Abstract
Activation of Gq protein-coupled receptors can be monitored by measuring the increase in intracellular calcium with fluorescent dyes. Recent advances in fluorescent kinetic plate readers and liquid-handling technology have made it possible to follow these transient changes in intracellular calcium in a 1,536-well plate format for high-throughput screening (HTS). Here, we have applied the latest generation of fluorescence kinetic plate readers to multiplex the agonist and antagonist screens of a G protein-coupled receptor (GPCR). This multiplexed assay format provides an efficient and cost-effective method for HTS of Gq-coupled GPCR targets.
Introduction
Guanosine triphosphate-binding protein (G-protein)-coupled receptors (GPCRs) are the largest family of membrane-signaling proteins and are important targets for drug development.1 Over 30% of marketed drugs mediate their actions through GPCRs.2 Various small-molecule modulators of GPCRs have been found to have wide therapeutic applications, including agonists, antagonists, inverse agonists, and allosteric modulators.3–5 GPCRs mainly signal through the Gs/i G-protein/cAMP and Gq G-protein/calcium pathways to regulate a variety of cellular functions. For the Gq-activated GPCRs, binding of an agonist results in an increase in intracellular calcium. In resting cells, the cytosolic calcium concentration is much lower (∼100–200 nM) than that in the extracellular environment (∼2 mM). When the cells are excited by the activation of GPCRs, the concentration of intracellular calcium can rapidly increase to ∼100 μM. The low basal intracellular calcium level and the rapid increase of cytosolic calcium upon receptor activation enable the use of fluorescent calcium dyes to measure transient changes of cytosolic calcium concentration. Because the calcium response is rapid and transient, hardware that supports kinetic measurements is needed.
Fura-2, a calcium dye, is excited at different wavelengths depending on whether it is bound to calcium, and has a common emission wavelength of 510 nm. In the presence of calcium, peak Fura-2 excitation is 340 nm, while in the absence of calcium it is 380 nm.6,7 The ratio of fluorescence emissions from excitations at 340 and 380 nm is used to quantify the increase in cytosolic calcium concentration. Fluo-3 and Fluo-4 are calcium dyes with a single excitation wavelength, and only fluoresce when calcium ions are bound to the dyes with an excitation peak at 480 nm and emission peak at 525 nm. Calcium dyes are commonly used in acetoxymethyl ester form, which facilitates the dyes crossing the cell membrane. Once inside a cell, intracellular esterases hydrolyze the esters, effectively trapping the calcium dye inside the cell.8–10 Remaining extracellular, dye needs to be washed away before agonist stimulation and any kinetic measurements in order to reduce the signal background. Recently, homogeneous calcium assay kits have become available that eliminate the cell wash step, simplifying the assay protocol. In the homogeneous calcium assay, a cell membrane-impermeable fluorescent quencher is added to the assay solution that suppresses fluorescent signal from extracellular calcium dye without affecting the intracellular fluorescence signal when the assay plate is detected in the bottom reading mode.11–13
In the past 10 to 15 years, instruments for the kinetic measurement of calcium fluorescence intensity have evolved from initial cuvette-based detectors to plate-based readers including Fluorescent Imaging Plate Reader (FLIPR)14,15 and Functional Drug Screening System (FDSS). The excitation light source in these kinetic fluorescent plate readers has progressed from laser to more durable and broad spectrum lights such as light-emitting diode (LED) and xenon lamp arrays. The well density of assay plates has also increased from 96- to 384- and even 1,536-well format, which has greatly increased the screening throughput and at the same time reduced screening costs. However, the 1,536-well plate format calcium assay using the previous versions of instruments is not optimal due to the limitations in liquid-handling systems and tip wash stations.16 Recently, a new version of FDSS instrument has become available with an expanded liquid-handling system for 1,536-well plates and a more sensitive CCD camera for luminescence. We have applied this new fluorescence kinetic plate reader to the high-throughput screening (HTS) of GPCRs and ion channel assays in 1,536-well plate format. We report here a multiplex calcium assay for identification of GPCR agonists and antagonists. This assay should markedly improve the screening efficiency and expand the assay design options of calcium-based assays for GPCRs using the fluorescence kinetic plate reader.
Materials and Methods
Materials
The neuropeptide S (NPS) peptide was synthesized by BiomerTech (Pleasanton, CA). The 1,536-well tissue culture-treated, clear-bottom black plates were purchased from Kalypsys (San Diego, CA). The no-wash PBX calcium assay kit was purchased from BD Biosciences (Rockville, MD). A Chinese hamster ovary (CHO) cell line expressing the murine M1 muscarinic acetylcholine receptor (CHO-M1, catalog # M1WT3) was obtained from American Type Culture Collection (ATCC, Manassas, VA).
Cell Culture
All cell culture reagents were purchased from Invitrogen (Carlsbad, CA) unless noted otherwise below. Chinese hamster ovary (CHO, Criscetulus griseus) cells were maintained in F12 Kaighn's media supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin at 37°C, 5% CO2 in a humidified atmosphere. For the frozen cell preparation, 5 million cells in 60 mL of media were seeded in each Nunclon triple layer flask (Nalge Nunc International, Rochester, NY) and were cultured for 3 to 4 days to reach 90%–95% confluence. The cells were then detached by incubation with 15 mL 0.25% trypsin at 37°C for 3 min and centrifuged at 1,000 rpm to remove the trypsin solution. The resulting cell pellet was resuspended in the cell freezing media containing 10% dimethyl sulfoxide (DMSO; Invitrogen, Carlsbad, CA) at a density of 4 × 107 cells/mL. One triple layer flask (500 cm2) yielded approximately 4–5 × 107 cells. Aliquots of cells with 0.5–1 mL per vial were put in a Cryo 1°C freezing container (Nalgene Nunc) and slowly frozen down overnight in a −80°C freezer at 1°C/min. The frozen cells were then transferred to a −140°C freezer for storage until use.
Instruments for Liquid Handling
Cells and dye-loading buffer were dispensed into 1,536-well plates by either a solenoid valve-based dispenser BioRAPTR FRD (Beckman, Fullerton, CA) or a Multidrop Combi dispenser (Thermo Fisher Scientific Inc., Waltham, MA). The control compounds were serially diluted in DMSO in 384-well plates manually and then reformatted into 1,536-well plates at 7 μL/well using Cybi-well dispensing station (Cybio, Inc., Woburn, MA). The 20 nL pins mounted on the 1,536-well pin tool head in FDSS7000 for compound transferring were purchased from V&P Scientifics (San Diego, CA). The final DMSO concentration in the assay plate is under 0.5% in the 1,536-well plate assay with the use of the 20 nL pins.
The 1,536-Well Tip Pipette Head Calibration
The 1 μM fluorescein (Sigma, St. Louis, MO) was used to determine the accuracy and precision of the 1,536-well tip pipette head. The 1,536-well tip pipette head was used on the FDSS7000 to transfer 1 to 4 μL of 1 μM fluorescein from a reservoir to 4 μL/well PBS in a 1,536-well assay plate. For each volume, the transferring test was repeated twice. A curve of relative fluorescence units (RFU) vs. volumes of fluorescein (μL) was constructed from these data, and a linear regression was obtained (RFU = 419 × [volume of fluorescein, μL] + 48.8, R2 = 0.9986). To determine the precision of the tip transfer, the average, standard deviation, and coefficient of variance of all well RFU data were calculated for each assay plate. Fidelity of the tip transfer was also evaluated by calculating the average, standard deviation, and coefficient of variance of 2 different assay plates.
GPCR Calcium Assay
On the day before the assay, frozen cells were resuspended in fresh media and seeded at 3 μL/well with 1,500 cells in black, tissue culture-treated, clear-bottom 1,536-well plates. Cells were cultured overnight at 37°C with 5% CO2. The calcium dye was prepared according to the manufacturer's instruction with 2 mM probenecid and loaded into cells at 3 μL/well. The assay plates were incubated at 37°C for 1 h followed by 20 min incubation at room temperature. Assay plates were then placed onto the FDSS7000 kinetic fluorescence plate reader for measuring the changes of intracellular free calcium in response to the GPCR activation. The basal fluorescence signal was recorded for 10 s at 1 Hz followed by an addition of 20 nL of compounds by pin tool and 210 s continuously recording at 1 Hz. Two microliters of agonist prepared in HBSS buffer supplemented with 0.1% BSA was added and the antagonist response of compounds was recorded for 140 s. The CCD binning was set to 2 × 2.
HTRF cAMP Assay
The HTRF cAMP immunoassay kit was obtained from Cisbio (Bedford, MA) and the experiment was carried out according to the manufacturer's instruction.
Radioactive Ligand-Binding Assay
The radioactive ligand-binding assay was performed as previously described.17
Data Analysis and Statistics of Assay Data
Time-course fluorescence responses for the calcium assay were expressed in terms of fluorescent change over background. For both agonist (1–220 s) and antagonist (221–400 s) phases, the maximal fluorescent response was exported into 2 text files using the instrument's software data export utility. Concentration–response curves were fitted and EC50/IC50s were calculated with the GraphPad Prism® software (GraphPad, San Diego, CA). The primary screening data was analyzed using software developed internally in NIH Chemical Genomics Center.18
Results
The Kinetic Fluorescence Plate Reader
Multiple liquid dispensing heads
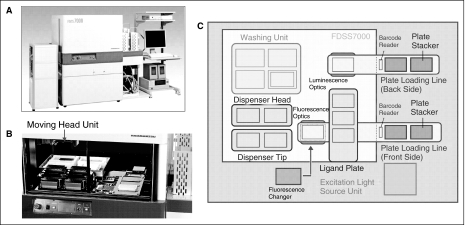
FDSS7000 is a newly improved version of the fluorescence kinetic plate reader (FDSS series). It is designed for assays with high well density and complicated protocols (Fig. 1). The most significant improvement in this version of instrument lies in the liquid-handling system. It is equipped with a moving head unit (Fig. 1B), which can pick up the desired head with tips or pin tool and add agonists or compound solution to the assay plate during the kinetic measurement. Moreover, the moving head unit can quickly wash the tips or pin tool and can also automatically switch between 2 different dispenser heads during one experiment, which enables this new plate kinetic reader for multiple tasks in compound screening experiments. In addition to the 96-well and 384-well tip heads, a new 1,536-tip pipette head with changeable plastic tips is available for 1–5 μL dispensing capacity as well as a 1,536-well pin tool head for 20 nL compound transfer. Two pipette heads such as a pin tool head and a tip-pipetting head can be placed in the stations and used for 1,536- or 384-well plate experiments (Fig. 1C). Two boxes of pipette tips can be placed on stations adjacent to the pipette head parking station, and can be automatically loaded and unloaded during the experiment (Fig. 1C).
Fig. 1.
(A) A picture of the Functional Drug Screening System (FDSS) 7000 system. (B) Inside view of the main station of FDSS7000. (C) Layout of the main station.
Precision test of 1,536-well pipette
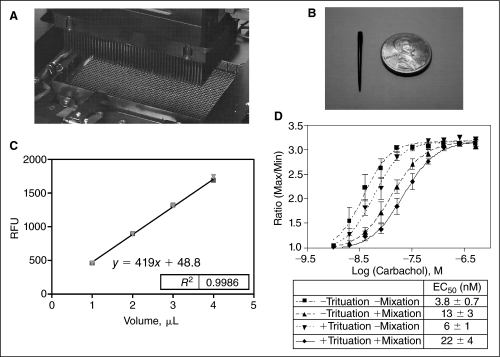
We have performed a precision test on the 1,536-tip pipetting head, which can transfer 1–5 μL solution from reagent reservoir to assay plate (Fig. 2). Fluorescein solution was transferred at various volumes to determine the accuracy and variability in transfer volumes within the same plate and between plates. A curve of relative fluorescence units (RFU) vs. volumes of fluorescein (μL) was constructed (Fig. 2C), and a linear regression was obtained (RFU = 419 × [volume of fluorescein, μL] + 48.8) with very good fit (R2 = 0.9986). The results showed that within a plate, percent coefficient of variance (CV) ranged from 4% to 6% for this 1,536-well plate pipette head. For the dispensing variation between plates, the CV was <2% for various dispensing volumes tested (Table 1), indicating that this 1,536-well pipette head was consistent and reliable for dispensing small volume of liquid.
Fig. 2.
(A) A close-up picture of the 1,536-tip pipette head. (B) A picture of the disposable tip for the 1,536-tip pipette head. (C) Relative fluorescence units (RFU) vs. volumes of fluorescein (μL) transferred by the 1,536-tip pipette head. (D) Trituation and plate mixing reversed the left shift of compound dose response caused by poor equilibration of pin tool transferring in calcium assay. Error bars represent standard error of n = 4 values.
Table 1.
Precision of Reagent Transfer Using a 1,536-well Pipette Head
| |
|
Plate Number |
|
|
|
|---|---|---|---|---|---|
| Volume (μL) | In Each Plate | 1 | 2 | Mean of Plate 1 and Plate 2 | %CV Between Plates |
| 1 | Mean (RFU) | 461.1 | 458.6 | 459.9 | 0.27 |
| CV (%) | 4.64 | 4.78 | |||
| 2 | Mean (RFU) | 896.4 | 893.4 | 894.9 | 0.17 |
| CV (%) | 4.83 | 5.89 | |||
| 3 | Mean (RFU) | 1,320 | 1,309 | 1,315 | 0.42 |
| CV (%) | 5.25 | 5.29 | |||
| 4 | Mean (RFU) | 1,690 | 1,744 | 1,717 | 1.57 |
| CV (%) | 4.73 | 5.01 | |||
Abbreviation: CV, coefficient of variance.
Compound transfer by 1,536-well pin tool head
It was previously reported that compound solutions transferred by pin tool were mixed incompletely with assay solutions.16 Once compound solutions (usually in DMSO) are transferred to assay plates, the high concentration compound solutions (usually 200-fold or more concentrated) often sink quickly from the pins through the assay buffer in wells to the bottom of the assay plate. These dense compound solutions can form a local high concentration zone on cells that usually adhere to the bottom of assay plates. The concentration–activity curves of these compounds can be shifted to left (5- to 8-fold more “potent”) due to the local high concentration of compounds on cells and this phenomena is more dominant in 1,536-well plate because of the narrow size of wells in that type of plates. To solve this problem, this instrument provides pin trituation and plate mixing functions to facilitate the rapid mixing of the transferred compound solution with the aqueous solution in wells. We then tested the effect of these 2 functions vs. the potency of carbachol in a 1,536-well format calcium assay (Fig. 2D). The addition of pin trituation or plate mixing to the pin-transferring protocol shifted the concentration–response curve and brought the potency values of carbachol closer to these reported previously in the 96- and 384-well plate assays (ref. 16; http://tools.invitrogen.com/content/sfs/brochures/583.pdf).
Tip wash modules with sonication bath
In order to sufficiently clean tips during experiments, this instrument is equipped with advanced tip wash/cleaning units. There are 4 modules including 3 tip wash modules and 1 tip-blotting module (Fig. 1C). Two tip wash modules are equipped with sonication baths, which provide efficient and rapid online tip washes during experiments. In our experiments, one tip wash module is filled with 50% DMSO and the other with H2O to accommodate the needs for washing off both hydrophobic and hydrophilic compounds. After washing, the tips are blotted on filter paper in the tip-blotting module to dry the tips and further remove potential compound residue. Both plastic tips in the pipette head and metal pins in the pin tool head are effectively cleaned with the combination of DMSO, water, and sonication baths plus the tip blotting in this instrument within 2 min (data not shown).
Plate and reagent delivery system
There are 2 sets of plate stackers in this instrument. One set is used for the assay plates and the other for the compound source plates. Each stacker can accommodate 28 standard 1,536-well plates (10 mm height). Inside the instrument, there is a ligand plate station, in which 3 additional control compound plates or reagent modules can be placed. An automated reagent feeding system is also available for continuous addition of the agonist into the internal reagent module. Thus, this instrument has sufficient plate and reagent supply systems for multiple-step assays performed continuously in a batch screen mode with 28 plates.
Optical and detection system
The excitation light source of 4 linked 150 W xenon lamps provides 600 W continuous excitation lights between 340 and 600 nm for the common fluorescence dyes as well as newly emerging ones. The optical system has been proved to be reliable, sufficient, and durable requiring minimal maintenance. For assay plate detection, this new instrument can have 2 CCD cameras, one for fluorescence assays and another for luminescence assays. For luminescence detection, a 2D photon-counting camera is used for flash luminescence applications such as the aequorin assay. High sensitivity in single photon light level can be achieved through a fiber optic bundle and a 2D photon-counting device that is connected to a real-time imaging processor.
Additional features
Other features include plate shaking function that is useful for quickly mixing of the reagent or compound added during the kinetic experiment with the well buffer of the assay plate, and a fast plate loading/moving system integrated with lid remover and barcode reader. This instrument can image assay plates at 1 Hz and the screen throughput could reach ∼10 to 20 plates per hour, suitable for both primary HTS and secondary screening for compound follow-up experiments.
Other improvements to be considered
We have only tested this instrument in stand-alone mode and have not used it to screen >28 plates at a time. It could be challenging to integrate the FDSS7000 with a robot for the fully automated compound library screening, which requires continuous and error-free running. Though the instrument has a sufficient liquid-handling system to add compound and agonist during the kinetic experiments, the fluorescent dye addition and incubation have to be performed outside before the assay plates are delivered to the instrument. In addition, several updates/changes may be considered for the FDSS7000 to make screening more smooth and efficient. A vacuum tip dryer can be added that dries the tips and pins more efficiently than the blotting papers. Second, a solution refilling system with the 4°C chilling capability is needed to provide continuous supply of reagents to the agonist reservoir. Third, when plates are stacked without lids water condensation frequently occurs on the bottom of assay plates and interferes with plate reading. To solve this problem, a small fan could be installed near the opening on instrument to blow off the moisture from the bottom of plates.
Multiplexed Measurement of Agonist and Antagonist Responses
Multiplex assay protocol
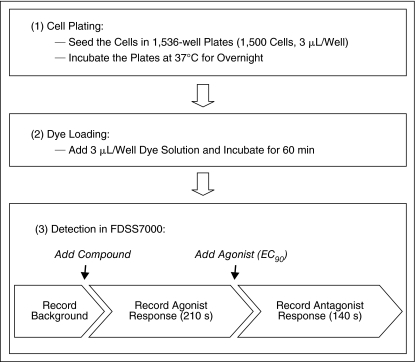
Both small-molecule GPCR agonists and antagonists are useful as research tools as well as chemical starting points for drug development. Compound screens for agonists and antagonists are commonly carried out in 2 separate experiments, doubling the numbers of cells, plates, reagents, and robotic running time for the screen. Multiplexing of agonist and antagonist screens using one assay plate in one screen can significantly reduce the cost of primary screening. We have established a protocol for the multiplex screen of GPCR agonists and antagonists with the fluorescent calcium assay (Fig. 3 and Table 2). In this assay format (Table 2), cells are seeded into 1,536-well plates 1 day before the experiment at a density of 1,500 cells/well in 3 μL/well. A no-wash calcium assay kit containing the cell membrane-impermeable fluorescence quencher is used and an equal volume of calcium dye mixture is loaded to the assay plates followed by 1 h incubation at 37°C. The multiplex assay is initiated inside the instrument with a 10 s baseline recording (1 Hz) of the assay plate at an excitation of 480 nm and an emission of 525 nm. The internal 1,536-well pin tool head delivers 20 nL/well DMSO compound solution to the assay plate while the plate is continuously read for 3.5 min at 1 Hz to record the agonist response of compounds. And then, an EC90 amount of agonist is added (2 μL/well) using the 1,536-well pipette head and the antagonist response of compounds is recorded for another 140 s at 1 Hz (Fig. 3). The total recording time for each plate is 6 min and the screen throughput of this assay format is ∼8 plates per hour including the plate delivery time.
Fig. 3.
Schema of the multiplex G protein-coupled receptor (GPCR) assay protocol. The final assay volumes were 6 μL for the agonist mode and 8 μL for the antagonist mode. The total recording time was 6 min for one plate.
Table 2.
Protocol of the Multiplexed Calcium Assay in 1,536-well Format
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Cells | 3 μL | 1,500 cells/well |
| 2 | Incubation | 16–20 h | 37°C, 5% CO2 |
| 3 | Reagent | 3 μL | No-wash calcium dye |
| 4 | Incubation | 60 min | 37°C, 5% CO2 |
| 5 | Incubation | 10 min | Room temperature |
| 6 | Detection | Basal signal | Read for 10 s in the FDSS7000 |
| 7 | Compounds | 20 nL | Added by pin tool in the instrument |
| 8 | Detection | Agonist response | Read for 3.5 min |
| 9 | Reagent | 2 μL | Add EC90 of agonist |
| 10 | Detection | Antagonist response | Read for 140 s |
Multiplex assay of M1 muscarinic acetylcholine receptor
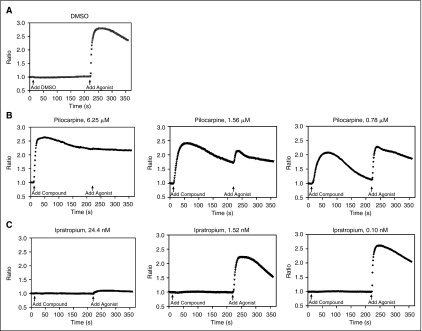
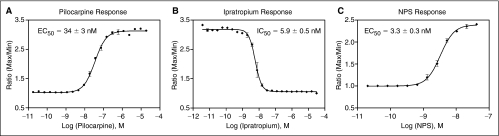
A CHO cell line stably expressing M1 muscarinic acetylcholine receptor was tested in this multiplex GPCR assay. Time-course calcium responses of pilocarpine, an M1 agonist, and ipratropium, an M1 antagonist, were shown in Figure 4. EC90 amount of carbachol was used to challenge the cells for the measurement of antagonist responses. After the experiments, the maxima of the agonist kinetic response and the antagonist kinetic response were separately exported into text files and used for the concentration–response curve fitting. As shown in Figure 4B, small-molecule agonists may act as weak antagonist in the antagonist screen segment of this multiplex assay. This is due to either GPCR desensitization where cells were not fully recovered from the agonist response, or an antagonist effect of a weak/partial agonist in response to stimulation by the full agonist. The EC50 value of the agonist pilocarpine was 34 nM as determined in agonist mode (Fig. 5A). Ipratropium, a M1 antagonist, displayed a concentration-dependent inhibition of the carbachol response in this calcium assay with an IC50 value of 5.9 nM (Fig. 5B).
Fig. 4.
Calcium response kinetics of (A) dimethyl sulfoxide (DMSO), (B) a M1 agonist pilocarpine, and (C) a M1 antagonist ipratropium in the multiplex GPCR assay. Fluorescence responses for the assay were expressed in terms of fluorescent change over background. Arrows mark the time of compound addition (t = 10 s) and addition of 2 μL of carbachol, a M1 agonist (t = 220 s). For (B) and (C), each fluorescent trace represents the calcium response resulting from a dose of a single concentration of pilocarpine or ipratropium transferred to a single well. The maxima of 2 different time periods (1–220 s and 221–360 s) of each trace were used dose–response curve construction for agonist and antagonist responses, respectively.
Fig. 5.
(A) Dose response of pilocarpine in the multiplex assay of muscarinic M1 receptor. Error bars represent standard error of n = 2 values. (B) Dose response of ipratropium in the multiplex assay of muscarinic M1 receptor. Error bars represent standard error of n = 2 values. (C) Dose response of neuropeptide S (NPS) in the multiplex assay of neuropeptide S receptor (NPSR). Error bars represent standard error of n = 4 values.
In order to determine whether the compound activities determined in this multiplex assay are similar to those measured in traditional single-step assays where agonist and antagonist activities are measured in separate assay plates, we tested 5 muscarinic acetylcholine antagonists in both multiplex assay and single-step assay formats. We observed that the potencies of these compounds determined by the multiplex assay followed the same rank-order as those determined in the standard assay format (Table 3). This indicated that the pharmacology of the antagonists was not changed in this multiplex assay.
Table 3.
Comparison of Muscarinic Acetylcholine Antagonists in the Multiplex Assay and the Single-step Assay
| Unit: nM | IC50 (multiplex assay) | IC50 (single-step assay) |
|---|---|---|
| Scopolamine hydrochloride | 7.17 | 12.6 |
| Propantheline bromide | 6.09 | 11.2 |
| l-Hyoscyamine | 7.11 | 11.2 |
| Atropine sulfate monohydrate | 14.2 | 10.0 |
| d,l-Trihexyphenidyl hydrochloride | 15.7 | 25.1 |
Multiplex screen of neuropeptide S receptor
The neuropeptide S receptor (NPSR), previously known as GPR154, is a recently de-orphanized GPCR.19,20 Its endogenous ligand, neuropeptide S (NPS), is a 20 amino acid peptide. The NPS receptor couples to both Gs and Gq G-proteins resulting in the activation of both intracellular calcium and cAMP signaling pathways. NPSR are highly expressed in brain areas that have been implicated in modulation of arousal, stress, and anxiety. Central administration of NPS in mice induces wakefulness and arousal, while at the same time suppresses anxiety. Therefore, selective small-molecule agonists and antagonists of NPS receptor are useful research tools for further studies of in vivo functions of this receptor, as well as being potential chemical leads for drug development.
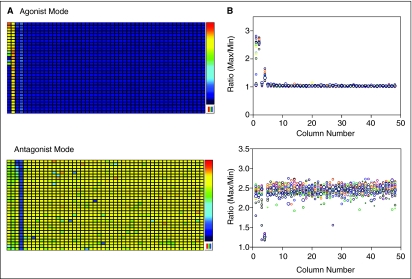
NPS showed a concentration-dependent increase in intracellular calcium release with an EC50 value of 3.3 nM in a CHO cell line permanently expressing NPSR (NPSR-CHO cells) (Fig. 5C). A DMSO plate was used to test the assay performance in this multiplex assay format. The results of 6-assay plates tested using the DMSO plate in the NPSR-CHO cell line are summarized in Table 4 with one representative plate shown in Figure 6. The signal-to-basal (S/B) ratios for agonist and antagonist modes were 2.5- and 2.1-folds, respectively. The Z′ factors in the agonist and antagonist modes were 0.71 and 0.59, respectively. As typically observed, the antagonist mode was noisier and less robust than the agonist mode, which might be due to the additional agonist dispensing step and the variable cell responses to the agonist at EC90 concentration. These results indicated that the embedded agonist and antagonist multiplex assay is acceptable for HTS though the well-to-well variation was larger in the antagonist mode.
Table 4.
Statistical Parameters of the DMSO Plate
| Agonista | Antagonistb | |
|---|---|---|
| Average signal-to-basal ratio | 2.51 ± 0.40 | 2.14 ± 0.36 |
| Average Z′ factor | 0.71 ± 0.11 | 0.59 ± 0.13 |
| Average %CV | 0.8 ± 0.4 | 7.3 ± 2.5 |
The means of averaged replicate values from 6 different assays were used for statistical calculations. Error reported as standard deviation.
For the agonist mode (0–220 s), signal and basal values were defined as the maximum response fluorescence resulting from EC100 and EC0 concentrations of NPS challenge, respectively.
For the antagonist mode (221–360 s), signal and basal values were defined as the maximum response fluorescence resulting from IC0 and IC100 concentrations of a NPSR antagonist in the presence of an EC90 challenge of NPS.
Fig. 6.
(A) Heat maps of a representative DMSO plate in the multiplexed GPCR assay. (B) Scatter plot of the DMSO plate. Upper panel results from the agonist responses; lower panel results from the antagonist responses. The plate was formatted as the following: column 1—dose response of NPS, column 2—EC100 of NPS, column 3—dose response of a known NPSR antagonist, column 4—IC100 of the known NPSR antagonist, columns 5 to 48—DMSO.
Compound library screen for NPS receptor in the multiplex assay format
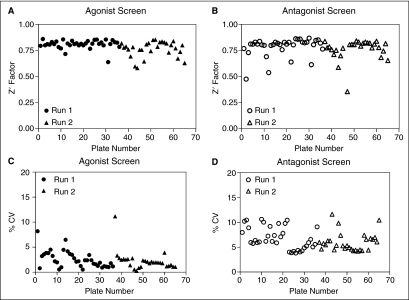
To validate the multiplex assay for agonist and antagonist discovery, a total of 37,888 compounds in 1,536-well plate format were screened. This primary screen was run twice to examine the reproducibility of the screening results. Both agonist and antagonist screens were acceptable for HTS because of the robust Z′ factors and %CV values (Table 5 and Fig. 7). The %CV values were 2%–3% for the agonist mode and 6%–7% for the antagonist mode in both screens. The average Z′ factors were greater than 0.7 in the 2 screens, for both agonist and antagonist outputs. Using a cutoff of 60% activation or inhibition in agonist and antagonist mode, respectively, the hit rates were 0.4% and below (Table 5).
Table 5.
Statistical Parameters of the Multiplex GPCR Screening for Agonists and Antagonists of NPSR
| |
First Run |
Second Run |
||
|---|---|---|---|---|
| Agonist | Antagonist | Agonist | Antagonist | |
| Plates screened | 29 | 29 | ||
| Total wells | 44,544 | 44,544 | ||
| Compounds tested | 37,888 | 37,888 | ||
| Compound final concentration | 8.25 μM | 6.25 μM | 8.25 μM | 6.25 μM |
| Average Z′ factor | 0.81 ± 0.04 | 0.78 ± 0.09 | 0.76 ± 0.08 | 0.73 ± 0.19 |
| %CV | 2.5 ± 1.7 | 6.8 ± 2.2 | 2.2 ± 1.9 | 6.0 ± 1.9 |
| Hit cutoff | 60% activation | 60% inhibition | 60% activation | 60% inhibition |
| Hit number | 46 | 124 | 37 | 172 |
| Hit rate | 0.1% | 0.3% | 0.1% | 0.4% |
Fig. 7.
Z′ factor (A and B) and % coefficient of variance (CV) (C and D) distribution for the high-throughput screening (HTS) screening of NPSR.
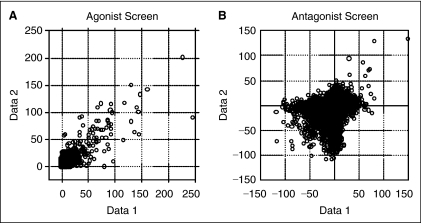
A gauge of assay reproducibility can be made by examining the concordance of the results from the 2 screen runs, and the confirmation of those hits. The agonist screening results are generally concordant, while the antagonist results appear to be less so (Fig. 8). Twenty nine of the agonist hits from the 46 hits in the first run and 37 hits from the second run were concordant (both results greater than 60% activation). However, none of these agonist hits were later confirmed to be real NPS receptor agonists because they were either the fluorescence compounds or nonspecific compounds. In contrast, only 6 of the antagonist hits from the 124 hits in the first run and 172 hits in the second run were concordant. However, we were able to confirm the antagonist activity of 4 out of 6 of the concordant hits in a confirmation run of the assay. The activity of one such compound is shown in Figure 9. So while the total hit rate for the antagonist screen was larger, many of the antagonist hits are likely false positives and true antagonists can be easily resolved by running the assay in replication or in quantitative HTS (qHTS) format21 (this is especially true given the low overall hit rate of the assay). Thus, the number of true antagonists is likely smaller than the number of hits from this compound set. The relatively lower reproducibility for the antagonist mode is consistent with the previous finding that the antagonist mode has higher variation and noise than the agonist mode. The subgroup of data points clustered along the X and Y axles in Figure 8B most likely originate from random variation during the tip dispense of the agonist to initiate an antagonist response and contribute to the majority of antagonist hits that are not concordant between different screen runs. Antagonist hits were randomly distributed within and across plate sets, and were consistent over time (which might otherwise indicate a mechanical failure in the liquid dispenser).
Fig. 8.
Correlation analysis of the 2 runs of NPSR screening with the multiplexed calcium assay. (A) Correlation analysis of the agonist mode. (B) Correlation analysis of the antagonist mode.
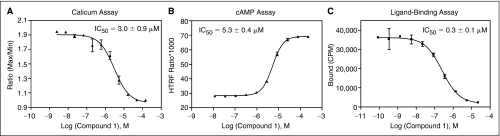
Fig. 9.
Dose responses of one hit compound identified from the screening of NPSR were examined in calcium assay (A), cAMP assay (B), and radioactive ligand-binding assay (C). In A, B, and C, error bars represent standard error of n = 4, 4, and 2 values, respectively.
Among the 6 hits from this antagonist primary screening, a top hit coincided with the best hit (Compound 1) in an independent NPSR HTS campaign using a cAMP assay (Fig. 9). Another high priority hit also shared structure similarity with that from the cAMP screening. Both compounds were tested in a ligand-binding assay, and both were able to compete with radiolabeled NPS, suggesting a direct interaction of the compounds with the receptor. Thus, despite a modest false positive rate, the multiplexed GPCR assay is able to identify true antagonists. These results indicate that this multiplex assay in the 1,536-well plate format for GPCRs is robust for HTS and is effective to identify both agonists and antagonists from the compound library.
Discussion
A critical requirement for multiplexing agonist and antagonist screens with the calcium assay is whether the reagent dispensing system is adequate in the fluorescence kinetic plate reader to function in HTS mode. Previous versions of fluorescence kinetic plate readers either had only one pipette head or lacked sufficient tip wash modules to rapidly remove residual compound from the pipette tips. The detection of agonist responses occurring in few seconds to a few minutes requires addition of test compound solution inside the instrument immediately after the baseline recording. In addition, the final DMSO (the compound vehicle) concentration in the assay plate usually must be <0.5% (v/v) to avoid the adverse solvent effect. Thus, the amount of compound solution added to the cells in the assay plates should be ≤200 nL for 384-well plates containing 40 μL of total volume or ≤25 nL for 1,536-well plates containing 5 μL of total volume. To handle such small volumes of compound solution rapidly and in parallel, the 384- or 1,536-well pin tool is clearly more appropriate than pipette tips because it can quickly and accurately transfer a fixed amount of compound solution (from 10 to 500 nL dependent on the size of pins selected).22 After pin tool addition of compounds and while the agonist response of compound is recording, a second pipette head with exchangeable tips is mounted to the moving head unit that then transfers the EC90 amount of agonist solution to the assay plate for the recording of antagonist response of the compounds. Use of 2 pipette heads for separate dispensing of compounds and agonist avoid the cross contamination of these 2 reagents in their source plates/reservoirs.
In order to quickly mix the compound DMSO solution with the buffer in wells of the assay plate, the pin tool “trituation” (rapid vertical movements within 1–5 mm distance adjustable by users) is used in combination with the quick rotational movement of the assay plate. For mixing the agonist solution with the buffer in assay plate, a ratio of 1:4 to 1:2 agonist solution (eg, 1–3 μL/well of agonist in a 1,536-well plate) has been used in combination with the rapid assay plate movement to facilitate mixing. As these agonist and antagonist responses of GPCR ligands are usually very rapid, both compound DMSO solution and EC90 agonist stimulation solution must be added to all wells in an assay plate simultaneously while the plate being read kinetically in the instrument. Our results have demonstrated that the FDSS7000 instrument has sufficient fluidic systems and mixing functions to accommodate the needs of multiple dispensing protocols, rapidly switching pipette heads and rapid tip washing to limit compound carryover.
The pipette tips on the 1,536-well head and the pins on the pin tool head need to be washed and cleaned within 1 to 2 min after each use for the continuous measurement. The choices of right solvents for the tip/pin wash are important for eliminating the compound carryover between plates. We found that one solution wash (eg, either DMSO or water) was insufficient to remove the residual compounds from tips and pins (data not shown). It can be explained by the diversity of compounds in the collection—some of them are lipophilic and others are hydrophilic. A combination of one DMSO bath and one water bath for wash with a brief sonication is an effective method to remove residual compounds on the tips or pins. A tip-blotting step in which the tips touch a layer of filter paper in a module after the tip wash steps wipes away the remaining liquid on the tips or pins. This cycle of tips washes, tip blotting, and pipetting head change can be completed within 2 min in this instrument and can be adjustably controlled by the operation software. Thus, this improved liquid-handling system in this kinetic plate reader enables the rapid and complex protocols that are needed for sequential agonist and antagonist screens in a single assay plate. However, we have observed the high irreproducible hit rate from the antagonist screen, indicating the uneven dispensing of agonist by the 1,536-well pipettor though the CV of dispensing may be still in the acceptable range of 5%–10%. Thus, the performance of this 1,536-well pipettor should be further optimized and improved by the manufacturer.
In the miniaturized assay format, it is a challenge to rapidly and completely mix the dispensed compound solution or agonist solution with the existing solution in a assay plate during a kinetic measurement because of the narrow and tall well dimension, ∼2 (w) × 2 (L) × 8 (H) mm for a standard 1,536-well plate. Gravity and diffusion are the main forces for the quick solution mixing in the 96-well plate assay but it takes longer time for the solution to be mixed in the higher well density plates.23 The higher gravity of compound DMSO solution may cause the compound sinking quickly to the bottom of plates resulting in a transit higher local concentration near cells attached on the bottom of assay plates.16 This high local concentration of compound resulting from insufficient mixing may lead to inaccurate compound activity. Or a concentration gradient of the added solution may be formed due to insufficient mixing in the higher well density assay plates that affects the accuracy of compound activity. In this new instrument, the combination of rapidly vertical pin movement and assay plate shaking functions can create effective and rapid mixing of the compound DMSO solution with the assay plate buffer while the plate is continuously read and recorded. We found that the local high concentration of compounds due to insufficient mixing can be avoided by using these functions in this instrument.
The main difference between GPCR agonist mode and antagonist mode in compound screens is the addition of an EC90 amount of a known agonist prior to recording the antagonist response. Thus, the antagonist response can be sequentially measured in the same assay plate after the agonist response is read and recorded by a rapidly addition of EC90 amount of a known agonist. A multiplex screen assay for GPCR agonist, potentiator, and antagonist in 384-well plate format was reported using a previous version FDSS6000 instrument.24 A dye removing step was required in that assay for the reduction of fluorescent background that could significantly decrease the screen throughput. Recent advances in the liquid-handling system in this kinetic plate reader in combination with the no-wash calcium dye contributed to the development of this multiplex agonist and antagonist assay in the 1,536-well plate format that simplifies the assay procedure, increases the screen throughput, and thus should be suitable for HTS. The fluorescence quenching dye in the no-wash calcium dye kit helps to eliminate the cell wash step after dye loading that enables an addition-only assay format for the calcium assay without affecting the pharmacology of agonist and antagonist. Our results from the validation tests using the muscarinic acetylcholine M1 receptor transduced cell line also demonstrated that the potencies of 5 known agonists and antagonists were similar in both the single mode and the sequential assay mode in the 1,536-well plate format.
In conclusion, the FDSS7000 fluorescence kinetic plate reader, a new generation model, has been equipped with a new liquid-handling system and sensitive optical detection train and can be used for more complicated and automated assay protocols. The multiple pipetting heads and tip washing modules are major enhancements that greatly improve cleaning efficiency and transfer times for pipette tips and pins as well as reducing the possibility of compound carryover. Our results also demonstrated that this fluorescence plate reader can be used for sequential screens of compounds for agonist and antagonist activities in a single 1,536-well assay plate. We believe that these characteristics will allow the full automation of such protocols in qHTS format, a future goal of the NCGC that should further improve the data quality in the primary compound screen.
ABBREVIATIONS
- FDSS
functional drug screening system
- GPCR
G protein-coupled receptor
- HTS
high-throughput screening
- NPS
neuropeptide S
Acknowledgments
This research was supported by the Molecular Libraries Initiative of the NIH Roadmap for Medical Research and the Intramural Research Programs of the National Human Genome Research Institute. This work was also supported by the intramural research programs of the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The authors thank Hui Sun and Anton Terasmaa for their technical assistance on the NPS cell line.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Pierce KL. Premont RT. Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins AL. Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 3.Eglen RM. Bosse R. Reisine T. Emerging concepts of guanine nucleotide-binding protein-coupled receptor (GPCR) function and implications for high throughput screening. Assay Drug Dev Technol. 2007;5:425–451. doi: 10.1089/adt.2007.062. [DOI] [PubMed] [Google Scholar]
- 4.May LT. Leach K. Sexton PM. Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 5.Strange PG. Antipsychotic drug action: antagonism, inverse agonism or partial agonism. Trends Pharmacol Sci. 2008;29:314–321. doi: 10.1016/j.tips.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Tsien RY. Rink TJ. Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985;6:145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- 7.Grynkiewicz G. Poenie M. Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 8.Gee KR. Brown KA. Chen WN. Bishop-Stewart J. Gray D. Johnson I. Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/ceca.1999.0095. [DOI] [PubMed] [Google Scholar]
- 9.Minta A. Kao JP. Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 10.Kao JP. Harootunian AT. Tsien RY. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989;264:8179–8184. [PubMed] [Google Scholar]
- 11.Li X. Llorente I. Brasch M. Improvements in live cell analysis of G protein coupled receptors using second generation BD calcium assay kits. Current Chemical Genomics. 2008;2:10–15. doi: 10.2174/1875397300802010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xin H. Wang Y. Todd MJ. Qi J. Minor LK. Evaluation of no-wash calcium assay kits: enabling tools for calcium mobilization. J Biomol Screen. 2007;12:705–714. doi: 10.1177/1087057107301522. [DOI] [PubMed] [Google Scholar]
- 13.Cassutt KJ. Orsini MJ. Abousleiman M. Colone D. Tang W. Identifying nonselective hits from a homogeneous calcium assay screen. J Biomol Screen. 2007;12:285–287. doi: 10.1177/1087057106298538. [DOI] [PubMed] [Google Scholar]
- 14.Zheng W. Spencer RH. Kiss L. High throughput assay technologies for ion channel drug discovery. Assay Drug Dev Technol. 2004;2:543–552. doi: 10.1089/adt.2004.2.543. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan E. Tucker EM. Dale IL. Measurement of [Ca2+] using the Fluorometric Imaging Plate Reader (FLIPR) Methods Mol Biol. 1999;114:125–133. doi: 10.1385/1-59259-250-3:125. [DOI] [PubMed] [Google Scholar]
- 16.Hodder P. Mull R. Cassaday J. Berry K. Strulovici B. Miniaturization of intracellular calcium functional assays to 1536-well plate format using a fluorometric imaging plate reader. J Biomol Screen. 2004;9:417–426. doi: 10.1177/1087057104264038. [DOI] [PubMed] [Google Scholar]
- 17.Xu YL. Reinscheid RK. Huitron-Resendiz S. Clark SD. Wang Z. Lin SH, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Southall NT. Jadhav A. Huang R. Nguyen T. Wang Y. Enabling the large scale analysis of quantitative high throughput screening data. In: Seethala R, editor; Zhang L, editor. Handbook of Drug Screening. 2nd. Informa Healthcare; New york: 2009. pp. 442–464. [Google Scholar]
- 19.Okamura N. Reinscheid RK. Neuropeptide S: a novel modulator of stress and arousal. Stress. 2007;10:221–226. doi: 10.1080/10253890701248673. [DOI] [PubMed] [Google Scholar]
- 20.Reinscheid RK. Neuropeptide S: anatomy, pharmacology, genetics and physiological functions. Results Probl Cell Differ. 2008;46:145–158. doi: 10.1007/400_2007_051. [DOI] [PubMed] [Google Scholar]
- 21.Inglese J. Auld DS. Jadhav A. Johnson RL. Simeonov A. Yasgar A, et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci USA. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleveland PH. Koutz PJ. Nanoliter dispensing for uHTS using pin tools. Assay Drug Dev Technol. 2005;3:213–225. doi: 10.1089/adt.2005.3.213. [DOI] [PubMed] [Google Scholar]
- 23.Yasgar A. Shinn P. Jadhav A. Auld D. Michael S. Zheng W, et al. Compound management for quantitative high-throughput screening. JALA Charlottesv Va. 2008;13:79–89. doi: 10.1016/j.jala.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niswender CM. Johnson KA. Weaver CD. Jones CK. Xiang Z. Luo Q, et al. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008;74:1345–1358. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]