Abstract
Energy deprivation in the myocardium is associated with impaired heart function and increased morbidity. LKB1 is a kinase that is required for activation of AMP-activated protein kinase (AMPK) as well as 13 AMPK-related protein kinases. AMPK stimulates ATP production during ischemia and prevents post-ischemic dysfunction. We used the Cre-Lox system to generate mice where LKB1 was selectively knocked out in cardiomyocytes and muscle cells (LKB1-KO) to assess the role of LKB1 on cardiac function in these mice.
Heart rates of LKB1-KO mice were reduced and ventricle diameter increased. Ex vivo, cardiac function was impaired during aerobic perfusion of isolated working hearts, and recovery of function after ischemia was reduced. Although oxidative metabolism and mitochondrial function were normal, the AMP/ATP ratio was increased in LKB1-KO hearts. This was associated with a complete ablation of AMPKα2 activity, and a stimulation of signaling through the mammalian target of rapamycin.
Our results establish a critical role for LKB1 for normal cardiac function under both aerobic conditions and during recovery after ischemia. Ablation of LKB1 leads to a decreased cardiac efficiency despite normal mitochondrial oxidative metabolism.
Keywords: Cardiac function, LKB1, AMP-activated-protein-kinase, mTOR
INTRODUCTION
Mortality from acute myocardial infarction has been reduced substantially over the last 25 years [32]. However, there has been a concomitant rise in mortality attributable to heart failure and today more than 2% of the U.S. population is affected by this condition [18]. Normal cardiac function requires a constant supply of nutrients and oxygen to generate ATP. Under physiological conditions, 60–70% of the ATP utilization goes towards the contractile apparatus [29]. When energy supply is restricted, such as during ischemia, ATP is primarily used to re-establish Ca2+ ionic homeostasis in an attempt to prevent ischemic injury [14]. As a result less ATP is available for contractile purposes and cardiac power and cardiac efficiency is reduced.
The regulation of cardiac ATP metabolism is complex and only partially elucidated. However, it is well established that the 5’-AMP-activated-protein-kinase (AMPK) is necessary for maintaining myocardial energy homeostasis during ischemia [34]. AMPK is a serine/threonine kinase that consists of three subunits, α, β and γ. In the heart, AMPK is phosphorylated and activated by an upstream kinase LKB1 [25]. LKB1 is a serine/threonine kinase that was originally identified as a tumor suppressor protein, and more recently, there has been considerable focus on LKB1 as a regulator of metabolism in liver and skeletal muscle [11,24,27]. LKB1 functions in a complex with the two subunits, STRAD and MO25. The activity of this complex is not regulated by ischemia but instead the complex appears to be constitutively active [25]. In addition to AMPK, LKB1 also phosphorylates 13 of 14 AMPK-related kinases [15]. Unlike AMPK, little is known about these additional LKB1 substrates, especially in the heart.
AMPK regulates energy metabolism in the heart by phosphorylation of key enzymes in the energy metabolism pathways. When activated, AMPK inhibits protein synthesis through regulation of mammalian target of rapamycin (mTOR) kinase activity (reviewed in [8]). This preserves ATP for the contractile apparatus and conserves cardiac function. Simultaneously, AMPK stimulates ATP production by promoting substrate oxidation [29]. AMPK stimulates glucose uptake by promoting translocation of the glucose transporter GLUT4 to the cell surface [21]. Fatty acid oxidation is stimulated by phosphorylation of acetyl-CoAcarboxylase (ACC) [1]. ACC regulates the production of malonyl-CoA, an allosterical inhibitor of carnitine palmitoyltransferase 1 (CPT-1) which controls transport of activated fatty acids into the mitochondria [1]. The combined outcome of AMPK activation is consequently stimulation of oxidative metabolism and inhibition of energy consumption.
Transgenic animals that express a kinase dead AMPK mutant in the heart have normal cardiac function under aerobic conditions [22,34]. However, when these hearts are subjected to ischemia, ATP regeneration and post-ischemic recovery of cardiac function are dependent on substrate availability [6,22,34]. This means AMPK is required for a normal response to ischemia but is not necessary for normal cardiac function under aerobic conditions. Less is known about the role of LKB1 in cardiac function. Several LKB1 transgenic mouse models have been created, a hypomorphic mouse model where muscle LKB1 expression is completely ablated while LKB1 expression is reduced by five- to ten-fold in other tissues [25], a muscle and heart-specific LKB1-KO mouse where LKB1 expression is completely ablated only in muscle tissue [11,30,31], and recently a cardiac-specific LKB1-KO mouse has been created [9]. Cardiac-specific LKB1-KO animals die prematurely and have poor cardiac performance [9], while muscle-specific LKB1 transgenic mice survive normally and display no obvious adverse phenotypes [25]. Unlike the AMPK transgenic animals, ACC phosphorylation is profoundly reduced in hearts from LKB1 transgenic mice [9,25,30] but surprisingly, malonyl-CoA content is normal in muscle-specific LKB1-KO hearts [30]. Hearts from the LKB1 hypomorphic mice have increased AMP/ATP ratios under aerobic conditions [25]. Whether this is due to impairments in substrate oxidation or due to an inability to suppress energy consuming processes is unknown.
The primary aim of the present study was to investigate the functional consequences of LKB1 deficiency under aerobic and post-ischemic conditions. In addition, we examined if ablation of LKB1 would affect energy substrate metabolism in the heart. For this purpose we studied hearts from mice where LKB1 was selectively deleted in muscle cells using the Cre-Lox system [11]. We found that knock out of LKB1 impairs cardiac function in vivo and ex vivo. Absolute glucose and fatty acid oxidation rates in LKB1-KO heart were similar to control hearts and mitochondrial function intact. However, cardiac efficiency in LKB1-KO hearts was impaired. This was associated with increased activity in the mTOR/p70S6 kinase pathway. These results establish a critical role for LKB1 in cardiac function and energy metabolism.
MATERIALS AND METHODS
Animals
Muscle and heart-specific LKB1-KO mice were generated as described previously [11]. Animals were housed in pathogen-free facilities, maintained on a 12-hour light cycle, and fed standard laboratory chow. Ten to twelve week-old mice were used for all experiments. All experimental procedures were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of the Joslin Diabetes Center.
Echocardiography was performed in non-anaesthetized mice with an Agilent Sonos 4500 ultrasound machine and a 12-MHz linear–array transducer, as previously described [26]. Electrocardiographic intervals were measured in 6 limb leads and 3 pre-cordial leads. Ventricular pre-excitation was diagnosed on the basis of a short PR with widened QRS interval.
Control and LKB1-KO mice were subjected to noninvasive physiological and behavioral characterization using the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH, USA) at the Physiology Core Laboratory of the Joslin Diabetes Center. The mice were monitored for 24 hours and assessed for oxygen consumption (VO2; ml/kg/h), carbon dioxide generation (VCO2; ml/kg/h), heat generation calculated from gas exchange data (kcal/h), respiratory exchange ratio calculated from gas exchange data, food consumption (grams/24h), water consumption (ml/24h), and locomotive activity evaluated by three-dimensional fixed point observation (counts/h). Monitoring started at 10:00 HR, and CLAMS assessment was made during both the light cycle (07:00 to 19:00 HR) and dark cycle (19:00 to 7:00 HR).
Isolated working mouse heart perfusions
Ex vivo perfused working mouse hearts from control and muscle-specific LKB1-KO mice were aerobically perfused as previously described [12]. Heart rates, pressures, and flows were also determined, as previously described [12]. Glucose oxidation rates were determined by measuring 14CO2 released from the metabolism of [U-14C]glucose, as described [13]. Palmitate oxidation rates were measured from the release of 3H2O, derived from the metabolism of [9,10-3H]palmitate, as described [13].
LKB1 signaling and protein expression
Animals were anaesthetized with i.p. injection of pentobarbital (100mg/kg). Hearts were either freeze-clamped in situ at baseline (basal conditions) or two minutes after cervical dislocation (ischemic conditions), a model previously shown to lead to rapid depletion of ATP and creatine phosphate in hearts [20].
Whole hearts were homogenized as previously described [7]. Western blot analyses were used to assess expression and phosphorylation levels of various proteins. Blots were developed using ECL reagents (Amersham, USA), and quantified using FluorChem 2.01(Alpha-Innotech-Corporation, USA).
LKB1 and CD36 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, USA). Antibodies to AMPKα1, and phospho-specific(Ser79)-ACC antibody were from Upstate (Charlottesville, USA). GLUT1 and GLUT4 antibodies were from Chemicon (Temecula, CA). Phospho-specific AMPK(Thr172), cytochrome C, Akt, phospho-specific Akt (Ser473 and Thr308), mTOR, phospho-specific mTOR (Ser2448), Tuberin/TSC and phospho-specific Tuberin/TSC (Thr1426) antibodies were from Cell Signaling (Beverly, USA). Antibodies to the beta subunit of ATP synthase was from (Abcam, USA). ACC expression was assessed using HRP-conjugated-streptavidin (Pierce Chemical, USA). Antibodies to AMPKα2, MalonylCoA-Decarboxylase (MCD) and TRB3 were generated as previously described [5,11,17].
Heart samples were homogenized and AMPKα1 and α2 activity was assayed as previously described [7,17]. A subset of lysates was assayed with and without AMP to determine AMP-dependency.
Citrate synthase activity, ACC activity, nucleotide content and glycogen measurements
Heart lysates were assayed for citrate synthase activity as described by Srere [28]. ACC activity was measured in protein lysates from perfused hearts in the absence or presence of 10 mM citrate as previously described [23]. AMP and ATP contents were measured using high-performance liquid chromatography (HPLC) as described by Merrill et al [16]. For glycogen measurements, heart muscle was hydrolyzed in 2 N HCl at 95°C for 2 h and then neutralized with 2 N NaOH. Glucose content was measured by a hexokinase method using a glucose HK reagent (Eagle Diagnostics, Desoto, Texas, USA).
Statistical analysis
Data are expressed as means ± SEM. Normality of the data was tested with the Kolmogorov-Smirnov test of normal distribution. Where p>0.20, the data was considered to be normally distributed. All normally distributed data were compared using Student’s t-test, one-way or two-way ANOVA. Data that was not normally distributed was compared using Mann-Whitney Rank Sum Test. The differences between groups were considered significant when p<0.05.
RESULTS
Creation of muscle-specific LKB1-KO mice
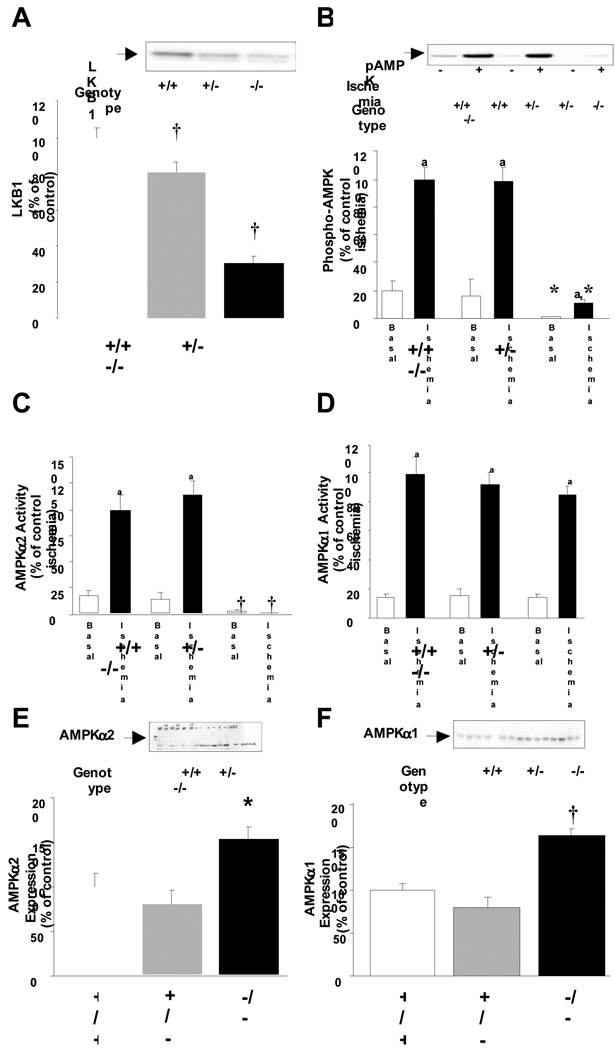
LKB1 expression in the whole heart was reduced by 25 and 70% in heterozygote and LKB1-KO mice, respectively (Fig 1A). This model is different from the hypomorphic LKB1 transgenic mouse where LKB1 expression in all tissues is reduced by five- to ten-fold [24] and the cardiac specific LKB1 knock out mouse where LKB1 expression in skeletal muscle is normal [9].
Fig 1.
A: There was a 70% reduction in LKB1 expression in LKB1-KO hearts (†: P<0.01 vs. control, N=8–18). The lox-sites did not affect LKB1 expression and Lox and Lox/+ mice were used as controls. B: Phosphorylation of AMPK-Thr172 was inhibited in LKB1-KO hearts under both basal and ischemic conditions (a: P<0.01 vs. basal.*: P<0.05 vs. control, N=5–8) C: This was associated with ablated AMPKα2-activity (a: P<0.01 vs. basal.†: P<0.01 vs. control, N=5–8) while D: Ischemia increased AMPKα1-activity among all genotypes (a: P<0.01 vs. basal, N=5–8). E and F: Knock out of LKB1 increased expression of AMPKα2 and α1 (*: P<0.05 and †: P<0.01 vs. control, N=8–18).
Knock out of LKB1 reduced basal and ischemia-induced phosphorylation of AMPKα-Thr172 by ~90%. AMPK phosphorylation in LKB1 heterozygote hearts was completely normal (Fig 1B). The decrease in AMPK phosphorylation corresponded to a complete inhibition of AMPKα2-activity (Fig 1C). Thus, knock out of LKB1 fully inhibited AMPKα2 activity despite a ~40% increase in expression of the protein. In contrast to AMPKα2, AMPKα1 activity was conserved in LKB1-KO hearts (Fig 1D). Furthermore, AMPKα1 activity from ischemic hearts was assayed ± AMP and this showed normal dependency of AMP in all genotypes (data not shown).
Heart characteristics
The whole body phenotype of muscle-specific LKB1-KO animals has been described previously [11]. LKB1-KO mice did not display any obvious cardiac phenotype. Spontaneous activity, heart weight and heart/body weight ratio was similar in LKB1-KO animals and their control littermates (Table 1). We did not observe enlarged atria in hearts from the muscle-specific LKB1-KO mice. Non-anaesthetized LKB1-KO mice and wild type littermates were evaluated by 2-dimensional/M-mode echocardiography. Heart rates in LKB1-KO mice were significantly decreased by ~10%. This was associated with increased left ventricular systolic diameter and diastolic diameter. There was a trend for a decreased fractional shortening, but this did not reach significance (Table 1). On electrocardiography analysis, there was no manifestation of ventricular pre-excitation or atrial fibrillation observed in any of the mice. There were also no differences of respiratory exchange ratio between genotypes in both fasted and fed conditions.
Table 1.
Heart characteristics.
| Genotype | +/+ | −/− |
|---|---|---|
| Heart weight (mg) | 107.0±5.5 | 116.6±12.0 |
| Heart weight/body weight (mg/g) | 4.2±0.1 | 4.3±0.2 |
| LV end diastolic diameter (mm) | 2.52±0.09 | 3.36±0.07** |
| LV end systolic diameter (mm) | 0.88±0.1 | 1.28±0.06* |
| Fractional shortening | 0.65±0.03 | 0.59±0.02 |
| Heart rate (min–1) | 724±19 | 627±16* |
| LV anterior wall thickness (mm) | 0.86±0.03 | 0.90±0.02 |
| LV posterior wall thickness (mm) | 0.88±0.04 | 0.94±0.01 |
| AMP/ATP-ratio (basal conditions) | 0.06±0.01 | 0.13±0.03* |
| AMP/AT- ratio (ischemic conditions) | 0.11±0.03 | 0.30±0.07* |
| Glycogen (nmol glucose/g (wet weight) | 15.0±1.14 | 13.3±1.1 |
| Citrate synthase activity (A.U.) | 100.0±4.5 | 93.0±4.7 |
| Cytochrome C expression (A.U.) | 100.0±7.3 | 91.9±6.2 |
| ATP synthase expression (A.U.) | 100.0±6.2 | 97.3±7.5 |
Values are mean±SEM, n=5–12,
P<0.05 and
P<0.01 vs. control
Nucleotide measurements revealed that LKB1-KO hearts were energy deprived in the basal state (Table 1). We therefore examined mitochondrial markers, and found that there were no difference in citrate synthase activity or cytochrome C and ATP synthase β-subunit expression (Table 1), all suggesting normal mitochondrial function.
Cardiac function and substrate metabolism in the perfused ex vivo working mouse heart
To assess the functional consequences of LKB1 deletion in the heart, we subjected control and LKB1-KO hearts to ex vivo aerobic perfusions. Similar to the in vivo observations, heart rates were significantly decreased in LKB1-KO hearts (Table 2). This was associated with an increase in developed pressure resulting in similar rate pressure product in control and LKB1-KO hearts. Unlike AMPK transgenic hearts, ablation of LKB1 was associated with a ~40% decrease in cardiac output and a ~30% decrease in cardiac power under aerobic conditions (Table 2). LKB1 is thus required for normal cardiac function under aerobic conditions.
Table 2.
Cardiac function in the aerobically perfused ex vivo working mouse heart
| Genotype | +/+ | −/− |
|---|---|---|
| Heart Rate (beats • min−1) | 285 ± 9 | 246 ± 17* |
| Rate pressure product (bpm • mmHg • 10−3) | 20 ± 0.5 | 18 ± 0.9 |
| Cardiac Work (ml • mmHg • min−1) | 6.5 ± 0.3 | 4.2 ± 0.6** |
| Cardiac Output (ml/min) | 9.3 ± 0.4 | 5.8 ± 0.7** |
| Aortic Output (ml/min) | 7.1 ± 0.4 | 3.7 ± 0.8** |
| Cardiac Power (mjoules • min-1) | 71.6 ± 3.3 | 47.6 ± 7.0** |
Values are mean ± SEM, n = 7–17,
P <0.05 vs. control,
P <0.01 vs. control
To investigate the role of LKB1 in post-ischemic recovery the hearts were subjected to 20 min of global ischemia followed by aerobic reperfusion. LKB1-KO hearts had significantly reduced recovery of function during reperfusion (Table 3). After 40 min reperfusion the rate pressure product was decreased by ~40% and cardiac output reduced ~70%. Together, these data show that LKB1 function is required for normal cardiac function under aerobic conditions and ablation of LKB1 aggravates post-ischemic cardiac dysfunction.
Table 3.
Post ischemic functional recovery in the aerobically perfused ex vivo working mouse heart after 20 min of ischemia.
| Genotype | +/+ | −/− |
|---|---|---|
| Heart Rate (beats • min−1) | 204 ± 11 | 177 ± 15 |
| Rate pressure product (bpm • mmHg • 10−3) | 12 ± 1.0 | 7 ± 1.3* |
| Cardiac Work (ml • mmHg • min−1) | 2.0 ± 0.4 | 0.5 ± 0.4** |
| Cardiac Output (ml/min) | 3.3 ± 0.7 | 0.9 ± 0.6* |
| Aortic Output (ml/min) | 1.8 ± 0.5 | 0.4 ± 0.3 |
| Cardiac Power (mjoules • min-1) | 31.2 ± 6.7 | 5.5 ± 3.9* |
Values are mean ± SEM, n = 7–17,
P <0.05 vs. control,
P <0.01
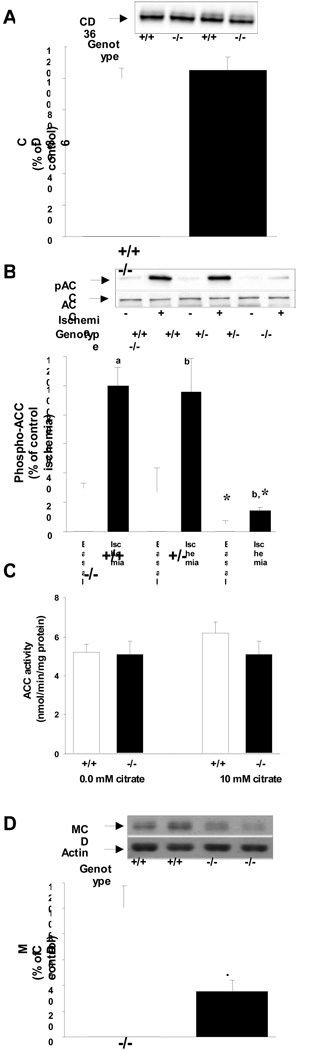
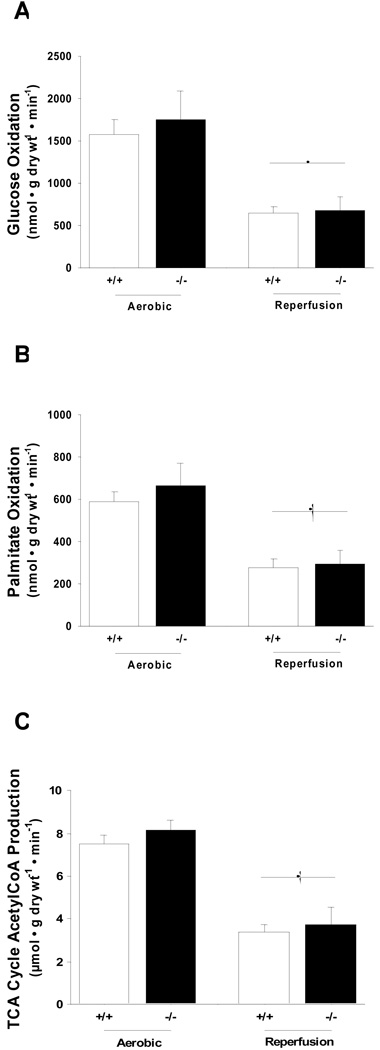
To determine if the compromised cardiac function in LKB1 deficient hearts was due to impairments in energy substrate metabolism we measured fatty acid metabolism. We did not detect any differences in the expression of the fatty acid transporter protein CD36 (Fig. 2A). We then determined phosphorylation and activity of ACC, a key regulator of malonyl-CoA production and fatty acid oxidation. The expression of ACC was similar among genotypes. In the basal state, ACC phosphorylation was reduced by 81% in LKB1-KO hearts while no difference was observed in heterozygote hearts (Fig 2B). During ischemia, ACC was phosphorylated in hearts from control and heterozygote animals, but in LKB1-KO hearts ACC phosphorylation was decreased by 85%. However, these differences in ACC phosphorylation did not translate into changes in ACC activity between knock out and control hearts (Fig 2C). We next measured MCD expression, the key enzyme involved in malonyl-CoA decarboxylation. In LKB1-KO hearts MCD protein expression was decreased by ~70% (Fig 2D). However, despite reduced MCD expression, fatty acid oxidation rates were similar between LKB1-KO and control animals (Fig 3A).
Fig 2.
A: Knock out of LKB1 did not affect expression of the fatty acid transporter protein CD36 (N=11–12). B: Phosphorylation of ACC was blunted in LKB1-KO hearts during both basal and ischemic conditions (a: P<0.01, b: P<0.05 vs. basal.*: P<0.05 vs. control, N=6–8). ACC protein-expression was similar among genotypes. C: The activity of ACC was measured in the presence or absence of 10 mM citrate in protein lysates from perfused hearts after reperfusion. There were no differences in activity among genotypes (N=5–6). D: Expression of MCD was reduced by ~70% in LKB1-KO hearts (P<0.05 vs. control (N=6).
Fig 3.
A and B: Knock out of LKB1 did not affect rates of glucose and palmitate oxidation or total during aerobic perfusion or reperfusion (†: P<0.01 vs. corresponding aerobic control group, N=7–11). C: Knock out of LKB1 also did not affect total tricarboxylic acid cycle acetyl-CoA production (†: P<0.01 vs. corresponding aerobic control group, N=5–12).
We next looked at glucose metabolism. In the basal state, glycogen levels were similar in LKB1-KO and control hearts (Table 1). Similar to CD36, expression of the glucose transporters GLUT1 and GLUT4 were normal in LKB1-KO hearts (data not shown). When glucose oxidation rates were measured ex vivo, we did not detect any differences between LKB1-KO and control hearts (Fig 3B). From these data, TCA cycle acetyl-CoA production was calculated and these data show that the impaired cardiac function in LKB1-KO hearts was not due to impaired oxidative metabolism (Fig 3C).
Mammalian target of rapamycin (mTOR) signaling
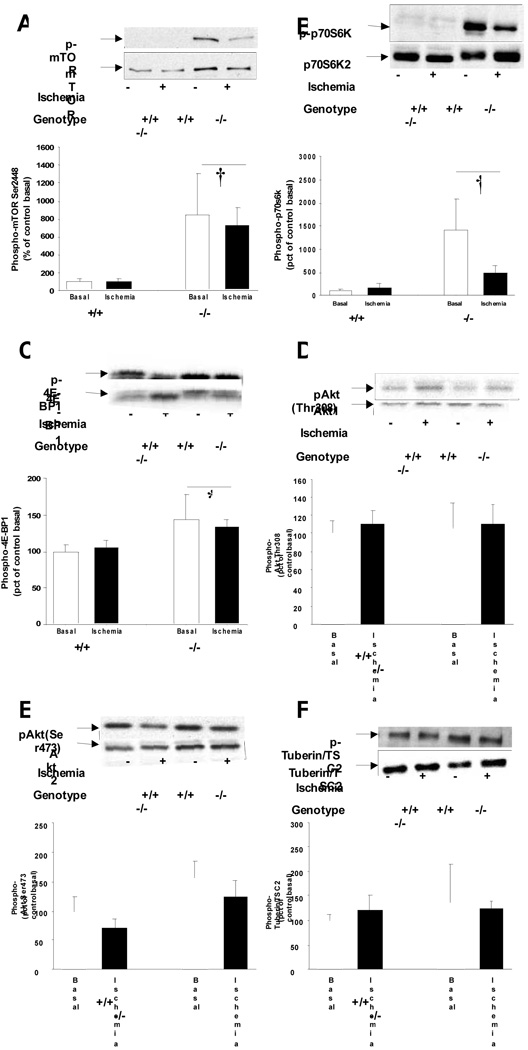
Measurements of oxidative metabolism did not provide an explanation for the impaired cardiac function. We therefore examined the regulation of energy consuming processes. When activated, AMPK inhibits protein syntheses and this process is impaired in cardiac-specific LKB1-KO hearts [9]. Consequently, we investigated signaling through mTOR, a master regulator of protein synthesis in the heart. Knock out of LKB1 increased cardiac mTOR protein expression by ~3 fold (Fig. 4A) and phosphorylation on the activating Ser2448 site by ~8 fold in the basal state with no further activation during ischemia (Fig. 4A). This was associated with increased phosphorylation of the downstream targets p70S6kinase and 4E-BP1 without changes in protein expression (Fig. 4B,C). Since insulin stimulates mTOR Ser2448 phosphorylation through Akt and tuberin/TSC2 phosphorylation, we investigated if these proteins were activated in LKB1-KO hearts. Interestingly, Akt2 expression was increased inLKB1-KO hearts by ~2 fold (Fig. 4E). Unlike skeletal muscle [11], this was not associated with decreased expression of TRB3 (data not shown). However, the increased Akt2 expression did not translate into increased phosphorylation of Akt on Ser473 and Thr408 (Fig. 4D,E) and no changes in tuberin/TSC2 expression or phosphorylation were observed (Fig 4F). Together these data show that ablation of LKB1 results in activation of mTOR in cardiomyocytes independently of Akt.
Fig 4.
A: Akt/mTOR/p70S6K signaling. A: Ablation of LKB1 increased mTOR Ser2448 phosphorylation independently of ischemia. B and C: The increased mTOR activity was associated with increased phosphorylation of p70S6 kinase and 4E-BP1. (*: P<0.05, †: P<0.01 vs. control, N=4–12). D and E: Knock out of LKB1 did not increase Akt phosphorylation or F: Akt signaling to the downstream target Tuberin/TSC2.
DISCUSSION
Our results establish that LKB1 is necessary for normal cardiac function. In vivo, there was limited cardiac dysfunction in the LKB1-KO mice. Heart rates were lower and ventricles dilated, but fractional shortening was not significantly reduced. However, ex vivo the heart was not able to compensate for the ablation of LKB1 and cardiac output was significantly reduced during aerobic perfusion. The impaired cardiac function was associated with an increased AMP/ATP ratio and decreased cardiac efficiency. This may provide the explanation for the impaired cardiac function since reductions in ATP availability impair contractile function and leave the hearts susceptible to ischemic injury. Indeed, when LKB1-KO hearts were subjected to ischemia ex vivo cardiac dysfunction was further aggravated. Whether this was a consequence of the poor function at baseline or if LKB1-KO hearts have a specific impairment in the response to ischemia remains to be investigated.
Deficiency of LKB1 resulted in up-regulation of mTOR/p70S6K signaling, suggesting an impaired ability to attenuate protein synthesis in these mice. The increased mTOR signaling was associated with a tendency for increased ventricle wall thickness and heart weights in our LKB1-KO animals, consistent with the mild [30] or more pronounced hypertrophy observed in other LKB1 genetic models [9,19,33]. However, unlike the cardiac-specific LKB1 KO model [9] we did not observe the severe morphometric phenotype with biatrial enlargement or atrial fibrillation in the muscle-specific LKB1 KO model. The mechanism for the increased mTOR signaling is not fully understood, but is likely due to the ablated AMPKα2 activity. AMPK can directly phosphorylate mTOR on Thr2446 [3] and tuberin/TSC2 [10] and thereby inhibit phosphorylation of the activating Ser2448 site on mTOR. If mTOR and tuberin/TSC2 phosphorylation are solely mediated through AMPKα2 and not AMPKα1 then this provides the mechanism by which knock out of LKB1 can increase mTOR signaling. Alternatively, one or more of the AMPK related kinases may be involved in the regulation of mTOR activity. Impaired ability to suppress the energy consuming processes regulated by mTOR would render less energy available for contractile purposes and provide explanation for the cardiac dysfunction in LKB1-KO hearts.
In vivo, we only observed a modest cardiac dysfunction as evidenced by a decreased heart rate but normal fractional shortening and this indicates that the ventricle maintained normal cardiac output by the Frank-Sterling mechanism. The echocardiographs were performed in awake mice and the stress from the procedure may have caused an elevation in catecholamines, which could have impaired the ability to detect left ventricular dysfunction. However, knock out of LKB1 did not reduce activity levels in the non-stressed environment of our Comprehensive Lab Animal Monitoring System (CLAMS). This does not exclude that under situations with more severe cardiac stress the compensation may be inadequate. Indeed, when LKB1-KO mice are challenged by exercise, voluntary running performance is significantly reduced (Koh and Goodyear unpublished observations and [31]), although this effect can be due to ablation of LKB1 in skeletal muscle, the heart or a combination of both. However, the data obtained from the muscle-specific LKB1-KO mice are not due to overt differences in activity levels.
Under non-ischemic conditions almost all (>95%) of ATP formation in the heart comes from oxidative metabolism in the mitochondria [29]. However, the compromised cardiac function in LKB1-KO hearts was not associated with reductions in oxidative rates or an impaired mitochondrial function. The impaired cardiac function and increased AMP/ATP ratio do therefore not appear to be caused by an inhibition of ATP production. AMPK has been suggested to play a major role in the regulation of metabolism in the heart through the regulation of malonyl-CoA levels [29]. ACC was one of the first identified substrates of AMPK [2] and our data confirm that in the heart phosphorylation of ACC on Ser79 is dependent on AMPKα2 activity. However, the reduced phosphorylation of ACC did not translate into changes in ACC activity and we did not observe decreased palmitate oxidation. ACC activity was measured at the end of the reperfusion period where no ACC phosphorylation could be detected in the LKB1-KO hearts. This observation is in agreement with the previous reports that demonstrate that malonyl-CoA levels in LKB1-KO hearts are similar to control hearts [30]. It is therefore clear that while ACC Ser79 phosphorylation can be used as an important marker of AMPKα2 activity it cannot be directly translated into changes in fatty acid oxidation in the heart. The exact explanation for the dissociation between ACC phosphorylation and activity is unknown. However, recent reports on the crystal structure of ACC have revealed that phosphorylation of ACC by AMPK disrupts the formation of active ACC polymers [4]. Whether alternative mechanisms to ACC phosphorylation can disrupt the formation of ACC polymers in LKB1-KO hearts is a topic of further investigation.
In contrast to AMPKα2, AMPKα1 activity was conserved in our LKB1-KO hearts. Similar observations have been made in the LKB1 hypomorphic mouse and has led to speculation as to whether the α1 subunit is solely expressed in non cardiomyocytes [25]. However, muscle specific knock out of LKB1 increases expression of AMPKα1 by ~50% in the heart. This effect could be a compensatory mechanism for the ablated α2 activity and would therefore indicate that the α1-subunit is indeed expressed in cardiomyocytes. If AMPKα1 is expressed in cardiomyocytes, the activity is regulated differently from AMPKα2 and is not dependent on LKB1 expression.
In conclusion, our findings demonstrate that LKB1 function is essential for normal contractile function in the heart. Ablation of the protein leads to decreased cardiac output under aerobic conditions and this functional defect is further aggregated after LKB1-KO hearts have been subjected to ischemia. The compromised cardiac function is not due to impaired oxidative metabolism but is associated with an increased mTOR signaling and may thus be due to an impaired ability to suppress energy consuming processes.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health grants to LJG (grant numbers: DK068626, AR45670). Additional funds to support this work were provided by the Danish Agency for Science Technology and Innovation to N Jessen (grant number: 271-07-0719), and from a Canadian Institutes of Health Research Grant to G. Lopaschuk. The authors thank C.R. Kahn and R.A. DePinho for providing the MCK-Cre and floxed LKB1 mice. We also thank N. Mukai for excellent technical assistance and Dr Ding An for valuable scientific input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bianchi A, Evans JL, Iverson AJ, Nordlund AC, Watts TD, Witters LA. Identification of an isozymic form of acetyl-CoA carboxylase. J Biol. Chem. 1990;265:1502–1509. [PubMed] [Google Scholar]
- 2.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 3.Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol. Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- 4.Cho YS, Lee JI, Shin D, Kim HT, Jung HY, Lee TG, Kang LW, Ahn YJ, Cho HS, Heo YS. Molecular mechanism for the regulation of human ACC2 through phosphorylation by AMPK. Biochem. Biophys. Res. Commun. 2010;391:187–192. doi: 10.1016/j.bbrc.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Dyck JR, Barr AJ, Barr RL, Kolattukudy PE, Lopaschuk GD. Characterization of cardiac malonyl-CoA decarboxylase and its putative role in regulating fatty acid oxidation. Am. J. Physiol. 1998;275:H2122–H2129. doi: 10.1152/ajpheart.1998.275.6.H2122. [DOI] [PubMed] [Google Scholar]
- 6.Folmes CD, Wagg CS, Shen M, Clanachan AS, Tian R, Lopaschuk GD. Suppression of 5'-AMP-activated protein kinase activity does not impair recovery of contractile function during reperfusion of ischemic hearts. Am. J. Physiol Heart Circ. Physiol. 2009;297:H313–H321. doi: 10.1152/ajpheart.01298.2008. [DOI] [PubMed] [Google Scholar]
- 7.Fujii N, Boppart MD, Dufresne SD, Crowley PF, Jozsi AC, Sakamoto K, Yu H, Aschenbach WG, Kim S, Miyazaki H, Rui L, White MF, Hirshman MF, Goodyear LJ. Overexpression or Ablation of JNK in Skeletal Muscle Has No Effect on Glycogen Synthase Activity. Am. J Physiol Cell Physiol. 2004 doi: 10.1152/ajpcell.00415.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hedhli N, Pelat M, Depre C. Protein turnover in cardiac cell growth and survival. Cardiovasc. Res. 2005;68:186–196. doi: 10.1016/j.cardiores.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR, Walsh K. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J. Biol. Chem. 2009;284:35839–35849. doi: 10.1074/jbc.M109.057273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 11.Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol Cell Biol. 2006;26:8217–8227. doi: 10.1128/MCB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuang M, Febbraio M, Wagg C, Lopaschuk GD, Dyck JR. Fatty acid translocase/CD36 deficiency does not energetically or functionally compromise hearts before or after ischemia. Circulation. 2004;109:1550–1557. doi: 10.1161/01.CIR.0000121730.41801.12. [DOI] [PubMed] [Google Scholar]
- 13.Larsen TS, Belke DD, Sas R, Giles WR, Severson DL, Lopaschuk GD, Tyberg JV. The isolated working mouse heart: methodological considerations. Pflugers Arch. 1999;437:979–985. doi: 10.1007/s004240050870. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Alaoui-Talibi Z, Clanachan AS, Schulz R, Lopaschuk GD. Uncoupling of contractile function from mitochondrial TCA cycle activity and MVO2 during reperfusion of ischemic hearts. Am J Physiol. 1996;270:H72–H80. doi: 10.1152/ajpheart.1996.270.1.H72. [DOI] [PubMed] [Google Scholar]
- 15.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO. J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 17.Musi N, Fujii N, Hirshman MF, Ekberg I, Froberg S, Ljungqvist O, Thorell A, Goodyear LJ. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001;50:921–927. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- 18.Neubauer S. The failing heart--an engine out of fuel. N. Engl. J. Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 19.Noga AA, Soltys CL, Barr AJ, Kovacic S, Lopaschuk GD, Dyck JR. Expression of an active LKB1 complex in cardiac myocytes results in decreased protein synthesis associated with phenylephrine-induced hypertrophy. Am. J. Physiol Heart Circ. Physiol. 2007;292:H1460–H1469. doi: 10.1152/ajpheart.01133.2006. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberger LB, Jacobs LW, Stanton HC. Evaluation of cardiac anoxia and ischemia models in the rat using calcium antagonists. Life. Sci. 1984;34:1379–1387. doi: 10.1016/0024-3205(84)90010-9. [DOI] [PubMed] [Google Scholar]
- 21.Russell RR, III, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 22.Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin. Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J. Biol. Chem. 1993;268:25836–25845. [PubMed] [Google Scholar]
- 24.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame HD, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO. J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, Bertrand L. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol. Metab. 2006;290:E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semsarian C, Ahmad I, Giewat M, Georgakopoulos D, Schmitt JP, McConnell BK, Reiken S, Mende U, Marks AR, Kass DA, Seidman CE, Seidman JG. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin. Invest. 2002;109:1013–1020. doi: 10.1172/JCI14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, DePinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- 29.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 30.Thomson DM, Brown JD, Fillmore N, Condon BM, Kim HJ, Barrow JR, Winder WW. LKB1 and the Regulation of Malonyl-CoA and Fatty Acid Oxidation in Muscle. Am J Physiol Endocrinol. Metab. 2007;293:E1572–E1579. doi: 10.1152/ajpendo.00371.2007. [DOI] [PubMed] [Google Scholar]
- 31.Thomson DM, Porter BB, Tall JH, Kim HJ, Barrow JR, Winder WW. Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am J Physiol Endocrinol. Metab. 2007;292:E196–E202. doi: 10.1152/ajpendo.00366.2006. [DOI] [PubMed] [Google Scholar]
- 32.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 33.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc. Natl. Acad. Sci. U S. A. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol. Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]