Abstract
In humans, absence of Fragile X mental retardation protein (FMRP), an RNA-binding protein, results in Fragile X syndrome (FXS), the most common inherited form of intellectual disability. Here we report through biochemical and electrophysiological studies that FMRP binds the C-terminus of the Slack sodium-activated potassium channel to activate the channel. The findings suggest that Slack activity may provide a link between patterns of neuronal firing and changes in protein translation.
The sodium-activated potassium channel (KNa) Slack-B is the largest known potassium channel subunit, consisting of an extended cytoplasmic C-terminal domain containing 913 amino acids1. To gain insight into potential functions of the Slack C-terminal domain, we carried out a yeast two-hybrid screen using this domain as bait. Several proteins that were identified repeatedly comprise components of an mRNA-protein regulatory complex that includes FMRP (Supplementary Fig. 1). FMRP is believed to exist as part of an mRNA-binding complex and is known to interact with target mRNAs, miRNAs, and other nuclear and cytosolic binding partners2, 3. FMRP is, however, not known to interact with any intrinsic membrane proteins. Here we report a role for FMRP as a potent activator of Slack-B.
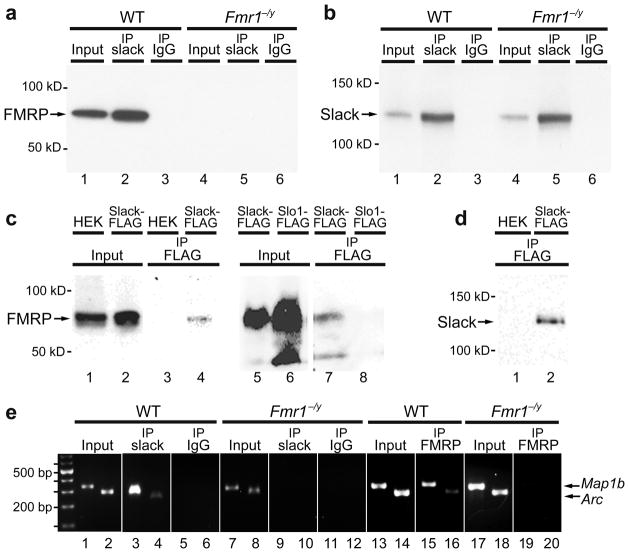
We confirmed the in vivo interaction of Slack with FMRP by co-immunoprecipitation of FMRP with Slack from synaptosomes prepared from olfactory bulb and brainstem regions of wild type (WT) mice (Fig. 1a and Supplementary Methods). The FMRP band that is co-immunoprecipitated with Slack is entirely absent in synaptosomal brain extracts from Fmr1−/y mice (lacking FMRP) (Fig. 1a). Slack-FMRP complexes also contained Map1b and Arc mRNA, known mRNA targets of FMRP4–6 that were detected by RT-PCR experiments on immunoprecipitates (Fig. 1e). No mRNA could be co-immunoprecipitated with Slack in synaptosomes from Fmr1−/y mice (Fig. 1e).
Figure 1.
FMRP directly binds to Slack channel subunits. (a,b) Slack antibodies co-immunoprecipitated FMRP from WT (a, lane 2) but not from Fmr1−/y mice synaptosomal samples (a, lane 5). Immunoblots were performed using FMRP (a) or Slack (b) antibodies (n=4). Input synaptosomal samples from WT or Fmr1−/y mice were isolated prior to co-immunoprecipitation (a, lanes 1, 4, b, lanes 1, 4). (c) FMRP was co-immunoprecipitated using anti-FLAG antibodies from Slack-FLAG HEK cells (c, lane 4) but not from untransfected HEK cells (c, lane 3) or Slo1-FLAG cells (c, lane 8, n=3). (d) The untransfected HEK and Slack-FLAG HEK immunopreciptates were also separately immunoblotted using anti-FLAG antibody detecting Slack-FLAG channels in transfected cells. (e) Slack-FMRP complexes contained known FMRP mRNA targets. Map1b and Arc mRNA were detected by isolating RNA after co-IP with anti-Slack antibodies followed by RT-PCR in WT samples (e, lanes 3, 4) but not Fmr1−/y samples (e, lanes 9, 10; n=3).
Specificity for interaction between Slack channels and FMRP was also demonstrated in vitro using FLAG-tagged Slack-B and FLAG-tagged Slo1 channels stably transfected in HEK cells, which express endogenous FMRP (Fig. 1c). When an anti-FLAG antibody was used to immunoprecipitate these FLAG-tagged channels, an FMRP immunoreactive band was present in the immunoprecipitate of Slack-B, but not Slo1 channels (Fig. 1c).
To probe the interaction between FMRP and Slack, we undertook electrophysiological studies to determine whether FMRP affects Slack-B channel activity. Single channel patch-clamp recordings from Xenopus oocytes expressing the Slack-B channel typically show large conductance channels (~180 pS) with frequent openings to subconductance states (substates, S)1, 7. Specifically, there are frequent transitions to a relatively long-lived substate with a conductance about one-third that of the fully-open state (Fig. 2a).
Figure 2.
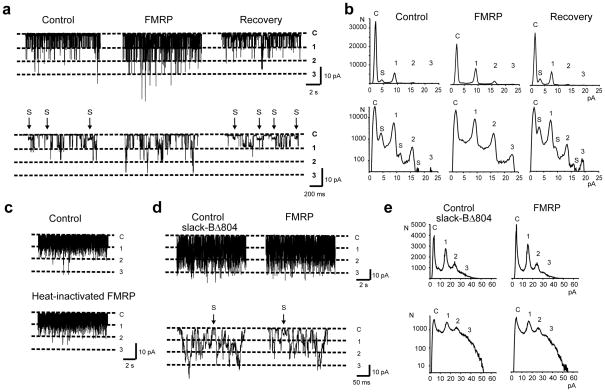
FMRP alters gating of full-length Slack-B channels, but not Slack-BΔ804, in inside-out patches. (a) Current recordings before (control), 20 seconds after application of 100nM FMRP(1–298) to the cytoplasmic face of the patch, and after washout of FMRP(1–298). Top traces show representative 10 second examples of recording at −40mV and bottom traces show expanded time views of the same patch. The increase in channel activity (top trace) and the suppression of substates, S, (bottom trace) are evident after FMRP reactivity. Dashed lines indicate closed state and number of channels. (b) All-points amplitude histograms plotted on linear (top) and logarithmic scale (bottom), of control, FMRP(1–298) treated, and recovery (after washout). (c) Application of heat-inactivated FMRP(1–298) had no effect on Slack-B channel activity; n=5. (d) Top traces show representative 10 second recording at −80 mV and bottom traces show expanded time views of Slack-BΔ804 channel activity before (control) and after application of FMRP(1–298). There are no changes in NPo or in the occurrence of substates, S. (e) All-points amplitude histograms show no significant changes in Slack-BΔ804 channel activity in response to FMRP(1–298).
Due to the reported difficulties8, 9 in synthesizing full-length FMRP protein (632aa), we used a commercially available recombinant FMRP containing amino acids 1–298. Importantly, FMRP(1–298) contains the N-terminal domain (NDF; aa1–134) of FMRP which serves as a platform for protein-protein interactions10. We used only stable patches that showed no changes in channel activity or kinetics over 2 minutes. The control trace shows frequent openings to the substate from both open and closed states. Addition of FMRP(1–298) produced a dramatic effect on channel gating within 20 seconds; a near-complete elimination of the substates and a strong activation of channel activity (n=5). The effect of FMRP(1–298) was reversible on washout (Fig. 2a).
All-points amplitude histograms show that intermediate peaks representing the substates are strongly suppressed after application of FMRP(1–298) (Fig. 2b). In addition, there was an increase in channel openings evidenced by decreased closed times, and increased simultaneous openings of two or three channels, which is particularly evident when the histograms are plotted on a logarithmic scale (Fig. 2b). The actions of FMRP(1–298) on the gating of Slack channels differs from that observed on increasing cytoplasmic Na+ levels in that elevations of Na+ do not eliminate substates11. Heat-inactivated FMRP(1–298) had no effect on Slack-B channel properties (Fig. 2c). The mean increase in NPo (Number of channels × probability of opening) produced by FMRP(1–298) was 112±12.4%, (n=5, p<0.0001 compared to −8.4±4.1% produced by heat-inactivated FMRP(1–298)).
A peptide array assay confirmed that FMRP(1–298) binds selectively to sequences at the Slack C-terminus, whereas no binding was detected to Slo1 and Kv2.1 potassium channels (Supplementary Fig. 2). To test the hypothesis that the Slack C-terminus is responsible for channel modulation, we constructed a Slack-B truncation mutant (Slack-BΔ804) lacking amino acids 804–1237 and tested whether application of FMRP altered channel activity.
While the gating behaviour of Slack-BΔ804 channels was more rapid than full-length Slack-B channels, substates were detected in the recordings although discrete substate peaks were attenuated in all-points amplitude histograms (Fig. 2e). In contrast to full-length Slack-B, application of FMRP(1–298) to the cytoplasmic face of Slack-BΔ804 channels had no effect on NPo or the occurrence of substates (Fig. 2d) (mean change in NPo = 8.4±4.0%, n=4).
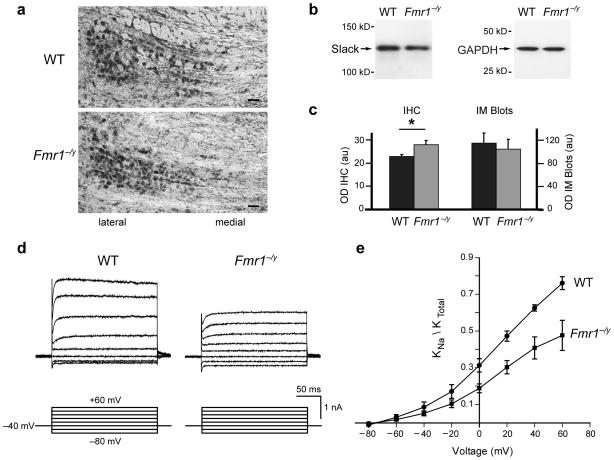
Previous work has demonstrated that KNa channels account for a major component of the total K+ current in principal neurons of the medial nucleus of the trapezoid body (MNTB) allowing these cells to fire at high rates with temporal fidelity11. If FMRP acts as an activator of native Slack channels, the loss of FMRP, as occurs in FXS, would be predicted to reduce whole-cell KNa currents. We first determined the distribution of Slack in Fmr1−/y mice using the anti-Slack-B antibody in synaptosomes and brain sections. Synaptosomal samples prepared from the brainstem and olfactory bulb regions of either WT or Fmr1−/y mice brains contain similar amounts of Slack channels (Fig. 3b). Slack is expressed at high levels in neurons of the lateral MNTB (Fig. 3a) and there was a small increase in Slack immunoreactivity in the MNTB of Fmr1−/y mice compared to WT mice (Fig. 3c).
Figure 3.
IK(Na) is reduced in Fmr1−/y MNTB neurons. (a) Images of Slack-B immunoreactivity in MNTB of WT and Fmr1−/y mice (scale bar=40μm) (b) Immunoblots for Slack-B in synaptosomal samples of WT and Fmr1−/y mice. As a loading control, the same samples were probed with antibodies against GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) (c) Quantification of Slack immunoreactivity. MNTB sections (n=63) were quantified using the mean optical density of pixels above threshold (OD IHC±s.e.m.). There was a 15% increase in Slack immunoreactivity in MNTB sections from Fmr1−/y mice compared to WT (p=0.02, n=3). Synaptosomes from WT and Fmr1−/y mice contained similar amounts of Slack (OD IM±s.e.m.; n=3). (d) Representative traces of IK(Na) in lateral MNTB neurons from WT and Fmr1−/y mice (20mM internal Na+). Outward currents evoked by voltage steps between −80 to +60mV in 20mV increments from a holding potential of −40mV. 1μM TTX and 20μM ZD-7288 were included in the bath to block Na+ and hyperpolarization-activated cation currents (IH), respectively. (e) I–V plots of KNa as a proportion of total K+ currents. The KNa component of current from Fmr1−/y mice (n=5) was significantly reduced relative to WT mice (n=4, p=0.02; two-sample t-test).
To compare the proportion of total K+ current that can be attributed to KNa channels (IK(Na)) in WT versus Fmr1−/y mice, we recorded whole-cell currents in lateral MNTB neurons with 20mM internal Na+ 11. Cells were held at −40mV for at least 2 min to inactivate low-threshold voltage-gated Kv1 channels12. We then applied the K+ channel blocker TEA (1mM) to record the IK(Na)-containing component of current 11(Fig. 3d). This component of current was significantly reduced in Fmr1−/y mice relative to WT (Fig. 3e). These results are consistent with a role for FMRP in regulating the activity of native Slack channels in the MNTB.
Loss of function of FMRP is the major cause of inherited intellectual disability. The present findings are the first demonstration of the interaction of FMRP with a membrane protein. It is known that activation of Slack channels contributes to the firing patterns of a wide variety of neurons, and, in auditory brainstem neurons may serve to maintain temporal accuracy of firing during high frequency stimulation11. Thus, the present data suggest that some of the neuronal abnormalities in FXS could represent altered patterns of neuronal firing that are distinct from the role of FMRP as a regulator of mRNA localization and translation. Moreover, in analogy with Slo1 channels13, the C-terminus of Slack channels is likely to undergo a conformational change during channel activation in response to repetitive neuronal stimulation. This raises the possibility that the interaction of Slack channels with FMRP serves to regulate the proposed functions of FMRP in mRNA trafficking and translation.
Supplementary Material
Acknowledgments
This research was supported by NIH grants NS61479 (M.R.B.), DC008449 (J.K.), DC01919, and the FRAXA Research Foundation (L.K.K.).
Footnotes
Author Contributions. M.R.B., J.K., L.K.K. designed experiments and wrote the manuscript. M.R.B., J.K., V.G., Y.C., J.G.S., D.N. performed experiments. F.J.S. contributed HEK-channel cell lines. All authors discussed the results and reviewed the manuscript.
References
- 1.Bhattacharjee A, et al. J Neurosci. 2003;23:11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeiffer BE, Huber KM. Neuroscientist. 2009;15:549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zukin RS, Richter JD, Bagni C. Front Neural Circuits. 2009;3:14. doi: 10.3389/neuro.04.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R, et al. Proc Natl Acad Sci U S A. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zalfa F, et al. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YQ, et al. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 7.Dryer SE. Trends Neurosci. 1994;17:155–160. doi: 10.1016/0166-2236(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 8.Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. Cell. 1993;74:291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- 9.Sjekloca L, et al. Biochem J. 2009;419:347–357. doi: 10.1042/BJ20082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos A, et al. Structure. 2006;14:21–31. doi: 10.1016/j.str.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Desai R, Kaczmarek LK. J Neurosci. 2007;27:2617–2627. doi: 10.1523/JNEUROSCI.5308-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LY, Gan L, Forsythe ID, Kaczmarek LK. J Physiol. 1998;509(Pt 1):183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Sigworth FJ. Nature. 2009;461:292–295. doi: 10.1038/nature08291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.