.3109/10409239509083488. [DOI] [PubMed] [Google Scholar]
- 65.Zhou GP. An intriguing controversy over protein structural class prediction. Journal of Protein Chemistry. 1998;17:729–738. doi: 10.1023/a:1020713915365. [DOI] [PubMed] [Google Scholar]
- 66.Chen K, Kurgan LA, Ruan J. Prediction of protein structural class using novel evolutionary collocation-based sequence representation. J Comput Chem. 2008;29:1596–1604. doi: 10.1002/jcc.20918. [DOI] [PubMed] [Google Scholar]
- 67.Jiang Y, Iglinski P, Kurgan L. Prediction of protein folding rates from primary sequences using hybrid sequence representation. J Comput Chem. 2008 doi: 10.1002/jcc.21096. [DOI] [PubMed] [Google Scholar]
- 68.Yang JY, Peng ZL, Yu ZG, Zhang RJ, Anh V, et al. Prediction of protein structural classes by recurrence quantification analysis based on chaos game representation. Journal of Theoretical Biology. 2009;257:618–626. doi: 10.1016/j.jtbi.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 69.He ZS, Zhang J, Shi XH, Hu LL, Kong XG, et al. Predicting drug-target interaction networks based on functional groups and biological features. PLoS ONE. 2010;5:e9603. doi: 10.1371/journal.pone.0009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This benchmark dataset S for Plant-mPLoc includes 1,055 plant protein sequences (978 different proteins), classified into 12 plant subcellular locations. Among the 978 different proteins, 904 belong to one subcellular location, 71 to two locations, and 3 to three locations. Both the accession numbers and sequences are given. None of the proteins has ≥25% sequence identity to any other in the same subset (subcellular location). See the text of the paper for further explanation.
(0.78 MB PDF)
The degenerate testing dataset used for comparing the performance between TargetP (Emanuelsson, et al. J. of Mol. Biol. 2000, 300: 1005–1016) and Plant-mPLoc of this paper. The dataset contains 1,775 plant proteins classified into 2 subcellular locations: (1) chloroplast, and (2) mitochondrion. To avoid bias, none of the proteins included here occurs in the training dataset of TargetP, nor in the training dataset of Plant-mPLoc. See the text of the paper for further explanation.
(0.91 MB PDF)
List of the results predicted by TargetP (Emanuelsson et al. J. Mol. Biol. 2000, 300: 1005–1016) and Plant-mPLoc on the 1,775 independent proteins in the Table S2, and their experimental subcellular locations as annotated in Swiss-Prot databank (version 55.3 released on 29-Apr-2008). Note for TargetP outputs, “C” means “Chloroplast”, “M” means “Mitochondrion”, “S” means “Secretory pathway”, and “_” means “Any other location”.
(0.41 MB PDF)
The degenerate testing dataset used for comparing the performance between Predotar (Small et al., Proteomics 2004, 4: 1581–1590) and Plant-mPLoc of this paper. The dataset contains 381 plant proteins classified into 3 subcellular locations: (1) endoplasmic reticulum, (2) mitochondrion, and (3) plastid. To avoid bias, none of the proteins included here occurs in the training dataset of TargetP, nor in the training dataset of Plant-mPLoc. See the text of the paper for further explanation.
(0.25 MB PDF)
List of the results predicted by Predotar (Small et al., Proteomics 2004, 4:1581–90) and Plant-mPLoc on the 381 independent proteins in the Table S4, and their experimental subcellular locations as annotated in Swiss-Prot databank (version 55.3 released on 29-Apr-2008). Note for the Predotar output, “ER” means “Endoplasmic reticulum”.
(0.16 MB PDF)