Abstract
Chronic adrenalectomy (ADX) causes a gradual and selective loss of granule cells in the dentate gyrus (DG) of the rat. Here, we administered replacement corticosterone to rats beginning 10 wk after ADX. We then tested them in three discrimination tasks based on object novelty, location, or object/context association. Only during testing of the object/context association did ADX rats demonstrate deficits. These findings add to a body of evidence that the hippocampus is necessary when contextual information is important. We also confirm that memory deficits after chronic adrenalectomy are not a result of loss of corticosterone per se.
Research with rats (Sutherland and McDonald 1990; Kim and Faneslow 1992; Anagnostaras et al. 2001; Lehmann et al. 2009), nonhuman primates (Machado and Bachevalier 2006; Pascalis et al. 2009), and humans (Alvarez et al. 2008; Marschner et al. 2008) shows that hippocampal damage can disrupt the ability to recall or express information about context. Furthermore, several reports suggest that the hippocampus is important for creating flexible representations of context and object associations. Studies in rats (Mumby et al. 2002; O’Brien et al. 2006) and humans (Pascalis et al. 2009) show that lesions specific to the hippocampus produce deficits in object recognition only when contextual information is altered.
Within the hippocampus, the dentate gyrus (DG) is important for certain aspects of memory (Xavier et al. 1999; Garthe et al. 2009). Chronic adrenalectomy (ADX) causes a gradual and selective loss of granule cells in the DG of the rat (Sloviter et al. 1989). This loss of cells is attributed to a lack of circulating corticosterone (CORT) (Sloviter et al. 1989; Woolley et al. 1991). Behavioral deficits as a result of chronic ADX have been reported in the Morris water task (Armstrong et al. 1993; Roozendaal et al. 1998; Spanswick et al. 2007) and open-field task (Islam et al. 1995). There has been debate as to whether the deficits experienced by ADX rats are a result of lost CORT or due to the depletion of the granule cell layer itself. Conrad and Roy (1995) and McCormick et al. (1997) report that acute CORT replacement is sufficient to alleviate some of the deficits in spatial tasks associated with chronic ADX. These findings have led some to conclude that the removal of CORT is responsible for the behavioral deficits experienced by ADX rats and not the loss of granule cells per se. In direct contrast to these findings, Spanswick et al. (2007) report that administration of CORT after 6 wk of ADX does not alleviate spatial deficits in a moving-platform version of the Morris water task. Also in opposition to Conrad and Roy (1995) and McCormick et al. (1997), studies utilizing colchicine as a method to remove granule cells report spatial deficits as a result of cell loss (Sutherland et al. 1983; Xavier et al. 1999; Jeltsch et al. 2001).
Here, we show using three versions of a novelty-preference task (novel object, novel place, and object/context mismatch) that lesions limited to the granule cell layer of the DG produce deficits that are specific to detecting an object/context mismatch. Our results support the idea that the hippocampus (specifically the DG) is critical in situations in which contextual discrimination is important. We also provide evidence that the behavioral deficits associated with long-term ADX are not a result of loss of CORT per se.
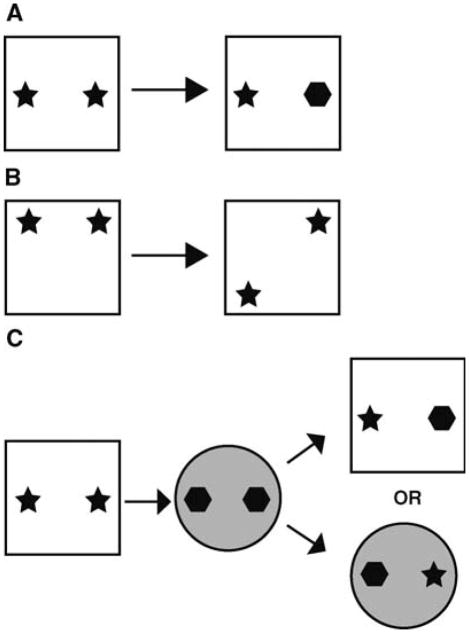
Adult male rats (n = 52) underwent ADX or sham surgery (Spanswick et al. 2007). Rats remained in their home cages for 10 wk to allow for degeneration of the granule cell layer. Six weeks after surgery, blood samples were collected and CORT levels were analyzed utilizing an EIA kit (Cayman Chemical) to determine the effectiveness of ADX. Starting at 10 wk, for 1 wk prior to and during testing, ADX rats received a daily oral administration of replacement CORT. We have previously demonstrated that this method of CORT delivery is sufficient to produce a diurnal rhythm in ADX rats that is similar to intact animals (Spanswick et al. 2007). Behavior was assessed after 1 wk of CORT replacement. Rats were pre-exposed to the discrimination context once a day for 10 min, for a total of 2 d prior to testing. The context consisted of a white, square plastic box ~60 cm × 60 cm with standard housing bedding on the floor. On day 3, behavior was assessed. For novel object preference, rats were placed in the context and allowed to explore two identical objects for 5 min (learning phase). Rats were removed from the context for 10 min and one of the objects was replaced with an object the rat had not previously encountered (Fig. 1A). Rats were reintroduced to the context for 3 min and allowed to investigate (test phase). The novel place preference task was run similarly, except that one of the identical pair of objects was moved relative to its previous location (Fig. 1B). An investigation ratio was calculated for both tasks by dividing the time spent investigating the novel object/place by the total time spent investigating both objects. The object/context mismatch was performed in a similar fashion to that as originally described by Mumby et al. (2002). Pre-exposure for the object/context mismatch involved exposing rats to two different contexts for 10 min each, one immediately after the other, each day, for a total of 2 d. Context A was identical to that described above. Context B was a large, black, circular bin, 60 cm in diameter, and was housed in a different testing room. On test day, each context housed a unique pair of identical objects, and rats explored each context for 5 min, one immediately after the other (learning phases one and two). After exposure to both contexts and a 5-min delay, rats were reexposed to one of them, this time with one object from each (test phase, Fig. 1C). Rats were allowed to explore for 3 min and an investigation ratio was calculated. To determine the extent of DG granule cell loss, a series of tissue was labeled with 4′, 6-diamidino-2-phenylindole (DAPI, Sigma) and Cavalieri volume estimates were performed. A second series of tissue in a subset of control (n = 7) and ADX (n = 5) rats was labeled with DAPI and total cell number estimates in the DG granule cell layer were performed utilizing the optical fractionator technique.
Figure 1.
Examples of the object discrimination tasks. (A) Novel object preference. (B) Novel place preference. (C ) Object/context mismatch task.
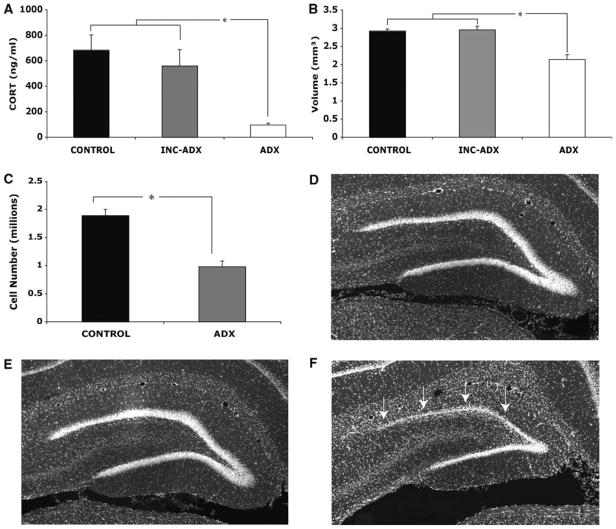
ADX significantly reduced CORT levels compared with control rats (F = 6.39, P < 0.01; Fig. 2A). A subset of ADX rats (n = 14) had CORT levels similar to controls, a result of incomplete ADX. Given that incomplete ADX does not reduce CORT levels enough to produce a loss of dentate granule cells (Sloviter et al. 1989) and incomplete ADX animals have been shown to act similarly to controls in various tests of memory (Islam et al. 1995; McCormick et al. 1997), we grouped incomplete ADX rats with controls. The combination of incomplete ADX and control rats was further supported by Cavalieri volume estimates (F(2,49) = 18.52, P < 0.001), revealing a significantly lower dentate granule cell layer volume in ADX rats compared both with controls (P < 0.001) and incomplete ADX animals (P < 0.001; Fig. 2B). No significant volume difference was noted between incomplete ADX and controls (P = 1.00). The pattern of granule cell loss in ADX rats was similar to previous reports (Conrad and Roy 1995; Roozendaal et al. 1998; Spanswick et al. 2007). Analysis of the optical fractionator data revealed a significant difference between control and ADX rats in the number of DG granule cells (F(1,11) = 34.84, P < 0.001; Fig. 2C). As one might expect, there was a significant correlation between DG volume and total cell number (r(12) = 0.904, P < 0.01).
Figure 2.
(A) Complete removal of the adrenal glands (ADX) resulted in a significant decrease in CORT. A subset of ADX rats (INC-ADX) did not differ from controls, suggesting the ADX was incomplete. (B) As determined by the Cavalieri method, ADX rats had a significantly smaller DG granule cell layer volume compared with both controls and incomplete ADX rats. Control rats and those with incomplete ADX did not differ significantly. (C ) Further analysis with the optical fractionator showed that ADX resulted in a significant loss of granule cells. (D – F) Representative DAPI-labeled sections taken from a control (D), incomplete ADX (E), and an ADX rat (F ). ADX-induced degeneration was most prevalent in the superior blade of the dentate gyrus (arrows).
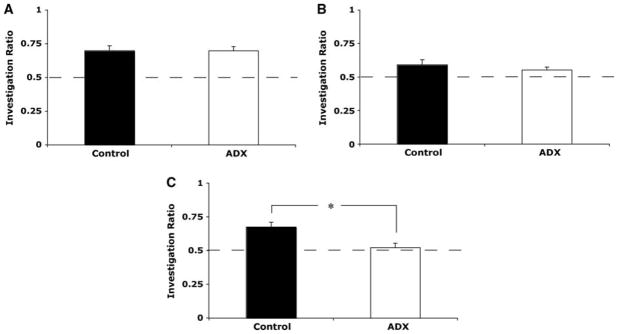
There was no significant difference between control (n = 12) and ADX (n = 6) rats in time spent investigating the identical object pair during the learning phase of novel object preference (F(1,16) = 0.214, P = 0.65). Mean investigation ratio for control rats during the test phase of novel object preference was 0.70 ± 0.04, while ADX rats had an average investigation ratio of 0.70 ± 0.03 (Fig. 3A). Investigation ratios for controls and ADX rats during novel object preference differed significantly from chance (0.5) (t(8) = 4.39, P < 0.01 and t(7) = 6.41, P < 0.01, respectively). There was no significant difference between investigation ratios for controls and ADX rats (F(1,16) = 0.003, P = 0.957).
Figure 3.
Performance of control and ADX rats was similar in novel object preference (A) and novel place preference (B); both groups discriminated the novel object or place at greater than chance levels. (C ) ADX animals were significantly impaired relative to controls and did not discriminate above chance levels during the object/context mismatch paradigm. (Dashed lines) Chance, (*) significant difference.
Time spent investigating the object pair during the learning phase of novel place preference did not differ significantly between groups (F(1,17) = 1.041, P = 0.322). The mean investigation ratio for control rats (n = 11) during the test phase of novel place preference was 0.59 ± 0.04 and differed significantly from chance (t(10) = 2.39, P < 0.05). ADX rats (n = 8) had a mean investigation ratio of 0.55 ± 0.02, which also differed significantly from chance (t(7) = 2.464, P < 0.05). The investigation ratio for controls and ADX rats did not differ significantly from one another (F(1,17) = 0.618, P = 0.443; Fig. 3B).
Time spent investigating the object pairs did not differ significantly between control (n = 10) and ADX rats (n = 6) during learning phase one (F(1,14) = 0.49, P = 0.495) or learning phase two (F(1,14) = 1.079, P = 0.316) during the object/context mismatch task. During the test phase of the object/context mismatch task, controls spent significantly more time investigating the novel context/object pairing (t(8) = 4.573, P < 0.05). ADX rats did not investigate the novel pairing greater than chance (t(6) = 1.15, P = 0.295). ANOVA revealed a significant difference between the investigation ratios of control and ADX rats, with controls investigating the out-of-context object more than ADX rats (F(1,14) = 7.29, P < 0.05; Fig. 3C). A significant correlation between investigation of the out-of-context object and granule cell layer volume was also detected (r(16) = 0.499, P < 0.05).
Control animals were able to successfully discriminate between a previously encountered and novel object, between objects that were in familiar vs. new locations, and between objects that were in expected vs. unexpected contexts. Importantly, ADX rats were similar to controls in both the novel object preference and novel place preference tasks. Only during the object/context mismatch task did ADX rats differ from controls, failing to discriminate between the out-of-context vs. in-context objects. Control and ADX rats investigated the objects during the learning phase of exploration similarly; thus, the deficits in novelty preference we observe are not due to lack of object investigation.
In agreement with previous reports (Sloviter et al. 1989; Conrad and Roy 1995; Roozendaal et al. 1998) we show that chronic ADX produces a loss of cells that is limited to the granule cell layer of the DG. We report an ~25% reduction in the volume of the dentate granule cell layer 10 wk after ADX. Further analysis using the optical fractionator method revealed that approximately half of the hippocampal granule cells were lost after ADX, confirming prior reports of ADX-induced granule cell loss (Sloviter et al. 1989, 1993). A subset of ADX animals did not show significantly decreased levels of CORT, nor a reduction in the volume of the granule cell layer in the DG. This is likely a result of incomplete ADX permitting secretion of CORT and preventing the degeneration associated with ADX (Sloviter et al. 1989). Given the lack of difference between these rats and controls, as well as evidence from previous research suggesting intact memory ability in incomplete ADX rats (McCormick et al. 1997), we grouped them together.
At the time of behavioral testing, our ADX rats were receiving replacement CORT. There is debate surrounding behavioral deficits as a result of chronic ADX; both removal of CORT and degeneration of the granule cells could be responsible for the observed deficits. Several studies have indicated that acute CORT replacement is sufficient to at least partially reverse behavioral deficits associated with ADX (Conrad and Roy 1995; McCormick et al. 1997). Our current findings, as well as our previous report (Spanswick et al. 2007), and those using colchicine as a method to ablate the DG (Sutherland et al. 1983; Xavier et al. 1999; Jeltsch et al. 2001) support the idea that granule cell loss contributes to the deficits.
A potential explanation for the disparity between the reports of ADX-induced deficits may be the sensitivity of the task used to assess behavior. Spanswick et al. (2007) utilized a multiple platform paradigm in the Morris water task, whereas Conrad and Roy (1995) and McCormick et al. (1997) employed a single platform location. The sensitivity of these differing versions of the Morris water task to detect behavioral deficits as a result of hippocampus damage may be very different. Here, we report a significant correlation between granule cell layer volume and performance in the object/context mismatch task, providing additional evidence for the idea that removal of CORT alone is insufficient to explain the observed deficits.
In this study we use a relatively short retention interval, similar to those previously employed for the object/context mismatch task (Mumby et al. 2002; O’Brien et al. 2006). Despite the short retention, the behavior of ADX rats was impaired specifically in the object/context mismatch task. Prior research has demonstrated that larger disruptions of DG function can also impair novel object preference (Lee et al. 2005; Jessberger et al. 2009) and spatial behavior (Sutherland et al. 1983; Xavier et al. 1999; Gilbert et al. 2001). A possible explanation for this disparity is that discriminations based upon context may typically require a higher degree of complexity in information load, resulting in a greater sensitivity to DG granule cell layer disruption. It is worthy to note that Lee et al. (2005) report deficits after colchicine infusions into dorsal DG (resulting in a 97% loss of granule cells in that region) in a novel object recognition paradigm. Lee and colleagues utilized a configuration of five objects; we employ only two, suggesting that information load may indeed explain the inconsistency. It is also important to note that despite control and ADX rats both discriminating at significantly greater than chance levels in the object/place task, the discrimination levels were lower than our other tasks, potentially obscuring a deficit.
A further possibility is that animals with DG granule cell loss are more susceptible to interference during the object/context mismatch task. Multiple theories have hypothesized that the hippocampus serves to reduce interference via a process referred to as pattern separation (Marr 1971; Shapiro and Olton 1994; McClelland et al. 1995; Rolls 1996). Given its sparse pattern of activity (Barnes et al. 1990; Chawla et al. 2005), particular attention has been paid to the DG as a potential mediator of pattern separation (Gilbert et al. 2001; Lee et al. 2004; Leutgeb et al. 2007; Clelland et al. 2009). A failure in pattern separation may provide an alternative explanation for the deficits we observe in our ADX rats.
Our finding that the selective loss of DG granule cells produces a deficit in object memory only when the rat must remember the context in which the object had been previously encountered adds to a converging body of evidence that suggests the hippocampus is essential in storing and retrieving certain types of context memories (Rudy 2009). Specifically, we show that hippocampal granule cells are necessary for supporting associations between objects and contexts. Furthermore, we find that adrenalectomy produces behavioral deficits in rats despite CORT replacement, clearly showing that loss of CORT does not alone account for the behavioral deficits associated with chronic adrenalectomy.
Acknowledgments
We thank grant sponsors CIHR (to R.J.S.) and AHFMR (to R.J.S and S.C.S).
References
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: Cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Armstrong JN, McIntyre DC, Neubort S, Sloviter RS. Learning and memory after adrenalectomy-induced hippocampal dentate granule cell degeneration in the rat. Hippocampus. 1993;3:359–371. doi: 10.1002/hipo.450030310. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL, Mizumori SJY, Leonard BW, Lin L-H. Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res. 1990;83:287–300. doi: 10.1016/s0079-6123(08)61257-1. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentate by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyres P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Roy EJ. Dentate gyrus destruction and spatial learning impairment after corticosteroid removal in young and middle-aged rats. Hippocampus. 1995;5:1–15. doi: 10.1002/hipo.450050103. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning. PLoS ONE. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Islam A, Henriksson B, Mohammed A, Winblad B, Adem A. Behavioral deficits in adult rats following long-term adrenalectomy. Neurosci Lett. 1995;194:49–52. doi: 10.1016/0304-3940(95)11724-b. [DOI] [PubMed] [Google Scholar]
- Jeltsch H, Bertrand F, Lazarus C, Cassel JC. Cognitive performances and locomotor activity following dentate granule cell damage in rats: Role of lesion extent and type of memory tested. Neurobiol Learn Mem. 2001;76:81–105. doi: 10.1006/nlme.2000.3986. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Faneslow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Lee I, Hunsaker MR, Kesner RP. The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Sparks FT, Spanswick SC, Hadikin C, McDonald RJ, Sutherland RJ. Making context memories independent of the hippocampus. Learn Mem. 2009;16:417–420. doi: 10.1101/lm.1385409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M-B, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;4:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus contextual fear conditioning. J Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psych Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McCormick CM, McNamara M, Mukhopadhyay S, Kelsey JE. Acute corticosterone replacement reinstates performance on spatial and nonspatial memory tasks 3 months after adrenalectomy despite degeneration in the dentate gyrus. Behav Neurosci. 1997;1111:518–531. doi: 10.1037//0735-7044.111.3.518. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien N, Lehmann H, Lecluse V, Mumby DG. Enhanced context-dependency of object recognition in rats with hippocampal lesions. Behav Brain Res. 2006;170:156–162. doi: 10.1016/j.bbr.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Hunkin NM, Bachevalier J, Mayes AR. Change in background context disrupts performance on visual paired comparison following hippocampal damage. Neuropsychologia. 2009;47:2107–2113. doi: 10.1016/j.neuropsychologia.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Sapolsky RM, McGaugh JL. Basolateral amygdala lesions block the disruptive effects of long-term adrenalectomy on spatial memory. Neuroscience. 1998;84:453–465. doi: 10.1016/s0306-4522(97)00538-1. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Olton DS. Hippocampal function and interference. In: Schacter DL, editor. Memory systems. MIT Press; Cambridge, MA: 1994. pp. 87–117. [Google Scholar]
- Sloviter RS, Valiquette G, Abrams GM, Ronk EC, Sollas AL, Paul LA, Neubort S. Selective loss of hippocampal granule cells in the mature rats brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dean E, Neubort S. Electron microscopic analysis of adrenalectomy-induced hippocampal granule cell degeneration in the rat: Apoptosis in the adult central nervous system. J Comp Neurol. 1993;330:337–351. doi: 10.1002/cne.903300305. [DOI] [PubMed] [Google Scholar]
- Spanswick SC, Epp JR, Keith JR, Sutherland RJ. Adrenalectomy-induced granule cell degeneration in the hippocampus causes spatial memory deficits that are not reversed by chronic treatment with corticosterone or fluoxetine. Hippocampus. 2007;17:137–146. doi: 10.1002/hipo.20252. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ. Hippocampus, amygdala, and memory deficits in rats. Behav Brain Res. 1990;37:57–79. doi: 10.1016/0166-4328(90)90072-m. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. A behavioral analysis of spatial localization following electrolytic, kainite- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;656:71–78. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Sakai RR, Spence RL, McEwen BS. Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res. 1991;554:312–315. doi: 10.1016/0006-8993(91)90207-c. [DOI] [PubMed] [Google Scholar]
- Xavier GF, Oliveira-Filho JB, Santos AMG. Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: Difficulties in “place strategy” because of a lack of flexibility in the use of environmental cues. Hippocampus. 1999;9:668–681. doi: 10.1002/(SICI)1098-1063(1999)9:6<668::AID-HIPO8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]