Abstract
Reactive oxygen species (ROS) are increasingly recognized as important signaling regulators. The family of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Noxs) is responsible for the production of most signaling ROS in cells. An emerging paradigm is that individual Nox family members are organized and activated at distinct subcellular locations for specific functions. Tyrosine kinase substrate (Tks) family adaptor proteins have now been identified as Nox organizer proteins that enhance the production of ROS at invadopodia and podosomes, which are subcellular adhesion structures associated with extracellular matrix degradation. ROS production is also shown to be required for invadopodia and podosome formation. These findings broaden the known signaling roles of ROS and identify a potential mechanism for the correlation of ROS production with cancer invasion.
Reactive oxygen species (ROS), such as superoxide (O2−) and peroxide (H2O2) were originally identified as toxic byproducts of metabolism (1). However, the discovery of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) and myeloperoxidase in phagocytes demonstrated that cells have evolved a system to manufacture ROS for specific purposes, such as killing bacteria (1). Nox proteins were subsequently identified in other cell types and tissues, suggesting that they have roles in more general cellular processes. Indeed, ROS have been shown to regulate various cellular functions in virtually every tissue in the body, including signal transduction, gene expression, cell growth, and apoptosis. ROS production is increased in cancer and linked to both transformation and metastasis (2–6), suggesting a subversion of normal signaling mechanisms. Two papers shed light on the role of ROS in invasion by demonstrating that Nox-produced ROS promote the formation of invadopodia, which are footlike cellular processes with associated matrix metalloproteinases (MMPs) (7, 8). Moreover, the authors show that the essential invadopodia scaffold proteins tyrosine kinase substrate 4 (Tks4) and Tks5 are in fact organizer proteins for Nox enzymes, suggesting that their function in invadopodia may be to localize ROS.
Invadopodia
Invadopodia are subcellular protrusions found in invasive cancer cells that possess extracellular matrix (ECM)–degrading activity (9, 10). Formation of invadopodia is associated with cancer invasiveness in various model systems, including metastasis in nude mice (11–13). Similar invasive structures with a more ringlike morphology are formed in Src kinase–transformed cells and are sometimes referred to as podosomes. Podosomes also form in normal cells that remodel ECM or migrate, such as osteoclasts and macrophages, respectively (14). Although there is an ongoing debate as to whether invadopodia and podosomes have distinct functions with regard to ECM adhesion (10, 14), they do share common molecular components and a similar ability to degrade ECM. Likewise, Tks proteins and ROS were shown to regulate the formation and function of both cancer cell invadopodia and podosomes in Src-transformed cells.
Several cellular activities converge at invadopodia and podosomes to promote ECM digestion, including cytoskeletal assembly, tyrosine kinase signaling, adhesion, and the vesicular trafficking of proteases (9). Many invadopodia proteins are either Src substrates or tyrosine kinase adaptor proteins, which is consistent with Src having a critical role in organizing these component processes (9). The Src substrates Tks4 and Tks5 are included in this list; however, unlike most invadopodia proteins that localize to and function in other subcellular structures, Tks4 and Tks5 appear to localize to invadopodia and podosomes (15, 16). Furthermore, they are essential for function, because knockdown of either protein abrogates the formation and function of invadopodia or podosomes, and coexpression of Tks5 with activated Src promotes the formation of invadopodia in epithelial cells (15, 16). The signals controlling the formation and turnover of invadopodia are largely unknown. Diaz, Shani, and colleagues show that ROS produced by NADPH oxidases of the Nox family are required for the formation of both podosomes and invadopodia (7).
Tks Proteins as Nox Organizers
Initial sequence analysis of Tks5 revealed homology with p47phox, a cytosolic regulator of the phagocyte NADPH oxidase Nox2 (17). Activation of phagocytes leads to the phosphorylation of p47phox and its translocation to the membrane-bound Nox2-p22phox complex (1). Because associated Nox activator proteins are also carried to the membrane, p47phox is considered to be an “organizer” protein that allows the activation of Nox2. In other cell types, the homologous protein NoxO1 functions in an analogous manner, activating Nox1 and Nox3 (1, 18). Gianni et al. show that Tks4 and Tks5 are Nox organizer proteins that can also support Nox1 and Nox3 activity (8). The selective localization of Tks proteins to invadopodia (15, 16) suggests that localization of ROS to invadopodia (7) is a critical function for Tks4 and Tks5 that is not shared with other Nox organizers. Consistent with that idea, overexpression of the organizer NoxO1 inhibits invadopodia formation in colon cancer cells (8).
Nox-produced ROS has a well-established role in signaling through the inhibition of phosphatases, activation of kinases, or regulation of ion channels (1). Molecular mechanisms include electron transport–dependent cell depolarization and ROS-dependent posttranslational modifications, such as cysteine oxidation and S-glutathiolation. In particular, inactivation of phosphatases occurs because of oxidation of redox-sensitive cysteine residues in the catalytic site (1). Thus, ROS can enhance tyrosine kinase signaling at various subcellular sites by the local removal of antagonistic phosphatase activity.
Because ROS are rapidly inactivated in cells, local generation of ROS is thought to be critical for the regulation of site-specific signaling activities (19), and one might expect colocalization of ROS production at major sites for tyrosine kinase activity. One such site occurs downstream of integrin activation at focal adhesions (FAs). The Nox organizer protein p47phox is targeted to FAs through interactions with the FA adaptor proteins Hic5 and TRAF4 (tumor necrosis factor receptor–associated factor 4) (20). TRAF4-activated ROS production leads to inactivation of the protein tyrosine phosphatase PEST (PTP-PEST) and promotes cellular migration.
Similar to focal adhesions, invadopodia are centers of tyrosine kinase activity. In particular, Src kinase activity is both necessary and sufficient for the formation and ECM-degrading activity of invadopodia structures (9). Invadopodia and the related podosome structures also share some molecular components with focal adhesions, including proteins such as Mena, vinculin, p130Cas, PTP-PEST, and Src (7, 21–25). As a potential mechanism for the effect of ROS on invadopodia and podosomes, Diaz, Shani, and colleagues demonstrate that knockdown of PTP-PEST dramatically increases the number of podosomes in Src-transformed cells (7). Thus, there are hints that ROS may regulate invadopodia, podosomes, and focal adhesions through a common molecular mediator.
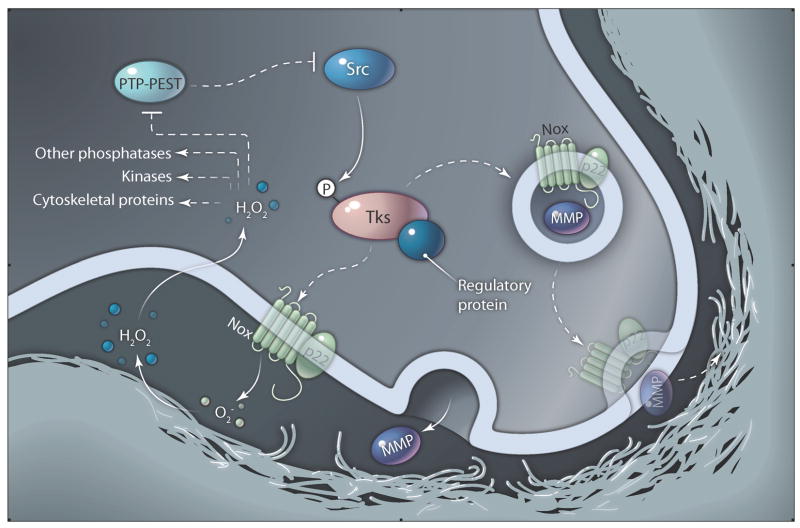
The signals upstream of ROS generation are varied. A paper by the Bokoch and Courtneidge groups demonstrated that Src promotes ROS formation in colon cancer cells (4). Because Tks4 and Tks5 are Src substrates, Src induction of ROS production may occur secondary to the phosphorylation of Tks4 and Tks5 and subsequent organization and activation of Nox enzymes at invadopodia (Fig. 1). Consistent with that possibility, knockdown of Tks5 ablated the increased ROS production caused by Src transformation of 3T3 fibroblasts and also reduced the total amount of ROS produced by human oral squamous cell carcinoma cells (7).
Fig. 1.
Potential ROS signaling pathways in invadopodia. Phosphorylation of Tks4 or Tks5 by Src may allow recruitment to plasma membrane-localized Nox enzymes at nascent invadopodia sites. Translocation of Nox regulatory proteins (X) associated with Tks proteins (Tks) leads to the binding of p22phox-Nox complexes, activation of Nox activity, and production of reactive oxygen species, such as O2− and H2O2. Oxidation of redox-sensitive amino acids in various invadopodia proteins, such as PTP-PEST, leads to enhanced formation and function of invadopodia. Feedback may occur from PTP-PEST to Src. Fusion of Nox-containing vesicles is also shown, in analogy to the fusion of phagosomes that occurs downstream of Nox2 activation in phagocytes. Although it is speculation at this time, an exciting possibility is that Nox activation could affect fusion of MMP-containing vesicles with the plasma membrane.
Gianni et al. and Diaz et al. make a critical first step in understanding ROS-mediated cancer invasion by demonstrating that ROS are essential for invadopodia formation and by establishing Tks proteins as Nox organizers at invadopodia. However, many questions remain. In particular, the mechanisms by which ROS regulate invadopodia formation remain to be determined (Fig. 1). Although it seems likely that inactivation of PTP-PEST is one function of invadopodia-localized ROS, there could be other targets, including other tyrosine phosphatases, lipid phosphatases, tyrosine and serine kinases, and cytoskeletal proteins (1, 6, 18, 19). Indeed, inhibition of ROS production leads to diminished tyrosine phosphorylation of Tks4 and Tks5, suggesting the decreased activity of an upstream tyrosine kinase. ROS may also regulate MMP expression to promote ECM degradation at invadopodia (1, 6). And analogously to the phagocyte oxidase system (1), activation of Nox could lead to the fusion of protease-containing vesicles at Tks-directed invadopodia sites. Future studies of this fascinating system are likely to shed new light on Src kinase signaling mechanisms, spatiotemporal regulation of ROS production, and mechanisms of cancer invasion.
Acknowledgments
The author would like to acknowledge funding from the National Institute of General Medical Sciences(1R01GM075126), National Cancer Institute (1R21DE018244), and American Cancer Society (RSG118085).
References and Notes
- 1.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 2.Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem Biophys Res Commun. 2009;379:445–450. doi: 10.1016/j.bbrc.2008.12.080. [DOI] [PubMed] [Google Scholar]
- 3.Ferraro D, Corso S, Fasano E, Panieri E, Santangelo R, Borrello S, Giordano S, Pani G, Galeotti T. Pro-metastatic signaling by c-Met through RAC-1 and reactive oxygen species (ROS) Oncogene. 2006;25:3689–3698. doi: 10.1038/sj.onc.1209409. [DOI] [PubMed] [Google Scholar]
- 4.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsushita J, Lambeth JD, Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004;64:3580–3585. doi: 10.1158/0008-5472.CAN-03-3909. [DOI] [PubMed] [Google Scholar]
- 6.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 7.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, Nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM. Novel p47phox-related organizers regulate NADPH oxidase 1 (Nox1) activity and localization. Sci Signal. 2009;2:ra54. doi: 10.1126/scisignal.2000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver AM. Invadopodia: Specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 10.Weaver AM. Invadopodia. Curr Biol. 2008;18:R362–R364. doi: 10.1016/j.cub.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 12.Coopman PJ, Do MT, Thompson EW, Mueller SC. Phagocytosis of cross-linked gelatin matrix by human breast carcinoma cells correlates with their invasive capacity. Clin Cancer Res. 1998;4:507–515. [PubMed] [Google Scholar]
- 13.Thompson EW, Paik S, Brünner N, Sommers CL, Zugmaier G, Clarke R, Shima TB, Torri J, Donahue S, Lippman ME, Martin GR, Dickson RB. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150:534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 14.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Courtneidge SA. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Lock P, Abram CL, Gibson T, Courtneidge SA. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 1998;17:4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terada LS. Specificity in reactive oxidant signaling: Think globally, act locally. J Cell Biol. 2006;174:615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Jr, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brabek J, Constancio SS, Shin NY, Pozzi A, Weaver AM, Hanks SK. CAS promotes invasiveness of Src-transformed cells. Oncogene. 2004;23:7406–7415. doi: 10.1038/sj.onc.1207965. [DOI] [PubMed] [Google Scholar]
- 23.Chellaiah MA, Biswas RS, Yuen D, Alvarez UM, Hruska KA. Phosphatidylinositol 3,4,5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J Biol Chem. 2001;276:47434–47444. doi: 10.1074/jbc.M107494200. [DOI] [PubMed] [Google Scholar]
- 24.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, Gertler FB. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–828. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinardi L, Rietdorf J, Nitsch L, Bono M, Tacchetti C, Way M, Marchisio PC. A dynamic podosome-like structure of epithelial cells. Exp Cell Res. 2004;295:360–374. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]