Abstract
We have recently shown that latent murine cytomegalovirus (MCMV) can influence murine transplant allograft acceptance. During these studies we became aware that vivarium-housed control mice can acquire occult MCMV infection. The purpose of this investigation was to confirm occult MCMV transmission and determine the timing, vehicle, and possible consequences of transmission. Mice arriving from a commercial vendor were negative for MCMV both by commercial serologic testing and by our nested PCR. Mice housed in our vivarium became positive for MCMV DNA 30-60 days after arrival, but remained negative for MCMV by commercial serologic testing. To confirm MCMV we sequenced PCR products for several genes and showed >99% homology to MCMV. Further sequence analyses show that the occult MCMV is similar to a laboratory strain of MCMV, but the vehicle of transmission remains unclear. Control tissues from historical experiments with unexplained graft losses were evaluated for occult MCMV, and mice with unexplained allograft losses showed significantly higher incidence of occult MCMV than did allograft acceptors. Deliberate infection with very low titer MCMV confirmed that viral transmission can occur without measurable virus specific antibody or T-cell responses. These data suggest that vivarium-housed mice can develop occult MCMV that is missed by currently available commercial serologic testing, and that these infections may influence transplant allograft acceptance.
1. Introduction
During our history of transplantation tolerance studies in laboratory mice [1-4], we have encountered intermittent periods of unexplained allograft failures in control mice. Because these failures were episodic and often involved entire cohorts within a single cage, we suspected an infectious etiology, yet commercial serologic evaluations of these and other sentinel mice were routinely negative for infectious pathogens (unpublished data). Recently, we have shown that murine cytomegalovirus (MCMV) can influence cardiac allograft acceptance [5]. During these studies we also became aware that vivarium-housed “naïve” mice can acquire MCMV. We therefore became suspicious that our colonies might intermittently be experiencing occult MCMV infections.
CMV is a beta-herpesvirus that is readily transmitted from infected to naïve hosts leading to endemic prevalence in both humans and wild mice [6, 7]. In immunocompetent hosts, primary CMV infection does not usually induce overt pathology, instead causing a selflimited flu-like illness. After acute infection the virus is not eradicated, and the infected host often sheds virus in saliva or urine, likely contributing to endemic spread. In mice, natural infections occur without clinical signs and thus without careful screening MCMV could go completely unnoticed.
MCMV is routinely included in commercially available murine health screening panels, although a recent publication suggests that the prevalence of MCMV in laboratory mice is extremely low (0.04%) [8]. This is in contrast to near 100% prevalence in wild mice [7]. Of note, laboratory mouse prevalence is based upon serologic testing for MCMV reactive antibodies. Despite these very low reported rates of MCMV in vivarium housed mice, our laboratory experience with highly sensitive PCR techniques led us to hypothesize that vivarium-housed mice might acquire MCMV that escapes commercially available serologic detection, a condition we term “occult” infection, and that occult MCMV infection might influence transplant allograft acceptance.
2. Materials and Methods
2.1 Animals
Female BALB/c and C57BL/6 mice (Harlan, Indianapolis IN) 6-8 weeks of age were used in this study. All animals were housed in an AAALAC-accredited animal facility, adhering to the Guide for the Care and Use of Laboratory Animals prepared by the National Research Council (NIH Publication No. 86-23, revised 1996) with approval of our Institutional Animal Care and Use Committee. All mice were housed in individually ventilated, autoclaved micro-isolator cages with automatic reverse osmosis water supply, Teklad corncob bedding and irradiated 7912 chow ad lib (Harlan, Indianapolis IN). Personnel are required to wear full barrier protection in the animal room, with all mouse manipulations done within a biosafety cabinet. SporKlenz disinfectant is used on all surfaces within the biosafety cabinet, as well as cages after use. Mice were euthanized by cervical dislocation under isoflurane inhalation anesthesia. Mouse tissues were dissected aseptically and frozen immediately in liquid nitrogen, then stored at −80°C. Tissues were procured similarly after euthanasia for wild mice, which were trapped in the vivarium building (hallway and office) as part of this facilities routine pest control.
2.2 Virus
Purified Smith strain (VR-1399) murine CMV obtained from ATCC (Rockville, MD) was used for positive controls for both PCR and sequencing. Occult/wild MCMV virus recovery was attempted by murine fibroblast culture with centrifugal enhancement of infectivity as previously described [9] using multiple tissues from study mice including lung, spleen, salivary gland, kidney, and bladder. For deliberate mouse infections, mice received 1, 10, or 106 pfu Smith MCMV via intraperitoneal injection.
2.3 PCR
Flanking and nested primers for MCMV Immediate Early 1 (IE1) described previously amplify respective sequences of 603 and 384 base pairs (bp) [10]. Primers used for β-Actin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and MCMV viral DNA polymerase (DPol) were previously published [10, 11]. Primers for maize high mobility gene a (HMGa) were forward - TGG ATT CCA TCA ATG CAA AA, reverse - GAG GAG CTC CAT CAC TCG TC. Flanking and nested primer sequences for MCMV glycoprotein B (GB) are available upon request (patent pending). DNA were extracted from tissues homogenates using DNeasy Blood & Tissue Kit (QIAGEN GmbH, Hilden, Germany). DNA were extracted from chow using QuickExtract Plant DNA extraction solution (EPICENTRE Biotechnologies, Madison WI) yielding concentrations of ~200 ng/μl. DNA were eluted in 100 μl of distilled water and stored at −20°C until analysis. DNA were amplified in a total volume of 25μl with 200 nM of each primer and 1.0 U of Taq DNA polymerase (GIBCO BRL) added in 2.5 μl of a PCR buffer (50 mM KCl, 20mM Tris-HCl (pH 8.4), and 1.5 mM MgCl2). PCR reactions were carried out using a Perkin Elmer 9700 thermocycler (PE Applied Biosystems, Foster City, CA), using the following program: initial denaturation 4 min at 94°C, 35 cycles-denaturation 30s at 94°C, annealing 30s at 53°C, elongation 30s at 72°C, followed by final elongation 7 min at 72°C, then hold at 4°C. β-actin or GAPDH transcripts served as cellular transcript controls. Amplification products were separated by electrophoresis in 1% agarose gels, and gels were stained with ethidium bromide.
2.4 Antibody detection
Sera were evaluated by Charles River Research Animal Diagnostic Services (Wilmington, MA) for MCMV reactive antibody by enzyme-linked immunosorbent assay ELISA.
2.5 DNA Sequencing and Analysis
PCR products were extracted using QIAquick Gel Extraction Kit (Qiagen). Products were eluted in 50 μl of extraction buffer, and 10 μl of this product was mixed with 2 μl of the appropriate PCR primer. These samples were analyzed using BigDye Terminator Reaction Chemistry v3.1 for sequence analysis on an Applied Biosystems 3730 DNA Analyzer. Further analyses of the sequences were performed using Bioedit Sequence Alignment Editor v7.0.9.0 (Ibis Biosciences, Carlsbad CA). Sequences obtained were compared to MCMV strain sequences referenced in GenBank and to sequenced laboratory strains of MCMV.
2.6 Cardiac Allograft Tissues
We have previously described a model of cardiac allograft acceptance [1]. Briefly, H2d cardiac allografts are heterotopically transplanted into H2b recipients and long term acceptance of these fully mismatched grafts is induced by gallium nitrate [1]. Impulses of these grafts are monitored after transplantation, and at studies conclusion grafts are procured aseptically and frozen. For the current study, we utilized historical tissues from mice with unexplained cardiac allograft failures (n=33, impulses of <2), and mice with successful graft acceptance (n=26, impulses ≥2). Cardiac allografts from these mice were evaluated by nested PCR for presence of MCMV-DNA.
2.7 Antibodies and flow cytometry
MCMV specific T-cells were identified using MHC-I tetramers specific for MCMV proteins pp89 (H2Ld-restricted 168YPHFMPTNL176 [12] and m164 (H2Dd-restricted 257AGPPRYSRI265 [13] as previously described [14]. Briefly, blood was collected via submandibular puncture into 30ul of ACD solution. RBC’s were lysed with ACK Lyses buffer (Biowhitaker) and peripheral blood mononuclear cells (PBMC) were divided and washed with FACs buffer. MHC class I peptide tetrameric complexes were produced and assembled as previously described [15]. PBMC were incubated with tetramers (37°C) for one hour followed by antibody surface staining (4°C) with fluorescent dye-conjugated antibody specific for CD8 for one hour (PerCP)(BD PharMingen, San Diego, CA). Cells were fixed and analyzed by flow cytometry (FACScalibur, Becton Dickinson, Mountain View, CA). Lymphocytes were gated by forward-side scatter and 5 × 105 events acquired for each specimen. Results were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
3. Results
3.1 Vivarium-housed mice can develop occult CMV
Our preliminary data suggested that mice housed in our vivarium can acquire occult MCMV infections. We therefore hypothesized that a) mice arrive from vendors already infected, or b) they acquire MCMV after delivery. To test these hypotheses, mice underwent serologic testing for MCMV by Charles River Laboratories as well as in-house salivary gland analysis for MCMV DNA. Submandibular salivary glands were chosen to evaluate for viral DNA because they are a major reservoir of MCMV [16]. These evaluations were performed immediately upon arrival and after 5 weeks of vivarium housing. We evaluated both MCMV “susceptible” (BALB/c) and MCMV “resistant” (C57BL/6) mouse strains.
As shown in Figure 1, mice arriving from our vendor were negative for MCMV DNA by PCR. In contrast, both susceptible and resistant mice housed in our vivarium for 5 weeks became MCMV DNA positive. Concomitantly performed commercial serologic antibody testing was negative for murine pathogens including MCMV (data not shown). Thus we confirm that mice housed in the barrier vivarium can develop occult MCMV infection that can be missed by commercially available serologic testing.
Figure 1. Development of occult murine cytomegalovirus (MCMV) in vivarium housed mice.
A. Two strains of mice (BALB/c and C57BL/6) were tested upon arrival and after 5 weeks of vivarium-housing. Nested PCR was performed on DNA extracted from salivary glands for MCMV glycoprotein B, and GAPDH was used as a positive control. Upon arrival (day 0) all mice tested were negative for MCMV DNA. After 5 weeks of vivarium-housing, all mice show MCMV DNA. Neg and Pos refer to negative and positive technique controls. Each lane represents results from single mice.
3.2 Timing of MCMV infection in vivarium
We hypothesized that viral transmission might be a consequence of animal handling, and therefore studied two additional cohorts of mice to determine the timing of occult MCMV infection in our facility. One cohort was managed by a single person whose sole vivarium responsibility was caring for this cohort. The second cohort was managed by vivarium staff responsible for the remaining mouse colonies. As shown in Figure 2, isolated care seemed to delay development of MCMV somewhat, but it did not prevent eventual viral transmission. All mice were serologically negative for MCMV antibody by commercial testing. We thus conclude that vivarium-housed mice can develop occult MCMV infection after 30-60 days of barrier housing even with dedicated handling.
Figure 2. Time-course of occult murine cytomegalovirus (MCMV) infection in vivarium housed mice.
Mice were divided into two cohorts after vendor delivery. The isolated group was handled by a single person caring only for this cohort, while the ULAR group was handled per protocol by vivarium staff. Months indicate months of housing before testing by nested PCR for MCMV glycoprotein B (GB) DNA and β-Actin (control) in salivary glands. Both cohorts tested negative after month 1. The ULAR group became positive during the second month, and the isolated group tested positive after the third month. Each lane represents results from a single mouse performed in duplicate.
3.3 Sequence confirmation of MCMV
Efforts to recover live virus from vivarium-housed mice in fibroblast cultures after spin inoculation were unsuccessful using salivary gland, spleen, lungs, kidneys, or bladder (not shown). This prompted some concern that this occult virus might not be MCMV, but some similar or previously uncharacterized herpes-family virus [17]. To confirm MCMV genes GB and IE1 were cloned and sequenced from mice with occult infection. We chose GB and IE-1 genes because both are highly conserved in laboratory and wild MCMV strains [18]. For a comparator we cloned and sequenced these gene regions from Smith strain MCMV, which were identical to those reported in GenBank (100% similarity). Sequences from vivarium-housed mice with occult infection were aligned and compared to Smith MCMV. Occult virus sequences had high similarity to Smith MCMV for both GB (98.2% of 236 bp) and IE (99.1% of 384 bp) genes. We therefore conclude that the occult infection observed in vivarium-housed mice is indeed MCMV.
3.4 Vehicle of transmission
During our studies two wild mice were captured by vivarium staff familiar with our studies. These appeared to be wild and not escaped vivarium mice based on their smaller size and brown coat color. Nested PCR performed on salivary gland DNA revealed that these wild mice harbored MCMV (Figure 3). These wild mice were thus a potential vehicle of MCMV transmission, but because ventilated micro-isolator barrier housing made direct contact impossible, we sought an alternate hypothesis. One common point of contact might be food, and we therefore hypothesized that wild mice could be contaminating vivarium chow that was subsequently distributed to the colony. Mouse chow from several locations within our vivarium was tested for MCMV DNA by nested PCR. Negative controls included chow from another vivarium, and unopened bags from our vivarium and a local vendor. Teklad 7912 chow contains significant maize (corn) substrate, so we amplified maize HMGa DNA for DNA controls. All chow tested positive for MCMV DNA (Figure 4), despite the source (potentially contaminated versus new).
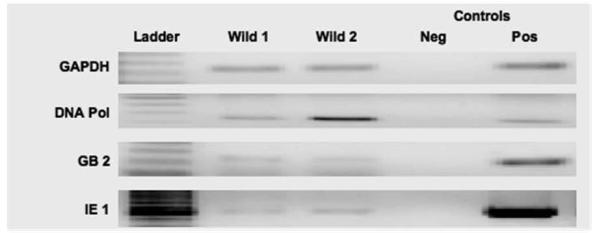
Figure 3. Wild mice trapped in vivarium harbor murine cytomegalovirus (MCMV).
Salivary glands from two wild mice were tested for MCMV DNA using nested PCR. GAPDH was used as a control. Both mice were positive for MCMV DNA Polymerase (DNA Pol), glycoprotein B (GB), and immediate early-1 (IE-1). Neg and Pos refer to negative and positive technique controls. Results were confirmed in triplicate (not shown).
Figure 4. Mouse chow analysis for murine cytomegalovirus (MCMV) DNA.
Mouse chow were evaluated for MCMV glycoprotein B (GB) DNA by nested PCR. Chow was obtained from several bins within the vivarium (lanes 1-3), freshly opened chow from two sources (lanes 4&5) and chow from a different vivarium (lane 6). Because mouse chow contains maize (corn), maize high mobility gene a (HMG) was used to confirm DNA extraction. All chow samples tested positive for MCMV GB DNA by nested PCR.
To test whether mouse chow might be our viral reservoir, PCR products for MCMV IE-1 (384 bp) from chow were sequenced and compared to sequences from vivarium-housed and wild mice. For illustrative purposes, a 40bp segment is shown in Figure 5, showing that wild mice and chow MCMV sequences had high similarity, but that both show important differences from occult MCMV isolates. Interestingly, vivarium mice with occult MCMV had sequences similar to laboratory MCMV. Because wild MCMV are known to have sequence variations when compared with lab strains [18], and the sequences for wild mice and chow are similar to each other and distinct from vivarium-housed mice with occult infection, we conclude that occult MCMV is not being transmitted to vivarium-housed mice either directly by wild mice or indirectly via chow.
Figure 5. Murine cytomegalovirus (MCMV) immediate early 1 (IE-1) gene sequence comparison.
PCR amplicons for IE-1 (384 bp) were sequenced by PCR gel isolation. DNA sequences from chow MCMV, wild MCMV, and vivarium MCMV were aligned and compared to lab MCMV (Smith strain). Shown is a 40bp sequence that highlights similarities and differences between the MCMV isolates. Dark gray indicates differences unique to Wild MCMV, and light gray differences seen in vivarium-acquired MCMV. Sequencing results were confirmed in triplicate (not shown).
3.5 MCMV in transplant recipients with unexpected allograft rejection
To determine if presence of occult MCMV has experimental relevance, we studied cardiac allograft acceptors and unexpected rejectors for evidence of occult MCMV. Frozen cardiac allografts from supposed MCMV - naïve mice from previous experiments were evaluated by nested PCR for presence of MCMV DNA. As shown in Table 1, twenty mice had occult MCMV detected in their allograft hearts, and mice with occult MCMV were twice as likely to experience graft failure than mice without occult MCMV (85% versus 41%, Fishers Exact Test, p=0.002). Put another way, of mice with unexplained graft failures, 52% had occult MCMV, compared with only 12% of mice with allograft acceptance. All tissues required nested-PCR to detect MCMV DNA, and it was impossible to quantitate viral load in these tissues (not shown). Unfortunately, sera were not available from these transplant mice for MCMV antibody testing.
Table 1.
Murine cytomegalovirus (MCMV) status for failed and accepted cardiac allografts.
| DNA Status | ||
|---|---|---|
| MCMV+ | MCMV− | |
| Failed | 17 | 16 |
| Accepted | 3 | 23 |
3.6 MCMV-specific antibody and T-cell responses after low titer infection
Taken together our results suggest that occult MCMV infections are very low titer infection and do not induce significant antibody responses. To test this hypothesis, we infected mice with very low doses of MCMV (100 and 101 pfu) and measured MCMV specific antibody responses over time. As shown in Figure 6A, MCMV antibody responses for 100 pfu were below the detection limit even 16 weeks after infection. Mice infected with 101pfu had antibody levels at the detection limits 4 weeks after infection, and these became and remained just above the detection limits after 16 weeks. In contrast there are robust antibody responses induced by 106 pfu infections.
Figure 6. Immune response to low titer murine cytomegalovirus (MCMV) infection.
Cohorts of n=5 BALB/c mice were infected with 100, 101, or 106 pfu Smith strain MCMV. Sera were serially obtained by venous puncture 4, 12, and 16 weeks after infection, and tissues were obtained 16 weeks after infection. A. MCMV-specific antibody responses to infections were measured by ELISA. B. Representative flow cytometry scatter plots for MCMV-m164 specific CD8 T-cells from peripheral blood mononuclear cells (PBMC). C. Summary of m164-specific T-cell response to infection from PBMC over time. D. Nested PCR for MCMV glycoprotein B (GB) from lungs 16 weeks after infection. For A&C, points/bars represent mean and standard errors from n=5 mice. For D, each lane represents an individual mouse, and NEG and POS refer to technique controls.
It has been shown for some viruses that T-cell responses might be a more sensitive indicator of infection [19], so we also measured MCMV specific T-cell responses to immunodominant epitope MCMV-m164[13, 14, 20]. As shown in Figure 6B&C this immunodominant peptide induces an “inflationary” T-memory response to 106 pfu MCMV by 16 weeks after infection that makes it particularly well suited for these studies. In contrast MCMV-specific T-cell responses to 100 pfu are not different from naïve mice even 16 weeks after infection. Infection with 101 pfu induces a modest but measurable MCMV-specific T-cell response. Identical results were observed for MCMV-pp89 (data not shown).
Most importantly, viral DNA was detectable in lungs (Figure 6D) and salivary gland (not shown).
4. Discussion
This study shows that vivarium-housed mice can develop occult MCMV that may be missed by currently available commercial serologic testing despite being in barrier housing. These MCMV-infected mice do not develop detectable MCMV-reactive antibody, but they do have MCMV DNA in their tissues. Their MCMV DNA concentrations are very low, requiring very sensitive nested PCR for detection, suggesting that these occult infections are very low titer. Indeed, we confirm that very low titer infections can occur without detectable antibody or T-cell responses. Despite our efforts, the vehicle of these occult infections remains unknown, but we feel that the association of occult MCMV with graft losses is very important to those involved in murine allograft transplantation or immunology studies.
Initially we were puzzled by the presence of viral DNA in absence of detectable antibody in vivarium-housed mice, but there is actually precedence for this in human CMV (HCMV). Similar to our observations in mice, some seronegative human patients have been found to harbor human HCMV DNA when tested using highly sensitive PCR based assays [21, 22]. In the present study we show directly that very low titer MCMV infections (100 pfu) do not elicit antibody responses detectable by commercially available serologic methods despite detectable DNA in tissues. Viral load in end organs after MCMV infection has been shown to correlate directly with the infecting inoculum [23]. We have previously shown that viral DNA correlates with viral load, and that after high titer infections MCMV can be detected by a single round of quantitative PCR [24]. Detection of all occult infections in the current report required a second nested-PCR reaction precluding quantitation, and these findings are consistent with extremely low DNA quantities. Likewise after deliberate low titer infections, CMV DNA was not detectable by quantitative PCR (not shown). Altogether these data suggest that occult MCMV infections are very low titer. Importantly for those utilizing murine transplant models, development of occult MCMV infections might elude detection by commercially available serologic methods, and suspicion of such infections should prompt evaluation of available tissues by nested PCR.
The most important question is whether occult MCMV infections are of any experimental relevance. CMV has an obvious well established association with graft loss following human transplantation (reviewed in [25]). Because we have recently shown that latent MCMV can influence allograft acceptance for murine cardiac transplantation [5], and others have shown accelerated cardiac allograft rejection in mice following acute infection [26, 27], we were interested to evaluate tissues from “naïve” mice with unexpected graft losses. There is a strikingly high rate of graft failure associated with occult MCMV suggested by the current report, although this finding is admittedly circumstantial. How occult infections that do not induce significant antibody or possibly T-cell responses can influence allograft acceptance is currently unclear and will require further substantiation. Such studies will also benefit from testing tissues from outside programs. Similarly, the clinical relevance of “occult” CMV in humans [21, 22] is not clear but with widespread availability of quantitative CMV assays these studies could easily be done.
If occult infections are confirmed to be experimentally relevant, one reason why some mice with occult MCMV did not lose their grafts might be explained by timing of occult infection. Others have shown that viral infections can have differential influence on allograft acceptance depending on when the infection occurs relative to transplantation [26-31]. Infections in the current report remained “occult” until post mortem, and unfortunately we have no way to pinpoint when the infections occurred (or to what titer). It is nonetheless clear from our kinetic analysis that vivarium housed non-immunosuppressed mice can acquire occult MCMV between 30-60 days after arrival. This easily overlaps the early time period after mice typically receive cardiac allografts in our program, a precarious period that may be particularly vulnerable to viral infection. We considered the possibility that occult MCMV might actually represent false positives from DNA contamination, but several observations suggest otherwise. First, mice arriving from the vendor were all MCMV negative, as was another cohort of MCMV “naïve” mice housed in another facility (data not shown). Second, our technique controls were consistently negative. Finally and perhaps most importantly, sequencing shows that the viruses isolated from chow and wild mice were significantly different from the occult MCMV strain isolated from vivarium-housed mice. If specimen contamination was occurring during processing, we would anticipate all of the sequences to be the same. Determining the vehicle of MCMV transmission in our vivarium has so far been unsuccessful. Although we experimentally study MCMV infection and reactivation in mice using a high titer (106 pfu) infection model, we maintain these experimentally infected mice within an animal facility geographically separate from our transplant mouse colonies to minimize risk of inadvertent transmission. Our sequencing results do not support chow or wild mouse contact as a route of transmission. In fact, our results suggest that vivarium-housed mice are somehow being infected with our laboratory MCMV strain. Possibilities include improper animal handling, inadequate disinfection of caging/supplies, or viral contamination of bedding, water, or personnel protective attire. In conclusion, these data confirm that hosts infected with low titer CMV may not develop appreciable CMV-specific antibody or T-cell immune responses. Mice housed in a barrier vivarium for more than 30-60 days can develop occult MCMV that may be missed by currently available commercial serologic testing. It is worrisome that these occult infections may significantly influence murine models of transplantation and completely escape notice. Although our results suggest that detection of occult MCMV infections requires more sensitive PCR-based techniques, more work is needed to confirm the significance of occult MCMV and whether traditional commercial serologic testing for antibody should be replaced by more sensitive PCR based methods.
Acknowledgements
This work was supported by NIH grants GM066115 & AI053094. This work was presented in part at the American Association for Laboratory Animal Science (AALAS) 60th Annual Meeting 11/09/2009. The authors wish to thank JJ Wang for performing cardiac allograft transplantations used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orosz CG, Wakely E, Sedmak DD, Bergese SD, VanBuskirk AM. Prolonged murine cardiac allograft acceptance: characteristics of persistent active alloimmunity after treatment with gallium nitrate versus anti-CD4 monoclonal antibody. Transplantation. 1997;63:1109–17. doi: 10.1097/00007890-199704270-00010. [DOI] [PubMed] [Google Scholar]
- 2.Bickerstaff A, Orosz C. Evidence for a limited contribution of immune regulation to cardiac allograft acceptance. Hum Immunol. 2002;63:935–47. doi: 10.1016/s0198-8859(02)00447-0. [DOI] [PubMed] [Google Scholar]
- 3.Bickerstaff AA, VanBuskirk AM, Wakely E, Orosz CG. Transforming growth factor-beta and interleukin-10 subvert alloreactive delayed type hypersensitivity in cardiac allograft acceptor mice. Transplantation. 2000;69:1517–20. doi: 10.1097/00007890-200004150-00055. [DOI] [PubMed] [Google Scholar]
- 4.Bickerstaff AA, Xia D, Pelletier RP, Orosz CG. Mechanisms of graft acceptance: evidence that plasminogen activator controls donor-reactive delayed-type hypersensitivity responses in cardiac allograft acceptor mice. J Immunol. 2000;164:5132–9. doi: 10.4049/jimmunol.164.10.5132. [DOI] [PubMed] [Google Scholar]
- 5.Cook CH, Bickerstaff AA, Wang JJ, Zimmerman PD, Forster MR, Nadasdy T, et al. Disruption of Murine Cardiac Allograft Acceptance by Latent Cytomegalovirus. Am J Transplant. 2009;9:42–53. doi: 10.1111/j.1600-6143.2008.02457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staras SAS, Dollard SC, Radford KW, Flanders W Dana, Pass RF, Cannon MJ. Seroprevalence of Cytomegalovirus Infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143–51. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 7.Smith AL, Singleton GR, Hansen GM, Shellam G. A serologic survey for viruses and Mycoplasma pulmonis among wild house mice (Mus domesticus) in southeastern Australia. J Wildl Dis. 1993;29:219–29. doi: 10.7589/0090-3558-29.2.219. [DOI] [PubMed] [Google Scholar]
- 8.Pritchett-Corning KR, Cosentino J, Clifford CB. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim. 2009;43:165–73. doi: 10.1258/la.2008.008009. [DOI] [PubMed] [Google Scholar]
- 9.Kurz SK, Rapp M, Steffens HP, Grzimek NK, Schmalz S, Reddehase MJ. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J Virol. 1999;73:482–94. doi: 10.1128/jvi.73.1.482-494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, Tumor Necrosis Factor Alpha, or Interleukin-1{beta} Triggers Reactivation of Latent Cytomegalovirus in Immunocompetent Mice. J. Virol. 2006;80:9151–58. doi: 10.1128/JVI.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook C, Zhang X, McGuinness B, Lahm M, Sedmak D, Ferguson R. Intra-abdominal Bacterial Infection Reactivates Latent Pulmonary Cytomegalovirus in Immunocompetent Mice. J Infect Dis. 2002;185:1395–400. doi: 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- 12.Del Val M, Volkmer H, Rothbard JB, Jonjic S, Messerle M, Schickedanz J, et al. Molecular basis for cytolytic T-lymphocyte recognition of the murine cytomegalovirus immediate-early protein pp89. J. Virol. 1988;62:3965–72. doi: 10.1128/jvi.62.11.3965-3972.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtappels R, Grzimek NKA, Simon CO, Thomas D, Dreis D, Reddehase MJ. Processing and Presentation of Murine Cytomegalovirus pORFm164-Derived Peptide in Fibroblasts in the Face of All Viral Immunosubversive Early Gene Functions. J. Virol. 2002;76:6044–53. doi: 10.1128/JVI.76.12.6044-6053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35:1113–23. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 15.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, et al. Phenotypic Analysis of Antigen-Specific T Lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 16.Collins T, Pomeroy C, Jordan MC. Detection of latent cytomegalovirus DNA in diverse organs of mice. J Infect Dis. 1993;168:725–9. doi: 10.1093/infdis/168.3.725. [DOI] [PubMed] [Google Scholar]
- 17.Ehlers B, Kuchler J, Yasmum N, Dural G, Voigt S, Schmidt-Chanasit J, et al. Identification of Novel Rodent Herpesviruses, Including the First Gammaherpesvirus of Mus musculus. J. Virol. 2007;81:8091–100. doi: 10.1128/JVI.00255-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith LM, McWhorter AR, Masters LL, Shellam GR, Redwood AJ. Laboratory Strains of Murine Cytomegalovirus Are Genetically Similar to but Phenotypically Distinct from Wild Strains of Virus. J. Virol. 2008;82:6689–96. doi: 10.1128/JVI.00160-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gujar SA, Michalak TI. Primary Occult Hepadnavirus Infection Induces Virus-Specific T Cell and Aberrant Cytokine Responses in the Absence of Anti-Viral Antibody Reactivity in the Woodchuck Model of Hepatitis B. J. Virol. 2009;83:3861–76. doi: 10.1128/JVI.02521-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, et al. Memory Inflation: Continuous Accumulation of Antiviral CD8+ T Cells Over Time. J Immunol. 2003;170:2022–29. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 21.Kotsimbos AThomas C, Sinickas V, Glare Eric M, Esmore Donald S, Snell Gregory I, Walters EH, et al. Quantitative Detection of Human Cytomegalovirus DNA in Lung Transplant Recipients. Am. J. Respir. Crit. Care Med. 1997;156:1241–46. doi: 10.1164/ajrccm.156.4.96-09106. [DOI] [PubMed] [Google Scholar]
- 22.Boeckh M, Huang M, Ferrenberg J, Stevens-Ayers T, Stensland L, Nichols WG, et al. Optimization of Quantitative Detection of Cytomegalovirus DNA in Plasma by Real-Time PCR. J. Clin. Microbiol. 2004;42:1142–48. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henson D, Smith RD, Gehrke J. Non-fatal mouse cytomegalovirus hepatitis. Combined morphologic, virologic and immunologic observations. Am J Pathol. 1966;49:871–88. [PMC free article] [PubMed] [Google Scholar]
- 24.Cook CH, Zimmerman P, Zhang Y, Chen L, Wen J, Trgovcich J, et al. CD28/B7-mediated costimulation is critical for early control of murine cytomegalovirus infection. Viral Immunology. 2009;22:91–103. doi: 10.1089/vim.2008.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streblow DN, Orloff SL, Nelson JA. Acceleration of allograft failure by cytomegalovirus. Curr Opin Immunol. 2007;19:577–82. doi: 10.1016/j.coi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlquist JF, Shelby J, Shao YL, Greenwood JH, Hammond ME, Anderson JL. Accelerated rejection of murine cardiac allografts by murine cytomegalovirus-infected recipients. Lack of haplotype specificity. J Clin Invest. 1993;91:2602–8. doi: 10.1172/JCI116499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao YL, Shelby J, Hisatake G, Kern ER, Nelson EW, Gay WA. Accelerated cardiac allograft rejection in murine cytomegalovirus-infected C3H recipients. Transplant Proc. 1991;23:129–30. [PubMed] [Google Scholar]
- 28.Williams MA, Onami TM, Adams AB, Durham MM, Pearson TC, Ahmed R, et al. Cutting edge: persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J Immunol. 2002;169:5387–91. doi: 10.4049/jimmunol.169.10.5387. [DOI] [PubMed] [Google Scholar]
- 29.Williams MA, Tan JT, Adams AB, Durham MM, Shirasugi N, Whitmire JK, et al. Characterization of Virus-Mediated Inhibition of Mixed Chimerism and Allospecific Tolerance. J Immunol. 2001;167:4987–95. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- 30.Welsh RM, Markees TG, Woda BA, Daniels KA, Brehm MA, Mordes JP, et al. Virus-Induced Abrogation of Transplantation Tolerance Induced by Donor-Specific Transfusion and Anti-CD154 Antibody. J. Virol. 2000;74:2210–18. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehl S, Hombach J, Aichele P, Rulicke T, Odermatt B, Hengartner H, et al. Viral and Bacterial Infections Interfere with Peripheral Tolerance Induction and Activate CD8+ T Cells to Cause Immunopathology. J. Exp. Med. 1998;187:763–74. doi: 10.1084/jem.187.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]