SUMMARY
Factor Inhibiting HIF-1α (FIH) is an asparaginal hydroxylase. Hydroxylation of HIF-α proteins by FIH blocks association of HIFs with the transcriptional co-activators CBP/p300, thus inhibiting transcriptional activation. We have created mice with a null mutation in the FIH gene, and found that it has little or no discernable role in mice in altering classical aspects of HIF function, e.g., angiogenesis, erythropoiesis, or development. Rather, it is an essential regulator of metabolism: mice lacking FIH exhibit reduced body weight, elevated metabolic rate, hyperventilation, improved glucose and lipid homeostasis, and are resistant to high fat diet-induced weight gain and hepatic steatosis. Neuron-specific loss of FIH phenocopied some of the major metabolic phenotypes of the global null animals: those mice have reduced body weight, increased metabolic rate, enhanced insulin sensitivity, and are also protected against high fat diet-induced weight gain. These results demonstrate that FIH acts to a significant degree through the nervous system to regulate metabolism.
INTRODUCTION
Metabolic response in animals is essentially the act of balancing demand against substrate availability. A key element of this balance for many animals and their constituent tissues is the variable of oxygen availability, which influences both rate and mechanism of substrate utilization for energy production (Semenza, 2007). The hypoxia inducible factors act as enhancers and regulators of transcriptional response to low oxygen. They are able to respond to a wide range of environmental oxygen concentrations, and coordinate responses at the cellular to organismal levels via alterations in at least 300 oxygen-responsive genes (Weidemann and Johnson, 2008).
The HIF response has a series of complex controls. A key mechanism of regulation, however, is through enzymatic, oxygen-dependent modification of the HIF-1α and HIF-2α proteins (Semenza, 2004). The post-translational events involved in this depend on the availability of oxygen for the catalysis of two different modifications of HIF-α proteins. The first category of oxygen-dependent covalent changes involves the prolyl hydroxylases, or PHDs. These are required for hydroxylation of highly conserved prolines in the HIF-α transcription factors; these in turn ultimately regulate ubiquitination and turnover of the HIF-α proteins (Semenza, 2007). The second category requires a factor termed FIH (encoded by the Hif1an gene), and it catalyzes an asparagine hydroxylation step that controls the association of HIF-α transcription factors with CBP/p300 transcriptional co-activators (Lando et al., 2002; Mahon et al., 2001). Genetic mutations in the PHDs and in the von Hippel-Lindau tumor suppressor gene (VHL) that mediate HIF-α ubiquitination have been described: null mutations in these genes in mice cause mid-gestation lethality when PHD2 and VHL are deleted (Gnarra et al., 1997; Takeda et al., 2006); mutations in the PHD1 and PHD3 genes have more subtle phenotypes (Aragones et al., 2008; Bishop et al., 2008), but all generally exhibit some of the archetypal features of increased HIF activation, e.g., increased vascularization and high levels of erythropoiesis (Takeda et al., 2008).
The FIH gene has been associated with hydroxylation events in a range of other substrates as well, including those in the Notch pathway (Coleman et al., 2007; Zheng et al., 2008) and the tankyrase protein (Cockman et al., 2009); given this, a genetic examination of the role of the FIH gene is essential to begin a determination of its actual role in animal physiology. We have found that loss of FIH results in a wide-ranging derangement of physiological response, causes a hypermetabolic phenotype, but results in few of the classical effects of HIF activation in vivo. These data also indicate that targeting of this pathway could provide a unique mechanism for manipulation of metabolism.
RESULTS
Targeting of the FIH gene creates a null allele
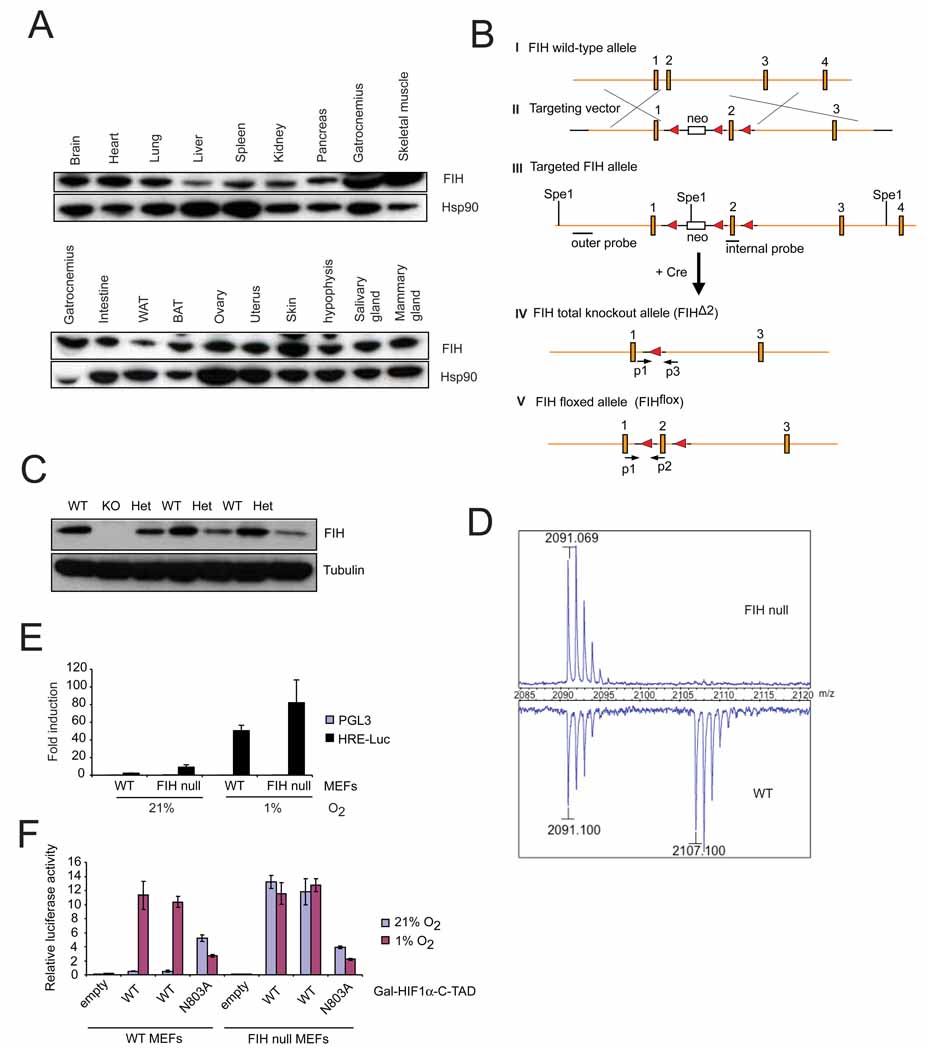
As can be seen in Figure 1A, the FIH gene product is expressed in a wide range of tissues. Therefore, to better understand the role of the FIH factor in the overall regulation of oxygen homeostasis, we created a conditional null mutation of the gene, as shown in Figure 1B. This deletion was engineered in embryonic stem cells, with a targeting efficiency of 2 clones generated with the designed event for approximately 1,800 ES cell clones screened (Figure S1A). Both isolated clones were able to produce germ line transmission of the altered allele. Following germline transmission of the conditional allele, animals were crossed with the EIIa-cre transgenic line in order to generate both complete nullizygous alleles (FIHΔ2) as well as to generate conditional alleles (FIHflox) as shown in (IV) and (V) of Figure 1B (Lakso et al., 1996).
Figure 1. Generation of FIH knockout mice.
- Immunoblotting for FIH in adult mouse tissues. Various organs from a 2-month old wild type female were harvested and frozen at −80°C before lysis. Whole cell extracts were obtained and immunoblotted for FIH. Hsp90 was used for the loading control.
- Strategy of targeting the FIH gene. The genomic wild-type FIH locus around exon 1–4 (filled boxes) is shown (I). A neo cassette (open box) and a loxP sites (filled triangle) from the targeting vector (II) were introduced upstream and downstream of exon 2 by homologous recombination (III), respectively. Restriction sites (SpeI) and positions of outer and internal probes for Southern blotting are indicated. After Cre-mediated excision, the FIH total knockout allele (FIHΔ2) (IV) and the FIH floxed allele (FIHflox) (V) were obtained. PCR primers used for genotyping (p1, p2, p3) are indicated (arrows).
- Immunoblot analysis of E12.5 whole embryo lysates of wild-type (WT), heterozygous (Het) and knockout (KO) using FIH and tubulin antibodies.
- MALDI-TOF-MS spectra of asparaginyl hydroxylation on HIF-1α in immortalized WT and FIH null MEFs under normoxia (21% O2).
- Luciferase assay using HRE (hypoxia response element) reporter in immortalized WT and FIH null MEFs under normoxia and hypoxia (1% O2) (n=3/each). Luciferase activity was measured 24hr following transfection.
-
Luciferase assay using Gal-HIF1α-C-TAD reporter in immortalized WT and FIH null MEFs under normoxia and hypoxia. WT is the wild type form of HIF1α-C-TAD. N803A is the mutant form in which asparagine 803 is mutated.Values in graphs are expressed as mean ± standard error of the mean (SEM).See also Figure S1.
Crosses of the resulting FIHΔ2 heterozygous animals resulted in production of animals homozygous for the FIH null allele, which were verified for loss of protein production from the deletion by immunoblotting of whole embryo lysates derived from heterozygous crosses, as shown in Figure 1C. As can be seen, embryos genotyped as heterozygous have clearly reduced levels of FIH, and the embryo genotyped as nullizygous for the gene lacks any detectable FIH protein (Figure 1C). Genotypes were also confirmed by PCR analysis (Figure S1B).
The FIH gene encodes an asparaginyl hydroxylase that acts on the C-terminal activation domain (C-TAD) of the HIF-α proteins (Lando et al., 2002). To determine whether deletion of the FIH gene results in elimination of hydroxylation of the C-TAD of the HIF-α proteins, a cloned and tagged C-TAD was stably transfected into MEFs generated from FIHdf embryos (exon2 of the FIH gene is flanked by two loxP sites). These cells were then treated with a Cre recombinase expression construct-containing adenovirus, and control and FIH null cells were created from untreated and treated cells, respectively. Mass spectrometric analysis of the C-TAD region was then carried out, and as seen in Figure 1D, loss of FIH completely eliminates detectable hydroxylation of the asparagine residue within the C-TAD. This indicates that no redundancy exists in the pathway regulating this hydroxylation step, and that FIH is essential for this posttranslational modification of the HIF-1α C-TAD.
Transcriptional activation of HIF target genes via C-TAD is controlled by FIH
Assays of gene expression from a hypoxia response element (HRE)-driven luciferase construct indicated that loss of FIH in MEFs caused modest increases in transcription from an HRE reporter in both normoxic and hypoxic conditions (Figure 1E). Luciferase expression driven by a Gal-HIF1α-CTAD construct, where expression is dependent on C-TAD activity, shows that loss of FIH eliminates differences in hypoxic versus normoxic expression (Figure 1F). This clearly occurs through a mechanism dependent on an intact asparagine at residue 803 in the C-TAD, as mutation of this residue makes the construct insensitive to the absence of FIH (Figure 1F). These data demonstrate that the FIH mutation described here eliminates negative regulation of HIF C-TAD driven gene expression.
Gene expression analysis demonstrates a selective clamp function for FIH
Gene expression levels for a large number of genes were surveyed, but generally, effects on expression levels of HIF target genes were modest in cells nullizygous for FIH (Figure 2A). As shown, the HIF target genes like vascular endothelial growth factor (Vegf), phosphoglycerate kinase (Pgk) and glucose transporter-1 (Glut-1) were all minimally increased at normoxia, and their increased expression at levels of 1% atmospheric oxygen were increased at roughly similar levels (Figure 2A). These data indicate that the FIH gene likely acts as a clamp on gene expression levels, which are additionally controlled by other factors, including the VHL-mediated turnover of gene expression acting via prolyl hydroxylases.
Figure 2. Synergistic effects of FIH and VHL on regulating HIF activity in vitro.
- Representative quantitative PCR (qPCR) analysis of mRNA levels for Vegf, Pgk and Glut-1. Immortalized WT and FIH null MEFs were treated under normoxia and hypoxia for 16 hours. Results were normalized to β-actin.
- Growth of WT and FIH null MEFs was examined under normoxia and hypoxia. Cells seeded at a low density were incubated in normoxic or hypoxic conditions and harvested for cell counts every 24hr. The average cell number of triplicates for each condition is shown.
- Immunoblotting for HIF-1α, FIH and VHL from whole cell lysates of immortalized WT, FIH null, VHL null and VHL/FIH double null MEFs. Cells were treated under normoxia or hypoxia for 16 hours before harvest. Tubulin was used as the loading control.
- and (E) Representative qPCR analysis of mRNA level for Vegf (D) and Car9 (E) in WT, VHL null and VHL/FIH null MEFs (n=3/genotype).
- and (G) mRNA levels of both Vegf (F) and Car9 (G) were restored by a triple deletion of VHL, FIH and HIF-1α. For (D–F), cells were cultured under normoxia. Primers specific for β-actin were used for normalization.
- Venn diagram showing the numbers of up and down-regulated genes in MEFs using a filter of >=2-fold change.
-
VHL and FIH have synergistic effects on regulating cell growth. They were restored by deletion of HIF-1αMEFs were seeded at a low density and incubated under normoxia or hypoxia for 48 hr. Cell numbers of each nullizygous MEFs were counted and normalized to relative wild-type MEFs (n=3/condition).Values in graphs are expressed as mean ± SEM. p values are from Student’s t-test.See also Table S1.
As can be seen in Figure 2B, loss of the gene results in a small but consistent decrease in cell growth, evident in growth curves at 72 hours post-seeding. Although this is seen under both normoxia and hypoxia, the difference between the wild type and mutant cells is similar under both conditions.
To determine the effect of the two factors together on this process, we crossed animals carrying the conditional allele of the VHL gene (VHLdf) (Haase et al., 2001), and animals carrying the conditional allele of the FIH gene (FIHdf), and then derived MEFs (as described above) from the resultant embryos with conditional alleles (VHLdf/FIHdf) of both the VHL and FIH genes. Figure 2C documents that overall effects on HIF-1α levels are as expected, with an increase in HIF-1α levels primarily determined by VHL; little effect on HIF-1α protein levels is seen in VHL/FIH double null cells.
Figures 2D and 2E show the effects on gene expression in normoxia of loss of the two factors, VHL and FIH, concurrently; and demonstrates that their deletion has at least two classes of outcome. The first, seen in the HIF target gene Vegf, is an approximately additive effect on gene expression of deletion of the two hydroxylase-mediated mechanisms of controlling gene expression (Figure 2D). The second and more dramatic difference is seen in a group of genes (Table S1) that includes the carbonic anhydrase 9 gene (Car9); here, loss of either VHL or FIH alone create a small increase in gene expression, but loss of the two in tandem causes an extraordinary alteration in gene control, with an approximate 200-fold increase in Car9 expression in the double deletion cells (Figure 2E). Figures 2F and 2G demonstrate that in the case of both the Vegf and Car9 genes, a triple deletion of VHL, FIH and HIF-1α restores to wild type levels the increased gene expression caused by loss of VHL and FIH. It is interesting, given that HIF-2α is also regulated by VHL and FIH, and known to effect HIF-mediated gene expression, that loss of HIF-1α in MEFṣalone represses the exaggerated gene expression caused by loss of VHL and FIH in tandem.
A gene expression matrix analysis was carried out, and the effects are shown graphically in Figure 2H. Here it is evident that loss of both factors does have an additive effect, and that there are both synergies and differential selectivity in the function of the two hydroxylase-dependent pathways of gene expression.
As shown in Figure 2I, loss of VHL has a deleterious effect on cell growth under both normoxia and hypoxia. As can be seen, loss of both factors has an additive negative effect on cell growth in normoxia and hypoxia. Here as well, the loss of HIF-1α in cells that contain conditional alleles for VHL and FIH (VHL/FIH/HIF-1α triple null cells) restores plating efficiency to wild type control levels, and indicates that it is the over-activation of HIF-1α that is primarily deleterious for cell survival.
Loss of FIH causes an increase in cellular ATP levels and suppresses AMPK activation
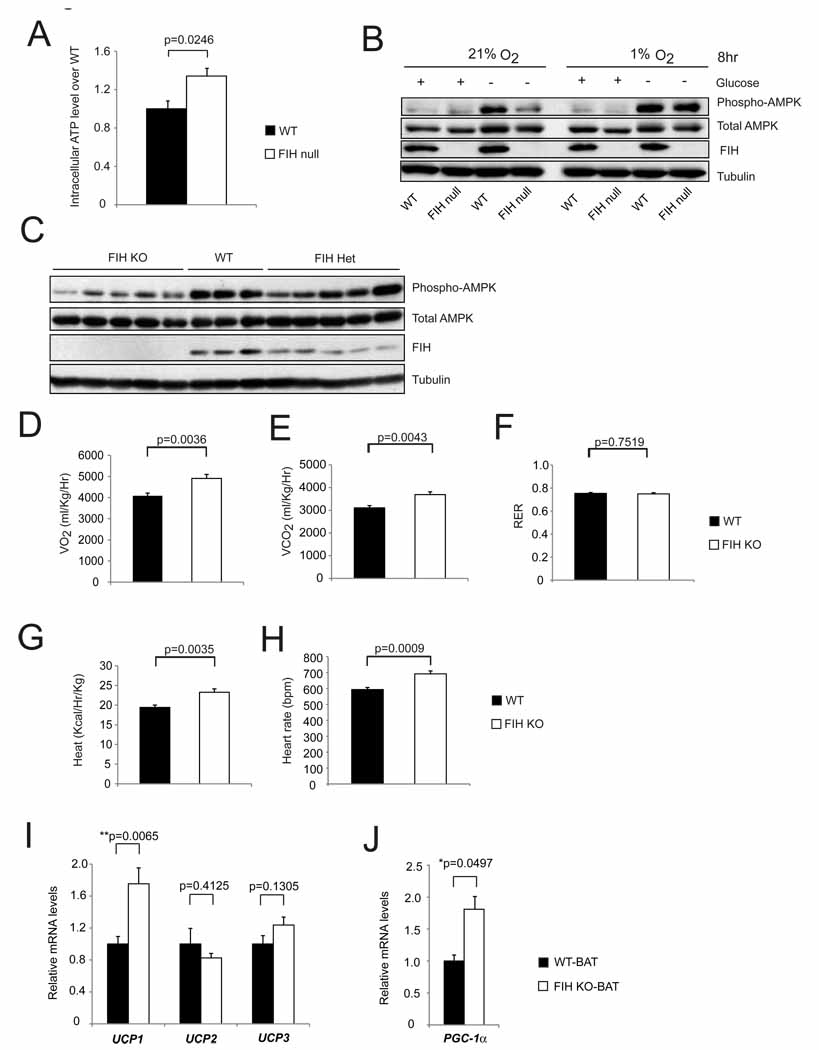
To investigate possible effects on cellular metabolism, we carried out assays on intracellular ATP levels in wild type and mutant cells maintained at normoxia and normoglycemia. As can be seen in Figure 3A, loss of FIH causes an increase in cellular ATP levels. Deletion of both FIH and HIF-1α results in decreased ATP levels (Figure S2A). To investigate further effects on cellular metabolism, total levels of the AMPK protein, as well as levels of activated AMPK were determined in normal as well as hypoxic and hypoglycemic cultures (Figure 3B and Figure S2B). This showed that loss of FIH acted to suppress AMPK activation under hypoglycemic conditions, whereas the suppression was diminished when both FIH and HIF-1α were deleted. A further investigation of AMPK activation during development showed that individual lysates of mid-gestation FIH null embryos also had lower levels of AMPK activation (Figure 3C); this indicates that the altered metabolic state which gives rise to increased cellular ATP in culture arises in development.
Figure 3. Hypermetabolism in FIH KO mice.
- Intracellular ATP levels in primary WT and FIH null MEFs under normoxia (n=3/group).
- Levels of phospho-AMPK and total AMPK were measured by immunoblotting with whole cell lysates from primary WT and FIH null MEFs treated with conditions as indicated.
- Immunoblotting analysis of whole cell lysates from E12.5 WT (n=3), FIH Het (n=5) and FIH KO (n=5) embryos using phospho-AMPK, total AMPK, FIH and tubulin antibodies.
- Resting whole-body O2 consumption (VO2) in 2-month old WT (n=7) and FIH KO (n=5) mice. Shown are the average value over 3 hr.
- Resting CO2 production (VCO2) in the same mice as in (D).
- RER (respiratory exchange ratio) were determined during the same period as in (D).
- Heat production was determined during the same measurement as in (D).
- Heart rates were measured in resting 2-month old WT (n=9) and FIH KO (n=5) mice under normoxic condition.
- UCP1, UCP2 and UCP3 mRNA levels in brown adipose tissue (BAT) from 5-month old mice under normal chow were measured by qPCR (n=6/genotype).
-
PGC-1α mRNA levels in BAT from 5-month old mice under normal chow were measured by qPCR (n=6/genotype).For gene expression analysis in (I) and (J), specific primers for β-actin were used for normalization.Values shown in graphs are expressed as mean ± SEM. p values are from Student’s t-test.See also Figures S2 and S3.
Loss of FIH causes a hypermetabolic state in vivo
We next investigated metabolic rates in adult FIH mutant mice. It has been found that activation of HIF in animals can increase glycolysis, reduce oxygen consumption and elevate the respiratory exchange ratio (RER=VCO2/VO2). However, we found that FIH mutant mice had an elevated consumption of oxygen and increased evolution of carbon dioxide under normoxic conditions (Figures 3D and 3E). RER is not changed in these mice, and this demonstrates that glycolysis has not increased in FIH mutant mice (Figure 3F).
This observation is important, as it indicates that the HIF-mediated control of glycolytic rates is not a key factor in the increased level of respiration in mutants; ratios of glycolysis relative to other metabolic pathways are unchanged (as indicated by the unchanged RER) in spite of the significant increase in mutant metabolism. As expected from elevations in oxygen consumption, loss of FIH causes an approximate 20% increase in heat production as expressed in calories per hour per unit body mass (Figure 3G). This is accompanied by an increased mutant heart rate (Figure 3H).
Increased oxygen consumption could be caused by changes in uncoupled mitochondrial respiration. We examined the expression levels of mitochondrial uncoupling genes in brown adipose tissue (BAT), white adipose tissue (WAT) and skeletal muscle (SM) and found that the uncoupling protein 1 (UCP1) mRNA level in BAT from FIH nullizygous mice was significantly increased (Figure 3I). Levels of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) mRNA were also increased in BAT (Figure 3J); PGC-1α is an important regulator of thermogenesis (Uldry et al., 2006). Expression levels of the uncoupling protein 2 (UCP2) and uncoupling protein 3 (UCP3) genes in BAT, WAT and SM and the PGC-1α gene in WAT were similar between genotypes (Figure 3I and Figures S3A, S3B and S3C).
Mice lacking FIH are hyperventilatory and exhibit alkalosis
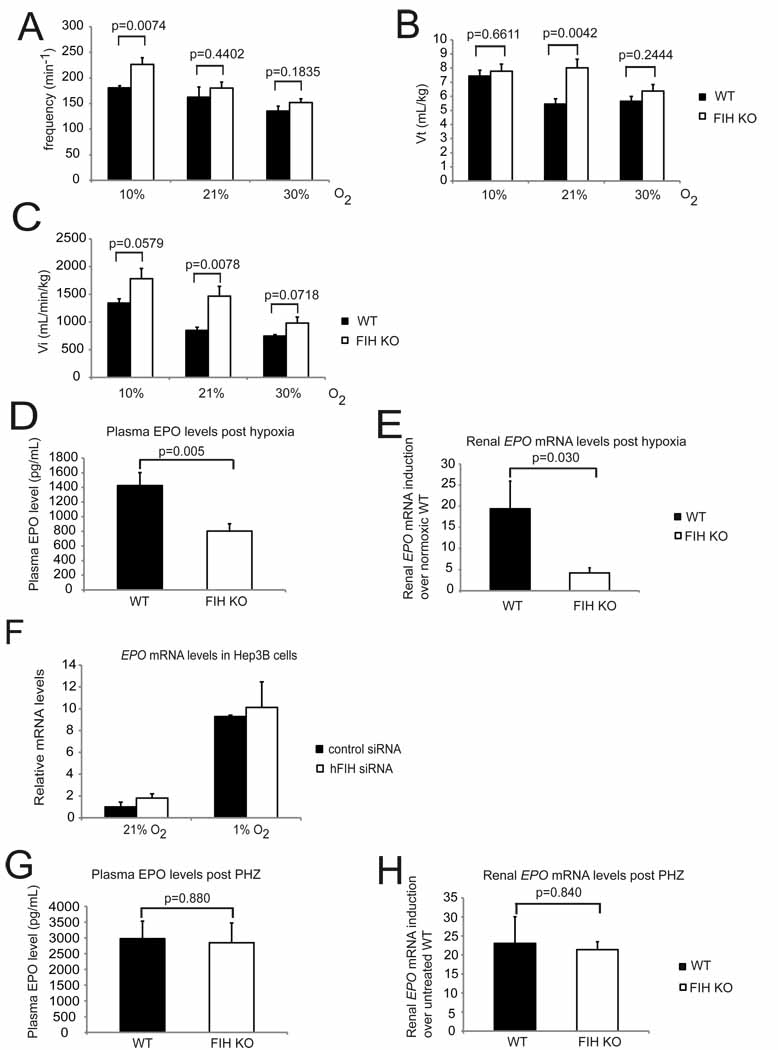
Ventilation is strongly affected in hypoxia; both tidal volume and respiratory frequency increase when mammals are exposed to low oxygen (Huey et al., 2000), and this can have significant effects on blood oxygenation. To investigate a potential role for FIH in hypoxic ventilation, we first examined global respiratory function in FIH null animals. In this context, we undertook plethysmography of the mutants (Figures 4A, 4B and 4C). These analyses demonstrated, first, that the frequency of breathing at normoxia is not elevated significantly in FIH mutants (Figure 4A), but that the tidal volume is significantly higher, with a mean increase of close to 30% (Figure 4B).
Figure 4. FIH KO mice are hyperventilatory.
- Respiration frequencies of unrestrained WT (n=7) and FIH KO (n=8) mice under acute hypoxia (10% O2), room air (21% O2), and hyperoxia (30% O2) were measured using barometric pressure plethysmography.
- Tidal volume (Vt) was measured in the same assay as in (A).
- Inspired minute ventilation (Vi) was determined in the same assay as in (A).
- Plasma EPO levels in WT and FIH KO mice following acute hypoxic exposure at 9% O2 for 14 hours (n=15/genotype).
- Analysis of renal EPO mRNA levels in hypoxia-treated WT (n=7) and FIH KO (n=8) mice (9% O2, 14 hr) by qPCR. Kidneys were collected immediately after hypoxia treatment, fast frozen in liquid N2 and stored at −80°C before RNA isolation.
- Representative qPCR analysis of mRNA levels for EPO in Hep3B cells following control or hFIH siRNA treatment. Cells were cultured under normoxic or hypoxic conditions for 16 hr before harvest (n=3/condition).
- Plasma EPO levels in WT (n=6) and FIH KO (n=5) mice after overnight PHZ treatment.
-
qPCR analysis of renal EPO expression in PHZ-treated mice (same as those in (G)).Results in (E), (F) and (H) were normalized to β-actin.Values shown in graphs are expressed as mean ± SEM. p values are from Student’s t-test.See also Table S2.
Taken together, these data argue that the large increase in tidal volume (Figure 4B) seen in wild type mice under hypoxic conditions is already present at normoxia in FIH mutants. Hyperoxia suppresses mutant tidal volume, such that at 30% oxygen their tidal volumes are not significantly different from those found in wild type mice. This indicates that FIH regulates a set point for mediating oxygen-regulated changes in tidal volume; and that this set point has been shifted in mutants to a higher oxygen concentration.
The elevation in frequency of respiration relative to wild type animals is only significant during hypoxia (Figure 4A); this indicates that a second level of compensation for hypoxia is triggered in FIH mutant animals, who presumably have little capacity to increase tidal volume further, and thus respond to decreased oxygenation with an increased respiratory frequency. This increase in respiratory frequency, in the presence of an increased tidal volume, would generate increased levels of blood oxygenation relative to wild type animals. Evidence of hyperoxygenation at normoxia is seen in Table S2, where both pO2 (Partial Pressure of Oxygen) and SO2 (oxygen saturation) are elevated in arterial blood gas analysis of mutants.
Analysis of minute volume (Vi, in Figure 4C), which is a measurement of total inspired air over time, and thus reflects both tidal volume and frequency of respiration, shows that the greatest discrepancy between wild types and mutants is at normoxia (Figure 4C). At hyperoxia, the genotypic differential in Vi is no longer statistically significant.
Increased ventilation can be due to hyperpnoea, or respiratory adaptation to an increased metabolic rate; this would be accompanied by a plasma acidosis or, if compensated, a normal blood pH. If hyperventilation is occurring, however, the increased exhalation of carbon dioxide in the absence of a metabolically driven production of CO2 causes a drop in pCO2 (Partial Pressure of Carbon Dioxide) and an accompanying alkalosis. This is seen in FIH null mutants, which have a clear hypocapnia (decreased pCO2) and respiratory alkalosis (increased blood pH) characteristic of hyperventilation (Table S2).
Mice lacking FIH have decreased EPO levels in response to hypoxia
To further determine how hypoxic physiology is regulated by the FIH enzyme, we undertook examination of a basal response to hypoxia, the increased expression of the erythropoietic hormone erythropoietin (EPO) (Semenza, 2009). Consistent with a lack of HIF-related changes in vascularization in the FIH null mutants (Figure S4C), basal levels of EPO, hematocrits, and reticulocyte counts are not different from those of wild type animals (data not shown). This also differs from a wide range of tissue-specific deletions of both the PHDs and VHL, which exhibit increased EPO expression and varying degrees of polycythemia (Boutin et al., 2008; Haase et al., 2001; Peyssonnaux et al., 2007; Rankin et al., 2007; Takeda et al., 2008).
We found that global loss of FIH does alter EPO expression after 14 hours of exposure to hypoxia (9% O2); levels of plasma EPO in FIH null animals are reduced by approximately 40% (Figure 4D). Renal expression of EPO mRNA correlates with the reduction in plasma EPO levels, and is reduced even more substantially, by approximately 75% relative to wild type levels post-hypoxia (Figure 4E).
To determine whether this was caused by a direct effect on gene expression, we examined EPO mRNA changes induced in an EPO-expressing cell line, the Hep3B cell line (Figure 4F). Suppression of FIH by siRNA in these cells did not, however, alter hypoxic induction of EPO expression significantly (Figure 4F).
To determine whether loss of EPO inducibility in mutants occurs in other contexts, we assayed plasma EPO levels following induction of experimental anemia (Figure 4G). This assay employs phenylhydrazine to induce red blood cell lysis; following treatment, the hematocrits in experimental animals drop, and the resulting anemia causes an increase in EPO expression and erythropoiesis. In these experiments, there were no significant changes in plasma EPO levels in mutants relative to wild type animals (Figure 4G) and no alteration in induction of renal EPO expression (Figure 4H). This demonstrates that the alteration in EPO expression that we see in FIH mutants is not related to direct effects on EPO expression in EPO-synthesizing tissues, but is correlated with altered respiration during hypoxia.
Loss of FIH causes decreased body mass and adiposity
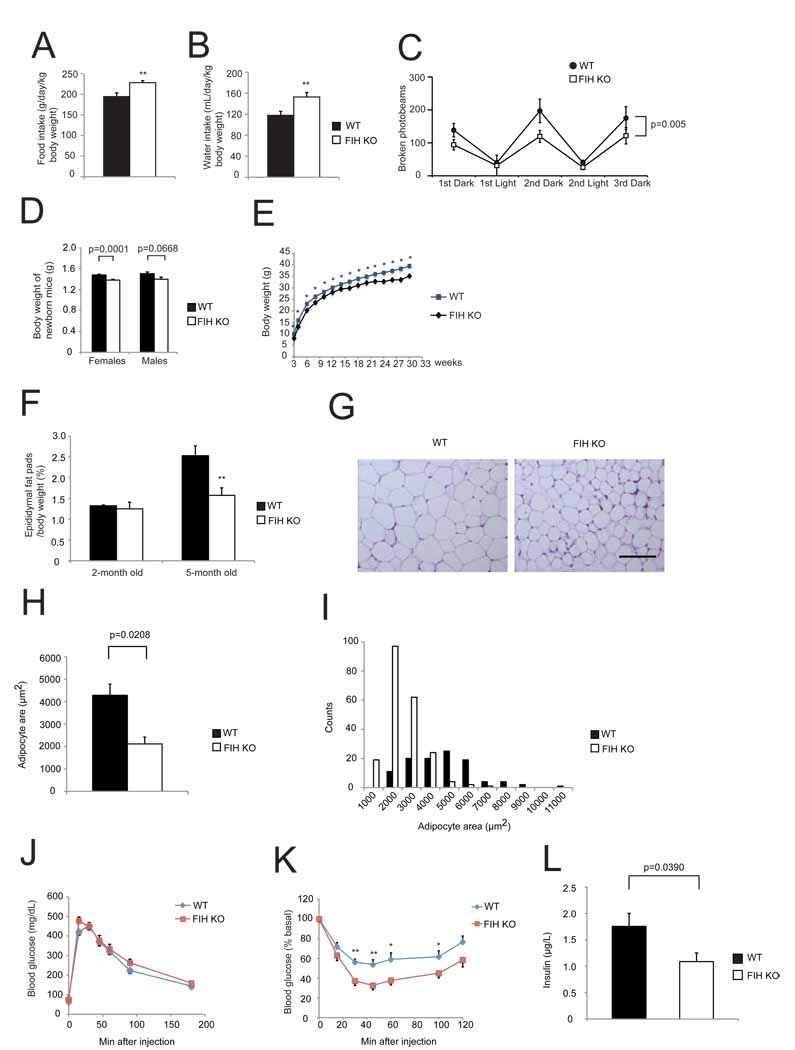
Loss of FIH causes an increase in both food and water consumption in conjunction with the increased metabolic rate described above (Figures 5A and 5B). Physical activity is a central aspect of energy expenditure, and so was measured together with O2 consumption. As shown in Figure 5C, a significantly higher O2 consumption in mutants accompanied a surprising hypoactivity, especially during dark cycles.
Figure 5. Global loss of FIH causes decreased body mass, decreased adiposity and increased insulin sensitivity.
- Food intake was measured in WT and KO mice (n=8/genotype) over 3 day/night cycles as monitored by CLAMS.
- Water intake was measured in the same assay as in (A).
- Physical activity was measured in the same assay as in (A) and (B). p=0.005 by two-way ANOVA.
- Body weights of newborn WT and KO mice. n=17–18 for females. n=11–14 for males, respectively.
- FIH KO mice are significantly smaller than their WT littermates. Growth curves show the average weights of WT (n=34) and KO (n=22) males. *p<0.05.
- Epididymal white fat pads weight normalized with body weight was measured from 2-month old and 5-month old WT and KO male mice. **p<0.01.
- Representative images of H&E stained epididymal WAT sections from 5-month old WT and KO mice fed with normal chow (NC). The scale bar represents 100µm.
- Epididymal adipocyte areas were measured from 5-month old WT and KO mice. n=3/genotype.
- Counts of adipocytes in different sizes from the same mice used in (H).
- Glucose tolerance test (GTT) was measured for 18hr-fasting 2-month old WT and KO mice fed with NC (2g D-glucose/kg body weight). n=10/genotype.
- Insulin tolerance test (ITT) was performed on 4hr-fasting 2-month old WT and KO mice fed with NC (0.75U/kg body weight). n=10–11/genotype. *p<0.05, **p<0.01.
-
Fed insulin levels were measured on 5-month old WT and KO mice. n=9/genotype.Values shown in graphs are expressed as mean ± SEM. p values are from Student’s t-test (except (C)).See also Figure S4, Table S3, and Table S4A.
Body weight represents to some extent a balance between energy intake and expenditure. Overall body mass in FIH nullizygous mice is lower at birth (Figure 5D) and throughout life (Figure 5E). Mutants also have a significantly reduced body length (Figures S4A and S4B). This is accompanied by a decrease in epididymal white adipose tissue (WAT) (Figure 5F). Mass ratios of other tissues, including liver, quadriceps, and kidney, are similar between genotypes (Table S4A). Adipocytes from FIH nullizygous mice are significantly smaller than those from control mice (Figures 5G–5I), demonstrating decreased adiposity.
To determine the effect of FIH deletion on glucose physiology, glucose and insulin tolerance tests (GTTs and ITTs) were carried out (Figures 5J and 5K). Loss of FIH had little effect on glucose clearance, but had a significant effect on insulin sensitivity (Figure 5K). Fasting blood glucose levels in mutants were lower (data not shown). Correlated with an enhanced insulin sensitivity was a significantly lower fed plasma insulin level in mutants (Figure 5L) and improved lipid homeostasis. Serum triglyceride (TG), high-density-lipoprotein (HDL) cholesterol, low-density-lipoprotein, very low-density-lipoprotein (LDL/VLDL) and total serum cholesterol levels were all significantly lower in mutant animals (Table S3).
Tissue-specific loss of FIH indicates that hypermetabolism is neuronally regulated under normal chow
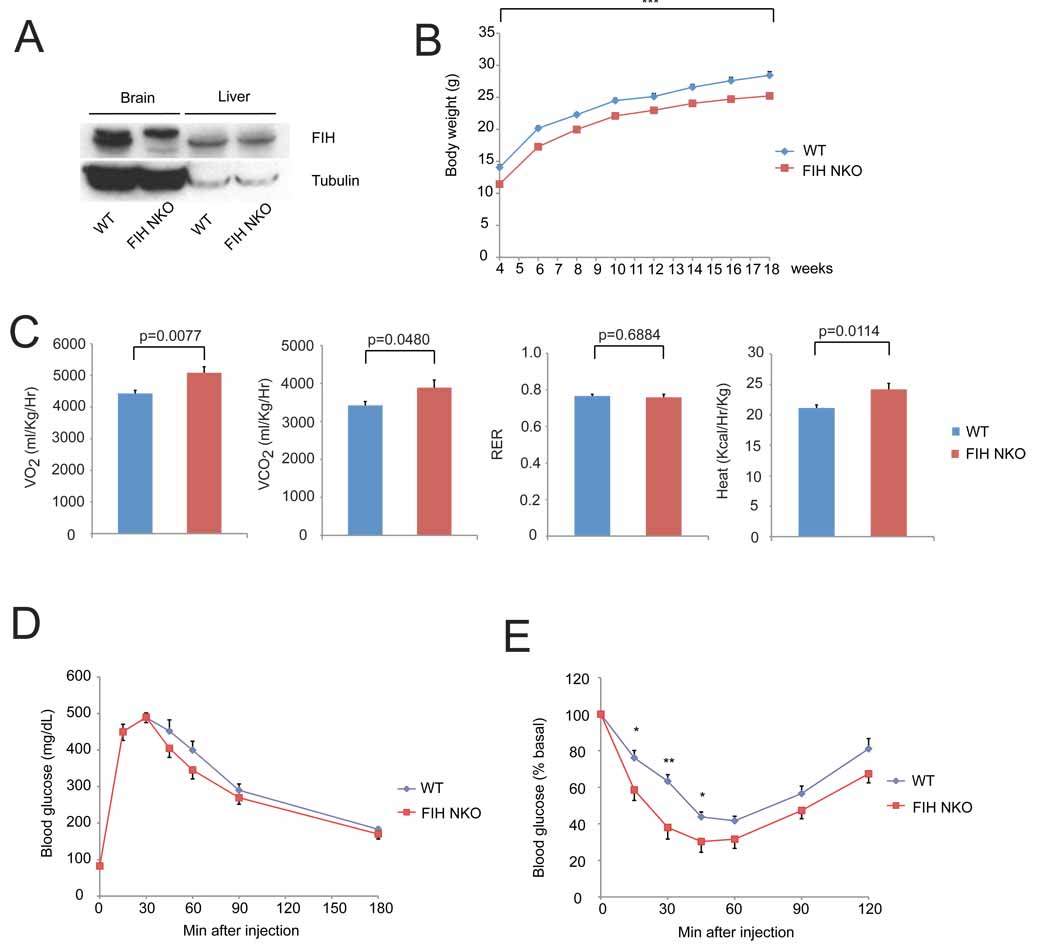
We next wished to determine whether the metabolic phenotypes described above could be ascribed to action of a single tissue, and began with the nervous system. We found that FIH is expressed at similar levels in different brain regions, i.e., cortex, hypothalamus and brain stem (Figure S5A). Animals were created with a pan-neuronal loss of FIH by crossing into a background of nestin promoter-driven cre expression. Specific deletion of FIH was confirmed by immunoblotting and qPCR (Figure 6A and Figure S5B). These tissue specific deletion mutants are significantly smaller than controls (Figure 6B) and phenocopy the increased metabolic rate seen in global FIH null animals (Figure 6C).
Figure 6. Neuron-specific loss of FIH indicates that hypermetabolism is neuronally regulated under normal chow.
- Immunoblot analysis of whole cell lysates of brain and liver samples from FIH neuron-specific knockout mice (FIH NKO) and WT mice using FIH and tubulin antibodies. The upper bands in brain samples are nonspecific.
- Growth curves of FIH NKO (n=17) and their WT (n=15) littermates under normal chow. ***p<0.001.
- Resting whole-body VO2, VCO2, RER and heat production in 2-month old WT (n=7) and FIH NKO (n=5) mice. Shown are the average value over 3 hr.
- GTTs were measured for 18hr-fasting 2-month old WT (n=6) and FIH NKO (n=6) mice (2g D-glucose/kg body weight).
-
ITTs were performed on 4hr-fasting 2-month old WT (n=7) and FIH NKO (n=6) mice (0.75 U/kg body weight). *p<0.05, **p<0.01.Values shown in graphs are expressed as mean ± SEM. p values are from Student’s t-test.See also Figure S5 and Figure S6.
To investigate whether loss of FIH in neurons affects glucose homeostasis, GTTs and ITTs were performed: neuronal FIH null mutants exhibited improved insulin sensitivity, although glucose tolerance was unchanged (Figures 6D and 6E). There was no significant difference in fasting blood glucose levels in 2-month old animals (Figure S5C), but fasting plasma insulin levels in mutants were lower (Figure S5D), which correlates with an enhanced insulin sensitivity.
In contrast, hepatic loss of FIH in an albumin-cre background had no effect on overall body weight and metabolic rate (Figures S6A, S6B and S6C); loss of FIH in liver did not affect glucose homeostasis (Figures S6D, S6E and S6F).
Expression of HIF-1α target genes, including Pgk, Vegf, Glut-1, Bnip3 and Car9 is not significantly induced in the brains of FIH nullizygous mice (Figure S5E), similar to results seen in other tissues examined in the mutants. This indicates that the neuronal FIH mutant phenotype is not directly related to a large and tissue-specific alteration in gene expression of HIF target genes in the central nervous system.
FIH KO mice are protected from high fat diet-induced weight gain and hepatic steatosis
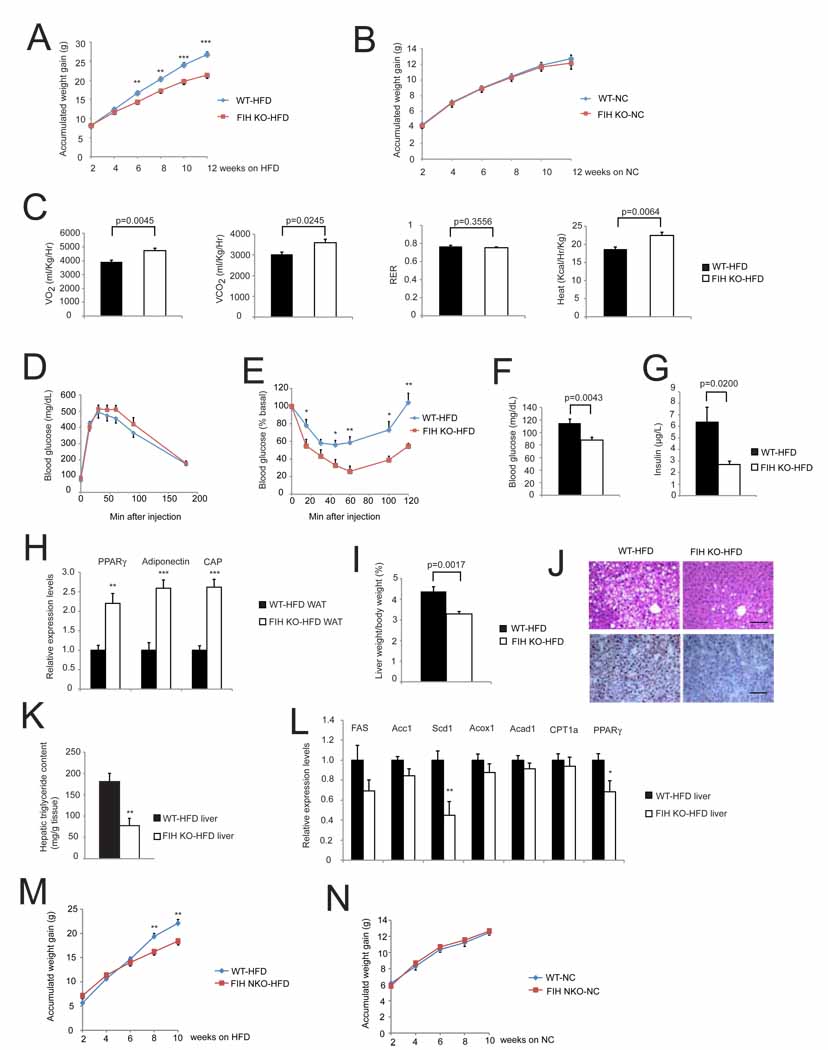
To determine whether global deletion of FIH influences the weight gain, insulin resistance and hepatic steatosis seen when mice are administered a high fat diet (HFD), we fed FIH null mice and wild type littermates for four months with a 60% fat diet. As can be seen in Figure 7A and Figure S7A, this caused weight gain in both wild type and mutant mice; however, mutants gained significantly less weight relative to wild type animals. This divergence is not seen over time when animals are fed normal chow (Figure 7B). These results demonstrate that there is a selective capacity for coping with high fat diet in these animals.
Figure 7. FIH KO mice are protected from high fat diet-induced weight again and hepatic steatosis.
- Accumulated weight gain of WT and KO mice fed with high fat diet (HFD) for 12-weeks. n=23 for WT mice. n=12 for FIH KO mice.**p<0.01. ***p<0.001.
- Accumulated weight gain of WT and KO mice fed with NC for 12-weeks. n=16 for WT mice. n=12 for FIH KO mice.
- Resting whole-body VO2, VCO2, RER and heat production in WT (n=6) and KO (n=8) fed with HFD for 12-weeks. Shown are the average value over 3 hr.
- GTTs were measured for 18hr-fasting WT (n=9) and KO (n=7) mice fed with HFD for 12-weeks (2g D-glucose/kg body weight).
- ITTs were performed on 4hr-fasting WT (n=7) and KO (n=5) mice fed with HFD for 12-weeks (1.0U/kg body weight). *p<0.05, **p<0.01.
- Fasting blood glucose levels were measured on 18 hr-fasting WT (n=20) and KO (n=16) mice fed with HFD for 12-weeks.
- Fed insulin levels were measured on WT and KO mice fed with HFD for 16-weeks. n=6/genotype.
- Expression levels of genes encoding PPARγ, Adiponectin and CAP were increased in epididymal WAT of KO mice fed with HFD. n=6/genotype. **p<0.01. ***p<0.001.
- Liver weight normalized with body weight from WT and KO mice fed with HFD. n=11–19/genotype.
- (Top) Representative images of H&E stained liver sections from WT and KO mice fed with HFD. (Bottom) Representative images of Oil Red O- stained liver sections. The scale bar represents 100µm.
- Hepatic triglyceride contents were measured in WT and FIH KO livers under HFD. n=6/genotype. **p<0.01.
-
Expression levels of genes encoding lipogenic enzymes (FAS, Acc1 and Scd1), those involved in β-oxidation (Acox1, Acad1 and CPT1a) and PPARγ in livers of WT and KO mice fed with HFD. n=6/genotype. *p<0.05. **p<0.01.WAT and liver samples used in (H) to (L) were from mice fed with HFD for 16-weeks.
- Accumulated weight gain of FIH neuronal KO (NKO) mice (n=6) and their WT littermates (n=11) fed with HFD for 10-weeks. **p<0.01.
-
Accumulated weight gain of FIH NKO (n=17) and their WT littermates (n=13) fed with NC for 10-weeks.Values shown in graphs are expressed as mean ± SEM. p values are from Student’s t-test.See also Figure S7, Table S3, and Table S4B.
We next measured individual tissue weights of liver, epididymal WAT, quadriceps, and kidney, and found that under a HFD, there were significant decreases in tissue/body mass ratios of liver and quadriceps (Table S4B). FIH global deletion mice also have higher metabolic rates under HFD (Figure 7C); this difference is similar to that seen with a normal diet. There was still no difference between WT and FIH nullizygous GTTs (Figure 7D and Figure S7C). ITTs of HFD animals demonstrate that the increase in insulin sensitivity evident in animals fed normal chow is maintained in mutants on a high fat diet (Figure 7E and Figure S7D). These were correlated with significantly lower levels of fasting plasma glucose (Figure 7F) and fed plasma insulin (Figure 7G) levels in mutants. In addition, although HFD increased serum cholesterol levels, FIH null mutants still showed significantly lower serum LDL/VLDL cholesterol levels (Table S3).
To examine gluconeogenic processes in the mutants, we measured expression levels of three genes involved in glucose homeostasis, glucokinase (GCK), phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase); these showed no significant changes in mRNA levels (Figure S7E). However, adiponectin, peroxisome proliferator-activated receptor γ (PPARγ, and c-Cbl-associated protein (CAP) mRNA levels in WAT were significantly induced in mutants (Figure 7H).
One of the other sequelae of a high fat diet is hepatic steatosis, or fatty liver. Overall liver/body mass ratio was significantly decreased in FIH nullizygous mice (Figure 7I, Figure S7B). As can be seen in Figure 7J, liver sections show loss of FIH causes a decrease in lipid droplets; quantification demonstrated that hepatic triglyceride content of FIH nullizygous mice was 60% lower (Figure 7K).
Reduced lipogenesis and increased β-oxidation are possible causes of reduced steatosis: we found no changes in mRNA levels of β-oxidation enzymes, but decreases in lipogenic enzymes (e.g., Scd1). Interestingly, we also detected significantly lower hepatic PPARγ expression in FIH nullizygous livers from high fat diet fed mice (Figure 7L); this is the opposite of the trend in PPARγ expression in WAT, and indicate that metabolic changes in PPARγ expression may help explain how FIH loss inhibits high fat diet-induced hepatic steatosis.
Finally, to examine whether a tissue-specific deletion of FIH in neurons also protects mutants from HFD-induced weight gain, we fed 4-week old FIH NKO mice and wild type littermates a HFD for 10 weeks. At 8-week and 10-week time points FIH NKO mice fed with HFD had gained significantly less weight (Figure 7M and 7N), consistent with a significant role for neuronal FIH expression in regulating HFD-induced weight gain.
DISCUSSION
Together, these data indicate that FIH, the asparaginyl hydroxylase, exerts a wide-ranging control over metabolism. This is surprising from many perspectives, particularly in reference to phenotypes of other mutations in the HIF pathway. Global and conditional mutations of genes regulating HIF are generally deleterious, causing embryonic lethality or significant changes in vascularization or erythropoiesis. The data presented here demonstrate that the two hydroxylase regulated HIF control pathways play very different roles in vivo.
The FIH enzyme also has a higher affinity for oxygen than that of the PHD enzymes (Koivunen et al., 2004; Peet et al., 2004). This may indicate that FIH could suppress HIF at different oxygen concentrations than those where the PHDs are most active. We find little evidence for this in vitro, where alterations in gene expression caused by FIH are for the most part small at both normoxia and hypoxia. It is clear from our observations, however, that some genes, e.g., Car9, are highly dependent on FIH control when VHL is absent; it is intriguing to speculate that further investigation of genes that show a requirement for both elements of hydroxylase control may unveil a functional relationship amongst such targets.
We have found that cellular metabolism is altered by deletion of FIH. Curiously, this alteration in metabolism, with an increase in intracellular ATP levels in MEFs, and a repression of AMP kinase activation in developing embryos, is not accompanied by the acidosis and increased glycolysis thought to typify increased HIF activation in animals. Analysis of whole body energy expenditure demonstrated that there is an acceleration of metabolic rate in FIH mutants, but there is no evidence of metabolic increases in glycolytic rate. Thus the altered metabolism of these mutants is not simply a change in HIF activation leading to increases in glycolysis and suppression of oxidative metabolism. UCP1 and PGC-1α expression in BAT were significantly elevated in FIH KO mice, indicating increased energy expenditure in FIH KO mice may be due to increased thermogenesis in BAT. Deletion of PHD1 in mice results in reduced O2 consumption by attenuating glucose oxidation in skeletal muscle; this was dependent on HIF function (Aragones et al., 2008; Aragones et al., 2009). As discussed above, different affinities of PHD1 and FIH for oxygen may represent a possible explanation for why they exhibit differential effects on energy expenditure, but further investigation is needed to understand this phenomenon.
Our data also connect the function of the FIH oxygen sensor with glucose and lipid metabolism in vivo. We have found that FIH null animals are smaller in size and weigh less when fed normal chow. They exhibit increased insulin sensitivity, which is correlated with significantly decreased plasma insulin levels. In contrast, there is no difference in glucose tolerance. FIH null mutants also show improved lipid homeostasis, with large reductions in serum triglyceride and cholesterol levels. Mice with tissue specific deletions of FIH in brain and liver demonstrate that deletion of FIH in neurons also leads to decreased body weight, increased energy expenditure and improved insulin sensitivity, and thus phenocopy global FIH null animals. Hepatic deletion animals did not have any of these metabolic phenotypes, arguing for a predominant role for FIH in the nervous system; clearly, further investigation of this is needed to isolate neuronal FIH pathways of metabolic control.
Deletion of FIH protects against high fat diet-induced weight gain and hepatic steatosis. The former may be explained by increased energy expenditure and improved insulin sensitivity in FIH global null animals. The latter is correlated with decreased expression of lipogenic genes in liver and increased adiponection expression in white adipose tissue. Interestingly, FIH neuronal null mice are also protected against HFD-induced weight gain. These findings implicate potential pharmaceutical application of FIH inhibitors, targeting insulin resistance and diet-induced obesity, although also indicate that these may need to be able to cross the blood/brain barrier.
The findings described here indicate a specific role for FIH in the process of respiratory control: maintenance of a set point for normoxic respiration. In the absence of FIH, tidal volumes at normoxia are equivalent to those seen in severely hypoxic wild type animals. This increased tidal volume is not constitutive: when breathing 30% oxygen, FIH mutant mice decrease tidal volumes to levels not significantly different from those of wild type mice. This indicates that loss of FIH has shifted oxygen-mediated control of respiration to a lower response point.
The regulation of chronic ventilatory adaptation has been shown to be a function of the HIF pathway in mice heterozygous for HIF-1α (Kline et al., 2002; Peng et al., 2006). Increased ventilation can be related to increases in metabolic rate; these would typically involve metabolically driven acidosis. In these mice, we see instead a respiratory alkalosis, which would indicate that some degree of hyperventilation is occurring. Hyperventilation could involve defective functioning of the carotid bodies, and the suppression of the hyperventilatory phenotype by hyperoxia in these mice could indicate an altered carotid body set point in the FIH null mice. The nestin gene, whose promoter drives cre recombinase expression in our neuronal FIH null mice, is expressed in carotid bodies (Izal-Azcárate et al., 2008). Thus preservation of the respiratory changes in neuronal FIH deletion could indicate that carotid body FIH is a key factor in regulating respiration. Further investigation is underway to determine this in isolated carotid bodies, with an ultimate goal of defining the mechanism of signaling controlled by FIH hydroxylation.
As discussed above, our results in global FIH null and neuron-specific FIH null animals reveal the role of this gene in regulation of metabolism. A key question is whether this is related to regulation of HIF function, or to altered activity of other putative FIH targets. Loss of VHL in hepatocytes results in a severe HIF-dependent steatosis, and deletion of HIF-1β in liver results in increased hepatic gluconeogenesis and lipogenic gene expression but reduced hepatic lipid storage (Rankin et al., 2009; Wang et al., 2009). These data indicate that HIF activation increases steatosis in the liver. However, we see reduction in steatosis following FIH deletion, which may indicate that the metabolic changes we are observing are not directly related to increased HIF activity.
Besides HIF, FIH has been shown to hydroxylate a range of other substrates in vitro; these include proteins containing ankyrin repeat domains (ARD), such as the intracellular domain of Notch receptors (Coleman et al., 2007; Zheng et al., 2008), p105, IkBα (Cockman et al., 2006), SOCS (suppression of cytokine signaling) box protein 4 (ASB4) (Ferguson et al., 2007), MYPT1 (Webb et al., 2009), Tankyrase-2, Rabankyrin-5 and RNase L (Cockman et al., 2009). This range of substrates has complicated the determination of direct FIH action, as the intersecting functional roles between these hydroxylation events are potentially large. As a further complication, one postulated role for the association of FIH with an ARD-domain-containing substrate has been that they act to sequester FIH from HIF, and thus potentiate HIF activation (Coleman and Ratcliffe, 2009; Wilkins et al., 2009). Our studies provide useful genetic mouse models for further investigating the physiological outcome of FIH hydroxylation on these substrates.
In conclusion, our data clearly show that the oxygen sensor FIH regulates respiration, energy balance, and lipid metabolism. They also demonstrate a novel role for neuronal FIH in regulation of body mass, energy expenditure and insulin sensitivity. Thus, FIH is a ready target for broad-spectrum pharmacological inhibition of hydroxylase activation, and in particular FIH-specific inhibitors (Banerji et al., 2005; Nagel, et al., 2010). Our data indicate that such inhibitors, especially those able to inhibit FIH function in neurons, might have significant therapeutic potential in reducing body weight and increasing insulin sensitivity.
EXPERIMENTAL PROCEDURES
See Supplemental Information for details.
HIGHLIGHTS
FIH is a non-redundant asparaginyl hydroxylase of HIF-1α.
FIH null mice exhibit elevated metabolic rate, increased insulin sensitivity and decreased adiposity.
Neuron-specific deletion of FIH gives rise to metabolic phenotypes identical to those found in global null animals.
Supplementary Material
ACKNOWLEDGEMENTS
We thank E. Kothari and Y.H. Zhu from UCSD transgenic core for assistance with gene targeting and chimera production. We thank K. Beck for helpful discussion on generation of knockout mice. We acknowledge L. Gapuz from UCSD histology core for histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures, four tables, Supplemental Experimental Procedures and Supplemental References.
We declare there are no conflicts of interest.
REFERENCES
- Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- Aragonés J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Banerji B, Conejo-Garcia A, McNeill LA, McDonough MA, Buck MR, Hewitson KS, Oldham NJ, Schofield CJ. The inhibition of factor inhibiting hypoxia-inducible factor (FIH) by beta-oxocarboxylic acids. Chem Commun (Camb) 2005:5438–5440. doi: 10.1039/b510707e. [DOI] [PubMed] [Google Scholar]
- Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega-Saenz P, Oster H, Wijeyekoon B, Sutherland AI, et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/−mice. Mol Cell Biol. 2008;28:3386–3400. doi: 10.1128/MCB.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, et al. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133:223–234. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML, Coles CH, Yu X, Hay RT, Ley SC, et al. Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH) Proc Natl Acad Sci U S A. 2006;103:14767–14772. doi: 10.1073/pnas.0606877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockman ME, Webb JD, Kramer HB, Kessler BM, Ratcliffe PJ. Proteomics-based identification of novel factor inhibiting hypoxia-inducible factor (FIH) substrates indicates widespread asparaginyl hydroxylation of ankyrin repeat domain-containing proteins. Mol Cell Proteomics. 2009;8:535–546. doi: 10.1074/mcp.M800340-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, McDonough MA, Hewitson KS, Coles C, Mecinovic J, Edelmann M, Cook KM, Cockman ME, Lancaster DE, Kessler BM, et al. Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor. J Biol Chem. 2007;282:24027–24038. doi: 10.1074/jbc.M704102200. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Ratcliffe PJ. Signalling Cross Talk of the HIF System: Involvement of the FIH Protein. Curr Pharm Des. 2009 doi: 10.2174/138161209789649448. [DOI] [PubMed] [Google Scholar]
- Ferguson JE, 3rd, Wu Y, Smith K, Charles P, Powers K, Wang H, Patterson C. ASB4 is a hydroxylation substrate of FIH and promotes vascular differentiation via an oxygen-dependent mechanism. Mol Cell Biol. 2007;27:6407–6419. doi: 10.1128/MCB.00511-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O'Connell PJ, Gonzalez FJ, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122:337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey KA, Low MJ, Kelly MA, Juarez R, Szewczak JM, Powell FL. Ventilatory responses to acute and chronic hypoxia in mice: effects of dopamine D(2) receptors. J Appl Physiol. 2000;89:1142–1150. doi: 10.1152/jappl.2000.89.3.1142. [DOI] [PubMed] [Google Scholar]
- Izal-Azcárate A, Belzunegui S, San Sebastián W, Garrido-Gil P, Vázquez-Claverie M, López B, Marcilla I, Luquin MA. Immunohistochemical characterization of the rat carotid body. Respir Physiol Neurobiol. 2008;161:95–99. doi: 10.1016/j.resp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci U S A. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem. 2004;279:9899–9904. doi: 10.1074/jbc.M312254200. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel S, Talbot NP, Mecinović J, Smith TG, Buchan AM, Schofield CJ. Therapeutic manipulation of the HIF hydroxylases. Antioxid Redox Signal. 2010;12:481–501. doi: 10.1089/ars.2009.2711. [DOI] [PubMed] [Google Scholar]
- Peet DJ, Lando D, Whelan DA, Whitelaw ML, Gorman JJ. Oxygen-dependent asparagine hydroxylation. Methods Enzymol. 2004;381:467–487. doi: 10.1016/S0076-6879(04)81031-0. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007 doi: 10.1126/stke.4072007cm8. cm8. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, Takeda H, Lee FS, Fong GH. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–3235. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Wang XL, Suzuki R, Lee K, Tran T, Gunton JE, Saha AK, Patti ME, Goldfine A, Ruderman NB, Gonzalez FJ, et al. Ablation of ARNT/HIF1beta in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009;9:428–439. doi: 10.1016/j.cmet.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JD, Muranyi A, Pugh CW, Ratcliffe PJ, Coleman ML. MYPT1, the targeting subunit of smooth-muscle myosin phosphatase, is a substrate for the asparaginyl hydroxylase factor inhibiting hypoxia-inducible factor (FIH) Biochem J. 2009;420:327–333. doi: 10.1042/BJ20081905. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- Wilkins SE, Hyvarinen J, Chicher J, Gorman JJ, Peet DJ, Bilton RL, Koivunen P. Differences in hydroxylation and binding of Notch and HIF-1alpha demonstrate substrate selectivity for factor inhibiting HIF-1 (FIH-1) Int J Biochem Cell Biol. 2009;41:1563–1571. doi: 10.1016/j.biocel.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Zheng X, Linke S, Dias JM, Gradin K, Wallis TP, Hamilton BR, Gustafsson M, Ruas JL, Wilkins S, Bilton RL, et al. Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc Natl Acad Sci U S A. 2008;105:3368–3373. doi: 10.1073/pnas.0711591105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.