Abstract
The Williams–Beuren syndrome (WBS) locus on human chromosome 7q11.23 is flanked by complex chromosome-specific low-copy repeats that mediate recurrent genomic rearrangements of the region. Common genomic rearrangements arise through unequal meiotic recombination and result in complex but distinct behavioural and cognitive phenotypes. Deletion of 7q11.23 results in WBS, which is characterised by mild to moderate intellectual disability or learning difficulties, with relative cognitive strengths in verbal short-term memory and in language and extreme weakness in visuospatial construction, as well as anxiety, attention-deficit hyperactivity disorder and overfriendliness. By contrast, duplication results in severely delayed speech and expressive language, with relative strength in visuospatial construction. Although deletion and duplication of the WBS region have very different effects, both cause forms of language impairment and suggest that dosage-sensitive genes within the region are important for the proper development of human speech and language. The spectrum and frequency of genomic rearrangements at 7q11.23 presents an exceptional opportunity to identify gene(s) directly involved in human speech and language development.
Although childhood disorders of cognition, language and behaviour are extremely common (WHO: http://www.who.int/en/), causative genes have remained particularly refractory to traditional genetic approaches since the disorders themselves are often sporadic in nature and their causes complex and both genetically and environmentally heterogeneous. Genomic disorders, caused by the gain, loss or inversion of specific chromosome regions, are often characterised by neurodevelopmental phenotypes and present a unique opportunity to identify genes and pathways that are necessary for proper brain development and function (Ref. 1).
One such region that is prone to genomic rearrangement is the Williams–Beuren syndrome (WBS) locus at chromosome 7q11.23. This 1.5 million base pair region has been shown to commonly undergo deletion, duplication or inversion through unequal meiotic recombination between highly similar flanking segments of DNA (Refs 2, 3, 4). Inversion of the region is not associated with any clinical features, but can predispose the chromosome to subsequent unequal recombination during the next round of meiosis. The inversion is estimated to be present at a frequency of 1 in 20, but there is a fivefold increase in carrier frequency in the parents of children with the WBS deletion (Refs 3, 5). Deletion and duplication of 7q11.23 result in distinct patterns of cognitive impairment, both of which impact on language and speech abilities, albeit in very different ways (Refs 4, 6, 7). The WBS deletion has an estimated frequency of between 1 in 7500 and 1 in 20 000 (Refs 8, 9). As a result of the mechanism of genomic rearrangement, duplication should occur at a similar frequency; however, owing to its very recent discovery, the population frequency has not yet been determined.
Genomic structure of 7q11.23
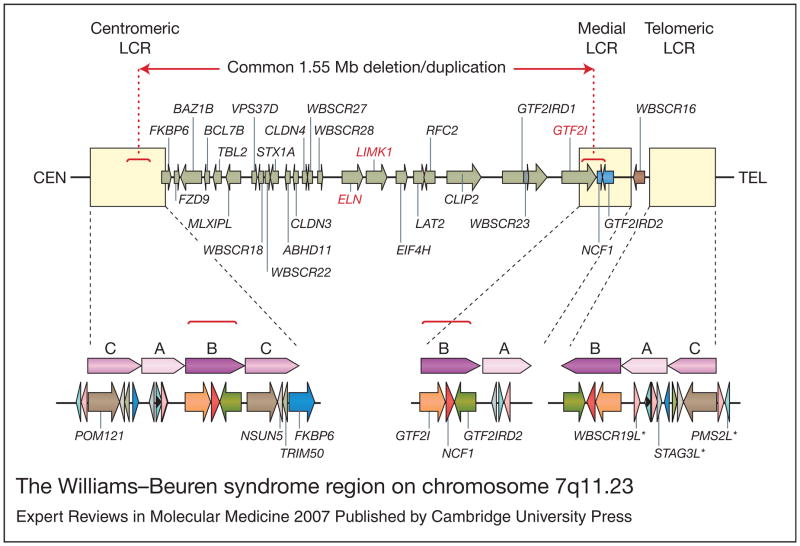
The WBS region lies on the long arm of chromosome 7, at 7q11.23 (Ref. 10). It is flanked by chromosome-specific, low-copy repeats (LCRs) that are thought to be directly responsible for the recurrent rearrangements (Ref. 2). Deletion of the region has been shown to occur as a result of unequal meiotic recombination between grandparental chromosomes in the transmitting parent (Refs 11, 12), and two-thirds are interchromosomal rearrangements (between the chromosome 7 homologues) while the remainder are intrachromosomal rearrangements (between sister chromatids of the same chromosome 7) (Ref. 13). It is predicted that this nonallelic homologous recombination is precipitated by the LCRs, which are made up of genes, pseudogenes and gene clusters that form highly homologous stretches of sequence (Refs 14, 15, 16). These regions have been broken down into blocks named A, B and C, which are present in the centromeric, medial and telomeric LCRs (Fig. 1). Each centromeric, medial and telomeric block can be distinguished by single nucleotide differences, but regions of greater than 99% sequence identity exist over distances of 100 kilobases or more, making detailed analysis of the LCRs extremely difficult (Ref. 5).
Figure 1. The Williams–Beuren syndrome region on chromosome 7q11.23.
Genes within the 1.55 Mb commonly deleted/duplicated region at 7q11.23 are represented by green arrows indicating the direction of transcription. ELN (elastin), LIMK (LIM domain kinase 1) and GTF2I (general transcription factor II, I) all of which have been linked to phenotypic aspects of Williams–Beuren syndrome, are highlighted in red. The two genes that are variably deleted/duplicated, depending upon the location of the telomeric breakpoint, are represented by blue arrows. WBSCR16, which lies outside the commonly deleted/duplicated region, is represented by a brown arrow. The flanking low-copy repeats (LCRs), which mediate meiotic rearrangement of the region, are represented by cream-coloured boxes. Each LCR is expanded beneath to show its composition of blocks of homology designated A, B and C. Approximately 95% of deletions occur between the highly similar B-blocks in the centromeric and medial LCRs, as indicated by the dotted red lines. Pseudogenes are indicated by an asterisk. For full versions of gene names see the HUGO Gene Nomenclature Committee website (http://www.gene.ucl.ac.uk/nomenclature/index.html). The figure was adapted from Ref. 113, with permission from Humana Press.
The common WBS deletion almost always occurs between the directly oriented B-blocks, although a small proportion of breakpoints reside in the A-blocks (Ref. 5). Both the high homology and high level of transcription from the LCRs are thought to contribute to the frequency of rearrangement in this region. The predominance of recombination between the B-blocks rather than the A-blocks is postulated to occur because of the higher sequence identity (99.6% versus 98.2%), more contiguous homology (there are two interstitial deletions of the medial A-block), and the shorter distance between the B-blocks (1.55 Mb versus 1.84 Mb). Duplication of the region has also recently been demonstrated, with the recombination breakpoints mapping to the same highly conserved B-blocks as those of the deletion (Ref. 4). As mentioned earlier, recombination can also occur between blocks in an inverted orientation with respect to each other, and this results in a common, nonpathogenic inversion of the WBS region (Refs 3, 5).
Deletion at 7q11.23
Clinical, cognitive and behavioural features of WBS
WBS is a multisystem disorder, resulting in physical, cognitive and behavioural features that together present a unique clinical picture. WBS is associated with a recognisable facies, and characteristic cardiovascular lesions – most frequently supravalvular aortic stenosis – are found in ~75% of patients. Other symptoms include hernias, visual impairment, hypersensitivity to sound, chronic otitis media, malocclusion, small or missing teeth, renal anomalies, constipation, vomiting, growth deficiency, infantile hypercalcaemia, musculoskeletal abnormalities, diabetes and a hoarse voice (Refs 17, 18, 19, 20).
Individuals with WBS usually have mild to moderate intellectual disability or learning difficulties. However, they characteristically exhibit distinct peaks and valleys of ability, with relative strengths in verbal short-term memory and in language, alongside extremely poor performance on visuospatial construction tasks (writing, drawing, pattern construction) (Ref. 21). On the Kaufman Brief Intelligence Test (KBIT; Ref. 22), the most commonly used IQ test in research studies of individuals with WBS, mean composite IQ is 69.32, with a range from 40 (lowest possible IQ on this measure) to 112 and a standard deviation of 15.36 (Ref. 20). This mean composite IQ value is approximately 2 standard deviations below that for the general population, but with the same amount of variability. The KBIT measures verbal ability and nonverbal reasoning ability but does not assess visuospatial construction. When a full-scale measure of intellectual ability that includes visuospatial construction is used, mean IQ is considerably lower. For example, on the Differential Ability Scales (DAS; Ref. 23), mean general conceptual ability (GCA; similar to IQ) is 58.29, with a range from 24 (lowest possible GCA) to 95 and a standard deviation of 12.77 (Ref. 20). However, for most children with WBS, GCA is not a valid indicator of intellectual ability because either their verbal standard score or their nonverbal reasoning standard score is significantly higher than expected given their GCA (Refs 20, 24).
The behavioural profile for WBS includes overfriendliness, empathy and anxiety (Ref. 25). Approximately 65% of children with WBS meet DSM-4 diagnostic criteria (Ref. 26) for attention-deficit hyperactivity disorder (ADHD); ~55% meet DSM-4 criteria for non-social specific phobia, and by early adolescence >20% meet DSM-4 criteria for generalised anxiety disorder, with a much higher proportion showing anticipatory worrying that is impairing but does not meet DSM-4 diagnostic criteria (Ref. 27). Hyperreactivity, sensory integration dysfunction, and multiple developmental motor disabilities affecting balance, strength, coordination and motor planning are also seen in WBS (Ref. 20).
Speech and language abilities in children with 7q11.23 deletion
Bellugi and her colleagues (e.g. Ref. 28) brought WBS to the attention of cognitive and language researchers with the argument that WBS was characterised by ‘intact’ language despite severe mental retardation. Thus, WBS was argued to provide strong evidence of cognitive modularity – in particular, the independence of language from other aspects of cognition. Nevertheless, most researchers who are currently studying WBS do not consider the language abilities of individuals with WBS to be independent of their other cognitive abilities (Refs 6, 29; for contrasting positions see Refs 28, 30, 31, 32, 33).
Early language
There has been very little research on speech ability in WBS. However, it is known that the onset of babbling is delayed (Ref. 34), perhaps due to delays in the onset of rhythmic banging, which Masataka has argued provides the motor substrate for canonical babble (strings of at least two syllables composed of a consonant and a vowel, such as baba and dadada), without which the production of words is largely impossible. Velleman and her colleagues found that at 18 months, children with WBS evidenced more immature babble patterns than typically developing children of the same age (Ref. 35). Early speech perception abilities are also delayed (Ref. 36), and Nazzi et al. have argued that difficulty segmenting words out of the ongoing speech stream likely plays a role in the language delay characteristic of WBS. Although there have been no studies of the articulation skills of school-age children with WBS, most children with this syndrome are easy to understand by the early school years, if not sooner.
Vocabulary
For most children with WBS, age of acquisition of an expressive vocabulary of 100 words is below the fifth percentile for typically developing children (Ref. 6, 37). At age 4 years, children with WBS who showed the nonlinear vocabulary growth pattern characteristic of typically developing children (slow early growth followed by considerably more rapid later growth) had considerably more advanced vocabulary, grammar, memory and nonverbal abilities than children who showed a slow linear pattern of growth (Ref. 6). For most older children and adults with WBS, receptive concrete vocabulary (understanding of words for objects, actions and descriptors) is their most advanced ability (Ref. 6). The same pattern holds for individuals with Down syndrome (Ref. 38), although consistent with the general finding that mean IQ is lower for Down syndrome than for WBS (Ref. 39), the level of receptive vocabulary is lower for individuals with Down syndrome than for individuals with WBS of the same chronological age (CA). The receptive conceptual/relational language (e.g. spatial, temporal, quantitative and dimensional terms) ability of individuals with WBS is considerably weaker than their receptive concrete vocabulary (Ref. 40) [closer to their level of visuospatial construction ability (Ref. 6)].
Grammar
By late childhood, most individuals with WBS speak in complete sentences that are typically grammatically correct, whereas CA- and IQ-matched individuals with Down syndrome rarely speak in complete sentences. This contrast was critical to Bellugi’s argument (Refs 28, 30, 32) that for individuals with WBS, language was independent of cognition. Bellugi’s finding that individuals with WBS use more complex grammar than CA- and IQ-matched individuals with Down syndrome has been replicated by several research groups (Refs 40, 41). However, when individuals with WBS are matched either to individuals with other aetiologies of intellectual disability for CA and IQ or to younger typically developing children for mental age, the grammatical abilities of the individuals with WBS are typically at the level of the contrast group [Refs 40, 42, 43, 44 (English); Ref. 45 (German); Ref. 46 (Hungarian); Refs 47, 48 (Italian)]. Thus, Bellugi’s original finding is currently interpreted as indicating the extreme difficulty that individuals with Down syndrome have with grammatical development, rather than that individuals with WBS have unusually good grammar relative to their overall intellectual abilities (Refs 6, 20, 49). Studies of morphological abilities, especially in languages that have more complex morphology than in English, have indicated that these abilities are at the same level or slightly lower than those of typically developing children matched for mental age [Ref. 50 (French); Ref. 51 (Hebrew); Ref. 46 (Hungarian)]. Grammatical ability is more closely linked to verbal working memory ability for children with WBS than for younger typically developing children matched for grammatical ability (Ref. 52).
Pragmatic/communicative aspects
Although children with WBS are highly approaching and overly friendly (Ref. 25), they have considerable difficulty with the pragmatic/communicative aspects of language. Toddlers with WBS are less likely to produce or comprehend referential gestures (e.g. points) or engage in triadic joint attention episodes (simultaneous attention to a communicative partner and an object or event) than are CA-and IQ-matched children with Down syndrome (Ref. 6) or younger typically developing children matched for mental age (Ref. 53). Although WBS is often argued, especially in the media, to be the clinical opposite of autism, the communicative difficulties identified in the studies of toddlers with WBS overlap those found in children who have autism. When preschoolers with WBS and limited language were assessed by the Autism Diagnostic Observation Schedule (ADOS; Ref. 54) Module 1, a semistructured measure designed to capture difficulties in sociocommunication for children with limited or no expressive language, more than half demonstrated significant difficulties with pointing, other gestures, giving, showing, and appropriate use of eye gaze (Ref. 55). Many also showed significant difficulties with joint attention and with the integration of gaze with other behaviours. Use of the Children’s Communication Checklist (CCC; Ref. 56) to evaluate the language and communicative abilities of a group of older children and young adults with WBS showed that the majority met the criterion for pragmatic language impairment (Ref. 57).
Summary of language abilities in children with 7q11.23 deletion
In summary, although the language abilities of individuals with WBS are considerably more advanced than those of individuals with Down syndrome, they are not more advanced than expected for overall level of intellectual ability. Verbal working memory ability is more strongly linked to grammatical ability for children with WBS than for typically developing children. Receptive concrete vocabulary is the strongest area of language ability, and receptive conceptual/relational language is the weakest area. Both are strongly related to other aspects of intellectual ability (Refs 20, 58). Although articulation abilities have not been explicitly studied for children with WBS, by the early school years the speech of most children with WBS is easy to understand. Individuals with WBS have significant pragmatic difficulties not only as very young children but also as adolescents and adults, even though by then their expressive language is typically grammatically correct and they have good concrete vocabularies.
Genotype–phenotype correlation
People with WBS
Unequal meiotic recombination between flanking repeats at 7q11.23 results in the hemizygous deletion of the same chromosome interval in almost all people with WBS. This interval spans at least 26 genes, although it is unlikely that all are contributing to the WBS phenotype, since not all genes are dosage-sensitive. Elastin is known to be the culprit for symptoms affecting elastic tissues, primarily causing cardiovascular stenoses and likely contributing to hypertension, diverticuli and the hoarse voice (Ref. 59). Point mutations of the elastin gene (ELN) have been identified in numerous cases with supravalvular aortic stenosis, but none of the additional, characteristic WBS features was present in these individuals (Refs 60, 61, 62). Further insight into which genes might be most important in the genetic basis of WBS has come from the study of a handful of individuals with smaller than usual deletions of 7q11.23. Individuals who have short deletions that include the common telomeric breakpoint appear to have classic WBS (Refs 63, 64), whereas many others exhibit only a few features of WBS and have a variety of smaller deletions, all including ELN (Refs 65, 66, 67, 68, 69, 70, 71, 72, 73). From these partial deletion cases, researchers have argued that LIMK1 (encoding LIM domain kinase 1) is involved in the visuospatial construction difficulties associated with WBS (Refs 65, 69; but see Ref. 66), that CLIP2 (encoding CAP-GLY domain containing linker protein 2) hemizygosity contributes to problems with motor coordination (Ref. 73), that GTF2IRD1 (encoding GTF2I repeat domain containing 1) is important for proper craniofacial development (Ref. 70) and that GTF2I (encoding general transcription factor II, I) (at the telomeric end of the deletion) is involved in the general intellectual disability/mental retardation (Ref. 69) and/or visuospatial construction difficulties (Refs 67, 68, 70, 74) associated with WBS.
Mouse models of WBS
In an attempt to understand more about the function of each of the genes from the WBS commonly deleted region, investigators have utilised mouse models. Several knock-out models have been generated and characterised, and some have semi-dominant phenotypes that suggest the gene may be haploinsufficient in WBS (Refs 75, 76, 77). Mice heterozygous for Clip2, for instance, show impairment in motor tasks, hippocampal dysfunction, and mild growth deficiencies, all of which are also seen in WBS (Refs 24, 73, 76). Studies of mice, although helpful, raise important questions about the parallels between humans and model organisms. Genes that are haploinsufficient in humans might not be so in mice, as a result of functional redundancy or the presence of alternative biological pathways. In addition, studies of characteristics such as language, emotion and behaviour can be extremely difficult in organisms other than humans, for obvious reasons.
Brain-imaging studies of individuals with WBS
Structural magnetic resonance imaging (MRI) studies, comparing adults with WBS who have normal intelligence to a group of CA- and IQ-matched adults in the general population, have indicated three regions in which there is reduced grey matter in WBS: in the intraparietal sulcus, the orbitofrontal cortex, and the region around the third ventricle (Ref. 78). The same reduced grey matter region has been identified in the intraparietal sulcus in children with WBS (Ref. 79). Reductions in intraparietal sulcus depth have been identified in individuals with WBS who have normal IQ (Ref. 80) as well as in individuals with WBS who have intellectual disability (Ref. 81). Path analyses using data from functional MRI studies of individuals with WBS and normal IQ, and an IQ-matched contrast group of individuals in the general population, have implicated the intraparietal sulcus in the visuospatial construction difficulties characteristic of individuals with WBS (Ref. 78), and have linked the reduced grey matter region in the orbitofrontal cortex with the hypersociability and non-social specific phobia characteristic of WBS (Ref. 82). Meyer-Lindenberg et al. (Ref. 78) hypothesised that the reduced grey matter region around the third ventricle may be associated with the hormonal disturbances characteristic of WBS (Refs 19, 83, 84). Multimodal imaging studies of the hippocampal formation indicated that although any structural changes were minimal, functional changes were significant. Positron emission tomography (PET) imaging indicated that baseline neurofunctional status was profoundly reduced. Proton magnetic resonance spectroscopy also indicated a reduced ratio of N-acetyl aspartate to creatine, indicating overall reduction in hippocampal energy metabolism and synaptic activity (Ref. 85). For a review of neuroimaging studies in WBS, see Ref. 24.
Duplication of 7q11.23
Although duplication of the WBS region had been predicted to occur at a similar frequency to deletion (based on the mechanism of rearrangement), duplications of this region were identified only recently. Hypotheses for the apparent lack of duplications were lethality, an absence of phenotypic consequence, or a clinical presentation that was unlike WBS, making screening of an appropriate patient population impossible. It turned out that the third hypothesis was correct, and that duplication of 7q11.23 results in a nonoverlapping clinical phenotype, with severe speech and expressive-language delay being the most prominent feature.
An initial paper describing the discovery of an 8-year-old boy with exact duplication of the WBS region reported that the most striking aspect of the phenotype was the severe delay in speech and expressive language, an area that is relatively spared in individuals with WBS compared with overall intellectual abilities (Ref. 4). The boy was able to correctly pronounce only a very small number of words, but scored at a much higher ability level on receptive vocabulary and on nonlanguage tasks.
Individuals with larger chromosome 7 duplications that include 7q11.23 also present with speech and language impairment, along with mild developmental delay (Refs 72, 86, 87, 88, 89, 90, 91). A review of these reported patients also suggests the presence of a subtle but recognisable facial phenotype, consisting of a high broad nose, posteriorly rotated ears, high arched palate and short philtrum. Since the initial report, eight additional children with a reciprocal duplication of the WBS region have been reported (Table 1; individuals with supernumerary ring chromosome 7 that includes the WBS locus are also described). Although only scant information concerning their phenotypic presentation is available for six of these cases, all have been diagnosed with speech delay (Refs 91, 92, 93, 94, 95, 96, 97). These duplication cases were identified during screens for copy number changes in regions of the genome known to undergo frequent rearrangements. The children were all part of cohorts with developmental delay, except one who was identified in a cohort with autism spectrum disorder. Developmental delay has been seen in each of the identified individuals with 7q11.23 duplication, suggesting it is likely part of the phenotype associated with this chromosome rearrangement.
Table 1.
Phenotypic characteristics of 7q11.23 duplication, showing common feature of expressive-language and speech delay
| Duplication | Language | IQ | Other | Ref. |
|---|---|---|---|---|
| Supernumerary ring 7a | ||||
| Mother | Poor articulationb | Low performancec | Mosaicism; facial dysmorphism | 86 |
| Daughter | Severe speech and language delay | Performance 73 Verbal 57 |
Mosaicism; facial dysmorphism | |
| Son | Severe speech and language delay | Mild DD | Mosaicism; facial dysmorphism | |
| Supernumerary ring 7a | Severe speech and expressive-language delay | 69 | Nonmosaicism; abnormalities of teeth, genitals and limbs | 87 |
| Supernumerary ring 7a | Expressive-language delayd | NA | Mosaicism; moderate hearing impairment; facial dysmorphism | 88 |
| Supernumerary ring 7a | Speech and expressive-language delay | 80 | Mosaicism; ADHD; facial dysmorphism | 88 |
| Supernumerary ring 7a | Severe speech and expressive-language delay | Average nonverbal IQe | Mosaicism | 89 |
| Supernumerary ring 7a | Speech delayf | DD | Mosaicism; CHD; facial dysmorphism; long digits | 90 |
| Supernumerary ring 7 | Severe language delayd | NA | Mosaicism; 47,XXY | 91 |
| >10 Mb duplication | ||||
| Sibling 1 | Significant language delayd | NA | Mosaicism; facial dysmorphism | 91 |
| Sibling 2 | Significant language delayd | NA | Mosaicism; facial dysmorphism | |
| >1.5 Mb duplication | Speech dyspraxiaf | NA | Craniofacial anomalies | 72 |
| 1.5 Mb duplication | Severe speech and expressive-language delay | Mild DD | Facial dysmorphism; ADHD | 4 |
| 1.5 Mb duplication | Severe speech delayb | Severe LD | Facial dysmorphism | 95 |
| 1.5 Mb duplication | Moderate language delayd | NA | 94 | |
| 1.5 Mb duplication | Severe speech and language delay | 53 | Cortical dysplasia of temporal lobe; facial dysmorphism | 96 |
| 1.5 Mb duplication | Speech delayb | NA | 92 | |
| 1.5 Mb duplication | Severe speech delayb | NA | Aicardi syndrome (X-linked); schizencephaly; polymicrogyria | 93 |
| 1.5 Mb duplication | Prominent language delayd | NA | 91 | |
| 1.5 Mb duplication | Prominent language delayd | NA | 91 | |
| 1.5 Mb duplication | Severe speech and language delay | Severe mental retardation | Autistism spectrum disorder; mild facial dysmorphism; ADHD; hyperphagia; paroxysmic episodes | 97 |
All cases of supernumerary ring chromosome 7 reported in this table have confirmed duplication of the WBS region.
No information on language ability reported.
Based on observation rather than formal assessment. No verbal or overall IQ reported.
No information reported re speech acquisition.
No verbal or overall IQ reported.
Too young to evaluate for language delay.
Abbreviations: ADHD, attention-deficit hyperactivity disorder; CHD, congenital heart disease; DD, developmental delay; LD, learning disability; NA, not available.
Speech and language abilities in children with 7q11.23 duplication
The five children with 7q11.23 duplication for whom published data are available [Refs 4, 94, 95, 96, 97; four other cases have been reported in conference proceedings (Refs 91, 92, 93)], have been characterised as having developmental delay, with greater delay in speech and expressive language than in general intellectual abilities. More detailed information was reported for three of the children. At age 8 years 10 months, the child reported by Somerville et al. (Ref. 4) was able to pronounce only a few words correctly. He communicated by a combination of vocalisations (including a number of words that family members were able to understand even though they were not clear to people who were not familiar with his speech), gestures, pantomime, manual signs, and drawing. Unlike children his age who have WBS, this child rarely produced word combinations. His performance on tests of expressive language was at floor (lowest possible standard score), although he performed in the low average range on tests of receptive vocabulary. His drawing ability was considerably stronger than that of children his age with WBS. He had good social interaction skills. Both he and his sister had ADHD; his parents were also reported to have had ADHD as children.
The child studied by Torniero et al. (Ref. 96) was identified with severe speech and language delay at age 4 years. At age 8 years, she was able to pronounce bisyllabic words but showed some consistent phonological substitutions. Testing indicated that she did not have orofacial apraxia. At age 12 years, she pronounced bisyllabic words correctly but only rarely produced multiword combinations. Overall intellectual functioning was in the range of moderate intellectual disability, with drawing ability stronger than verbal ability. Receptive vocabulary was in the range of mild disability. Spatial memory ability was in the low average range; in contrast, verbal memory ability was in the range of moderate to severe disability. She had good social interaction skills and did not have ADHD.
The child studied by Depienne et al. (Ref. 97) was found to have 7q11.23 duplication based on a screening for 7q11 rearrangements conducted on 206 individuals with autism spectrum disorder. This child was considerably more disabled than the previously identified individuals. He had severe intellectual disability, ADHD, severe speech delay, and talked in single words that were unintelligible out of context, although the language he did have was usually used appropriately. Although the speech and language characteristics of this child were similar to those of other individuals with 7q11.23 duplication, he had more severe intellectual disability, sudden outbursts of aggression (which were being treated with thioridazine), and hyperphagia, and had been diagnosed with autism – all characteristics that had not been reported previously for individuals with 7q11.23 duplication. This pattern suggests that this child may also have an additional genetic disorder.
MRI, electroencephalogram (EEG) and audiometry findings are available for all three children. The child studied by Torniero et al. (Ref. 96) had a normal EEG at age 4 years. However, at age 12 years she began having partial seizures, which were controlled by medication within a few months. At 13 years, structural MRI revealed cortical dysplasia of the left temporal lobe. Audiometry revealed normal hearing. The child reported by Depienne et al. had an MRI that showed mild dilatation of the left temporal horn and a small arachnoid cyst in the temporal fossa (Ref. 97). EEG recordings were inconclusive, with unstructured rhythms but no synchronised activity. Audiometry indicated normal hearing at 18 months of age. The child studied by Somerville et al. had a structural MRI at age 6 years and an EEG at age 7 years; no significant abnormalities were found on either (Ref. 4; supplementary appendix). Results of behavioural audiometry and otoacoustic emmisions indicated normal hearing (Ref. 4). Neuroimaging studies of additional children with duplications of the WBS region are needed to determine if the findings of Torniero et al. (Ref. 96) and/or Depienne et al. (Ref. 97) are typical for individuals with 7q11.23 duplication.
Implications for understanding speech and language development
Developmental speech/language impairments are estimated to affect 3–10% of children and have been shown to be highly heritable (Ref. 98). A study of 2-year-olds found a heritability of 0.73 (Ref. 99) and twin studies have reported monozygotic concordance of 70% and dizygotic concordance of 45% (Refs 100, 101, 102) (for a single gene disorder with complete penetrance, monozygotic concordance would be 1.0 and dizygotic concordance 0.5). The underlying genetic bases for the majority of cases of speech/language impairments have been postulated to be complex, involving several loci that interact with each other and the environment to produce an overall susceptibility (Ref. 103). Linkage analysis has identified several possible contributing loci (Refs 104, 105), but so far only a single gene –the transcription factor FOXP2 on chromosome 7q31 – has been implicated in the aetiology of developmental speech/language impairments, and only in a few cases (Refs 106, 107, 108, 109, 110, 111). Disruption of FOXP2 results in reduced functional dosage and produces deficits in both expressive and receptive language in addition to orofacial dyspraxia that impairs the coordination of complex fine motor movements of the lower face for speech and nonspeech purposes (Ref. 106).
The recent identification of individuals with an exact duplication of the WBS region and severe speech and language delay defines 7q11.23 as a new locus for expressive language disorder and speech impairment (Refs 4, 94, 95, 96, 97). Although the common duplication interval spans at least 26 genes, it might be predicted that, as is the case with deletions of the region in WBS, only one or a few of these genes are playing a prominent role in the language-impairment phenotype. The prospect of evaluating two dozen genes, rather than the 40 000 or so in the entire human genome is certainly appealing. In addition, we might also expect to identify individuals with smaller duplications of the region that will enable genotype–phenotype correlation and narrow the critical interval even further. Since research into 7q11.23 duplication is in its infancy, there are no clues as to which gene(s) are likely to be implicated at this time. Analysis of gene expression in lymphocytes from one child with a 7q11.23 duplication revealed increased expression of all but one gene from the duplicated interval (Ref. 4).
Finding the gene(s) responsible for this expressive-language and speech impairment will shed new light on the molecular genetics of speech and language and on the physiological basis of expressive language. Although the children with 7q11.23 duplication identified so far have not undergone detailed orofacial movement and oral praxis testing, they do not appear to show any obvious problem with motor coordination of the lower face muscles or tongue, although mild gross motor coordination difficulties were reported in some. This is in contrast to most patients with disruption of FOXP2, who have problems with sneezing, throat clearing and coughing and are sometimes unable to blow their noses (Refs 106, 108, 110). Other reported oromotor problems arising from FOXP2 disruption include difficulties with lip protrusion, tongue elevation and lateralisation, and rapid alternating movements, as well as difficulties with chewing, gagging and swallowing in their early years (Refs 108, 110). The underlying basis for expressive-language impairment in children with 7q11.23 duplication is, therefore, potentially unrelated to that in children with FOXP2 disruption.
The study of WBS is unlikely to be as fruitful as the study of 7q11.23 duplication syndrome for advancing our understanding of the genetics of language impairment. Although the language abilities of most children with WBS are lower than would be expected for their CA, these abilities are typically at or slightly above the level that would be expected for their overall intellectual ability (Refs 6, 20). Individuals with shorter deletions that do not include GTF2I typically have stronger language abilities than individuals with classic WBS deletions. However, the overall intellectual ability of individuals with shorter deletions that do not include GTF2I is also considerably higher than that of individuals with classic WBS deletions; performance is higher not only for language but also for nonverbal reasoning and for visuospatial construction, suggesting that the stronger language abilities are probably related to the higher level of general intellectual ability rather than reflecting an independent improvement in language (Refs 69, 73).
Research in progress and outstanding research questions
Although the frequency of 7q11.23 duplication is estimated at between 1 in 7500 and 1 in 20 000 live births based on the frequency of 7q11.23 deletion, the actual number of children carrying this genomic rearrangement remains unknown. Furthermore, the usual or ‘classic’ clinical presentation has not yet been established, because of the small number of individuals identified so far. It is not known whether a severe delay in speech and expressive language is always associated with 7q11.23 duplication, or whether some level of developmental delay is always present. The answers to these phenotypic questions will not become available until a much larger number of children with the duplication have undergone detailed developmental assessment. Since children with 7q11.23 duplications have received a primary diagnosis of speech and expressive-language delay, and of developmental delay, individuals with either of these diagnoses are the focus of duplication-screening efforts.
To dissect the molecular basis for language impairment in children with 7q11.23 duplication, it will probably be necessary not only to use genotype–phenotype comparisons in humans but also to utilise animal models. Since we would predict that it is the overexpression of a particular gene(s) that is contributing to the speech and expressive language delay, increasing the dosage of candidate genes in the mouse may help uncover the precise genetic basis. This can be done either by duplicating the same set of genes, which conveniently lie together in the same order on mouse chromosome 5, or by adding individual genes one at a time. It may seem strange to consider modelling a speech disorder in an animal, but such models present attractive tools for understanding the neurogenetic and physiological mechanisms by which developmental speech/language impairments arise. They also provide access to vital information about the temporal and spatial expression of genes and allow us to study the development of neural networks both in the normal state and in genetically manipulated models of the human condition.
The Foxp2 gene has been disrupted in a mouse model of developmental dyspraxia, with fascinating results. Neonatal pups homozygous for the Foxp2 disruption showed a complete absence of ultrasonic vocalisation on being separated from their mother, along with severe motor impairment and premature death, while pups heterozygous for the disruption (as is the case in humans with FOXP2 disruption) showed significantly reduced vocalisation (Ref. 112). Clues to the origin of the phenotype in these mice included cerebellar abnormalities such as a disorganised Purkinje cell layer and abnormal migration of granule-cell progenitors.
By combining studies in humans and animal models, it will be possible to identify the genetic basis for speech and expressive-language delay that resides at 7q11.23, and to understand more about the physiological and neurological processes that mould speech and language development. Should 7q11.23 duplication prove to contribute significantly to the aetiology of speech and expressive-language delay, testing for this genomic arrangement in children with a characteristic phenotype can be easily integrated into the existing molecular genetics screening panel, allowing for earlier diagnosis and implementation of intensive speech and language therapy.
Further reading, resources and contacts.
Publications
- Somerville MJ, et al. Severe expressive-language delay related to duplication of the Williams-Beuren locus. N Engl J Med. 2005;353:1694–1701. doi: 10.1056/NEJMoa051962. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper was the first description of an individual with duplication of 7q11.23.
- Fisher SE. On genes, speech, and language. N Engl J Med. 2005;353:1655–1657. doi: 10.1056/NEJMp058207. [DOI] [PubMed] [Google Scholar]; This is an informative editorial discussing the genetic basis of speech and language in relation to chromosome 7.
Website
- A database collating clinical information about chromosomal microdeletions/duplications/insertions, translocations and inversion: http://www.sanger.ac.uk/PostGenomics/decipher/
Acknowledgments
The authors’ research is supported by grants from the Canadian Institutes of Health Research (L.R.O), Sick Kids Foundation (L.R.O), Genome Canada/Ontario Genomics Institute (L.R.O), the National Institute of Neurological Disorders and Stroke (R01 NS35102 – C.B.M) and the National Institute of Child Health and Development (R37 HD29957 – C.B.M).
References
- 1.Inoue K, Lupski JR. Molecular mechanisms for genomic disorders. Annu Rev Genomics Hum Genet. 2002;3:199–242. doi: 10.1146/annurev.genom.3.032802.120023. [DOI] [PubMed] [Google Scholar]
- 2.Valero MC, et al. Fine-scale comparative mapping of the human 7q11.23 region and the orthologous region on mouse chromosome 5G: the low-copy repeats that flank the Williams-Beuren syndrome deletion arose at breakpoint sites of an evolutionary inversion(s) Genomics. 2000;69:1–13. doi: 10.1006/geno.2000.6312. [DOI] [PubMed] [Google Scholar]
- 3.Osborne LR, et al. A 1.5 million-base pair inversion polymorphism in families with Williams-Beuren syndrome. Nat Genet. 2001;29:321–325. doi: 10.1038/ng753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somerville MJ, et al. Severe expressive-language delay related to duplication of the Williams-Beuren locus. N Engl J Med. 2005;353:1694–1701. doi: 10.1056/NEJMoa051962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayes M, et al. Mutational mechanisms of Williams-Beuren syndrome deletions. Am J Hum Genet. 2003;73:131–151. doi: 10.1086/376565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mervis CB, Becerra A. Language and communicative development in Williams syndrome. Ment Retard Dev Disabil Res Rev. 2007;13:3–15. doi: 10.1002/mrdd.20140. [DOI] [PubMed] [Google Scholar]
- 7.Mervis CB, Klein-Tasman BP. Williams syndrome: cognition, personality, and adaptive behavior. Ment Retard Dev Disabil Res Rev. 2000;6:148–158. doi: 10.1002/1098-2779(2000)6:2<148::AID-MRDD10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg F. Williams syndrome professional symposium. Am J Med Genet Suppl. 1990;6:85–88. doi: 10.1002/ajmg.1320370617. [DOI] [PubMed] [Google Scholar]
- 9.Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002;17:269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- 10.Ewart AK, et al. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet. 1993;5:11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- 11.Dutly F, Schinzel A. Unequal interchromosomal rearrangements may result in elastin gene deletions causing the Williams-Beuren syndrome. Hum Mol Genet. 1996;5:1893–1898. doi: 10.1093/hmg/5.12.1893. [DOI] [PubMed] [Google Scholar]
- 12.Urban Z, et al. 7q11.23 deletions in Williams syndrome arise as a consequence of unequal meiotic crossover. Am J Hum Genet. 1996;59:958–962. [PMC free article] [PubMed] [Google Scholar]
- 13.Baumer A, et al. High level of unequal meiotic crossovers at the origin of the 22q11.2 and 7q11.23 deletions. Hum Mol Genet. 1998;7:887–894. doi: 10.1093/hmg/7.5.887. [DOI] [PubMed] [Google Scholar]
- 14.Osborne LR, et al. PMS2-related genes flank the rearrangement breakpoints associated with Williams syndrome and other diseases on human chromosome 7. Genomics. 1997;45:402–406. doi: 10.1006/geno.1997.4923. [DOI] [PubMed] [Google Scholar]
- 15.Perez Jurado LA, et al. A duplicated gene in the breakpoint regions of the 7q11.23 Williams-Beuren syndrome deletion encodes the initiator binding protein TFII-I and BAP-135, a phosphorylation target of BTK. Hum Mol Genet. 1998;7:325–334. doi: 10.1093/hmg/7.3.325. [DOI] [PubMed] [Google Scholar]
- 16.DeSilva U, et al. Molecular characterization of the mouse p47-phox (Ncf1) gene and comparative analysis of the mouse p47-phox (Ncf1) gene to the human NCF1 gene. Mol Cell Biol Res Commun. 2000;3:224–230. doi: 10.1006/mcbr.2000.0214. [DOI] [PubMed] [Google Scholar]
- 17.Pober BR, Dykens EM. Williams syndrome: an overview of medical, cognitive, and behavioral features. Child Adolesc Psych Clinics N Am. 1996;5:929–943. [Google Scholar]
- 18.Morris CA, et al. Natural history of Williams syndrome: physical characteristics. J Pediatr. 1988;113:318–326. doi: 10.1016/s0022-3476(88)80272-5. [DOI] [PubMed] [Google Scholar]
- 19.Morris CA. The dysmorphology, genetics, and natural history of Williams-Beuren syndrome. In: Morris CA, Lenhoff H, Wang P, editors. Williams-Beuren Syndrome: Research, Evaluation, and Treatment. Johns Hopkins University Press; Baltimore, MD: 2006. pp. 3–17. [Google Scholar]
- 20.Mervis CB, Morris CA. Williams syndrome. In: Mazzocco MMM, Ross J, editors. Neurogenetic Developmental Disorders: Variation of Manifestation in Childhood. MIT Press; Cambridge, MA: 2007. pp. 199–262. [Google Scholar]
- 21.Mervis CB, et al. The Williams syndrome cognitive profile. Brain Cogn. 2000;44:604–628. doi: 10.1006/brcg.2000.1232. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman A, Kaufman N. Kaufman Brief Intelligence Test. American Guidance Services; Circle Pines, MN: 1990. [Google Scholar]
- 23.Elliott C. Differential Ability Scales. Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- 24.Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci. 2006;7:380–393. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- 25.Klein-Tasman BP, Mervis CB. Distinctive personality characteristics of 8-, 9-, and 10-year-olds with Williams syndrome. Dev Neuropsychol. 2003;23:269–290. doi: 10.1080/87565641.2003.9651895. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 27.Leyfer OT, et al. Prevalence of psychiatric disorders in 4 to 16-year-olds with Williams syndrome. Am J Med Genet B Neuropsychiatr Genet. 2006;141:615–622. doi: 10.1002/ajmg.b.30344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellugi U, et al. Dissociation between language and cognitive functions in Williams syndrome. In: Bishop D, Mogford K, editors. Language Development in Exceptional Circumstances. Churchill Livingstone; London: 1988. pp. 177–189. [Google Scholar]
- 29.Mervis CB. Williams syndrome: 15 years of psychological research. Dev Neuropsychol. 2003;23:1–12. doi: 10.1080/87565641.2003.9651884. [DOI] [PubMed] [Google Scholar]
- 30.Bellugi U, Wang P, Jernigan T. Williams syndrome: an unusual neuropsychological profile. In: Broman S, Grafman J, editors. Atypical Cognitive Deficits in Developmental Disorders: Implications for Brain Function. Erlbaum; Hillsdale, NJ: 1994. pp. 23–56. [Google Scholar]
- 31.Clahsen H, Almazan M. Syntax and morphology in Williams syndrome. Cognition. 1998;68:167–198. doi: 10.1016/s0010-0277(98)00049-3. [DOI] [PubMed] [Google Scholar]
- 32.Bellugi U, et al. I. The neurocognitive profile of Williams Syndrome: a complex pattern of strengths and weaknesses. J Cogn Neurosci. 2000;12(Suppl 1):7–29. doi: 10.1162/089892900561959. [DOI] [PubMed] [Google Scholar]
- 33.Clahsen H, Ring M, Temple C. Lexical and morphological skills in English-speaking children with Williams syndrome. Essex Research Reports in Linguistics. 2003;43:1–27. [Google Scholar]
- 34.Masataka N. Why early linguistic milestones are delayed in children with Williams syndrome: late onset of hand banging as a possible rate-limiting constraint on the emergence of canonical babbling. Developmental Science. 2001;4:158–164. [Google Scholar]
- 35.Velleman S, et al. In: Horga D, Mildner V, editors. Phonological development in Williams syndrome; Proceedings of the International Clinical Phonetics and Linguistics Association; 31 May– 3 June 2006; Dubrovnik, Croatia. Zagreb, Croatia: FF Press; 2006. [Google Scholar]
- 36.Nazzi T, Paterson S, Karmiloff-Smith A. Early word segmentation by infants and toddlers with Williams syndrome. Infancy. 2003;4:251–271. [Google Scholar]
- 37.Mervis CB, Robinson BF. Expressive vocabulary ability of toddlers with Williams syndrome or Down syndrome: a comparison. Dev Neuropsychol. 2000;17:111–126. doi: 10.1207/S15326942DN1701_07. [DOI] [PubMed] [Google Scholar]
- 38.Glenn S, Cunningham C. Performance of young people with Down syndrome on the Leiter-R and British picture vocabulary scales. J Intellect Disabil Res. 2005;49:239–244. doi: 10.1111/j.1365-2788.2005.00643.x. [DOI] [PubMed] [Google Scholar]
- 39.Klein BP, Mervis CB. Contrasting patterns of cognitive abilities of 9- and 10-year-olds with Williams syndrome or Down syndrome. Dev Neuropsychol. 1999;16:177–196. [Google Scholar]
- 40.Mervis CB. Language ability es in Williams-Beuren syndrome. In: Morris CA, Lenhoff H, Wang P, editors. Williams-Beuren syndrome: Research, Evaluation, and Treatment. Johns Hopkins University Press; Baltimore, MD: 2006. pp. 159–206. [Google Scholar]
- 41.Vicari S, et al. Neuropsychological profile of Italians with Williams syndrome: an example of a dissociation between language and cognition? J Int Neuropsychol Soc. 2004;10:862–876. doi: 10.1017/s1355617704106073. [DOI] [PubMed] [Google Scholar]
- 42.Udwin O, Yule W. Expressive language of children with Williams syndrome. Am J Med Genet Suppl. 1990;6:108–114. doi: 10.1002/ajmg.1320370620. [DOI] [PubMed] [Google Scholar]
- 43.Grant J, Valian V, Karmiloff-Smith A. A study of relative clauses in Williams syndrome. J Child Lang. 2002;29:403–416. doi: 10.1017/s030500090200510x. [DOI] [PubMed] [Google Scholar]
- 44.Zukowski A. Investigating knowledge of complex syntax: insights from experimental studies of Williams syndrome. In: Rice M, Warren S, editors. Developmental Language Disorders: From Phenotypes to Etiologies. MIT Press; Cambridge, MA: 2004. pp. 99–119. [Google Scholar]
- 45.Gosch A, Stading G, Pankau R. Linguistic abilities in children with Williams-Beuren syndrome. Am J Med Genet. 1994;52:291–296. doi: 10.1002/ajmg.1320520308. [DOI] [PubMed] [Google Scholar]
- 46.Lukács Á. Language Abilities in Williams syndrome. Akadémiai Kiadó; Budapest, Hungary: 2005. [Google Scholar]
- 47.Volterra V, et al. Linguistic abilities in Italian children with Williams syndrome. Cortex. 1996;32:663–677. doi: 10.1016/s0010-9452(96)80037-2. [DOI] [PubMed] [Google Scholar]
- 48.Volterra V, et al. Early linguistic abilities of Italian children with Williams syndrome. Dev Neuropsychol. 2003;23:33–58. doi: 10.1080/87565641.2003.9651886. [DOI] [PubMed] [Google Scholar]
- 49.Mervis CB, et al. Language abilities of people with Williams syndrome. In: Abbeduto L, editor. International Review of Research in Mental Retardation. Academic Press; Orlando, FL: 2003. pp. 35–81. [Google Scholar]
- 50.Karmiloff-Smith A, et al. Language and Williams syndrome: how intact is “intact”? Child Dev. 1997;68:246–262. [PubMed] [Google Scholar]
- 51.Levy Y, Hermon S. Morphological abilities of Hebrew-speaking adolescents with Williams syndrome. Dev Neuropsychol. 2003;23:59–83. doi: 10.1080/87565641.2003.9651887. [DOI] [PubMed] [Google Scholar]
- 52.Robinson BF, Mervis CB, Robinson BW. The roles of verbal short-term memory and working memory in the acquisition of grammar by children with Williams syndrome. Dev Neuropsychol. 2003;23:13–31. doi: 10.1080/87565641.2003.9651885. [DOI] [PubMed] [Google Scholar]
- 53.Laing E, et al. Atypical development of language and social communication in toddlers with Williams syndrome. Developmental Science. 2002;5:233–246. [Google Scholar]
- 54.Lord C, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 55.Klein-Tasman BP, et al. Socio-communicative deficits in young children with Williams syndrome: Performance on the Autism Diagnostic Observation Schedule. Child Neuropsychology. doi: 10.1080/09297040601033680. in press. [DOI] [PubMed] [Google Scholar]
- 56.Bishop DV. Development of the Children’s Communication Checklist (CCC): a method for assessing qualitative aspects of communicative impairment in children. J Child Psychol Psychiatry. 1998;39:879–891. [PubMed] [Google Scholar]
- 57.Laws G, Bishop D. Pragmatic language impairment and social deficits in Williams syndrome: a comparison with Down’s syndrome and specific language impairment. Int J Lang Commun Disord. 2004;39:45–64. doi: 10.1080/13682820310001615797. [DOI] [PubMed] [Google Scholar]
- 58.Mervis CB. The Williams syndrome cognitive profile: strengths, weaknesses, and interrelations among auditory short term memory, language, and visuospatial constructive cognition. In: Winograd E, Fivush R, Hirst W, editors. Ecological Approaches to Cognition: Essays in Honor of Ulric Neisser. Erlbaum; Mahwah, NJ: 1999. pp. 193–227. [Google Scholar]
- 59.Curran ME, et al. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993;73:159–168. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- 60.Li DY, et al. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet. 1997;6:1021–1028. doi: 10.1093/hmg/6.7.1021. [DOI] [PubMed] [Google Scholar]
- 61.Tassabehji M, et al. Elastin: genomic structure and point mutations in patients with supravalvular aortic stenosis. Hum Mol Genet. 1997;6:1029–1036. doi: 10.1093/hmg/6.7.1029. [DOI] [PubMed] [Google Scholar]
- 62.Metcalfe K, et al. Elastin: mutational spectrum in supravalvular aortic stenosis. Eur J Hum Genet. 2000;8:955–963. doi: 10.1038/sj.ejhg.5200564. [DOI] [PubMed] [Google Scholar]
- 63.Botta A, et al. Detection of an atypical 7q11.23 deletion in Williams syndrome patients which does not include the STX1A and FZD3 genes. J Med Genet. 1999;36:478–480. [PMC free article] [PubMed] [Google Scholar]
- 64.Heller R, et al. Partial deletion of the critical 1.5 Mb interval in Williams-Beuren syndrome. J Med Genet. 2003;40:e99. doi: 10.1136/jmg.40.8.e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frangiskakis JM, et al. LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell. 1996;86:59–69. doi: 10.1016/s0092-8674(00)80077-x. [DOI] [PubMed] [Google Scholar]
- 66.Tassabehji M, et al. Williams syndrome: use of chromosomal microdeletions as a tool to dissect cognitive and physical phenotypes. Am J Hum Genet. 1999;64:118–125. doi: 10.1086/302214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gagliardi C, et al. Unusual cognitive and behavioural profile in a Williams syndrome patient with atypical 7q11.23 deletion. J Med Genet. 2003;40:526–530. doi: 10.1136/jmg.40.7.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirota H, et al. Williams syndrome deficits in visual spatial processing linked to GTF2IRD1 and GTF2I on chromosome 7q11.23. Genet Med. 2003;5:311–321. doi: 10.1097/01.GIM.0000076975.10224.67. [DOI] [PubMed] [Google Scholar]
- 69.Morris CA, et al. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am J Med Genet A. 2003;123:45–59. doi: 10.1002/ajmg.a.20496. [DOI] [PubMed] [Google Scholar]
- 70.Tassabehji M, et al. GTF2IRD1 in craniofacial development of humans and mice. Science. 2005;310:1184–1187. doi: 10.1126/science.1116142. [DOI] [PubMed] [Google Scholar]
- 71.Howald C, et al. Two high throughput technologies to detect segmental aneuploidies identify new Williams-Beuren syndrome patients with atypical deletions. J Med Genet. 2006;43:266–273. doi: 10.1136/jmg.2005.034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tassabehji M, et al. Gene dosage and Williams syndrome. Presented at the International Congress of Human Genetics; 6–10 August 2006; Brisbane, Australia. 2006. http://www.ichg2006.com/abstract/1137.htm. [Google Scholar]
- 73.van Hagen JM, et al. Contribution of CYLN2 and GTF2IRD1 to neurological and cognitive symptoms in Williams Syndrome. Neurobiol Dis. 2007;26:112–124. doi: 10.1016/j.nbd.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Edelmann L, et al. An atypical deletion of the Williams-Beuren Syndrome interval implicates genes associated with defective visuospatial processing and autism. J Med Genet. 2007;44:136–143. doi: 10.1136/jmg.2006.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li DY, et al. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102:1783–1787. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoogenraad CC, et al. Targeted mutation of Cyln2 in the Williams syndrome critical region links CLIP-115 haploinsufficiency to neurodevelopmental abnormalities in mice. Nat Genet. 2002;32:116–127. doi: 10.1038/ng954. [DOI] [PubMed] [Google Scholar]
- 77.Meng Y, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 78.Meyer-Lindenberg A, et al. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 79.Boddaert N, et al. Parieto-occipital grey matter abnormalities in children with Williams syndrome. Neuroimage. 2006;30:721–725. doi: 10.1016/j.neuroimage.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 80.Kippenhan JS, et al. Genetic contributions to human gyrification: sulcal morphometry in Williams syndrome. J Neurosci. 2005;25:7840–7846. doi: 10.1523/JNEUROSCI.1722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.American Academy of pediatrics committee on Genetics DC. Towards a quantitative, probabilistic neuroanatomy of cerebral cortex. Cortex. 2004;40:211–212. doi: 10.1016/s0010-9452(08)70954-7. [DOI] [PubMed] [Google Scholar]
- 82.Meyer-Lindenberg A, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- 83.American Academy of Pediatrics Committee on Genetics. Healthcare supervision for children with Williams syndrome. Pediatrics. 2001;107:1192–1204. [PubMed] [Google Scholar]
- 84.Cherniske EM, et al. Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet A. 2004;131:255–264. doi: 10.1002/ajmg.a.30400. [DOI] [PubMed] [Google Scholar]
- 85.Meyer-Lindenberg A, et al. Functional, structural, and metabolic abnormalities of the hippocampal formation in Williams syndrome. J Clin Invest. 2005;115:1888–1895. doi: 10.1172/JCI24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan-Sindhunata G, et al. Molecular cytogenetic characterization of a small, familial supernumerary ring chromosome 7 associated with mental retardation and an abnormal phenotype. Am J Med Genet. 2000;92:147–152. [PubMed] [Google Scholar]
- 87.Velagaleti GV, et al. De novo supernumerary ring chromosome 7: first report of a non-mosaic patient and review of the literature. Clin Genet. 2002;61:202–206. doi: 10.1034/j.1399-0004.2002.610306.x. [DOI] [PubMed] [Google Scholar]
- 88.Chantot-Bastaraud S, et al. Clinical findings and cytogenetic analysis of small supernumerary ring chromosomes 7: report of two new cases. Ann Genet. 2004;47:241–249. doi: 10.1016/j.anngen.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Lichtenbelt KD, et al. Supernumerary ring chromosome 7 mosaicism: case report, investigation of the gene content, and delineation of the phenotype. Am J Med Genet A. 2005;132:93–100. doi: 10.1002/ajmg.a.30408. [DOI] [PubMed] [Google Scholar]
- 90.von Beust G, et al. Molecular cytogenetic characterization of a de novo supernumerary ring chromosome 7 resulting in partial trisomy, tetrasomy, and hexasomy in a child with dysmorphic signs, congenital heart defect, and developmental delay. Am J Med Genet A. 2005;137:59–64. doi: 10.1002/ajmg.a.30835. [DOI] [PubMed] [Google Scholar]
- 91.Berg J, et al. Severe language delay associated with duplication of the Williams-Beuren critical region. Presented at The American Society of Human Genetics; 9–13 October 2006; New Orleans, LA, USA. 2006. http://www.ashg.org/genetics/ashg06s/index.shtml(Program number 86) [Google Scholar]
- 92.Golden D, et al. Array-CGH identifies submicroscopic duplication of common micodeletion regions 7q11.23, 16p13.3, and 22q11.2. Presented at the 31st Annual Meeting of the Association of Genetic Technologists; 9–11 June, 2006; Baltimore, MD, USA. 2006. http://www.unmc.edu/dept/mmi/index.cfm?L2_ID=63&L1_ID=23&CONREF=53. [Google Scholar]
- 93.Jayakar P, et al. A female patient with Aicardi syndrome and duplication of Williams syndrome critical region (7q11.23). Presented at The American Society of Human Genetics; 9–13 October 2006; New Orleans, LA, USA. 2006. http://www.ashg.org/genetics/ashg06s/index.shtml (Program number 793) [Google Scholar]
- 94.Kriek M, et al. Copy number variation in regions flanked (or unflanked) by duplicons among patients with developmental delay and/or congenital malformations; detection of reciprocal and partial Williams-Beuren duplications. Eur J Hum Genet. 2006;14:180–189. doi: 10.1038/sj.ejhg.5201540. [DOI] [PubMed] [Google Scholar]
- 95.Kirchhoff M, et al. MLPA analysis for a panel of syndromes with mental retardation reveals imbalances in 5.8% of patients with mental retardation and dysmorphic features, including duplications of the Sotos syndrome and Williams-Beurensyndromeregions. Eur J Med Genet. 2007;50:33–42. doi: 10.1016/j.ejmg.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Torniero C, et al. Cortical dysplasia of the left temporal lobe might explain severe expressive-language delay in patients with duplication of the Williams-Beuren locus. Eur J Hum Genet. 2007;15:62–67. doi: 10.1038/sj.ejhg.5201730. [DOI] [PubMed] [Google Scholar]
- 97.Depienne C, et al. Autism, language delay and mental retardation in a patient with 7q11 duplication. J Med Genet. 2007 Mar 30; doi: 10.1136/jmg.2006.047092. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bishop DVM. Uncommon Understanding: Development and Disorders of Language Comprehension in Children. Psychology Press; Hove, UK: 1997. [Google Scholar]
- 99.Dale PS, et al. Genetic influence on language delay in two-year-old children. Nat Neurosci. 1998;1:324–328. doi: 10.1038/1142. [DOI] [PubMed] [Google Scholar]
- 100.Lewis BA, Thompson LA. A study of developmental speech and language disorders in twins. J Speech Hear Res. 1992;35:1086–1094. doi: 10.1044/jshr.3505.1086. [DOI] [PubMed] [Google Scholar]
- 101.Bishop DV, North T, Donlan C. Genetic basis of specific language impairment: evidence from a twin study. Dev Med Child Neurol. 1995;37:56–71. doi: 10.1111/j.1469-8749.1995.tb11932.x. [DOI] [PubMed] [Google Scholar]
- 102.Tomblin JB, Buckwalter PR. Heritability of poor language achievement among twins. J Speech Lang Hear Res. 1998;41:188–199. doi: 10.1044/jslhr.4101.188. [DOI] [PubMed] [Google Scholar]
- 103.Fisher SE, Lai CS, Monaco AP. Deciphering the genetic basis of speech and language disorders. Annu Rev Neurosci. 2003;26:57–80. doi: 10.1146/annurev.neuro.26.041002.131144. [DOI] [PubMed] [Google Scholar]
- 104.SLI Consortium. A genomewide scan identifies two novel loci involved in specific language impairment. Am J Hum Genet. 2002;70:384–398. doi: 10.1086/338649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.SLI Consortium. Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. Am J Hum Genet. 2004;74:1225–1238. doi: 10.1086/421529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lai CS, et al. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 107.MacDermot KD, et al. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005;76:1074–1080. doi: 10.1086/430841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feuk L, et al. Absence of a paternally inherited FOXP2 gene in developmental verbal dyspraxia. Am J Hum Genet. 2006;79:965–972. doi: 10.1086/508902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shriberg LD, et al. Speech, prosody, and voice characteristics of a mother and daughter with a 7;13 translocation affecting FOXP2. J Speech Lang Hear Res. 2006;49:500–525. doi: 10.1044/1092-4388(2006/038). [DOI] [PubMed] [Google Scholar]
- 110.Zeesman S, et al. Speech and language impairment and oromotor dyspraxia due to deletion of 7q31 that involves FOXP2. Am J Med Genet A. 2006;140:509–514. doi: 10.1002/ajmg.a.31110. [DOI] [PubMed] [Google Scholar]
- 111.Lennon PA, et al. Deletion of 7q31.1 supports involvement of FOXP2 in language impairment: Clinical report and review. Am J Med Genet A. 2007;143:791–798. doi: 10.1002/ajmg.a.31632. [DOI] [PubMed] [Google Scholar]
- 112.Shu W, et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci U S A. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scherer SW, Osborne LR. Williams-Beuren syndrome. In: Stankiewicz PT, Lupski JR, editors. Genomic Disorders: The Genomic Basis of Disease. Humana Press; Totowa, NJ: 2007. pp. 221–236. [Google Scholar]