Abstract
Insulin is an important regulator of glucose, lipid and protein metabolism. It suppresses hepatic glucose and triglyceride production, inhibits adipose tissue lipolysis and whole-body and muscle proteolysis and stimulates glucose uptake in muscle. In this review we discuss what is currently known about the control of substrate metabolism by insulin in men and women. The data available so far indicate that women are more sensitive to insulin with regards to glucose metabolism (both in the liver and in muscle) whereas there are no differences between men and women in insulin action on lipolysis. Potential differences exist in the regulation of plasma triglyceride concentration and protein metabolism by insulin and in changes in insulin-action in response to stimuli (e.g., weight loss and exercise) that are known to alter insulin sensitivity. However, these areas have not been studied comprehensively enough to draw firm conclusions.
Keywords: glucose uptake, hepatic glucose production, lipolysis, triglyceride secretion, triglyceride clearance, proteolysis
Introduction
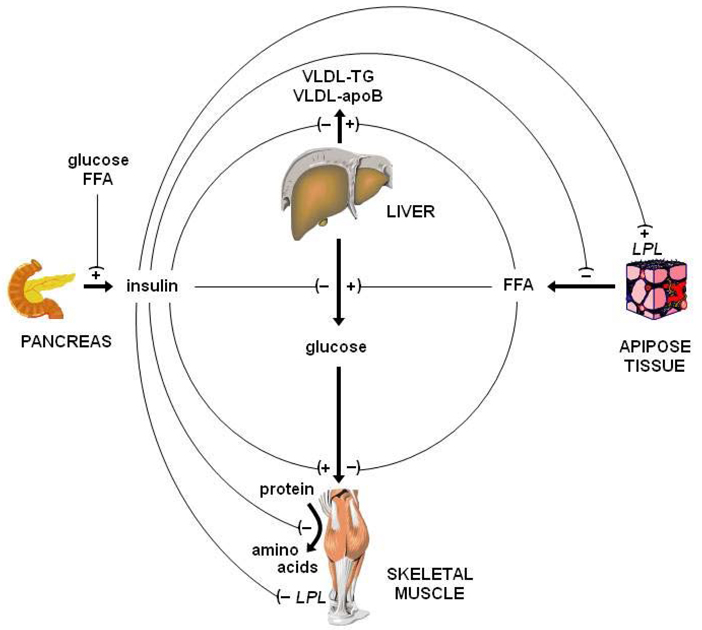
Here we summarize recent findings on differences between men and women in insulin action on glucose, lipid and protein metabolism. Although quite often insulin’s role is narrowly defined within the context of regulation of glucose metabolism (i.e., suppression of hepatic glucose production and stimulation of glucose uptake in muscle by insulin), its actions reach an array of metabolic pathways. Insulin is an important (if not the primary) inhibitor of adipose tissue lipolysis and fatty acid release into the blood stream [1,2]; it is involved in the regulation of hepatic lipoprotein metabolism (e.g., apolipoprotein and triglyceride secretion) [3,4] and blood lipid clearance (by stimulating lipoprotein lipase activity in adipose tissue [5]); and, it is a well-established and very potent inhibitor of protein breakdown [6–9] (Figure 1). It may also affect protein synthesis; however, the effect of insulin on protein synthesis cannot be generalized easily because it is largely dependent on the concomitant availability of amino acids [8–10], and potentially also the target protein of interest [11,12]. Accordingly, resistance to the actions of insulin will result in a multitude of metabolic abnormalities. Furthermore, resistance to the actions of insulin is not a global feature as there are cases of selective insulin resistance (e.g., in the vasculature, insulin resistance affects the PI3 kinase pathway but not other pathways of insulin signaling including the MAPK pathway [13,14]). In addition, there appears to be highly specific regulation of insulin action as evidenced by different sensitivities of different metabolic pathways to the action of insulin (e.g., glucose uptake vs. glucose production etc).
Figure 1.
Summary of the major metabolic actions of insulin. Insulin suppresses hepatic glucose production, stimulates glucose uptake in muscle, suppresses adipose tissue lipolysis and fatty acid release into the blood stream; suppresses hepatic apolipoprotein B-100 and triglyceride secretion, stimulates lipoprotein lipase activity in adipose tissue, and inhibits protein breakdown. Adequate insulin action on adipose tissue lipolysis prevents fatty acid-induced insulin resistance in β-cells, muscle and the liver.
Between group differences in the metabolic actions of insulin can occur through alterations at multiple levels, including insulin secretion from β-cells, insulin delivery and trans endothelial transport, insulin receptor expression and signal transduction in the target tissue [15,16]. We have learned a lot about the mechanism(s) responsible for the regulation of insulin action, both at the whole-body and the cellular level. From a metabolic point of view, it is now well established that cross-talk between adipose tissue and the sites of insulin production and action plays a central role in regulating insulin action. Free fatty acids released during adipose tissue lipolysis inhibit glucose uptake in muscle, and increase glucose production from the liver [17,18]. On the other hand, free fatty acids stimulate insulin secretion from β-cells [19], which may help overcome the negative effects of FFA on insulin action (Figure 1). Interestingly, free fatty acids do not appear to inhibit proteolysis [20]. However, this conclusion was based on proteolytic enzyme expression only. The interaction between free fatty acids and insulin on proteolysis has not been evaluated to date. Adipokines can also affect insulin sensitivity [21]. In addition, insulin may indirectly affect substrate metabolism by acting as a vasodilator thereby regulating nutrient and insulin delivery to tissues [16].
Sex differences in the regulation of substrate metabolism by insulin have started to be recognized only fairly recently and the results from the relatively few studies in this area are not always conclusive. The best, although still understudied area, is the control of glucose metabolism by insulin in men and women. Interest in sexual dimorphism in lipoprotein metabolism was fueled by the well-established differences in the plasma lipid profile [39,103–105], whereas interest in sex differences in lipid metabolism in adipose tissue and protein metabolism in skeletal muscle has arisen due to the obvious differences in body composition between the sexes. For the most part, the data available to date which cover these topics do not extend beyond the results from initial observational studies and there is a lot to discover with regards to the sexually dimorphic control mechanisms responsible for the observed differences. Here we discuss what is currently known about the control of substrate metabolism by insulin in men and women and potential differences between the sexes in changes in insulin-action in response to stimuli (e.g., weight loss and exercise) that are known to alter insulin sensitivity.
Sex differences in the control of glucose metabolism by insulin
Insulin is a major regulator of plasma glucose concentration; it reduces endogenous glucose production (>90% from the liver) and stimulates peripheral glucose uptake. Suppression of endogenous glucose production occurs maximally at relatively low plasma insulin concentrations (<60 mU/l) [22,23], whereas stimulation of whole-body and skeletal muscle glucose uptake occurs maximally at much higher insulin concentrations (e.g., >120 mU/l in healthy, lean subjects) [24].
Fasting plasma glucose and insulin concentrations [25,26] and the basal rates of endogenous glucose production and whole-body glucose disposal, expressed per kg of body weight or lean mass, are typically not different between healthy adult men and women [27–31]; although there are some reports of slightly greater (10–20%) glucose turnover rates in women than in men [32,33]. Total endogenous glucose production is greater in men than women [31,34] due to differences in body size between men and women. No sex differences in endogenous glucose production have been observed during exogenous insulin and dextrose infusion to achieve euglycemia and plasma insulin concentrations of ~50–120 mU/l [27–29]. However, during insulin infusion alone (to induce hypoglycemia) at rates that raise plasma insulin concentrations 20 to ~15–20 mU/l, women exhibited a greater and more prolonged suppression of endogenous glucose production rate than men [30]. And, the suppression of endogenous glucose production after a mixed meal (adjusted for body weight) which raised plasma insulin to peak concentrations of ~50 mU/l was also found to be greater in women than in men [33]. Considering that the insulin-mediated suppression of endogenous glucose production is a measure of hepatic insulin sensitivity [24], women therefore appear to be more sensitive to the effects of insulin in the liver and suppress their endogenous glucose production to a greater extent than men at low plasma insulin concentrations whereas maximal insulin-mediated suppression of endogenous glucose production appears to be the same in men and women.
The results from studies in which the hyperinsulinemic-euglycemic clamp procedure was combined with isotope labeled tracers to evaluate the effect of insulin on glucose rate of disappearance from plasma provide no evidence of sex differences in the insulin-mediated stimulation (percent increase from basal) of whole-body glucose uptake at plasma insulin concentrations between ~50 and 120 mU/l [27–29,35]. Although, there are some dissonant reports. Insulin infusion alone at a rate that raised plasma insulin concentrations to ~15–20 mU/l with a concomitant drop in plasma glucose concentration stimulated glucose uptake in men but failed to do so in women [30] suggesting that women are less insulin sensitive than men. On the other hand, Shadid et al. [34] found no differences in glucose disposal between men and women although plasma insulin concentrations were significantly greater in men than in women, suggesting that men are less insulin sensitive than women. In contrast, studies that measured glucose uptake by the leg/forearm (by using the arteriovenous balance technique or positron emission tomography) during systemic insulin infusion and euglycemia, or following glucose ingestion, have consistently demonstrated a significantly greater insulin-mediated glucose uptake rate per kg of skeletal muscle tissue in women than in men [36–38]. Glucose uptake in skeletal muscle therefore appears to be more sensitive to insulin in women than in men. This is interesting because free fatty acids and increased adipose tissue accumulation is associated with a decrease in insulin sensitivity [17,18] and women are fatter than men and relative to lean tissue mass have greater FFA release into the circulation [39–41]. The reasons for the apparent discrepancy between studies at the whole-body level and across a limb (mostly muscle) are unclear. It is unlikely that adipose tissue is responsible for this discrepancy. Although we are not aware of studies that evaluated insulin-mediated glucose uptake by adipose tissue in vivo in human subjects, the results from studies performed in vitro indicate that adipocytes from female rodents and humans are more insulin-sensitive than those from males with respect to glucose transport and utilization [42–44].
Recently, attempts have been made to unravel the mechanisms responsible for the greater skeletal muscle insulin sensitivity in women than in men; so far with little conclusive evidence other than the fact that differences in insulin receptor and glucose transporter expression, proximal insulin signaling intermediates, intramuscular triacylglycerol, ceramide and diacylglycerol concentrations are not likely the candidates mediating the differences in the response of skeletal muscle to insulin in men and women [37,45]. Furthermore there is no indication that differences in the sex hormone milieu are responsible for the differences between men and women in insulin-mediated glucose disposal. There is no evidence that menstrual cycle phase affects basal glucose metabolism [46–50] or insulin’s action on endogenous glucose production or whole-body glucose disposal [49–52], although greater rates of glucose disposal during the follicular than the luteal phase of the menstrual cycle have been observed during a hyperglycemic (blood glucose > 200 mg/dl) hyperinsulinemic clamp [53]. Furthermore, there is no evidence that treatment with low-dose oral contraceptives [54] or estradiol or progesterone or a combination of both [55] affects basal glucose metabolism and insulin’s action on endogenous glucose production and peripheral glucose uptake; although in cross-sectional studies decreased insulin sensitivity was reported in women who took oral contraceptive pills [27,46]. Moreover, pharmacological suppression of endogenous ovarian hormone secretion [52] and physiological loss of ovarian function during menopause [56,57] is not accompanied by differences in insulin-mediated whole-body glucose disposal. On the other hand, treatment with testosterone reduces insulin-mediated glucose disposal in women [58] and hyperandrogenemia might be the major culprit for the insulin resistance in women with polycystic ovary syndrome [59].
Results from studies that used the hyperinsulinemic (plasma insulin concentrations between 50 and 120 mU/l) euglycemic clamp technique without tracers to evaluate potential sex differences in the regulation of glucose metabolism in men and women provide contrasting results: in most studies no differences in glucose uptake per kg of body weight or lean mass were found between men and women [27–29,60–64], whereas some investigators report greater [36,45,65–67] and others found smaller [35,68,69] insulin-mediated glucose disposal rates in women than in men. However, interpretation of these results is somewhat difficult because this method does not account for potential differences in the contribution of endogenous glucose production to total glucose uptake. Similarly, the results from studies that relied on the oral glucose tolerance test (OGTT) to evaluate insulin sensitivity are equivocal [66,68,70–73] and difficult to interpret because the OGTT does not take into account differences in body size between men and women.
Initial investigations into the interaction of free fatty acids in plasma and the regulation of glucose metabolism by insulin indicated that women [28] do not exhibit free fatty acid-induced insulin resistance, which confirmed earlier works on rats [74]. This observation was later overturned by two independent groups of investigators who found that fatty acids do inhibit insulin-mediated glucose uptake and endogenous glucose production in women both during hyperglycemia-hyperinsulinemia and euglycemia-hyperinsulinemia [75,76]. It should be noted, however, that in the early work by Frias and coworkers [28] plasma insulin concentration was clamped at ~120 mU/l whereas plasma insulin concentration was clamped at 50 mU/l [75] and 85 mU/l [76] in subsequent studies. It is therefore possible that increased free fatty acid availability does not interfere with near-maximal or maximal insulin-stimulated glucose uptake but does reduce the responsiveness to insulin. Only recently Vistisen and colleagues [45] directly compared the lipid-induced inhibition of insulin-mediated glucose disposal in men and women and found it was the same. However, these findings are somewhat difficult to interpret because plasma free fatty acid concentrations were raised to ~2.4 mM in women and 3.7 mM in men; although this difference was not statistically significant, it is big enough to account for possible confounding of the results.
Three studies evaluated the effect of exercise on insulin-mediated glucose uptake in men and women; the results are equivocal. Perreault et al. [77] report that a single prolonged (90 min) bout of moderate intensity exercise augments insulin-mediated glucose disposal to a greater extent in lean women than in lean men. On the other hand, Vistisen and colleagues [45], who evaluated the effect of 30 min of low-intensity exercise on insulin-mediated glucose uptake during concomitant lipid infusion, found that the exercise-induced increase in glucose uptake was not different in obese men and women. And, O’Leary and colleagues [78] report no sex difference in the exercise training-induced improvement in whole-body glucose disposal in obese older adults but the number of subjects in the study was small (7 men), which may have limited statistical power. Clearly more rigorous evaluation of potential sex differences in the exercise-mediated changes in insulin action is needed. Only one study evaluated the effect of weight loss on insulin action in men and women separately and found that women appear to experience lesser improvements (~20%) in insulin-mediated glucose uptake than men (~40% increase in glucose disposal rate during the clamp) [79,80]; however, the study was not specifically designed to evaluate differences in men and women and the difference did not reach statistical significance, most likely due to a type-II error. These are nonetheless important observations and warrant future research to determine whether exercise or weight loss should be emphasized differently in men and women to reverse the negative impact of obesity on glucose metabolism.
Sex differences in the control of adipose tissue lipolysis by insulin
Insulin availability is one of the primary factors regulating adipose tissue lipolysis [1] and free fatty acid release into plasma [2]: an increase in insulin concentration (e.g., after meal ingestion) suppresses lipolytic rates and decreases plasma free fatty acid concentrations [81,82], whereas a decrease in insulin concentration (e.g., during fasting) leads to accelerated lipolysis and increased plasma free fatty acid concentrations [83,84]. Adipose tissue lipolysis and free fatty acid release into plasma are exquisitely sensitive to insulin and half-max suppression of lipolysis occurs within the range of normal fasting plasma insulin concentrations (<15 mU/l) [2,85,86] whereas suppression of basal insulin secretion approximately doubles the rate of lipolysis [2].
During the postabsorptive state, total free fatty acid rate of appearance (Ra) into plasma is generally not different between men and women [34,39,40]. However, because women have more body fat and less fat-free mass than men, basal free fatty acid Ra in relation to the amount of tissues that consume free fatty acids as a fuel and have high energy requirements is ~40–50% greater in women than in men [39–41] whereas free fatty acid Ra in relation to fat mass is the same in men and women [41].
An insulin dose-response study revealed that the plasma insulin concentration at which free fatty acid release into plasma is half-maximally suppressed is not different between men and women (both lean and obese) [85]. Furthermore, two studies evaluated plasma free fatty acid kinetics in men and women after consumption of an energy-adjusted mixed meal and found that the relative (to basal values) meal-induced decrease in free fatty acid Ra was the same in men and women [87,88]. Similarly, the suppression of plasma free fatty acid concentration following ingestion of a mixed meal adjusted for sex differences in energy requirements was found to be the same in men and women [89–91]. However, the suppression of plasma free fatty acid concentration after a standard OGTT is typically greater in women than in men [38,66,73,92,93], most likely because the greater glucose/insulin challenge in women. In addition, there is no evidence for differences between men and women in near-maximal or maximal suppression of plasma free fatty acid concentration and/or free fatty acid Ra during insulin or glucose infusion [27,28,34,45,93–95]. Thus, the response of adipose tissue lipolysis to insulin appears to be the same in the two sexes. Curiously however, the increase in glycerol Ra into plasma (index of adipose tissue lipolysis) during prolonged (~22 h) fasting has been reported to be smaller in women than in men despite a greater decrease in plasma insulin concentration [31], whereas the ability of insulin to suppress lipolysis and plasma free fatty acid concentrations after prolonged fasting (~38 h) is greater in women than in men [96]. It is therefore possible that stimuli that alter insulin sensitivity might affect the insulin response to a different degree in men and women.
Although data is limited, there is so far no evidence that menstrual cycle phase affects basal postabsorptive FFA kinetics [46–48,97,98] or meal fatty acid disposal [99]. The effect of sex hormones on insulin-mediated suppression of lipolysis has to our knowledge not been studied in vivo in human subjects.
Sex differences in the control of plasma triglyceride metabolism by insulin
Insulin is an important regulator of plasma triglyceride homeostasis. Insulin reduces plasma triglyceride and VLDL-apoB-100 concentrations [100] by inhibiting hepatic VLDL-triglyceride and VLDL-apoB-100 production [3,4] and increasing LPL activity in adipose tissue [5]. Some but not all of the insulin-mediated suppression of hepatic VLDL-triglyceride and apoB-100 secretion is due to insulin mediated suppression of adipose tissue lipolysis and fatty acid release into the circulation. Plasma free fatty acid availability is a major regulator of VLDL-TG and VLDL-apoB-100 secretion, most likely by providing substrate for hepatic triglyceride formation [3,4,102]. Nonetheless, it has been demonstrated in mice that the secretion of VLDL-triglyceride by the liver is less sensitive to the inhibitory effect of insulin when compared to endogenous (hepatic) glucose production or insulin-mediated suppression of plasma free fatty acid concentration [101]. In fact, plasma insulin concentration at which VLDL-triglyceride secretion is half-maximally suppressed was similar to that at which whole-body glucose uptake was stimulated to half-maximal values and approximately twice as high as the plasma insulin concentration at which both endogenous glucose production and suppression of plasma free fatty acid concentration occurred [101].
Although there are well-established differences between men and women in basal VLDL-triglyceride kinetics [39,103–105] and in the plasma lipid profile both during fasted and fed conditions [39,89,106], few studies have examined potential sex differences in the metabolic control of VLDL kinetics, including potential sex differences in the sensitivity of VLDL-triglyceride metabolism to insulin. The results from these studies are inconclusive. It has been demonstrated that the relative decrease in total plasma triglyceride concentration after an oral glucose load is greater in women than in men at similar post-glucose challenge insulin concentrations (~20–30 mU/l) in men and women [92]. On the other hand, hyperinsulinemia (>50 mU/l) induced via glucose [107] or insulin and concomitant glucose infusion [28,45] suppressed total plasma triglyceride concentrations similarly in men and women. Furthermore, in two separate studies, one conducted in lean women, the other in lean men, it was found that infusion of insulin to achieve plasma insulin concentrations of ~65 mU/l during euglycemia (plasma glucose concentration at ~5.0 mM) reduces hepatic VLDL-triglyceride and VLDL-apoB-100 secretion rates both in lean men [108] and lean women [3] by ~65% (triglyceride) and ~50% (apoB-100); however, direct comparison of the response in men and women is lacking. We have recently found that moderate prolonged hyperinsulinemia (20–40 mU/l) in response to constant intravenous glucose infusion to achieve modest hyperglycemia (plasma glucose concentration ~7 mM) suppresses hepatic VLDL-triglyceride secretion to the same extent (~45%) in lean men, lean women and obese men [95]; obese women, on the other hand, were resistant to the inhibitory effect of hyperglycemia-hyperinsulinemia on VLDL-triglyceride secretion [95]. Similarly, it was reported that obese compared with lean women are resistant to the insulin-mediated suppression of VLDL-apoB-100 secretion [3]; although the insulin-mediated (at plasma insulin concentrations of ~65 mU/l.) suppression of VLDL-triglyceride secretion appeared to be the same as in lean women. Carefully planned studies are necessary to help put these findings into context and firmly establish or rule out differences between the sexes in the control of triglyceride homeostasis by insulin.
Sex differences in the control of protein metabolism by insulin
Insulin is a potent inhibitor of whole-body and muscle protein breakdown. At the whole-body level, insulin suppresses whole-body protein breakdown (measured as leucine flux) in a dose-dependent manner to approximately 70% of basal values at supraphysiological plasma insulin concentrations. The plasma insulin concentration at which leucine flux was half-maximally suppressed was the same (~35 µU/ml) as the insulin concentration at which whole-body glucose disposal is half-maximally stimulated; however, the corresponding insulin effect in absolute terms corresponded to only ~15% suppression of leucine flux in contrast to a ~2.5 fold increase in glucose disposal [6]. Whole-body protein breakdown is therefore much less responsive to insulin than glucose disposal. On the other hand, muscle protein breakdown is maximally (~50%) suppressed at a plasma insulin concentration of 15–30 µU/ml [7,9]. The effects of insulin on muscle protein synthesis are still not entirely clear but they seem to depend a lot on the availability of amino acids for protein synthesis (which themselves stimulate protein synthesis [8,109]) and may be indirect through insulin’s effect on blood flow and consequently amino acid delivery [9,10,110,111].
Evidence for potential sex differences in protein metabolism is emerging [112–116] – the interest being stimulated in large part by the obvious differences in lean body and muscle mass between men and women [117–120]. Although to date there is too little data to draw firm conclusions and many investigators do not observe differences in the basal rate of muscle protein turnover between men and women [115,121–123]; interestingly, those that do find differences, report a greater rate of muscle protein synthesis in women than in men [112,113]. This is against expectations because of the well-established anabolic effects of testosterone [124] which stimulates muscle protein synthesis and muscle hypertrophy [125–127] whereas female sex steroids inhibit muscle protein synthesis and muscle growth in rodents [128,129]. Data on potential differences between men and women in the control of protein metabolism by insulin is scarce. In a study of 30 y old men and women it was observed that the inhibitory effect of insulin on whole-body proteolysis (assessed during a hyperinsulinemic-euglycemic-isoaminoacidemic clamp) was not different between the sexes but women appeared to be resistant to the stimulatory effect of insulin on whole-body protein synthesis resulting in a smaller net anabolic response to hyperinsulinemia in women than in men [114]. This difference at the whole-body level was most likely not attributable to differences in muscle protein metabolism, which accounts for ~20–40% of a healthy person’s whole-body protein turnover rate [130,131], because we have recently demonstrated that the stimulatory effect of insulin in the context of moderate hyperaminoacidemia (via intravenous amino acid infusion) on muscle protein synthesis is not different in 25–45 year old men and women [121]. On the other hand, we observed anabolic resistance with regards to the stimulatory effect of feeding which resulted in modest hyperinsulinemia, hyperaminoacidemia, and hyperglycemia in 65–80 year old women compared with age-matched men [113]. Future work in this area should be encouraged and will likely yield novel insights.
Summary and Conclusion
In summary: i) women appear to be more sensitive to insulin with regards to glucose metabolism (both in the liver and in muscle); ii) there are no differences in insulin action on lipolysis in men and women; iii) the data available on the regulation of triglyceride and protein metabolism by insulin in men and women is too scarce to draw firm conclusions; and iv) there might be differences in the insulin-sensitizing effects of exercise and weight loss in men and women.
ACKNOWLEDGEMENTS
During the preparation of this work, the authors have received support from National Institutes of Health (NIH) grants AR 49869, HD 057796, AG 031297, DK 56341 (Clinical Nutrition Research Unit) and grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research and the Longer Life Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BM is responsible for the conception of the project, and FM, XW, and BM are jointly responsible for the collection of information and the writing of the manuscript. The final manuscript has been seen and approved by all authors and that they have taken due care to ensure the integrity of their work and their personal scientific reputation.
REFERENCES
- 1.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–297. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38:1595–1601. doi: 10.2337/diab.38.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 1993;42:833–842. doi: 10.2337/diab.42.6.833. [DOI] [PubMed] [Google Scholar]
- 4.Lewis GF, Zinman B, Uffelman KD, Szeto L, Weller B, Steiner G. VLDL production is decreased to a similar extent by acute portal vs. peripheral venous insulin. Am J Physiol. 1994;267:E566–E572. doi: 10.1152/ajpendo.1994.267.4.E566. [DOI] [PubMed] [Google Scholar]
- 5.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med. 2002;80:753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 6.Fukagawa NK, Minaker KL, Rowe JW, Goodman MN, Matthews DE, Bier DM, Young VR. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985;76:2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest. 1992;90:2348–2354. doi: 10.1172/JCI116124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rennie MJ. Body maintenance and repair: how food and exercise keep the musculoskeletal system in good shape. Exp Physiol. 2005;90:427–436. doi: 10.1113/expphysiol.2005.029983. [DOI] [PubMed] [Google Scholar]
- 9.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. Faseb J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahlman B, Charlton M, Fu A, Berg C, O'Brien P, Nair KS. Insulin's effect on synthesis rates of liver proteins. A swine model comparing various precursors of protein synthesis. Diabetes. 2001;50:947–954. doi: 10.2337/diabetes.50.5.947. [DOI] [PubMed] [Google Scholar]
- 12.Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes. 2001;50:2652–2658. doi: 10.2337/diabetes.50.12.2652. [DOI] [PubMed] [Google Scholar]
- 13.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes. 2009;58:2238–2245. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 16.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boden G. Free fatty acids (FFA), a link between obesity and insulin resistance. Front Biosci. 1998;3:d169–d175. doi: 10.2741/a272. [DOI] [PubMed] [Google Scholar]
- 18.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden G. Free fatty acids and insulin secretion in humans. Curr Diab Rep. 2005;5:167–170. doi: 10.1007/s11892-005-0004-5. [DOI] [PubMed] [Google Scholar]
- 20.Turpin SM, Ryall JG, Southgate R, Darby I, Hevener AL, Febbraio MA, Kemp BE, Lynch GS, Watt MJ. Examination of 'lipotoxicity' in skeletal muscle of high-fat fed and ob/ob mice. J Physiol. 2009;587:1593–1605. doi: 10.1113/jphysiol.2008.166033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyck DJ. Adipokines as regulators of muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab. 2009;34:396–402. doi: 10.1139/H09-037. [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo RA. Use of the splanchnic/hepatic balance technique in the study of glucose metabolism. Baillieres Clin Endocrinol Metab. 1987;1:837–862. doi: 10.1016/s0950-351x(87)80008-3. [DOI] [PubMed] [Google Scholar]
- 23.Prager R, Wallace P, Olefsky JM. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J Clin Invest. 1986;78:472–481. doi: 10.1172/JCI112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes. 2004;53:2042–2050. doi: 10.2337/diabetes.53.8.2042. [DOI] [PubMed] [Google Scholar]
- 25.Francois A, Maumus S, Vincent-Viry M, Gueguen R, Siest G, Visvikis S. Age- and sex-related reference values for serum insulin concentration and its biological determinants in a French healthy population. The STANISLAS cohort. Clin Chem Lab Med. 2004;42:1140–1149. doi: 10.1515/CCLM.2004.233. [DOI] [PubMed] [Google Scholar]
- 26.Foster KJ, Alberti KG, Hinks L, Lloyd B, Postle A, Smythe P, Turnell DC, Walton R. Blood intermediary metabolite and insulin concentrations after an overnight fast: reference ranges for adults, and interrelations. Clin Chem. 1978;24:1568–1572. [PubMed] [Google Scholar]
- 27.Perseghin G, Scifo P, Pagliato E, Battezzati A, Benedini S, Soldini L, Testolin G, Del Maschio A, Luzi L. Gender factors affect fatty acids-induced insulin resistance in nonobese humans: effects of oral steroidal contraception. J Clin Endocrinol Metab. 2001;86:3188–3196. doi: 10.1210/jcem.86.7.7666. [DOI] [PubMed] [Google Scholar]
- 28.Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT. Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes. 2001;50:1344–1350. doi: 10.2337/diabetes.50.6.1344. [DOI] [PubMed] [Google Scholar]
- 29.Koska J, Stefan N, Permana PA, Weyer C, Sonoda M, Bogardus C, Smith SR, Joanisse DR, Funahashi T, Krakoff J, Bunt JC. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr. 2008;87:295–302. doi: 10.1093/ajcn/87.2.295. [DOI] [PubMed] [Google Scholar]
- 30.Amiel SA, Maran A, Powrie JK, Umpleby AM, Macdonald IA. Gender differences in counterregulation to hypoglycaemia. Diabetologia. 1993;36:460–464. doi: 10.1007/BF00402284. [DOI] [PubMed] [Google Scholar]
- 31.Mittendorfer B, Horowitz JF, Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab. 2001;281:E1333–E1339. doi: 10.1152/ajpendo.2001.281.6.E1333. [DOI] [PubMed] [Google Scholar]
- 32.Koska J, Stefan N, Votruba SB, Smith SR, Krakoff J, Bunt JC. Distribution of subcutaneous fat predicts insulin action in obesity in sex-specific manner. Obesity (Silver Spring) 2008;16:2003–2009. doi: 10.1038/oby.2008.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza RA. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55:2001–2014. doi: 10.2337/db05-1692. [DOI] [PubMed] [Google Scholar]
- 34.Shadid S, Kanaley JA, Sheehan MT, Jensen MD. Basal and insulin-regulated free fatty acid and glucose metabolism in humans. Am J Physiol Endocrinol Metab. 2007;292:E1770–E1774. doi: 10.1152/ajpendo.00655.2006. [DOI] [PubMed] [Google Scholar]
- 35.Pereira S, Marliss EB, Morais JA, Chevalier S, Gougeon R. Insulin resistance of protein metabolism in type 2 diabetes. Diabetes. 2008;57:56–63. doi: 10.2337/db07-0887. [DOI] [PubMed] [Google Scholar]
- 36.Nuutila P, Knuuti MJ, Maki M, Laine H, Ruotsalainen U, Teras M, Haaparanta M, Solin O, Yki-Jarvinen H. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes. 1995;44:31–36. doi: 10.2337/diab.44.1.31. [DOI] [PubMed] [Google Scholar]
- 37.Hoeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JF, Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol. 2009;107:824–831. doi: 10.1152/japplphysiol.91382.2008. [DOI] [PubMed] [Google Scholar]
- 38.Paula FJ, Pimenta WP, Saad MJ, Paccola GM, Piccinato CE, Foss MC. Sex-related differences in peripheral glucose metabolism in normal subjects. Diabete Metab. 1990;16:234–239. [PubMed] [Google Scholar]
- 39.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab. 2007;92:1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;111:981–988. doi: 10.1172/JCI16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.224. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58:803–812. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerre-Millo M, Leturque A, Girard J, Lavau M. Increased insulin sensitivity and responsiveness of glucose metabolism in adipocytes from female versus male rats. J Clin Invest. 1985;76:109–116. doi: 10.1172/JCI111932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen O, Hjollund E, Lindskov HO. Insulin binding and action on fat cells from young healthy females and males. Am J Physiol. 1982;243:E158–E167. doi: 10.1152/ajpendo.1982.243.2.E158. [DOI] [PubMed] [Google Scholar]
- 45.Vistisen B, Hellgren LI, Vadset T, Scheede-Bergdahl C, Helge JW, Dela F, Stallknecht B. Effect of gender on lipid-induced insulin resistance in obese subjects. Eur J Endocrinol. 2008;158:61–68. doi: 10.1530/EJE-07-0493. [DOI] [PubMed] [Google Scholar]
- 46.Corssmit EP, Stouthard JM, Romijn JA, Endert E, Sauerwein HP. Sex differences in the adaptation of glucose metabolism to short-term fasting: effects of oral contraceptives. Metabolism. 1994;43:1503–1508. doi: 10.1016/0026-0495(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 47.Heiling VJ, Jensen MD. Free fatty acid metabolism in the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab. 1992;74:806–810. doi: 10.1210/jcem.74.4.1548345. [DOI] [PubMed] [Google Scholar]
- 48.Zderic TW, Coggan AR, Ruby BC. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J Appl Physiol. 2001;90:447–453. doi: 10.1152/jappl.2001.90.2.447. [DOI] [PubMed] [Google Scholar]
- 49.Diamond MP, Jacob R, Connolly-Diamond M, DeFronzo RA. Glucose metabolism during the menstrual cycle. Assessment with the euglycemic, hyperinsulinemic clamp. J Reprod Med. 1993;38:417–421. [PubMed] [Google Scholar]
- 50.Toth EL, Suthijumroon A, Crockford PM, Ryan EA. Insulin action does not change during the menstrual cycle in normal women. J Clin Endocrinol Metab. 1987;64:74–80. doi: 10.1210/jcem-64-1-74. [DOI] [PubMed] [Google Scholar]
- 51.Yki-Jarvinen H. Insulin sensitivity during the menstrual cycle. J Clin Endocrinol Metab. 1984;59:350–353. doi: 10.1210/jcem-59-2-350. [DOI] [PubMed] [Google Scholar]
- 52.Cooper BC, Sites CK, Casson PR, Toth MJ. Ovarian suppression with a gonadotropin-releasing hormone agonist does not alter insulin-stimulated glucose disposal. Fertil Steril. 2007;87:1131–1138. doi: 10.1016/j.fertnstert.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diamond MP, Simonson DC, DeFronzo RA. Menstrual cyclicity has a profound effect on glucose homeostasis. Fertil Steril. 1989;52:204–208. [PubMed] [Google Scholar]
- 54.Scheen AJ, Jandrain BJ, Humblet DM, Jaminet CB, Gaspard UJ, Lefebvre PJ. Effects of a 1-year treatment with a low-dose combined oral contraceptive containing ethinyl estradiol and cyproterone acetate on glucose and insulin metabolism. Fertil Steril. 1993;59:797–802. doi: 10.1016/s0015-0282(16)55862-2. [DOI] [PubMed] [Google Scholar]
- 55.Thornton KL, DeFronzo RA, Sherwin RS, Diamond MP. Micronized estradiol and progesterone: effects on carbohydrate metabolism in reproductive-age women. J Soc Gynecol Investig. 1995;2:643–652. doi: 10.1016/1071-5576(95)00012-4. [DOI] [PubMed] [Google Scholar]
- 56.Toth MJ, Sites CK, Eltabbakh GH, Poehlman ET. Effect of menopausal status on insulin-stimulated glucose disposal: comparison of middle-aged premenopausal and early postmenopausal women. Diabetes Care. 2000;23:801–806. doi: 10.2337/diacare.23.6.801. [DOI] [PubMed] [Google Scholar]
- 57.Muscelli E, Kozakova M, Flyvbjerg A, Kyriakopoulou K, Astiarraga BD, Glintborg D, Konrad T, Favuzzi A, Petrie J. The effect of menopause on carotid artery remodeling, insulin sensitivity, and plasma adiponectin in healthy women. Am J Hypertens. 2009;22:364–370. doi: 10.1038/ajh.2009.16. [DOI] [PubMed] [Google Scholar]
- 58.Zang H, Carlstrom K, Arner P, Hirschberg AL. Effects of treatment with testosterone alone or in combination with estrogen on insulin sensitivity in postmenopausal women. Fertil Steril. 2006;86:136–144. doi: 10.1016/j.fertnstert.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 59.Giallauria F, Palomba S, Vigorito C, Tafuri MG, Colao A, Lombardi G, Orio F. Androgens in polycystic ovary syndrome: the role of exercise and diet. Semin Reprod Med. 2009;27:306–315. doi: 10.1055/s-0029-1225258. [DOI] [PubMed] [Google Scholar]
- 60.Donahue RP, Prineas RJ, DeCarlo Donahue R, Bean JA, Skyler JS. The female 'insulin advantage' in a biracial cohort: results from the Miami Community Health Study. Int J Obes Relat Metab Disord. 1996;20:76–82. [PubMed] [Google Scholar]
- 61.Rattarasarn C, Leelawattana R, Soonthornpun S, Setasuban W, Thamprasit A. Gender differences of regional abdominal fat distribution and their relationships with insulin sensitivity in healthy and glucose-intolerant Thais. J Clin Endocrinol Metab. 2004;89:6266–6270. doi: 10.1210/jc.2004-0209. [DOI] [PubMed] [Google Scholar]
- 62.Marcell TJ, McAuley KA, Traustadottir T, Reaven PD. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005;54:533–541. doi: 10.1016/j.metabol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Yki-Jarvinen H. Sex and insulin sensitivity. Metabolism. 1984;33:1011–1015. doi: 10.1016/0026-0495(84)90229-4. [DOI] [PubMed] [Google Scholar]
- 64.Manco M, Nolfe G, Calvani M, Natali A, Nolan J, Ferrannini E, Mingrone G. Menopause, insulin resistance, and risk factors for cardiovascular disease. Menopause. 2006;13:809–817. doi: 10.1097/01.gme.0000233492.38638.74. [DOI] [PubMed] [Google Scholar]
- 65.Borissova AM, Tankova T, Kirilov G, Koev D. Gender-dependent effect of ageing on peripheral insulin action. Int J Clin Pract. 2005;59:422–426. doi: 10.1111/j.1368-5031.2005.00209.x. [DOI] [PubMed] [Google Scholar]
- 66.Sumner AE, Kushner H, Sherif KD, Tulenko TN, Falkner B, Marsh JB. Sex differences in African-Americans regarding sensitivity to insulin's glucoregulatory and antilipolytic actions. Diabetes Care. 1999;22:71–77. doi: 10.2337/diacare.22.1.71. [DOI] [PubMed] [Google Scholar]
- 67.Ferrara CM, Goldberg AP, Nicklas BJ, Sorkin JD, Ryan AS. Sex differences in insulin action and body fat distribution in overweight and obese middle-aged and older men and women. Appl Physiol Nutr Metab. 2008;33:784–790. doi: 10.1139/h08-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falkner B, Hulman S, Kushner H. Gender differences in insulin-stimulated glucose utilization among African-Americans. Am J Hypertens. 1994;7:948–952. doi: 10.1093/ajh/7.11.948. [DOI] [PubMed] [Google Scholar]
- 69.Falkner B, Sherif K, Kushner H. Gender differences in the relationship between insulin-mediated glucose utilization and sex hormones in young African-Americans. J Gend Specif Med. 2000;3:60–65. [PubMed] [Google Scholar]
- 70.Boyns DR, Crossley JN, Abrams ME, Jarrett RJ, Keen H. Oral glucose tolerance and related factors in a normal population sample. I. Blood sugar, plasma insulin, glyceride, and cholesterol measurements and the effects of age and sex. Br Med J. 1969;1:595–598. doi: 10.1136/bmj.1.5644.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donahue RP, Bean JA, Donahue RA, Goldberg RB, Prineas RJ Miami Community Health Study. Insulin response in a triethnic population: effects of sex, ethnic origin, and body fat. Diabetes Care. 1997;20:1670–1676. doi: 10.2337/diacare.20.11.1670. [DOI] [PubMed] [Google Scholar]
- 72.Potteiger JA, Jacobsen DJ, Donnelly JE, Hill JO. Glucose and insulin responses following 16 months of exercise training in overweight adults: the Midwest Exercise Trial. Metabolism. 2003;52:1175–1181. doi: 10.1016/s0026-0495(03)00146-x. [DOI] [PubMed] [Google Scholar]
- 73.Sumner AE, Kushner H, Tulenko TN, Falkner B, Marsh JB. The relationship in African-Americans of sex differences in insulin-mediated suppression of nonesterified fatty acids to sex differences in fasting triglyceride levels. Metabolism. 1997;46:400–405. doi: 10.1016/s0026-0495(97)90055-x. [DOI] [PubMed] [Google Scholar]
- 74.Hevener A, Reichart D, Janez A, Olefsky J. Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes. 2002;51:1907–1912. doi: 10.2337/diabetes.51.6.1907. [DOI] [PubMed] [Google Scholar]
- 75.Shah P, Vella A, Basu A, Basu R, Adkins A, Schwenk WF, Johnson CM, Nair KS, Jensen MD, Rizza RA. Elevated free fatty acids impair glucose metabolism in women: decreased stimulation of muscle glucose uptake and suppression of splanchnic glucose production during combined hyperinsulinemia and hyperglycemia. Diabetes. 2003;52:38–42. doi: 10.2337/diabetes.52.1.38. [DOI] [PubMed] [Google Scholar]
- 76.Homko CJ, Cheung P, Boden G. Effects of free fatty acids on glucose uptake and utilization in healthy women. Diabetes. 2003;52:487–491. doi: 10.2337/diabetes.52.2.487. [DOI] [PubMed] [Google Scholar]
- 77.Perreault L, Lavely JM, Bergman BC, Horton TJ. Gender differences in insulin action after a single bout of exercise. J Appl Physiol. 2004;97:1013–1021. doi: 10.1152/japplphysiol.00186.2004. [DOI] [PubMed] [Google Scholar]
- 78.O'Leary VB, Jorett AE, Marchetti CM, Gonzalez F, Phillips SA, Ciaraldi TP, Kirwan JP. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab. 2007;293:E421–E427. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 79.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. Faseb J. 1999;13:2051–2060. doi: 10.1096/fasebj.13.14.2051. [DOI] [PubMed] [Google Scholar]
- 80.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 81.Abbasi F, McLaughlin T, Lamendola C, Reaven GM. The relationship between glucose disposal in response to physiological hyperinsulinemia and basal glucose and free fatty acid concentrations in healthy volunteers. J Clin Endocrinol Metab. 2000;85:1251–1254. doi: 10.1210/jcem.85.3.6450. [DOI] [PubMed] [Google Scholar]
- 82.Campbell PJ, Carlson MG, Hill JO, Nurjhan N. Regulation of free fatty acid metabolism by insulin in humans: role of lipolysis and reesterification. Am J Physiol. 1992;263:E1063–E1069. doi: 10.1152/ajpendo.2006.263.6.E1063. [DOI] [PubMed] [Google Scholar]
- 83.Bergman BC, Cornier MA, Horton TJ, Bessesen DH. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol Endocrinol Metab. 2007;293:E1103–E1111. doi: 10.1152/ajpendo.00613.2006. [DOI] [PubMed] [Google Scholar]
- 84.Klein S, Sakurai Y, Romijn JA, Carroll RM. Progressive alterations in lipid and glucose metabolism during short-term fasting in young adult men. Am J Physiol. 1993;265:E801–E806. doi: 10.1152/ajpendo.1993.265.5.E801. [DOI] [PubMed] [Google Scholar]
- 85.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism. 2007;56:68–76. doi: 10.1016/j.metabol.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 86.Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72:96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- 87.Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest. 1995;96:2297–2303. doi: 10.1172/JCI118285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen TT, Mijares AH, Johnson CM, Jensen MD. Postprandial leg and splanchnic fatty acid metabolism in nonobese men and women. Am J Physiol. 1996;271:E965–E972. doi: 10.1152/ajpendo.1996.271.6.E965. [DOI] [PubMed] [Google Scholar]
- 89.Horton TJ, Commerford SR, Pagliassotti MJ, Bessesen DH. Postprandial leg uptake of triglyceride is greater in women than in men. Am J Physiol Endocrinol Metab. 2002;283:E1192–E1202. doi: 10.1152/ajpendo.00164.2002. [DOI] [PubMed] [Google Scholar]
- 90.Burdge GC, Powell J, Calder PC. Lack of effect of meal fatty acid composition on postprandial lipid, glucose and insulin responses in men and women aged 50–65 years consuming their habitual diets. Br J Nutr. 2006;96:489–500. [PubMed] [Google Scholar]
- 91.Koutsari C, Zagana A, Tzoras I, Sidossis LS, Matalas AL. Gender influence on plasma triacylglycerol response to meals with different monounsaturated and saturated fatty acid content. Eur J Clin Nutr. 2004;58:495–502. doi: 10.1038/sj.ejcn.1601836. [DOI] [PubMed] [Google Scholar]
- 92.McKeigue PM, Laws A, Chen YD, Marmot MG, Reaven GM. Relation of plasma triglyceride and apoB levels to insulin-mediated suppression of nonesterified fatty acids. Possible explanation for sex differences in lipoprotein pattern. Arterioscler Thromb. 1993;13:1187–1192. doi: 10.1161/01.atv.13.8.1187. [DOI] [PubMed] [Google Scholar]
- 93.Sumner AE, Kushner H, Lakota CA, Falkner B, Marsh JB. Gender differences in insulin-induced free fatty acid suppression: studies in an African American population. Lipids. 1996;31 Suppl:S275–S278. doi: 10.1007/BF02637090. [DOI] [PubMed] [Google Scholar]
- 94.Carpentier A, Patterson BW, Leung N, Lewis GF. Sensitivity to acute insulin-mediated suppression of plasma free fatty acids is not a determinant of fasting VLDL triglyceride secretion in healthy humans. Diabetes. 2002;51:1867–1875. doi: 10.2337/diabetes.51.6.1867. [DOI] [PubMed] [Google Scholar]
- 95.Mittendorfer B, Patterson BW, Klein S, Sidossis LS. VLDL-triglyceride kinetics during hyperglycemia-hyperinsulinemia: effects of sex and obesity. Am J Physiol Endocrinol Metab. 2003;284:E708–E715. doi: 10.1152/ajpendo.00411.2002. [DOI] [PubMed] [Google Scholar]
- 96.Soeters MR, Sauerwein HP, Groener JE, Aerts JM, Ackermans MT, Glatz JF, Fliers E, Serlie MJ. Gender-related differences in the metabolic response to fasting. J Clin Endocrinol Metab. 2007;92:3646–3652. doi: 10.1210/jc.2007-0552. [DOI] [PubMed] [Google Scholar]
- 97.Magkos F, Patterson BW, Mittendorfer B. No effect of menstrual cycle phase on VLDL-triglyceride and VLDL-apolipoprotein B-100 metabolism. Am J Physiol Endocrinol Metab. 2006;291:E1243–E1249. doi: 10.1152/ajpendo.00246.2006. [DOI] [PubMed] [Google Scholar]
- 98.Horton TJ, Miller EK, Bourret K. No effect of menstrual cycle phase on glycerol or palmitate kinetics during 90 min of moderate exercise. J Appl Physiol. 2006;100:917–925. doi: 10.1152/japplphysiol.00491.2005. [DOI] [PubMed] [Google Scholar]
- 99.Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol Endocrinol Metab. 2005;288:E547–E555. doi: 10.1152/ajpendo.00340.2004. [DOI] [PubMed] [Google Scholar]
- 100.Bioletto S, Golay A, Munger R, Kalix B, James RW. Acute hyperinsulinemia and very-low-density and low-density lipoprotein subfractions in obese subjects. Am J Clin Nutr. 2000;71:443–449. doi: 10.1093/ajcn/71.2.443. [DOI] [PubMed] [Google Scholar]
- 101.den Boer MA, Voshol PJ, Kuipers F, Romijn JA, Havekes LM. Hepatic glucose production is more sensitive to insulin-mediated inhibition than hepatic VLDL-triglyceride production. Am J Physiol Endocrinol Metab. 2006;291:E1360–E1364. doi: 10.1152/ajpendo.00188.2006. [DOI] [PubMed] [Google Scholar]
- 102.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 103.Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr. 2003;77:573–579. doi: 10.1093/ajcn/77.3.573. [DOI] [PubMed] [Google Scholar]
- 104.Nikkila EA, Kekki M. Polymorphism of plasma triglyceride kinetics in normal human adult subjects. Acta Med Scand. 1971;190:49–59. doi: 10.1111/j.0954-6820.1971.tb07395.x. [DOI] [PubMed] [Google Scholar]
- 105.Olefsky J, Farquhar JW, Reaven GM. Sex difference in the kinetics of triglyceride metabolism in normal and hypertriglyceridaemic human subjects. Eur J Clin Invest. 1974;4:121–127. doi: 10.1111/j.1365-2362.1974.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 106.Magkos F, Mohammed BS, Mittendorfer B. Effect of obesity on the plasma lipoprotein subclass profile in normoglycemic and normolipidemic men and women. Int J Obes (Lond) 2008;32:1655–1664. doi: 10.1038/ijo.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Riemens SC, Ligtenberg JJ, Dullaart RP. Hyperglycemia-induced hyperinsulinemia acutely lowers plasma apolipoprotein B but not lipoprotein (a) in man. Clin Chim Acta. 1997;261:149–158. doi: 10.1016/s0009-8981(97)06528-5. [DOI] [PubMed] [Google Scholar]
- 108.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 110.Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and Insulin-Like Growth Factor-I Enhance Human Skeletal Muscle Protein Anabolism during Hyperaminoacidemia by Different Mechanisms. Journal of Clinical Investigation. 1995;96:1722–1729. doi: 10.1172/JCI118217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin's effect to stimulate protein synthesis in the human forearm. Am J Physiol. 1998;274:E1067–E1074. doi: 10.1152/ajpendo.1998.274.6.E1067. [DOI] [PubMed] [Google Scholar]
- 112.Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O'Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. Faseb J. 2009;23:631–641. doi: 10.1096/fj.08-117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith GI, Atherton P, Villareal DT, Frimel T, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS ONE. 2008;3:1875–1883. doi: 10.1371/journal.pone.0001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chevalier S, Marliss EB, Morais JA, Lamarche M, Gougeon R. The influence of sex on the protein anabolic response to insulin. Metabolism. 2005;54:1529–1535. doi: 10.1016/j.metabol.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 115.Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol. 2001;91:1041–1047. doi: 10.1152/jappl.2001.91.3.1041. [DOI] [PubMed] [Google Scholar]
- 116.Pannemans DL, Halliday D, Westerterp KR, Kester AD. Effect of variable protein intake on whole-body protein turnover in young men and women. Am J Clin Nutr. 1995;61:69–74. doi: 10.1093/ajcn/61.1.69. [DOI] [PubMed] [Google Scholar]
- 117.Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism. 1970;19:653–663. doi: 10.1016/0026-0495(70)90062-4. [DOI] [PubMed] [Google Scholar]
- 118.Mingrone G, Marino S, DeGaetano A, Capristo E, Heymsfield SB, Gasbarrini G, Greco AV. Different limit to the body's ability of increasing fat-free mass. Metabolism. 2001;50:1004–1007. doi: 10.1053/meta.2001.25650. [DOI] [PubMed] [Google Scholar]
- 119.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 120.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–672. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- 121.Smith GI, Atherton PJ, Reeds DN, Mohammed BS, Jaffrey H, Rankin D, Rennie MJ, Mittendorfer B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.00348.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab. 1999;84:1007–1010. doi: 10.1210/jcem.84.3.5522. [DOI] [PubMed] [Google Scholar]
- 123.Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007;292:E77–E83. doi: 10.1152/ajpendo.00173.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63:280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 125.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 126.Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003;88:358–362. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- 127.Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275:E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- 128.Toth MJ, Poehlman ET, Matthews DE, Tchernof A, MacCoss MJ. Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. Am J Physiol Endocrinol Metab. 2001;280:E496–E501. doi: 10.1152/ajpendo.2001.280.3.E496. [DOI] [PubMed] [Google Scholar]
- 129.Fisher JS, Hasser EM, Brown M. Effects of ovariectomy and hindlimb unloading on skeletal muscle. J Appl Physiol. 1998;85:1316–1321. doi: 10.1152/jappl.1998.85.4.1316. [DOI] [PubMed] [Google Scholar]
- 130.Long CL, Birkhahn RH, Geiger JW, Blakemore WS. Contribution of skeletal muscle protein in elevated rates of whole body protein catabolism in trauma patients. Am J Clin Nutr. 1981;34:1087–1093. doi: 10.1093/ajcn/34.6.1087. [DOI] [PubMed] [Google Scholar]
- 131.Morais JA, Gougeon R, Pencharz PB, Jones PJ, Ross R, Marliss EB. Whole-body protein turnover in the healthy elderly. Am J Clin Nutr. 1997;66:880–889. doi: 10.1093/ajcn/66.4.880. [DOI] [PubMed] [Google Scholar]