Abstract
Elucidating the underlying mechanisms that govern microglial activation and survival is essential for the development of new treatment strategies for neurodegenerative disorders, since microglia serve not only as guardian sentries of the nervous system, but also play a significant role in determining neuronal and vascular cell fate. Here we show that endogenous and exogenous Wnt1 in inflammatory microglial cells is necessary for the prevention of apoptotic early membrane phosphatidylserine exposure and later DNA degradation, since blockade of Wnt1 signaling abrogates cell survival during oxidative stress. Wnt1 prevents apoptotic demise through the post-translational phosphorylation and maintenance of FoxO3a in the cytoplasm to inhibit an apoptotic cascade that relies upon the loss of mitochondrial membrane permeability, cytochrome c release, Bad phosphorylation, and activation of caspase 3 and caspase 1 as demonstrated by complimentary gene knockdown studies of FoxO3a. Furthermore, subcellular trafficking and gene knockdown studies of NF-κB p65 illustrate that microglial cell survival determined by Wnt1 during oxidative stress requires NF-κB p65. Our work highlights Wnt1 and the control of novel downstream transcriptional pathways as critical components for the oversight of nervous system microglial cells.
Keywords: apoptosis, cell demise, microglia, wingless, Wnt
1. Introduction
Inflammatory cells influence multiple systems throughout the body, but none maybe more influential than microglia that continually monitor the central nervous system. During periods of inflammatory cell activation, microglia require the activation of endogenous cytoprotective pathways to proliferate and remove injured cells that are no longer functional [1]. Microglia allow for the repair of neuronal and vascular tissues in the nervous system by performing immune surveillance for toxins [2], such as β-amyloid [3], initiating the release of neurtrophins [4], and blocking the entrance of foreign microorganisms [5]. However, the regenerative processes governed by microglia also can initiate the release of reactive oxygen species [6], activate cytokines [7], and eventually lead to the demise of cells in the nervous system [8, 9].
Given the vital role microglia provide in the protection and maintenance of the central nervous system, identifying and targeting cellular pathways that govern microglial survival and proliferation become vital for the development of novel strategies to reverse or arrest neurodegenerative disorders. Wnt1, a cysteine-rich glycosylated protein derived from the Drosophila Wingless (Wg) and the mouse Int-1 genes, may represent one such pathway since Wnt proteins determine multiple cellular functions that include stem cell development, vascular regeneration, and maturation of the nervous system [10-13]. Wnt signaling has been associated with neurodegeneration in models of frontotemporal dementia [14] and late onset Alzheimer’s disease [15]. In addition, stimulation of the Wnt pathway may provide alternative treatments for Alzheimer’s disease [16]. In regards to Wnt1 and its attributes, Wnt1 can function through cytoprotective agents such as erythropoietin [17, 18], to protect vascular cells against elevated glucose in models of diabetes [19]. Furthermore, Wnt1 has been shown to prevent apoptotic neuronal injury during β-amyloid exposure and block microglial activation [20].
Here we show that exogenous and endogenous Wnt1 control early PS membrane and late DNA fragmentation apoptotic injury in microglia as well as the activation and proliferation of these inflammatory cells. Endogenous Wnt1 provides a vital component for the protection of microglia during oxidative stress, since blockade of Wnt1 signaling intensifies cell injury. Furthermore, Wnt1 employs the post-translational phosphorylation and inhibition of FoxO3a to prevent the trafficking of the “pro-apoptotic” FoxO3a from the cytoplasm to the nucleus of microglia. Gene knockdown studies of FoxO3a further support that loss of FoxO3a activity is a significant component for Wnt1 to block microglial injury during oxidative stress. Control of microglial survival by Wnt1 ultimately relies upon the maintenance of mitochondrial membrane permeability, cytochrome c release, Bad phosphorylation, the activation of caspase 3 and caspase 1, and the nuclear trafficking and preservation of nuclear factor-κB (NF-κB p65). Our work identifies Wnt1 and its regulation of central apoptotic pathways as primary targets for the development of novel therapeutic strategies for neurodegenerative disorders.
2. Materials and methods
2.1 Microglia cell cultures
The microglial cell line EOC 2 was obtained from American Type Culture Collection (ATTC, Manassas, VA.). Cells were maintained in Dulbecco’s modified Eagle medium (ATTC, Manassas, VA), supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 50 μg/ml penicillin and streptomycin and 20% media from the LADMAC cell line (ATCC, Manassas, VA) which contains colony stimulating factor-1 (CSF-1) secreted by LADMAC cells. Cells were seeded onto 24-well plates or 35 mm culture dishes at a density of 1.5 × 106 cells per well or 4 × 106 cells per dish.
2.2 Experimental treatments
Oxygen-glucose deprivation (OGD) in microglia was performed by replacing the media with glucose-free HBSS containing 116 mmol/l NaCl, 5.4 mmol/l KCl, 0.8 mmol/l MgSO4, 1 mmol/l NaH2PO4, 0.9 mmol/l CaCl2, and 10 mg/l phenol red (pH 7.4) and cultures were maintained in an anoxic environment (95% N2 and 5% CO2) at 37 °C per the experimental paradigm. For treatments applied prior to OGD, human recombinant Wnt1 protein (R&D Systems, Minneapolis, MN) or mouse monoclonal anti body against Wnt1 (R&D Systems, Minneapolis, MN) were continuous.
2.3 Assessment of cell survival
Microglial injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 24 hours following treatment with OGD per our previous protocols [9, 21, 22]. The mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 10-20 cells (viable + non-viable). Each experiment was replicated 6 times independently with different cultures.
2.4 Assessment of DNA fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay [9, 17]. Briefly, microglial cells were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde and the 3′-hydroxy ends of cut DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA).
2.5 Assessment of membrane phosphatidylserine (PS) residue externalization
Phosphatidylserine (PS) exposure was assessed through the established use of annexin V. Per our prior protocols [9, 20-22], a 30 μg/ml stock solution of annexin V conjugated to phycoerythrin (PE) (R&D Systems, Minneapolis, MN) was diluted to 3 μg/ml in warmed calcium containing binding buffer (10 mmol/l Hepes, pH 7.5, 150 mmol/l NaCl, 5 mmol/l KCl, 1 mmol/l MgCl2, 1.8 mmol/l CaCl2). Plates were incubated with 500 μl of diluted annexin V for 10 minutes. Images were acquired with “blinded” assessment with a Leitz DMIRB microscope (Leica, McHenry, IL) and a Fuji/Nikon Super CCD (6.1 megapixels) using transmitted light and fluorescent single excitation light at 490 nm and detected emission at 585 nm.
2.6 Assessment of microglial activation and proliferation
Proliferating cell nuclear antigen (PCNA) expression for microglial activation [23] and bromodeoxyuridine (BrdU) uptake for microglial proliferation [24] was performed with anti-mouse monoclonal antibody PCNA (1:1000) or BrdU (1:6000) (Sigma, St Louis, MO) conjugated with biotinylated anti-mouse IgG (1:100) and visualized through fluorescein avidin (1:100) for PCNA and Texas Red streptavidin (Vector laboratories, Burlingame, CA) for BrdU. BrdU (10 μM) and fluorodexyuridine (1μM) (Sigma, St. Louis, MO) were applied 1 hour prior to the time of fixation.
2.7 Expression of Wnt1, phosphorylated FoxO3a, total FoxO3a, phosphorylated Bad, active caspase 1 and 3, and NF-κB p65
Cells were homogenized and each sample (50 μg/lane) was subjected to SDS-polyacrylamide gel electrophoresis (7.5% FoxO3a, NF-κB; 12.5% Wnt1, caspase 1 and 3). After transfer, the membranes were incubated with a rabbit polyclonal antibody against Wnt1 (1:1000, R&D Systems, Minneapolis, MN), a rabbit polyclonal antibody against a rabbit antibody against phospho-FoxO3a (1:1000) (p-FoxO3a, Ser253, Cell Signaling, Beverly, MA), a rabbit antibody against total FoxO3a, a rabbit monoclonal antibody against phospho-Bad (Ser136, 1:1000, Cell Signaling, Beverly, MA), a rabbit antibody against cleaved (active) caspase 1 (20 kDa) (1:1000), a rabbit antibody against cleaved (active) caspase 3 (17 kDa) (1:1000) (Cell signaling Technology, Beverly, MA), or a primary rabbit anti-NF-κB p65 antibody (1:200) (Santa Cruz Biotechnologies, Santa Cruz, CA). Following washing, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody goat anti-rabbit IgG (1:2000, Zymed Laboratories, Carlsbad, CA). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
2.8 Gene knockdown of FoxO3a and NF-κB with small interfering RNA (siRNA)
To silence FoxO3a gene expression, the following sequences were synthesized (Applied Biosystems, Foster City, CA): the FoxO3a siRNA sense strand 5′-CGGACAAACGGCUCACUUUtt-3′ and the antisense strand 5′-AAAGUGAGCCGUUUGUCCGgg-3′. To silence NF-kB gene expression, the following sequences were synthesized (Applied Biosystems, Foster City, CA): the NF-kB siRNA sense strand 5′-CUUGGUCAAUCUCAAGAUAtt-3′ and the antisense strand 5′-UAUCUUGAGAUUGACCAAGca-3′. Transfection of siRNA duplexes was performed with Lipofectamine 2000 reagent according to manufacturer guidelines (Invitrogen, Carlsbad, CA). Experimental assays were performed 72 hours post-transfection. For each siRNA assay, positive controls contain multiple siRNAs including the target siRNA and negative controls are absent of the target siRNA.
2.9 Assessment of mitochondrial membrane potential
The fluorescent probe JC-1 (Molecular Probes, Eugene, OR), a cationic membrane potential indicator, was used to assess the mitochondrial membrane potential. Microglia in 35 mm dishes were incubated with 2 μg/ml JC-1 in growth medium at 37 °C for 30 min. The cultures were washed three times using fresh growth medium. Mitochondria were then analyzed immediately under a Leitz DMIRB microscope (Leica, McHenry, IL, USA) with a dual emission fluorescence filter with 515-545 nm for green fluorescence and emission at 585-615 nm for red fluorescence [9].
2.10 Preparation of mitochondria for the analysis of cytochrome c release
After washing once with ice-cold PBS, cells were harvested at 10,000g for 15 min at 4°C and the resulting pellet was re-suspended in buffer A (20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 phenylmethylsulfonylfluoride) containing 250 mM sucrose and used as the mitochondrial fraction. The supernatant was subjected to ultracentrifugation at 50,000 g for 1 hour at 4 °C with the resultant supernatant used as the cytosolic fraction [9].
2.11 Immunocytochemistry for FoxO3a, NF-κB, caspase 3, and caspase 1
For immunocytochemical staining of FoxO3a, NF-κB, cleaved caspase 3 (active form), or cleaved caspase 1 (active form), microglia were fixed with 4% paraformaldehyde and permeabilized using 0.2% Triton X-100. Cells were then incubated with rabbit anti-FoxO3a (1:100, Cell Signaling Technology, Beverly, MA), rabbit anti-cleaved caspase 3 (1:200, Cell Signaling Technology, Beverly, MA), or rabbit anti-cleaved caspase 1 (1:200, Cell Signaling Technology, Beverly, MA) over night at 4°C and then with biotinylated anti-rabbit IgG (1:50, Vector laboratories) for 2 hours followed by Texas Red streptavidin (1:50, Vector laboratories) for 1 hour. Cells were washed in PBS, then stained with DAPI (Sigma, St. Louis, MO) for nuclear identification. FoxO3a, NF-κB, caspase 3, and caspase 1 proteins were imaged with fluorescence at the wavelengths of 565 nm (red) and 400 nm (DAPI nuclear staining).
2.12 Subcellular translocation of FoxO3a or NF-κB by western analysis
Microglial cells were initially homogenized. The cytoplasmic and nuclear proteins were subsequently prepared by using NE-PER nuclear and cytoplasmic extraction reagents according to the instructions of the manufacturer (Pierce, Rockford, IL). The expression of FoxO3a or NF-κB in the microglial nucleus and cytoplasm was determined by Western analysis. Each sample (50 μg/lane) was subjected to 7.5% SDS-polyacrylamide gel electrophoresis. After transfer, the membranes were incubated with a primary rabbit antibody against FoxO3a (1:1000) (Cell Signaling, Beverly, MA) or a primary rabbit anti-NF-κB p65 antibody (1:200, Santa Cruz Biotechnology, Santa Cruz, CA). After washing, the membranes were incubated with a horseradish peroxidase conjugated with a secondary antibody (goat anti-rabbit IgG, 1:2000) (Invitrogen, Carlsbad, CA). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
2.13 Statistical analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from 6 replicate experiments with the post-hoc Dunnett’s test. Statistical significance was considered at p<0.05.
3. Results
3.1 Oxygen-glucose deprivation (OGD) leads to reduced Wnt1 expression in microglia
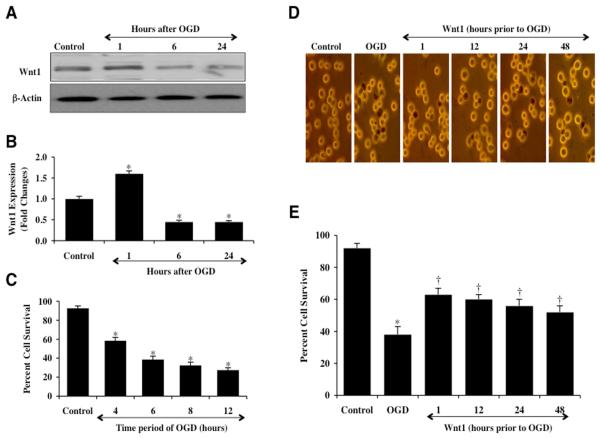
Western blot assay was performed for Wnt1 protein expression at 1, 6, and 24 hours following exposure to OGD that was applied for a 6 hour period. As shown in Fig. 1A, OGD initially increased the expression of Wnt1 at 1 hour when compared to control, but a significant decrease in Wnt1 expression in microglia was observed within 6 hours following OGD exposure that was less than expression during untreated control studies. Within 24 hours post OGD exposure, expression of Wnt1 was further reduced when compared to untreated control cell levels (Fig. 1A), suggesting that Wnt1 is degraded during OGD exposure. Quantitative results demonstrate that Wnt1 expression is significantly increased 1.7 fold at 1 hour following OGD, but is significantly decreased to approximately 0.5 fold below control levels at 6 hours and 24 hours after OGD exposure (Fig. 1B).
Fig. 1. Exogenous Wnt1 is protective against OGD injury in microglia that blocks endogenous Wnt1 expression.
(A and B) Microglial protein extracts (50 μg/lane) were immunoblotted with anti-Wnt1 (Wnt1) at 1, 6, and 24 hours following OGD exposure. Wnt1 expression is initially elevated at 1 hour, but then progressively and significantly is reduced at 6 and 24 hours following OGD exposure (*P<0.01 vs. control). (C) Microglia were exposed to progressive durations of OGD at 4, 6, 8, and 12 hours and microglial survival was determined 24 hours later by trypan blue dye exclusion assay. Microglial survival was significantly decreased to 58 ± 4% (4 hours), 38 ± 4% (6 hours), 32 ± 4% (8 hours), and 27 ± 3% (12 hours) following OGD exposure when compared with untreated control cultures (92 ± 3%, *P <0.01 vs. Control). Each data point represents the mean and SEM from 6 experiments. (D and E) Wnt1 (100 ng/ml) was administered 1, 12, 24, or 48 hours prior to a 6 hour period of OGD. Cell survival was determined 24 hours after OGD exposure through the trypan blue dye exclusion method. Representative images illustrate decreased trypan blue staining during Wnt1 application at each time period. Quantification of data demonstrates that OGD significantly decreased percent cell survival when compared to the control cells. Wnt1 significantly increased cell survival at each time period, but application of Wnt1 closest to the point of injury at the 1 hour period yielded the greatest degree of cytoprotection for microglia (*P<0.01 vs. control; †P<0.01 vs. OGD). Each data point represents the mean and SEM from 6 experiments. Control = untreated microglia.
3.2 Wnt1 protein protects microglial cell injury against OGD during abbreviated as well as during prolonged application periods
We initially investigated microglial survival after exposure to OGD at various periods of exposure of 4 hours, 6 hours, 8 hours, and 12 hours. Cell survival was assessed with trypan blue exclusion 24 hours after OGD exposure. As shown in Fig. 1C, microglial survival was significantly reduced over progressive times following OGD application to 58 ± 4% (4 hours), 34 ± 4% (6 hours), 32 ± 4% (8 hours), and 27 ± 3% (12 hours) when compared with untreated control cultures (92 ± 3%, p<0.01). Since OGD exposure for a period of 6 hours resulted in survival rate of approximately 35-40% (a 60% microglial cell loss), this duration of OGD was used for the reminder of the experimental paradigms.
We next examined the ability of Wnt1 to alter microglial cell injury following OGD exposure. Administration of Wnt1 protein (100 ng/ml), a concentration that is minimally sufficient for cytoprotection in other cell systems [19] and leads to the induction of Wnt signaling pathways [19, 25], at 48 hours, 24 hours, 12 hours, and 1 hour prior to OGD exposure significantly reduced trypan blue uptake in microglia 24 hours following OGD administration (Fig. 1D). On further analysis, Wnt1 increased cell survival to 63 ± 4% at 1 hour prior to OGD, to 60 ± 3% at 12 hours prior to OGD, to 56 ± 4% at 24 hours prior to OGD, and to 52 ± 4% at 48 hours to OGD (Fig. 1E), demonstrating that applications of Wnt1 closest to the point of OGD exposure yielded the greatest degree of cytoprotection for microglia although exposure to Wnt1 48 hours prior to OGD also continued to provide significant protection. We therefore utilized a 1 hour application of Wnt1 prior to OGD exposure for subsequent studies.
3.3 Wnt1 is necessary for protection against cell injury and apoptotic early phosphatidylserine (PS) exposure and later nuclear DNA degradation following OGD exposure
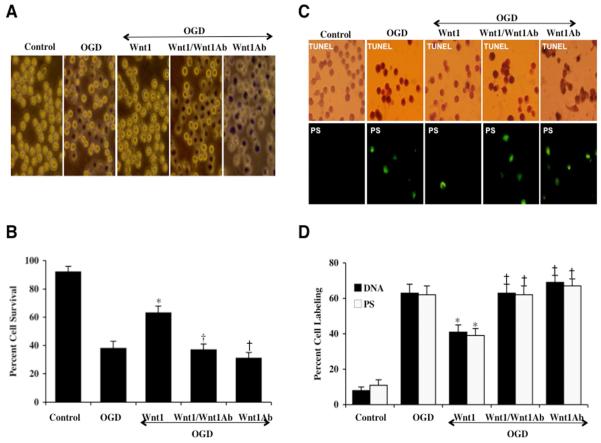
In representative Figs. 2A and 2C, untreated control microglia do not exhibit trypan cell injury, DNA fragmentation assessed by TUNEL, or PS externalization assessed by annexin V. Following exposure to OGD for a 6 hour period, significant trypan blue cell injury, DNA fragmentation (TUNEL), and membrane PS exposure (annexin V) is present 24 hours after exposure in microglia. In Figs. 2B and 2D, twenty-four hours after OGD exposure led to a significant loss in cell survival (38 ± 5%), percent DNA fragmentation (62 ± 5%), and membrane PS exposure (62 ± 5%) when compared to untreated control cultures for cell survival (92 ± 4%), DNA fragmentation (8 ± 2%), and for PS externalization (11 ± 3%) respectively. During Wnt1 protein (100 ng/ml) administration 1 hour prior to OGD, cell survival was markedly increased and DNA fragmentation and membrane PS exposure were significantly reduced with cell survival equal to 63 ± 5% and DNA fragmentation decreasing from 63 ± 5% (OGD alone) to 41 ± 4% and with apoptotic PS exposure decreasing from 62 ± 5% (OGD alone) to 39 ± 4% (Figs 2A – 2D).
Fig. 2. Blockade of Wnt1 worsens microglial injury while application of exogenous Wnt1 prevents apoptotic early phosphatidylserine (PS) exposure and subsequent nuclear DNA degradation.
(A and B) Microglial cells were exposed to a 6 hour period of OGD and microglial survival was determined 24 hours later by trypan blue assay. Representative images illustrate increased trypan blue staining during OGD and during blockade of Wnt1 with Wnt1Ab (1 μg/ml) and combined Wnt1 (100 ng/ml) administration. Wnt1 (100 ng/ml) administration alone significantly increased microglial survival during OGD. In addition, Wnt1Ab (1 μg/ml) alone markedly decreased microglial survival during OGD to a greater degree than OGD alone, suggesting that an endogenous level of Wnt1 in microglia is protective against cell injury. In all cases control = untreated microglia (*P<0.01 vs. OGD; †P <0.01 vs. Wnt1/OGD). Each data point represents the mean and SEM from 6 experiments. (C and D) Representative images illustrate that Wnt1 (100 ng/ml) administration during OGD significantly blocks microglial genomic DNA degradation assessed by TUNEL and membrane PS externalization assessed by annexin V phycoerythrin (green fluorescence). In contrast, blockade of Wnt1 with Wnt1Ab (1 μg/ml) resulted in increased DNA fragmentation and membrane PS exposure and higher apoptotic injury than OGD alone in the presence of Wnt1Ab (1 μg/ml) only, suggesting that an endogenous level of Wnt1 also provides protection against apoptotic early and late programs. Quantification of data illustrates that DNA fragmentation and membrane PS externalization were significantly increased following OGD for a 6 hour period when compared to untreated microglial control cultures, but Wnt1 (100 ng/ml) prevents DNA fragmentation and membrane PS exposure during OGD (*P<0.01 vs. OGD; †P <0.01 vs. Wnt1/OGD). Inhibition of Wnt1 with Wnt1Ab (1 μg/ml) significantly worsens apoptotic injury. Each data point represents the mean and SEM from 6 experiments.
We next examined whether specific antagonism against exogenous Wnt1 application with the Wnt1Ab could neutralize the protective capacity of Wnt1 during OGD. In the presence of the Wnt1Ab (1 μg/ml), the protective capacity of Wnt1 was significantly reduced yielding microglial survivals of 37 ± 4% (p<0.01) when compared to a survival of 63 ± 5% in microglia with Wnt1 only treatment 24 hours following OGD (Figs. 2A and 2B). In these studies with exposure to OGD, application of the Wnt1Ab 1 μg/ml alone also significantly decreased microglial survival when compared to cultures treated with OGD alone, suggesting that endogenous Wnt1 also provides a minimum level of protection to microglia. In a similar manner, co-application of the Wnt1Ab (1 μg/ml) with Wnt1 resulted in an increase in percent DNA fragmentation and percent membrane PS exposure in microglia during OGD, illustrating the necessity of the Wnt1 pathway for protection in microglia (Figs. 2C, 2D). Furthermore, application of Wnt1Ab (1 μg/ml) alone significantly increased trypan blue (TB) and DNA fragmentation (DNA) labeling when compared to OGD exposure alone, illustrating the cytoprotective capacity of endogenous Wnt1 in microglia. Administration of an antibody to Wnt1 (Wnt1Ab, 1 μg/ml) alone did not significantly alter microglial survival when compared to untreated control cultures (data not shown).
3.4 Wnt1 maintains inhibitory phosphorylation of p-FoxO3a and governs subcellular trafficking of FoxO3a
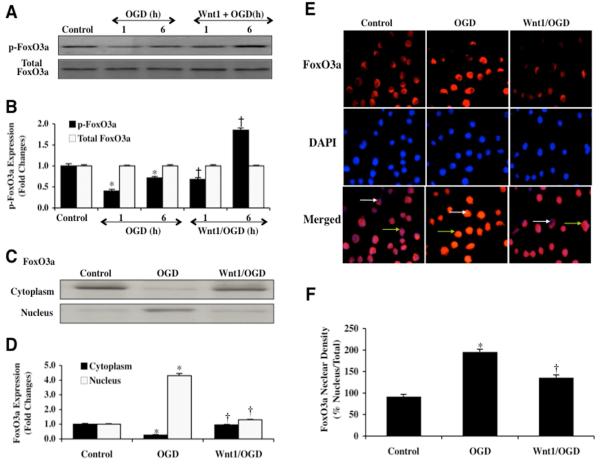
Western blot assay was performed for phosphorylated FoxO3a (p-FoxO3a) at the preferential phosphorylation site of Ser253 for protein kinase B (Akt), a regulatory pathway for FoxO3a that is controlled by Wnt1 [20, 26], and as well as for the expression of total FoxO3a (Figs. 3A and 3B). Following OGD exposure, expression of phosphorylated (inactive) p-FoxO3a was decreased at 1 and 6 hours, suggesting increased activity of FoxO3a. Total FoxO3a expression remained unchanged. However, application of Wnt1 protein (100 ng/ml) increased expression of phosphorylated (inactive) p-FoxO3a significantly when compared to OGD exposure alone, especially at the 6 hour time period, illustrating that Wnt1 maintains expression of inactive p-FoxO3a during OGD.
Fig. 3. Wnt1 maintains inhibitory phosphorylation of p-FoxO3a and prevents nuclear translocation of FoxO3a in microglia during OGD.
In A and B, microglial protein extracts (50 μg/lane) were immunoblotted with anti-phosphorylated-FoxO3a (p-FoxO3a, Ser253) or anti-total FoxO3a at 1 and 6 hours following OGD exposure. Quantification of western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image). During OGD, phosphorylated (inactive) FoxO3a (p-FoxO3a) expression is significantly decreased at 1 hour and 6 hours following OGD, but total FoxO3a expression not affected illustrating that FoxO3a protein is intact but post-translational phosphorylation has been changed (*P<0.01 vs. control). Wnt1 (100 ng/ml) administration increases phosphorylation of FoxO3a at 1 hour when compared to this time period with OGD only and significantly increases inhibitory phosphorylation of FoxO3a at 6 hours following OGD exposure (*P<0.01 vs. untreated microglia = Control; †P <0.01 vs. OGD at 1 hour). In C and D, equal amounts of cytoplasmic (cytoplasm) or nuclear (nucleus) protein extracts (50 μg/lane) were immunoblotted with anti-FoxO3a at 6 hours following administration of OGD. At 6 hours following OGD alone, FoxO3a translocates from the cytoplasm to the nucleus. In contrast, Wnt1 (100 ng/ml) prevents trafficking of FoxO3a from the cytoplasm to the cell nucleus in microglia (*P<0.01 vs. untreated microglia = Control; †P <0.01 vs. OGD). In E and F, microglia were imaged 6 hours following OGD with immunofluorescent staining for FoxO3a (Texas-red streptavidin). Nuclei of microglia were counterstained with DAPI. In merged images, untreated control microglia have visible nuclei (dark blue in color, white arrows) that illustrate absence of FoxO3a in the nucleus. Merged images after OGD demonstrate microglia with red cytoplasm (green arrows) and no visible nucleus with DAPI illustrating translocation of FoxO3a to the nucleus. Wnt1 (100 ng/ml) application during OGD maintains FoxO3a in the cytoplasm of microglia (*P<0.01 vs. untreated microglia = Control; †P <0.01 vs. OGD). In D and F, quantification of the intensity of FoxO3a nuclear staining or FoxO3a western expression was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image). Each data point represents the mean and SEM from 6 experiments.
Post-translational phosphorylation of FoxO3a results in the association with 14-3-3 proteins to sequester FoxO3a in the cytoplasm and block the nuclear transcription of “pro-apoptotic” proteins [27, 28]. Since Wnt1 can maintain the post-translational phosphorylation of FoxO3a, we next examined whether Wnt1 controls subcellular trafficking of FoxO3a. We performed immunofluorescent staining for FoxO3a and DAPI nuclear staining to follow the subcellular translocation of FoxO3a 6 hours after OGD (Figs. 3E and 3F). During OGD exposure alone, immunofluorescent staining for FoxO3a in the nucleus of microglia is significant. This is evident by the inability to visualize DAPI nuclear staining (blue in color) in cells during merged images since prominent FoxO3a staining (red in color) is present in the nucleus (Figs. 3E and 3F). Yet, administration of Wnt1 (100 ng/ml) prevents the translocation of FoxO3a to the cell nucleus and maintains FoxO3a in the cytoplasm of cells similar to untreated control cells demonstrating minimal nuclear staining as shown with DAPI staining (blue nuclei in color) in the nucleus merged images (Figs. 3E and 3F). We complemented the immunofluorescent studies with assessment of FoxO3a subcellular translocation from the cell cytoplasm to the nucleus through western analysis (Figs. 3C and 3D). Consistent with our immunofluorescent work, administration of Wnt1 (100 ng/ml) maintains FoxO3a in the cytoplasm of microglia similar to untreated control microglia.
3.5 Gene knockdown of FoxO3a prevents microglial injury and increases survival similar to Wnt1 during OGD
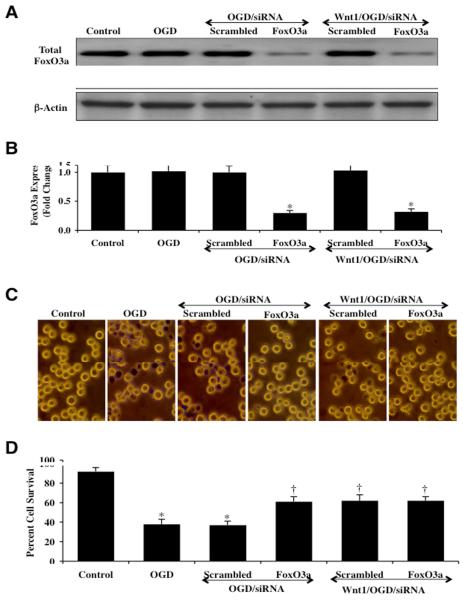
Microglia were transfected with FoxO3a siRNA and the expression of total FoxO3a protein was documented with Western blot analysis 6 hours following OGD exposure (Figs. 4A and 4B). Transient gene knockdown of FoxO3a in cells exposed to OGD alone or OGD with Wnt1 (100 ng/ml) administration resulted in markedly reduced or absent expression of total FoxO3a (Figs. 4A and 4B). As a control, non-specific scrambled FoxO3a siRNA did not alter total FoxO3a expression in cells exposed to OGD alone or OGD with Wnt1 (100 ng/ml) administration. Representative figures illustrate significant trypan blue staining in microglia 24 hours after OGD exposure alone or with OGD during scrambled (non-specific) siRNA (Fig. 4C). In contrast, significantly reduced trypan blue uptake is present in microglia following OGD with FoxO3a siRNA transfection or during Wnt1 (100 ng/ml) administration with gene knockdown of FoxO3a (Fig. 4C), demonstrating that the presence of FoxO3a contributes to microglial injury during OGD. On further analysis in Fig. 4D, microglial survival was increased from 39 ± 5% during OGD alone to 61 ± 5% (P<0.01) during gene knockdown of FoxO3a and to 62 ± 4% (P<0.01) during gene knockdown of FoxO3a with Wnt1 (100 ng/ml) administration. Transfection with scrambled siRNA did not alter microglial injury during OGD or during OGD with Wnt1 (100 ng/ml) administration.
Fig. 4. Gene knockdown of FoxO3a increases microglial survival similar to Wnt1 during OGD.
In A and B, microglial protein extracts (50 μ/lane) were immunoblotted with anti-total FoxO3a at 6 hours following OGD. Gene knockdown of FoxO3a was performed with transfection of FoxO3a siRNA (siRNA). FoxO3a siRNA significantly reduced expression of total FoxO3a following a 6 hour period of OGD or during Wnt1 (100 ng/ml) application with OGD, but non-specific scrambled siRNA did not alter total FoxO3a expression (*P<0.01 vs. OGD). Quantification of the western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image). In C and D, gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly increased microglial survival and decreased microglial membrane injury assessed by trypan blue staining 24 hours after OGD in representative figures and quantitative analysis. In addition, significantly increased microglial cell survival is present during Wnt1 (100 ng/ml) administration with gene knockdown of FoxO3a similar to gene knockdown of FoxO3a alone during OGD. FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not protect cells during OGD (*P<0.01 vs. untreated microglia = Control; †P <0.01 vs. OGD). Each data point represents the mean and SEM from 6 experiments.
3.6 Wnt1 blocks apoptotic early phosphatidylserine (PS) exposure and later nuclear DNA degradation and relies upon p-FoxO3a inhibition for microglial protection
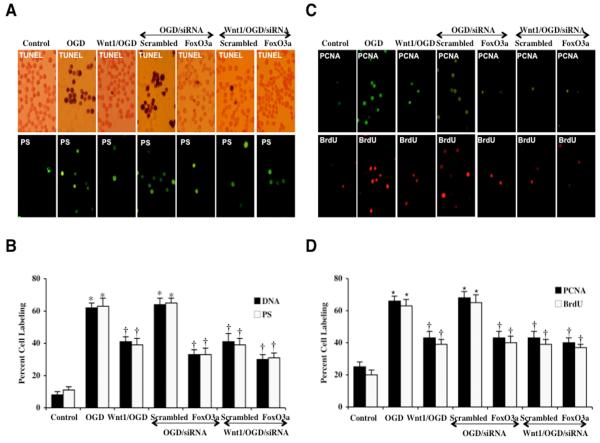
In representative Fig. 5A, untreated control microglia were without DNA fragmentation assessed by TUNEL or significant PS externalization assessed by annexin V. In microglia exposed to OGD, significant DNA fragmentation (TUNEL) and membrane PS exposure (annexin V) occurs 24 hours later. In Figs. 5A and 5B, OGD results in a significant increase in percent DNA fragmentation and membrane PS exposure in microglia 24 hours after OGD compared to untreated control cultures for DNA (8 ± 2%) and for PS (11 ± 2%) respectively. However, during gene knockdown of FoxO3a with siRNA, microglial DNA fragmentation and membrane PS exposure were significantly prevented, demonstrating that FoxO3a is necessary for apoptotic programs during OGD (Figs. 5A and 5B). In addition, combined administration of Wnt1 (100 ng/ml) during gene knockdown of FoxO3a also resulted in a similar degree of protection against DNA fragmentation and membrane PS exposure in microglia during OGD when compared to gene knockdown of FoxO3a alone, suggesting that Wnt1 requires FoxO3a inhibition for the prevention of apoptotic programs (Figs. 5A and 5B). Transfection with non-specific scrambled siRNA did not alter DNA fragmentation or PS exposure in microglia during OGD alone or during Wnt1 administration.
Fig. 5. Gene knockdown of FoxO3a prevents apoptotic DNA fragmentation and phosphatidylserine (PS) exposure through FoxO3a during OGD.
In A, representative images illustrate gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly blocks microglial genomic DNA degradation assessed by TUNEL and membrane PS externalization assessed by annexin V phycoerythrin (green fluorescence) 24 hours after OGD. Non-specific scrambled siRNA did not alter DNA fragmentation or membrane PS exposure. In addition, combined administration of Wnt1 (100 ng/ml) during gene knockdown of FoxO3a also resulted in a similar degree of protection against DNA fragmentation and membrane PS exposure in microglia when compared to gene knockdown of FoxO3a alone, suggesting that Wnt1 requires FoxO3a inhibition for the prevention of apoptotic programs. In B, quantification of data illustrates that DNA fragmentation and membrane PS externalization were significantly increased following OGD when compared to untreated microglial control cultures, but transfection of FoxO3a siRNA alone or in combination with Wnt1 (100 ng/ml) prevents DNA fragmentation and membrane PS exposure during OGD (*P<0.01 vs. untreated microglia = Control; †P <0.01 vs. OGD). FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not protect cells during OGD. Each data point represents the mean and SEM from 6 experiments. In C, representative images illustrate that PCNA and BrdU expression is significantly and rapidly increased in microglia at 6 hours after OGD. Wnt1 (100 ng/ml) alone or in combination with gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly decreases the expression of PCNA and the uptake of BrdU at 6 hours after OGD. In D, quantification of data demonstrates that PCNA and BrdU were significantly increased following OGD (*p<0.01 vs. untreated microglia = control). In addition, Wnt1 (100 ng/ml) alone or in combination with gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) markedly reduces the expression of PCNA and the uptake of BrdU at 6 hours after OGD (*P<0.01 vs. untreated microglia = control; †P <0.01 vs. OGD). FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not alter PCNA expression or BrdU uptake during OGD (†P <0.01 vs. OGD). In all cases, control = untreated cells. Each data point represents the mean and SEM from 6 experiments.
3.7 Loss of FoxO3a can block the early activation and proliferation of microglia
In Fig. 5C, a significant increase in microglial activation and proliferation following OGD at 6 hours is evident through increased PCNA expression and BrdU uptake. In contrast, untreated control cells are without significant PCNA expression or BrdU uptake. In Fig. 5D, quantification of PCNA labeling demonstrates significant expression in PCNA at 6 hours (66 ± 3%, p<0.01) compared to control microglia (25 ± 3%). BrdU uptake also was significantly increased to 63 ± 4% at 6 hours following OGD compared to untreated control cells (20 ± 3%). Yet, PCNA expression and BrdU uptake was significantly prevented in microglia exposed to Wnt1 protein (100 ng/ml) administration 1 hour prior to OGD (Figs. 5C and 5D). Wnt1 decreased PCNA expression from 66 ± 3% (OGD alone) to 43 ± 4% during OGD and decreased BrdU uptake from 63 ± 4% (OGD alone) to 39 ± 3% during OGD, demonstrating the ability of Wnt1 to limit the activation and proliferation of microglia.
We next examined the role of FoxO3a during microglial activation and proliferation. In Figs. 5C and 5D, representative figures demonstrate a significant decrease in microglial activation with minimal PCNA expression and a marked reduction in microglial proliferation with minimal BrdU uptake during FoxO3a siRNA transfection and OGD exposure. Furthermore, application of Wnt1 (100 ng/ml) during gene knockdown of FoxO3a led to a similar level of blockade for microglial activation and proliferation when compared to gene knockdown of FoxO3a alone, illustrating that Wnt1 employs the inhibition of FoxO3a to control the early activation and proliferation of microglia (Figs. 5C and 5D). Non-specific scrambled siRNA transfection did not alter PCNA expression or BrdU uptake during OGD or during Wnt administration, illustrating the ability of FoxO3a to lead to early activation and proliferation in microglia.
3.8 Wnt1 through FoxO3a prevents mitochondrial depolarization, cytochrome c release, and activation of Bad
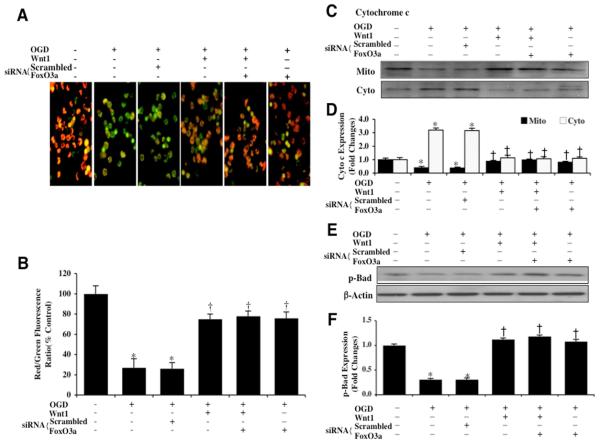
During OGD exposure, mitochondrial depolarization in microglia was assessed by the cationic membrane potential indicator JC-1. In Figs. 6A and 6B, OGD exposure produced a significant decrease in the microglial mitochondrial red/green fluorescence intensity ratio within 6 hours (27 ± 9%) when compared to untreated control mitochondria (100 ± 8%), demonstrating that OGD results in mitochondrial membrane depolarization. Administration of Wnt1 (100 ng/ml) in the presence of OGD significantly increased the red/green fluorescence intensity of the mitochondria to 75 ± 5%, illustrating that Wnt1 can significantly improve mitochondrial permeability transition pore membrane potential (Figs. 6A and 6B). Furthermore, gene knockdown of FoxO3a in microglia alone or during Wnt1 (100 ng/ml) administration significantly increased the red/green fluorescence intensity of the mitochondria (siRNA FoxO3a + Wnt1, 78 ± 5%), indicating that mitochondrial permeability transition pore membrane potential was markedly improved during OGD to a similar degree when compared with Wnt1 administration alone (Figs. 6A and 6B). In contrast, non-specific scrambled siRNA during OGD did not prevent mitochondrial membrane depolarization, suggesting that FoxO3a is required for OGD to lead to mitochondrial membrane depolarization.
Fig. 6. Wnt1 through FoxO3a prevents mitochondrial depolarization, blocks the release of cytochrome c, and maintains activation of Bad.
In A, OGD leads to a significant decrease in the red/green fluorescence intensity ratio of mitochondria using the cationic membrane potential indicator JC-1 within 6 hours when compared with untreated control microglial cells, demonstrating that OGD leads to mitochondrial membrane depolarization. Application of Wnt1 (100 ng/ml) during OGD significantly increased the red/green fluorescence intensity of mitochondria in microglia, illustrating that mitochondrial membrane potential was restored. Furthermore, transfection of FoxO3a siRNA alone or in combination with Wnt1 (100 ng/ml) also maintained mitochondrial membrane potential similar to Wnt1 administration alone. Transfection with non-specific scrambled siRNA did not prevent mitochondrial membrane depolarization during OGD. In B, the relative ratio of red/green fluorescent intensity of mitochondrial staining in untreated control microglia, in microglia exposed to a 6 hour period of OGD, during Wnt1 administration or during Wnt1 administration with gene knockdown of FoxO3a, or during FoxO3a gene knockdown alone was measured in 6 independent experiments with analysis performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) (untreated microglia = Control vs. OGD, *P<0.01; Wnt1 or Wnt1 plus siRNA FoxO3a vs. OGD, †P<0.01). In C, equal amounts of mitochondrial (mito) or cytosol (cyto) protein extracts (50 μg/lane) were immunoblotted demonstrating that Wnt1 (100 ng/ml) administration alone, in combination with gene knockdown of FoxO3a, or gene knockdown of FoxO3a alone significantly prevented cytochrome c release from mitochondria 6 hours after OGD. Non-specific scrambled siRNA did not prevent cytochrome c release during OGD. In D, quantification of the western band intensity was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image) and demonstrates that significant release of cytochrome c occurs 6 hours following OGD, but Wnt1 (100 ng/ml) administration alone or during Wnt1 administration with gene knockdown of FoxO3a prevents cytochrome c release from microglial mitochondria. Non-specific scrambled siRNA was ineffective during OGD to prevent cytochrome c release (untreated microglia = Control vs. OGD, *P<0.01; Wnt1 or Wnt1 plus siRNA FoxO3a vs. OGD, †P<0.01). Each data point represents the mean and SEM from 6 experiments. In E and F, primary microglial protein extracts (50 μ/lane) were immunoblotted with anti-phosphorylated-Bad (p-Bad, Ser136) at 6 hours following OGD. Phosphorylated Bad (p-Bad) expression is promoted by Wnt1 (100 ng/ml) administration alone, during Wnt1 administration with gene knockdown of FoxO3a, or during gene knockdown of FoxO3a alone, but is significantly diminished during OGD alone. Non-specific scrambled siRNA during OGD did not change Bad phosphorylation and was similar to Bad phosphorylation during OGD alone (untreated microglia = Control vs. OGD, *P<0.01; Wnt1 or Wnt1 plus siRNA FoxO3a vs. OGD, †P<0.01).
OGD also produced a significant release of cytochrome c from the mitochondria to a greater than 3.0 fold increase when compared to untreated control mitochondria using western analysis during OGD (Figs. 6C and 6D). Wnt1 (100 ng/ml) administration prevented the release of cytochrome c during OGD exposure. Interestingly, gene knockdown of FoxO3a alone or during Wnt1 application also prevented cytochrome c release to a similar degree that occurs with Wnt1 administration alone, illustrating that Wnt1 maintains mitochondrial membrane function through blockade of FoxO3a (Figs. 6C and 6D).
Western blot assay was performed for phosphorylated Bad (p-Bad) at the preferential phosphorylation site of Ser136 for Akt, a principal modulatory pathway for Wnt1 [20] (Figs. 6E and 6F). At 6 hours following OGD exposure, Bad phosphorylation was significantly reduced. Administration of Wnt1 (100 ng/ml) in the presence of OGD significantly increased the phosphorylation of Bad at 6 hours. Furthermore, loss of FoxO3a during gene knockdown alone or in combination with Wnt1 continued to maintain the phosphorylation of Bad similar to Wnt1 alone during exposure to OGD, suggesting that Wnt1 through inhibition of FoxO3a activity controls Bad (Figs. 6E and 6F).
3.9 Wnt1 governs caspase 3 and caspase 1 activation during OGD that depends upon FoxO3a inhibition
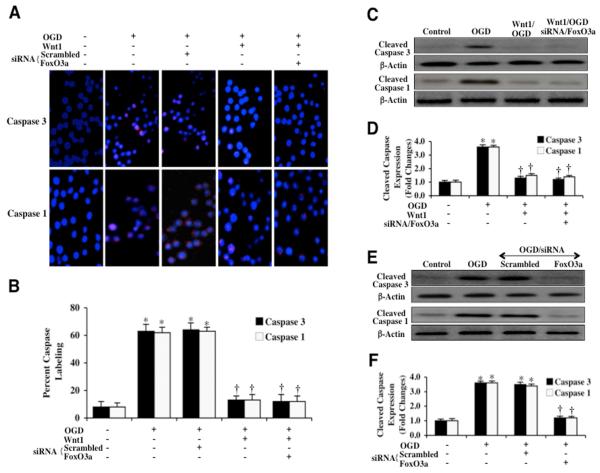
Within 6 hours following OGD exposure, immunocytochemistry reveals significant cleaved (active) caspase 3 (blue/red staining) (Figs. 7A and 7B) or cleaved (active) caspase 1 (blue/red staining) (Figs. 7A and 7B) during OGD. In contrast, Wnt1 (100 ng/ml) administration in the presence of OGD significantly blocks caspase 3 activity (Figs. 7A and 7B) and caspase 1 activity (Figs. 7A and 7B) as evidenced by primarily blue immunocytochemical staining and by reducing the percentage of cleaved caspase 3 labeling to 13 ± 3% from 63 ± 3% (Figs. 7A and 7B) or by reducing the percentage of cleaved caspase 1 labeling to 13 ± 4% from 62 ± 4% (Figs. 7A and 7B). In addition, loss of FoxO3a with siRNA transfection and combined administration of Wnt1 (100 ng/ml) resulted in a similar degree of caspase 3 (Figs. 7A and 7B) or caspase 1 (Figs. 7A and 7B) inhibition compared to Wnt1 application alone, suggesting that Wnt1 requires inhibition of FoxO3a to prevent caspase 3 activity during OGD.
Fig. 7. Wnt1 controls caspase 1 and caspase 3 during OGD that is dependent upon FoxO3a.
In A, microglia were exposed to OGD and caspase 3 and caspase 1 activation were determined 6 hours after OGD period through immunocytochemistry with antibodies against cleaved active caspase 3 (17 kDa) and cleaved active caspase 1 (20 kDa). Representative images illustrate active caspase 3 staining or caspase 1 staining (red) in cells following OGD, but cellular red staining is almost absent during Wnt1 (100 ng/ml) administration alone or during Wnt1 administration with gene knockdown of FoxO3a. Non-specific scrambled siRNA did not eliminate caspase 3 or caspase 1 activity during OGD. In B, quantification of caspase 3 and caspase 1 immunocytochemistry was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) and demonstrates that OGD significantly increased the expression of cleaved (active) caspase 3 or caspase 1 when compared to untreated control cells. Expression of cleaved (active) caspase 3 or caspase 1 was significantly limited during Wnt1 (100 ng/ml) administration alone or during Wnt1 administration with gene knockdown of FoxO3a. (*P <0.01 vs. untreated microglia = Control; †P<0.01 vs. OGD). In C, microglial protein extracts (50 μg/lane) were immunoblotted with anti-cleaved caspase 3 product (active caspase 3, 17 kDa) and with anti-cleaved caspase 1 product (active caspase 1, 20 kDa) at 6 hours following OGD. OGD markedly increased cleaved caspase 3 and caspase 1 expression, but Wnt1 (100 ng/ml) administration alone or during Wnt1 administration with gene knockdown of FoxO3a significantly blocked cleaved caspase 3 and caspase 1 expression 6 hours after OGD. Non-specific scrambled siRNA did not eliminate caspase 3 or caspase 1 activities during OGD. In D, quantification of western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) and demonstrates that Wnt1 (100 ng/ml) application alone or Wnt1 administration in combination with gene knockdown of FoxO3a prevents cleaved caspase 3 and caspase 1 expression 24 hours after OGD (*P <0.01 vs. untreated microglia = Control; †P<0.01 vs. OGD). Each data point represents the mean and SEM from 6 experiments. In E, microglial protein extracts (50 μg/lane) were immunoblotted with anti-cleaved caspase 3 product (active caspase 3, 17 kDa) and with anti-cleaved caspase 1 product (active caspase 1, 20 kDa) at 24 hours following OGD. OGD markedly increased cleaved caspase 3 and caspase 1 expression, but gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly blocks cleaved (active) caspase 3 and caspase 1 activities 24 hours after OGD. Non-specific scrambled siRNA did not eliminate caspase 3 or caspase 1 activities during OGD. In F, quantification of western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) and demonstrates that gene knockdown of FoxO3a prevents cleaved caspase 3 and caspase 1 expression 6 hours after OGD. In addition, non-specific scrambled siRNA did not change caspase 1 and caspase 3 activities when compared to OGD alone (*P <0.01 vs. untreated microglia = Control; †P<0.01 vs. OGD). Each data point represents the mean and SEM from 6 experiments.
In Figs. 7C and 7D, the expression of cleaved (active) caspase 3 and caspase 1 on western analysis were assessed and demonstrate significant caspase 3 and caspase 1 activities at 24 hours following OGD exposure. Wnt1 (100 ng/ml) administration significantly prevented the expression of cleaved caspase 3 and caspase 1 during OGD (Figs. 7C and 7D). Loss of FoxO3a during gene knockdown in the presence of Wnt1 (100 ng/ml) also blocked the expression of cleaved caspase 3 and caspase 1 during OGD similar to Wnt1 alone, suggesting the inhibition of FoxO3a by Wnt1 to control caspase activity (Figs. 7C and 7D).
We also investigated the ability of FoxO3a to control caspase activity at the 24 hour period following OGD in microglia. In Figs. 7E and 7F, expression of cleaved active caspase 3 and caspase 1 (Figs. 7E and 7F) is elevated almost 4 fold over untreated control microglial levels following OGD, but transfection with FoxO3a siRNA significantly blocks cleaved active caspase 3 and caspase 1 activities. Non-specific scrambled siRNA was not effective in reducing caspase 3 or caspase 1 activity during OGD, supporting the specific role for FoxO3a to control caspase 3 and caspase 1 activity during OGD in microglia (Figs. 7E and 7F).
3.10 Wnt1 fosters nuclear translocation of NF-κB that occurs during FoxO3a inhibition and employs NF-κB for cytoprotection
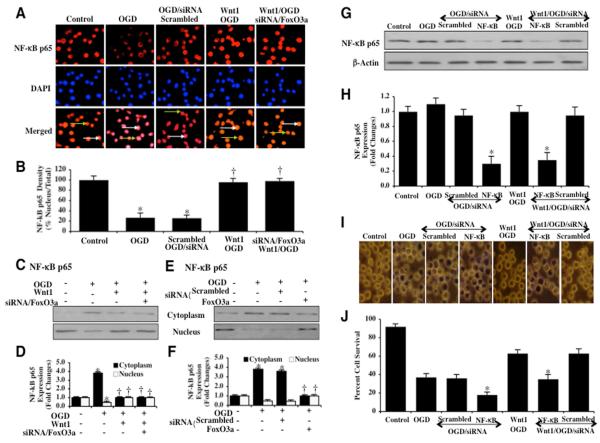
The phosphorylation of IκB proteins by the IκB kinase (IKK) and their subsequent degradation that lead to the release of NF-κB for its translocation to the nucleus to initiate gene transcription [29]. Yet, primary gene activation is controlled by the p65 member that consists of two potent transactivation domains within its C terminus [30]. As a result, we chose to examine whether OGD altered subcellular localization of NF-κB p65 in microglia. We used immunofluorescent staining for NF-κB p65 and DAPI nuclear staining to follow the translocation of NF-κB 6 hours after OGD. Untreated control microglia in merged images do not have visible nuclei (red in color, white arrows) that illustrate predominately nuclear localization of NF-κB. During OGD exposure, NF-κB was significantly confined to the cytoplasm of microglia shown by minimal nuclear staining with DAPI staining (pink nuclei in color) in the nucleus in microglia in merged images (Figs. 8A and 8B). In contrast, Wnt1 (100 ng/ml) administration during OGD promoted the translocation of NF-κB from the cytoplasm to the nucleus. To a similar degree, Wnt1 (100 ng/ml) with gene knockdown of FoxO3a also fostered the translocation of NF-κB from the cytoplasm to the nucleus, suggesting the common ability of Wnt1 or the absence of FoxO3a to traffic NF-κB to the nucleus (Figs. 8A and 8B). We also assessed NF-κB p65 subcellular translocation from the cell cytoplasm to the nucleus through western analysis (Figs. 8C, 8D, 8E, and 8F). During Wnt1 (100 ng/ml) administration with OGD (Figs. 8C and 8D), during gene knockdown of FoxO3a alone with OGD (Figs. 8E and 8F), or during Wnt1 (100 ng/ml) administration with gene knockdown of FoxO3a alone in the presence of OGD (Figs. 8C and 8D), NF-κB was maintained in the nucleus of microglia. In contrast, during OGD alone or during non-specific scrambled siRNA transfection, NF-κB remained in the cytoplasm of microglia (Figs. 8C, 8D, 8E, and 8F).
Fig. 8. Wnt1 promotes nuclear translocation of NF-κB that occurs with FoxO3a inhibition and relies upon NF-κB for cytoprotection.
In A and B, microglia were imaged 6 hours following OGD with immunofluorescent staining for NF-κB p65 (Texas-red streptavidin). Nuclei of microglia were counterstained with DAPI. In merged images, untreated control microglia do not have visible nuclei (red in color, white arrows) that illustrate the presence of NF-κB p65 in the nucleus. Merged images after OGD demonstrate microglia with visible nuclei (light pink in color, white arrows) and red cytoplasm (green arrows) demonstrating that NF-κB p65 is retained in the cytoplasm. Wnt1 (100 ng/ml) administration during OGD or during Wnt1 administration with gene knockdown of FoxO3a during OGD fosters the translocation of NF-κB p65 to the cell nucleus. Non-specific scrambled siRNA does not alter NF-κB p65 subcellular trafficking during OGD (*P<0.01 vs. untreated microglia = Control; †P <0.01 vs. OGD). In C and D, equal amounts of cytoplasmic (cytoplasm) or nuclear (nucleus) protein extracts (50 μg/lane) were immunoblotted with anti- NF-κB p65 at 6 hours following administration of OGD. NF-κB p65 is maintained in the cytoplasm of microglia during OGD. In contrast, Wnt1 (100 ng/ml) administration with OGD or during Wnt1 administration with gene knockdown of FoxO3a with OGD allows trafficking of NF-κB p65 from the cytoplasm to the cell nucleus in microglia (*P<0.01 vs. untreated microglia = Control; †P <0.01 vs. OGD). In E and F, equal amounts of cytoplasmic (cytoplasm) or nuclear (nucleus) protein extracts (50 μg/lane) were immunoblotted with anti-NF-κB p65 at 6 hours following administration of OGD. As previously shown, NF-κB p65 is maintained in the cytoplasm of microglia during OGD. Yet, gene knockdown of FoxO3a during OGD fosters trafficking of NF-κB p65 from the cytoplasm to the cell nucleus in microglia. Non-specific scrambled siRNA did not alter NF-κB p65 translocation during OGD alone (*P<0.01 vs. untreated microglia = Control; †P <0.01 vs. OGD). In B, D, and F, quantification of the intensity of FoxO3a nuclear staining or NF-κB p65 expression on western was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image). Each data point represents the mean and SEM from 6 experiments. In G and H, microglial protein extracts (50 μ/lane) were immunoblotted with anti- NF-κB p65 at 6 hours following OGD. Gene knockdown of NF-κB p65 was performed with transfection of NF-κB p65 siRNA (siRNA). NF-κB p65 siRNA significantly reduced expression of NF-κB p65 following a 6 hour period of OGD or during Wnt1 (100 ng/ml) application with OGD, but non-specific scrambled siRNA did not alter NF-κB p65 expression (*P<0.01 vs. OGD). Quantification of the western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image). In I and J, representative images and quantitative analysis show that gene knockdown of NF-κB p65 with NF-κB p65 siRNA (siRNA) significantly decreased microglial survival and increased microglial membrane injury assessed by trypan blue staining 24 hours after OGD. Loss of microglial cell survival also is present during Wnt1 (100 ng/ml) administration with gene knockdown of NF-κB p65 during OGD. In addition, gene knockdown of NF-κB during OGD alone resulted in increased cell injury when compared to microglial injury during OGD alone, illustrating that endogenous levels of NF-κB are cytoprotective during oxidative stress. Transfection with scrambled siRNA did not alter microglial injury during OGD or during OGD with Wnt1 (100 ng/ml) administration (*P<0.01 vs. OGD).
In Figs. 8G, 8H, 8I, and 8J, microglia were transfected with NF-κB p65 siRNA prior to OGD and western blot analysis for NF-κB p65 was performed 6 hours following OGD. Gene knockdown of NF-κB p65 in cells exposed to OGD alone or OGD with Wnt1 (100 ng/ml) administration resulted in significantly diminished or absent expression of NF-κB p65 (Figs. 8G and 8H). As a control, non-specific scrambled NF-κB siRNA did not alter NF-κB p65 expression in cells exposed to OGD alone or OGD with Wnt1 (100 ng/ml) administration. With representative figures and analysis of cell survival (Figs. 8I and 8J), microglial cell survival was significantly decreased by NF-κB p65 gene silencing during OGD exposure, demonstrating that NF-κB p65 is an important component for endogenous microglial cell protection during OGD injury. Furthermore, microglial survival was decreased from 63 ± 4% during Wnt1 (100 ng/ml) plus OGD to 35 ± 5% (P<0.01) during gene knockdown of NF-κB, illustrating that Wnt1 uses NF-κB for cytoprotection in microglia during OGD (Figs. 8I and 8J). In addition, gene knockdown of NF-κB during OGD alone resulted in increased cell injury when compared to microglial injury during OGD alone, demonstrating that endogenous levels of NF-κB are cytoprotective during oxidative stress (Figs. 8I and 8J). Transfection with scrambled siRNA did not alter microglial injury during OGD or during OGD with Wnt1 (100 ng/ml) administration.
4. Discussion
Inflammatory cells have a vital role in the recognition and disposal of non-functional neuronal and vascular cells to maintain and repair injured tissue in the central nervous system [2, 4, 29, 31-33]. As a result, elucidating novel pathways, such as Wnt1, that control the survival of microglial cells may foster the development of treatments for a host of neurodegenerative disorders. In general, Wnt proteins serve a variety of cellular functions that include the proliferation and enhancement of cellular survival [12, 16, 32, 34, 35]. Wnt signaling pathways also have been described in astrocytic cells [36], but the function that Wnt1 plays during microglial survival and proliferation remains unclear. During periods of cell injury, Wnt signaling can be depressed in bone tissue during ethanol-induced oxidative stress [37], in endothelial cells during elevated glucose [19], and in neurons during β-amyloid toxicity [20]. We demonstrate that Wnt1 expression is initially up-regulated within 1 hour following OGD in microglia, suggesting that an endogenous cellular response exists to increase Wnt1 expression during an insult from oxidative stress. However, at 6 and 24 hours following OGD, endogenous Wnt1 expression is significantly lost that correlates with injury in microglial cells over a 24 hour period.
In contrast, microglial cell injury can be prevented during the exogenous administration of the recombinant Wnt1 protein. Prior work has linked Wnt signaling to cellular survival and proliferation in several cell systems. These studies include the over-expression of Wnt1 in fibroblasts to protect lymphoid cells from apoptosis [38] as well as work that demonstrates that loss of the Wnt-β-catenin pathway results in increased infarct size during cerebral ischemia [39], cell death during excitotoxicity [40], and inhibition of cell proliferation during cancer [13]. In addition, Wnt1 over-expression blocks β-amyloid toxicity in neuronal cells [20] and exogenous recombinant Wnt1 protects cells against apoptosis during elevated glucose in endothelial cells [19]. Interestingly, we show that administration of Wnt1 closest to the point of OGD exposure yields the greatest degree of microglial protection, suggesting that resolution of early signal transduction pathways leading to cell injury by Wnt1 are most advantageous and can afford enhanced cellular survival. We also illustrate that Wnt1 can prevent both early apoptotic early PS externalization in neurons as well as genomic DNA degradation. The ability of Wnt1 to stop these apoptotic programs suggests an important checkpoint for the use of Wnt signaling to combat multiple disorders affected by programmed cell death [12]. Apoptotic externalization of membrane PS residues occurs significantly earlier than nuclear DNA degradation and promotes hypercoagulable states that lead to injury throughout the body [5].
Our work also demonstrates that Wnt1 is necessary for the protection of microglial cells during oxidative stress. Blockade of Wnt1 signaling with a Wnt1Ab abrogates protection by Wnt1 during OGD, illustrating that Wnt1 is required, at least as one component, to prevent cell injury, apoptotic PS exposure and DNA degradation. Furthermore, loss of Wnt1 signaling alone during OGD with administration of the Wnt1Ab worsens survival and apoptotic injury when compared to treatment with OGD alone, demonstrating that endogenous Wnt1 in microglial cells provides an important level of protection against oxidative stress.
One potential pathway for Wnt to oversee microglial cell survival during oxidative stress may involve the control of the activity and subcellular trafficking of the apoptotic forkhead transcription factor FoxO3a [26]. Post-translational phosphorylation of FoxO3a prevents association of FoxO3a with 14-3-3 proteins to allow FoxO3a to translocate to the cell nucleus and initiate a “pro-apoptotic” program [27, 28]. Since unphosphorylated FoxO3a can translocate to the cell nucleus, we investigated the ability of Wnt1 to control phosphorylation of FoxO3a at 1 and 6 hours following OGD exposure. In prior studies using 293T cells that were co-transfected with FoxO3a and Wnt3a, Wnt3a led to the exclusion of FoxO3a from the cell nucleus during oxidative stress [41]. We demonstrate that minimally phosphorylated (inactive) p-FoxO3a is present within 1 hour and slightly increases at 6 hours following OGD, suggesting that the active (unphosphorylated) FoxO3a form is also present since total FoxO3a expression remains unchanged and prominent. Yet, Wnt1 administration is able to significantly enhance the phosphorylation of FoxO3a at 1 hour and significantly at 6 hours following OGD to inhibit FoxO3a activity throughout this period.
Phosphorylation and inhibition of FoxO3a activity by Wnt1 also correlates with the subcellular trafficking of FoxO3a and the ability to protect microglial cells. Wnt1 controls the subcellular localization of FoxO3a in microglia to maintain FoxO3a in the cytoplasm during OGD. In untreated control microglia, FoxO3a is in the cytoplasm, but FoxO3a translocates to the microglial cell nucleus during OGD. Administration of Wnt1 during OGD prevents the trafficking of FoxO3a from the cytoplasm to the nucleus in microglia. Furthermore, elimination of FoxO3a through gene knockdown significantly increases microglial cell survival and prevents membrane PS exposure and DNA degradation during OGD similar to work that demonstrates FoxO3a controls microglial survival during other injury paradigms, such as β-amyloid toxicity, elevated glucose, and oxidative stress [21, 22, 42]. However, gene knockdown of FoxO3a during recombinant Wnt1 administration also significantly enhances cell survival and blocks apoptosis to a similar degree compared to the administration of Wnt1 alone, suggesting that Wnt1 relies upon the inhibition of FoxO3a activity to foster cytoprotection.
Since oxidative stress and apoptotic membrane PS residue exposure in other injured cells can lead to the activation of microglia and the subsequent phagocytosis of these injured cells, we investigated whether Wnt1 could provide an additional level of protection through the modulation of microglial activation and proliferation. Within 6 hours of OGD exposure, microglia have a significant increase in activation (PCNA expression) and proliferation (BrdU uptake) when compared to microglia not exposed to OGD. Yet, PCNA expression and BrdU uptake in microglia were markedly reduced during Wnt1 administration or during FoxO3a gene knockdown. Transfection with scrambled siRNA did not alter PCNA expression or BrdU uptake during OGD, illustrating that the ability to block microglial activation and proliferation was dependent upon the removal of FoxO3a. In addition, administration of Wnt1 in conjunction with transfection of FoxO3a siRNA also yielded a similar degree of inhibition for PCNA expression and BrdU uptake, demonstrating that Wnt1 employs inhibition of FoxO3a to down-regulate microglial activation and proliferation during OGD exposure.
Mitochondrial membrane permeability and the release of cytochrome c are closely aligned to apoptotic cell injury [43-45]. In addition, Wnt signaling pathways have been associated with impaired mitochondrial signaling [20, 46]. We demonstrate that mitochondrial membrane depolarization and the release of cytochrome c occur in microglia following OGD exposure. Yet, administration of recombinant Wnt1 prevents mitochondrial depolarization and cytochrome c release during OGD. In addition, gene knockdown of FoxO3a alone or in conjunction with Wnt1 administration provides the same level of protection against mitochondrial membrane permeability loss and cytochrome c release as administration of Wnt1 alone, indicating that Wnt1 depends upon inhibition of FoxO3a to maintain mitochondrial permeability during OGD exposure.
Since Wnt1 employs protein kinase B (Akt1) in its signal transduction pathways [20] and relies upon the control of FoxO3a to govern apoptotic programs in microglia, we hypothesized that Wnt1 also may govern Bad that is localized in the outer mitochondrial membrane and when phosphorylated by Akt1, similar to FoxO3a [27, 28], can bind to protein 14-3-3 to release Bcl-xL and prevent apoptosis [47, 48]. We now show that Wnt1 through the inhibition of FoxO3a activity controls Bad phosphorylation. Wnt1 increases the phosphorylation of Bad during OGD and FoxO3a gene knockdown alone or in combination with Wnt1 maintains this phosphorylation of Bad to a similar degree as during Wnt1 administration alone.
Given that Wnt1 in conjunction with FoxO3a controls mitochondrial membrane permeability in microglia, we next investigated whether Wnt1 also affects apoptotic caspase 3 and caspase 1 activity. Mitochondrial release of cytochrome c leads to apoptotic caspase 3 and caspase 1 activation [22, 42, 49-52]. We show that significant activation of caspase 3 and caspase 1 occur as early as 6 hours and can extend through a 24 hour course following OGD exposure, but administration of Wnt1 alone or in combination with the gene knockdown of FoxO3a markedly attenuates caspase 3 and caspase 1 activation, illustrating that Wnt1 uses the loss of FoxO3a activity to control caspase 1 and caspase 3. Our subsequent studies further support this premise and are consistent with prior work illustrating the role of FoxO3a during caspase activity [21, 22, 42, 53] by demonstrating that transfection of FoxO3a siRNA alone in microglia also prevents caspase 3 and caspase 1 activation similar to the application of recombinant Wnt1 alone during OGD exposure.
In addition to the examination of an apoptotic mitochondrial-caspase pathway, we also considered the role of NF-κB p65, a cytoprotective pathway [47, 54, 55] that has been previously reported to be necessary for microglial cell survival [1, 56]. In addition, pathways that involve Wnt signaling have been shown to activate NF-κB during serum deprivation or ultraviolet radiation to preserve cell survival [57, 58]. We show that OGD sequesters NF-κB p65 in the cytoplasm of microglia. However, administration of Wnt1, application of Wnt1 in combination with gene knockdown of FoxO3a, or during gene knockdown of FoxO3a alone promotes the translocation of NF-κB p65 from the cytoplasm to the nucleus in microglia during OGD exposure, illustrating that Wnt1 through FoxO3a can traffic NF-κB p65 to the microglial cell nucleus during oxidative stress. More importantly, gene knockdown of NF-κB p65 during Wnt1 administration significantly decreased the cytoprotective ability of Wnt1, demonstrating the reliance of Wnt1 upon NF-κB p65 to protect microglia. Furthermore, microglial cell survival was markedly decreased by NF-κB p65 gene silencing during OGD to a greater degree than during OGD exposure alone, demonstrating that NF-κB p65 also independently provides endogenous protection in microglia.
5. Conclusions
Microglia are beneficial as guardians in the nervous system against toxins and invasion by infectious agents, but also serve to eliminate injured or non-function neuronal and vascular cells [31, 59, 60]. As a result, it becomes vital to understand the cellular mechanisms responsible for microglial survival and proliferation as a means to target multiple disorders within the nervous system. Our present work demonstrates that both exogenous Wnt1 and endogenous Wnt1 in microglia control early PS membrane and late DNA fragmentation apoptotic injury programs as well as the activation and proliferation of microglia during oxidative stress. Furthermore, Wnt1 relies upon the post-translational modification and subcellular trafficking of FoxO3a to inhibit its activity to block an apoptotic cascade that involves the loss of mitochondrial membrane permeability, cytochrome c release, Bad phosphorylation, and activation of caspase 3 and caspase 1. Wnt1 through FoxO3a oversight, also fosters the nuclear translocation of NF-κB p65 during oxidative stress to promote microglial survival that requires the presence of NF-κB p65. Our studies identify Wnt1 and its control of critical apoptotic downstream pathways in inflammatory microglial cells as potential targets for the development of novel therapeutic strategies to treat immune mediated neurodegenerative disorders.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), NIH NIA, NIH NINDS, and NIH ARRA.
Abbreviations
- (BrdU)
bromodeoxyuridine
- (OGD)
oxygen-glucose deprivation
- (PI 3-K)
phosphatidylinositol 3-kinase
- κB
nuclear factor
- (PS)
phosphatidylserine
- (PCNA)
proliferating cell nuclear antigen
- (TUNEL)
terminal deoxynucleotidyl transferase nick end labeling
- (Wnt1Ab)
Wnt1 antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose.
References
- [1].Chong ZZ, Li F, Maiese K. Int J Mol Med. 2007;19(2):263–72. [PMC free article] [PubMed] [Google Scholar]
- [2].Madinier A, Bertrand N, Mossiat C, Prigent-Tessier A, Beley A, Marie C, Garnier P. PLoS ONE. 2009;4(12):e8101. doi: 10.1371/journal.pone.0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Salminen A, Kaarniranta K. J Mol Med. 2009;87(7):697–701. doi: 10.1007/s00109-009-0472-1. [DOI] [PubMed] [Google Scholar]
- [4].Liang J, Takeuchi H, Jin S, Noda M, Li H, Doi Y, Kawanokuchi J, Sonobe Y, Mizuno T, Suzumura A. Brain Res. 2010;1322:8–23. doi: 10.1016/j.brainres.2010.01.083. [DOI] [PubMed] [Google Scholar]
- [5].Maiese K, Chong ZZ, Hou J, Shang YC. Oxid Med Cell Longev. 2009;2(5):279–90. doi: 10.4161/oxim.2.5.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Arch Biochem Biophys. 1998;353(2):312–21. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- [7].Dello Russo C, Lisi L, Tringali G, Navarra P. Biochem Pharmacol. 2009;78(9):1242–51. doi: 10.1016/j.bcp.2009.06.097. [DOI] [PubMed] [Google Scholar]
- [8].Bureau G, Longpre F, Martinoli MG. J Neurosci Res. 2008;86(2):403–10. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- [9].Kang JQ, Chong ZZ, Maiese K. Mol Pharmacol. 2003;64(3):557–69. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- [10].Alexander SP, Mathie A, Peters JA. Br J Pharmacol. 2008;153(Suppl 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Binet R, Ythier D, Robles AI, Collado M, Larrieu D, Fonti C, Brambilla E, Brambilla C, Serrano M, Harris CC, Pedeux R. Cancer Res. 2009;69(24):9183–91. doi: 10.1158/0008-5472.CAN-09-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maiese K, Li F, Chong ZZ, Shang YC. Pharmacol Ther. 2008;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang LH, Xu HT, Han Y, Li QC, Liu Y, Zhao Y, Yang ZQ, Dong QZ, Miao Y, Dai SD, Wang EH. Mol Cancer. 2010;9:25. doi: 10.1186/1476-4598-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wiedau-Pazos M, Wong E, Solomon E, Alarcon M, Geschwind DH. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Saez K, Henriquez JP, Zhao A, Wollmer MA, Nitsch RM, Hock C, Morris CM, Hardy J, Moon RT. Proc Natl Acad Sci U S A. 2007;104(22):9434–9. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mercado-Gomez O, Hernandez-Fonseca K, Villavicencio-Queijeiro A, Massieu L, Chimal-Monroy J, Arias C. Neurochem Res. 2008;33(8):1599–609. doi: 10.1007/s11064-008-9714-9. [DOI] [PubMed] [Google Scholar]
- [17].Chong ZZ, Kang JQ, Maiese K. Circulation. 2002;106(23):2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- [18].Vogiatzi G, Briasoulis A, Tousoulis D, Papageorgiou N, Stefanadis C. Expert Opin Biol Ther. 2010;10(2):251–64. doi: 10.1517/14712590903547819. [DOI] [PubMed] [Google Scholar]
- [19].Chong ZZ, Shang YC, Maiese K. Curr Neurovasc Res. 2007;4(3):194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chong ZZ, Li F, Maiese K. Cell Signal. 2007;19(6):1150–62. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shang YC, Chong ZZ, Hou J, Maiese K. Curr Neurovasc Res. 2009;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shang YC, Chong ZZ, Hou J, Maiese K. Curr Neurovasc Res. 2009;6(4):223–38. doi: 10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Williams K, Schwartz A, Corey S, Orandle M, Kennedy W, Thompson B, Alvarez X, Brown C, Gartner S, Lackner A. Am J Pathol. 2002;161(2):575–85. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Martinez-Contreras A, Huerta M, Lopez-Perez S, Garcia-Estrada J, Luquin S, Beas Zarate C. J Neurosci Res. 2002;67(2):200–10. doi: 10.1002/jnr.10093. [DOI] [PubMed] [Google Scholar]
- [25].Salazar KD, Lankford SM, Brody AR. Am J Physiol Lung Cell Mol Physiol. 2009;297(5):L1002–11. doi: 10.1152/ajplung.90347.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maiese K, Chong ZZ, Shang YC. Trends Mol Med. 2008;14(5):219–27. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chong ZZ, Maiese K. Br J Pharmacol. 2007;150(7):839–50. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhu W, Bijur GN, Styles NA, Li X. Brain Res Mol Brain Res. 2004;126(1):45–56. doi: 10.1016/j.molbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]
- [29].Maiese K, Chong ZZ, Li F, Shang YC. Prog Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schmitz ML, Baeuerle PA. Embo J. 1991;10(12):3805–17. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gilfillan AM, Rivera J. Immunol Rev. 2009;228(1):149–69. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Maiese K. Biomed Pharmacother. 2008;62(4):218–32. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Teng FY, Hor CH, Tang BL. Differentiation. 2009;77(2):121–7. doi: 10.1016/j.diff.2008.09.013. [DOI] [PubMed] [Google Scholar]
- [34].Espada J, Calvo MB, Diaz-Prado S, Medina V. Clin Transl Oncol. 2009;11(7):411–27. doi: 10.1007/s12094-009-0380-4. [DOI] [PubMed] [Google Scholar]
- [35].Kikuchi A, Yamamoto H, Sato A. Trends Cell Biol. 2009;19(3):119–29. doi: 10.1016/j.tcb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [36].Lee HN, Jeon GS, Kim DW, Cho IH, Cho SS. Neurochem Res. 2010;35(1):114–21. doi: 10.1007/s11064-009-0036-3. [DOI] [PubMed] [Google Scholar]
- [37].Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ. J Bone Miner Res. 2010 doi: 10.1002/jbmr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Doubravska L, Simova S, Cermak L, Valenta T, Korinek V, Andera L. Apoptosis. 2008;13(4):573–87. doi: 10.1007/s10495-008-0191-z. [DOI] [PubMed] [Google Scholar]
- [39].Lei ZN, Zhang LM, Sun FY. Neurosci Lett. 2008;435(2):108–12. doi: 10.1016/j.neulet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- [40].Lee JH, Lee EO, Kang JL, Chong YH. J Neurochem. 2008;106(3):1066–77. doi: 10.1111/j.1471-4159.2008.05444.x. [DOI] [PubMed] [Google Scholar]
- [41].Dehner M, Hadjihannas M, Weiske J, Huber O, Behrens J. J Biol Chem. 2008;283(28):19201–10. doi: 10.1074/jbc.M710366200. [DOI] [PubMed] [Google Scholar]
- [42].Hou J, Chong ZZ, Shang YC, Maiese K. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lee I, Pecinova A, Pecina P, Neel BG, Araki T, Kucherlapati R, Roberts AE, Huttemann M. Biochim Biophys Acta. 2010;1802(2):275–83. doi: 10.1016/j.bbadis.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maiese K, Li F, Chong ZZ. Jama. 2005;293(1):90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Su Y, Sun H, Fang J, Hu G, Xiao M. Neurochem Res. 2010;35(3):399–404. doi: 10.1007/s11064-009-0068-8. [DOI] [PubMed] [Google Scholar]
- [46].Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, Delafontaine P, Chandrasekar B. Cell Signal. 2010;22(5):809–20. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chong ZZ, Li F, Maiese K. Curr Neurovasc Res. 2005;2(5):387–99. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schlecht-Bauer D, Antier D, Machet MC, Hyvelin JM. J Cardiovasc Pharmacol. 2009 doi: 10.1097/FJC.0b013e3181b04d01. [DOI] [PubMed] [Google Scholar]
- [49].Astiz M, de Alaniz MJ, Marra CA. Ecotoxicol Environ Saf. 2009;72(7):2025–32. doi: 10.1016/j.ecoenv.2009.05.001. [DOI] [PubMed] [Google Scholar]
- [50].Hao J, Shen W, Tian C, Liu Z, Ren J, Luo C, Long J, Sharman E, Liu J. J Cell Mol Med. 2009;13(4):701–11. doi: 10.1111/j.1582-4934.2008.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Maiese K, Hou J, Chong ZZ, Shang YC. ScientificWorldJournal. 2009;9:1072–104. doi: 10.1100/tsw.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Petit-Paitel A, Brau F, Cazareth J, Chabry J. PLoS ONE. 2009;4(5):e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gomez-Gutierrez JG, Souza V, Hao HY, Montes de Oca-Luna R, Dong YB, Zhou HS, McMasters KM. Cancer biology & therapy. 2006;5(7):875–83. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- [54].Jung KJ, Lee EK, Yu BP, Chung HY. Free Radic Biol Med. 2009;47(7):983–91. doi: 10.1016/j.freeradbiomed.2009.07.009. [DOI] [PubMed] [Google Scholar]
- [55].Kuhad A, Bishnoi M, Tiwari V, Chopra K. Pharmacol Biochem Behav. 2009;92(2):251–9. doi: 10.1016/j.pbb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- [56].Li F, Chong ZZ, Maiese K. Curr Neurovasc Res. 2006;3(3):187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bournat JC, Brown AM, Soler AP. J Neurosci Res. 2000;61(1):21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [58].Lee JL, Chang CJ, Chueh LL, Lin CT. Breast Cancer Res Treat. 2006;100(1):49–58. doi: 10.1007/s10549-006-9233-9. [DOI] [PubMed] [Google Scholar]
- [59].Chong ZZ, Li F, Maiese K. Prog Neurobiol. 2005;75(3):207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- [60].Martin I, Andres CR, Vedrine S, Tabagh R, Michelle C, Jourdan ML, Heuze-Vourc’h N, Corcia P, Duittoz A, Vourc’h P. Brain Res. 2009;1284:22–30. doi: 10.1016/j.brainres.2009.05.070. [DOI] [PubMed] [Google Scholar]