Abstract
Aims
Cardiovascular disease (CVD) is the leading cause of death among Native Hawaiians. In this article, all-cause and cardiovascular mortality rates among Native Hawaiians are examined, along with associated CVD risk factors.
Methods
A total of 855 Native Hawaiians (343 men and 512 women, ages 19–88) were examined as participants of the Cardiovascular Risk Clinics program (1992–1998) and underwent surveillance through September 2007. Cause of each death was determined by review of medical records, death certificates, newspapers, and through queries to community members.
Results
CVD accounted for 55% of deaths. Coronary heart disease (CHD) accounted for the majority of CVD deaths. CVD increased with age and was higher in those with diabetes, hypertension, or high low-density lipoprotein cholesterol (LDL-C). CVD rates were higher in men than in women and 4-fold higher in those with diabetes. In addition to age, diabetes, hypertension, and elevated LDL-C were major risk factors.
Conclusions
Diabetes is a major determinant of CVD in this population and most of the CVD is occurring in those with diabetes. Strategies to prevent diabetes and manage blood pressure and lipids should reduce CVD rates in Native Hawaiians.
Keywords: Native Hawaiians, cardiovascular diseases, diabetes-related complications
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death among Native Hawaiians [1,2], but data to date have been obtained from state health records that are based solely on death certificates. The only systematic population-based study of Native Hawaiian adults, the Native Hawaiian Health Research Project, did not examine mortality records to determine cause of death [3]. More information can be obtained by reviewing cause of death in medical records, where standardized methods allow for comparisons with other populations [4,5,6,7]. This analysis was conducted to provide systematic data on CVD mortality in Native Hawaiians and to examine the roles of potential CVD risk factors.
From 1992 to 1998, the Cardiovascular Risk Clinic (CRC) program on the island of Molokài examined a population-based sample of Native Hawaiians [8]. Information on physiologic and lifestyle risk factors was obtained using systematic methods, and all deaths in this population since the beginning of the CRC program have been reviewed, with cause of death adjudicated using standardized criteria. In this article, the data on all-cause and cardiovascular mortality rates in this population, along with associated CVD risk factors, will be presented.
SUBJECTS
The CRC was a screening program initiated in 1992 and implemented by Na Pùuwai, the Native Hawaiian Health Care System serving the island of Molokài, to identify adults at risk for CVD and refer them to health care services [9]. Native Hawaiian male and non-pregnant female residents ≥ age 18 were recruited for participation. Recruitment strategies involved mailings and direct contact by community health workers. The CRCs were conducted in the main town and also in rural areas to accommodate those residing in remote communities on the island. Although the recruitment was not systematically population-based, efforts were made to identify and contact all Native Hawaiian residents and accommodations made to ensure participation regardless of socio-economic status or health condition. The only data available to allow comparisons between our study population and the population of Molokài as a whole are from the U.S. census. However, the population covered by the U.S. census differs from our population in age, proportion of self-identified Native Hawaiians, and average household income [10,11].
The CRC program examined 947 men and women > age 18 between 1992 and 1998, approximately half of the adult Native Hawaiian population of the island in that age range. After excluding 80 participants who identified themselves as non-Native Hawaiian, 5 participants whose diabetes status could not be determined, 3 decedents whose families declined permission for follow up, and 4 decedents who had no medical records and for whom the state of Hawaii could not provide death certificates, the current cohort consisted of 855 participants (343 men and 512 women, ages 19–88). Surveillance of this cohort continued through September 2007.
MATERIALS AND METHODS
Examination
The baseline examination consisted of a questionnaire evaluating behavioral risk factors, including smoking, alcohol use, physical activity, and diet. Measurements of height, weight, waist and hip circumference, and blood pressure (BP) were made by trained observers. A morning urine specimen was obtained for protein and glucose, and a fasting blood sample was obtained for cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), hemoglobin A1c, and glucose. BP was recorded as the average of the second and third consecutive resting measures taken with a standard arm cuff and aneroid sphygmomanometer. Participants were considered hypertensive if they reported a previous diagnosis of hypertension, reported taking antihypertensive medication, or had a systolic BP (SBP) ≥ 140 mm Hg or a diastolic BP (DBP) ≥ 90 mm Hg. Body mass index (BMI) was calculated by the equation (weight (lb) / [height (in)]2] × 703). Total cholesterol (TC), HDL-C, TG, and glucose concentrations were determined by enzymatic methods using a Roche Hitachi 747 chemistry analyzer and consistent, standardized reagents (Boehringer Mannheim, Indianapolis, IN). LDL-C was calculated in those with TG < 400 mg/dL using the equation (TC-HDL-(TG/5)). For the 42 people with TG > 400 mg/dL and for whom LDL-C was not calculated, LDL-C values were imputed using the mean method.
Diabetes was identified by self-report or fasting glucose (FG) ≥ 126 mg/dL. Use of hypoglycemic agents was not ascertained. Diabetes duration was determined via questionnaire.
Follow-up
All Native Hawaiian CRC participants were eligible for the follow-up surveillance and written informed consent was obtained from them or, if deceased, from the next of kin. The primary objective of the surveillance of the CRC cohort was to collect all-cause mortality data and relate them to information obtained in the baseline exam; information on vital status was available for all participants. Retrospective surveillance began from the date of the baseline (CRC) examination and continued until September 30, 2007, thereby providing approximately 15 years of surveillance of this cohort.
Deaths occurring in the cohort since the baseline exam were ascertained by queries to community members and local leaders and through community newspapers and notices. Copies of all death certificates were obtained from the State Department of Health.
Cause of each death was determined by review of medical records and other available information. The medical record was reviewed, and pertinent information was abstracted and de-identified by a trained medical records abstractor. Discharge summaries, examination reports, and, in the case of potential CVD deaths, procedures, laboratory test results, and other relevant materials were photocopied.
Records for all deaths (N=69) were reviewed by two trained physician reviewers, and cases with ambiguous causes of death were adjudicated by a third reviewer or by an adjudication discussion. Deaths were classified as CVD; malignant neoplasm; infection (including pneumonia, influenza, septicemia, and HIV/AIDS); other chronic condition (chronic obstructive pulmonary disease, diabetes, liver disease/cirrhosis, nephritis, nephritic syndrome, and end-stage renal disease); or trauma (unintentional injury, motor vehicle accident, homicide, or suicide). CVD deaths were further classified as myocardial infarction (MI), coronary heart disease (CHD), stroke, heart failure, or other CVD using standardized criteria from the MESA study [12]. These criteria were derived primarily from the International Diagnostic Criteria and the ARIC and Framingham studies [13,14].
Data Analysis
Incidence rates per 100 for total mortality and major causes of death -- cardiovascular, cancer, others -- were computed by gender; average follow-up was 11.9 years (range 0.44–15.75 years). Rate ratios with 95% confidence intervals and incidence rates per 1000 person years were computed to evaluate the relative differences in rates of mortality and causes of death in men versus women. Univariate assessment of the association between risk factors and CVD mortality was performed by Cox proportional hazard regression among the entire cohort and among only the participants with diabetes. Adjustment for covariates was not possible because of the small number of endpoints. Hazard ratios and 95% confidence intervals for major CVD risk factors are reported.
RESULTS
Characteristics of the study population are shown in Table 1. Average age was 46, and 46.2% were obese with central fat distribution. BP and lipid values varied widely. Average age and BMI in men and women were similar, but men had slightly higher BP, lower HDL-C, and were more likely to smoke. Diabetes prevalence was 23.3% in men and 20.3% in women; average duration (self-reported) was approximately 8 years.
Table 1.
Baseline characteristics of Native Hawaiians examined as participants of the Cardiovascular Risk Clinic program, 1992–1998
| Characteristic | Men % or Mean (range) | Women % or Mean (range) | Total % or Mean (range) |
|---|---|---|---|
| N | 343 | 512 | 855 |
| Age (years) | 45 (19–85) | 46 (19–88) | 46 (19–88) |
| Body mass index (BMI) (kg/m ) | 32.7 (19.4–69.0) | 32.6 (18.3–88.1) | 32.6 (19.4–88.1) |
| Waist circumference (inches) | 40 (17–77) | 38 (20–80) | 39 (17–80) |
| Current smoker (%) | 29.2 | 25.1 | 26.7 |
| Hypertensive (%)1 | 46.4 | 37.7 | 41.2 |
| Systolic blood pressure (mm Hg) | 130 (57–214) | 127 (73–220) | 128 (57–220) |
| Diastolic blood pressure (mm Hg) | 85 (45–129) | 81 (33–131) | 82 (33–131) |
| LDL cholesterol (mg/dL) | 132 (41–400)* | 133 (48–400)* | 132 (41–400)* |
| HDL cholesterol (mg/dL) | 40 (19–74) | 45 (23–100) | 43 (19–100) |
| Triglycerides (mg/dL) | 206 (38–999)** | 146 (30–997) | 170 (30–999)** |
| Fasting plasma glucose (mg/dL) | 126.1 (61–475) | 112.8 (47–387) | 118.1 (47–475) |
| HbA1c*** | 9.2 (5.2–16.2) | 8.3 (5.3–15.3) | 8.7 (5.2–16.2) |
|
| |||
| Diabetes (%)2 | 23.3 | 20.3 | 21.5 |
HDL = high-density lipoprotein cholesterol; LDL = low-density lipoprotein cholesterol.
Defined as systolic/diastolic ≥140/90 mm Hg or self-report of previous diagnosis of hypertension or self-report of taking antihypertensive medication.
Defined by American Diabetes Association criteria.
Upper lab limit = 400 mg/dL.
Upper lab limit = 999 mg/dL.
Based on 78 values (35 in men and 43 in women)
Cause-specific mortality rates are shown in Table 2. The CVD mortality/1000 person-years was 3.73 (4.46 in men and 3.25 in women). CVD accounted for 55% of deaths and malignant neoplasm for 23%. Infections represented approximately 10% and rates of death from other chronic conditions and trauma were low. Rates in men and women were similar. MI and CHD accounted for the majority of the CVD deaths (Table 2). Five deaths were due to stroke and four deaths to heart failure. Although not significant, a trend was observed in gender differences, with higher rates of MI and CHD in men.
Table 2.
Mortality rates in Native Hawaiians participating in the Cardiovascular Risk Clinic program, average follow-up 11.9 years, n = 855
| Men N(%) | Rate (/1000 py) | Women N(%) | Rate (/1000 py) | Rate ratio (95% CI) Men/Women | |
|---|---|---|---|---|---|
| All-cause mortality | 32 (9.33) | 7.93 | 37 (7.23) | 6.01 | 1.32 (0.82–2.10) |
| Cardiovascular disease | 18 (5.25) | 4.46 | 20 (3.91) | 3.25 | 1.37 (0.72–2.57) |
| MI | 3 (0.87) | 0.74 | 4 (0.78) | 0.65 | 1.14 (0.25–5.06) |
| CHD | 10 (2.92) | 2.48 | 7 (1.37) | 1.14 | 2.18 (0.82–5.67) |
| Stroke | 2 (0.58) | 0.50 | 3 (0.59) | 0.49 | 1.02 (0.17–6.03) |
| HF | 2 (0.58) | 0.50 | 2 (0.39) | 0.33 | 1.52 (0.21–10.72) |
| Other CVD | 1 (0.29) | 0.25 | 4 (0.78) | 0.65 | 0.38 (0.04–3.38) |
| Malignant neoplasm | 7 (2.04) | 1.73 | 9 (1.76) | 1.46 | 1.19 (0.44–3.15) |
| Infections* | 3 (0.87) | 0.74 | 4 (0.78) | 0.65 | 1.14 (0.25–5.06) |
| Other chronic | 1 (0.29) | 0.25 | 2 (0.39) | 0.33 | 0.76 (0.07–8.34) |
| conditions** | |||||
| Other/undetermined | 1 (0.29) | 0.25 | 2 (0.39) | 0.33 | 0.76 (0.07–8.34) |
| Trauma*** | 2 (0.58) | 0.50 | 0 (0) | - | - |
CHD = coronary heart disease, HF = heart failure; MI = myocardial infarction; py = person years.
Pneumonia, influenza, septicemia, HIV/AIDS.
Chronic obstructive pulmonary disease (COPD), diabetes, liver disease/cirrhosis, nephritis, nephritic syndrome, nephrosis, end-stage renal disease.
Unintentional injury, motor vehicle accidents, homicide and legal intervention, suicide.
Univariate assessment of associations between CVD death and standard risk factors (Table 3) showed that CVD increased with age (11.2% in those > age 50 compared with 0.9% in those < age 50) and was higher in those with diabetes (Figure 1), hypertension, or high LDL-C. Among participants with diabetes, the association with age (P<0.0001) remained significant.
Table 3.
CVD hazard ratios by risk factor, in the entire cohort and among participants with diabetes.
| % of total | HR; 95% CI (P) Entire Cohort | HR; 95% CI (P) Participants with DM | ||
|---|---|---|---|---|
| Gender | Women | 3.91 | ||
| Men | 5.25 | 1.37; 0.72, 2.58 (0.3373) | 1.06; 0.42, 2.697 (0.8997) | |
|
| ||||
| Age | 35–49 | 0.92 | ||
| ≥50 | 11.24 | 13.53; 4.75, 38.58 (<0.0001) | 12.93; 2.72, 97.49 (0.0130) | |
|
| ||||
| BMI | <29.9 | 4.80 | ||
| ≥30.0 | 3.82 | 0.78; 0.40, 1.50 (0.4557) | 0.41; 0.16, 1.03 (0.0574) | |
|
| ||||
| Diabetes | No | 2.98 | ||
| Yes | 9.78 | 3.57; 1.89, 6.76 (<0.0001) | - | |
|
| ||||
| Hypertensive | No | 2.39 | ||
| Yes | 7.39 | 3.24; 1.64, 6.43 (0.0007) | 2.57; 0.85, 8.81 (0.0958) | |
|
| ||||
| LDL-C | < 130 | 3.05 | ||
| ≥ 130 | 6.06 | 2.07; 1.05, 4.09 (0.0360) | 1.75; 0.56, 5.42 (0.3329) | |
|
| ||||
| HDL-C | ≥40 men; ≥50 women | 3.91 | ||
| <40 men; <50 women | 4.84 | 1.23; 0.62, 2.44 (0.3579) | 1.16; 0.38, 3.52 (0.7937) | |
|
| ||||
| TG | <150 | 3.97 | ||
| ≥150 | 5.20 | 1.31; 0.69, 2.48 (0.4040) | 0.93; 0.35, 2.47 (0.8788) | |
|
| ||||
| Current smoker | No | 4.39 | ||
| Yes | 3.02 | 0.68; 0.28, 1.66 (0.3945) | 1.64; 0.55, 4.89 (0.3750) | |
Abbreviations: BMI = body mass index; CI = confidence interval; HDL = high-density lipoprotein cholesterol; HR = hazard ratio; LDL = low-density lipoprotein cholesterol; TG = triglycerides.
Figure 1.
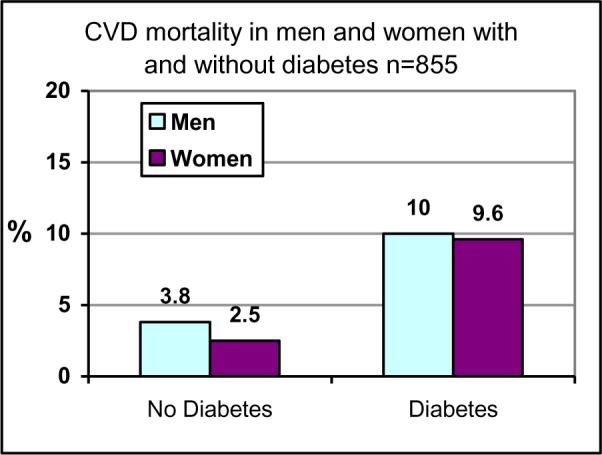
Diabetes-specific rates of CVD mortality.
DISCUSSION
This longitudinal analysis in a population-based cohort was undertaken to provide systematic data on CVD mortality in Native Hawaiians and to examine the roles of potential CVD risk factors. CVD was the largest cause of mortality in this population, accounting for 56% of deaths in men and 54% in women; cancer accounted for only 10%. CVD rates were higher in men than in women, and the majority of CVD deaths were due to MI and CHD. In addition to age and gender, diabetes, hypertension, and elevated LDL-C were major risk factors.
Reports of increasing CVD mortality in Native Hawaiians have been published, but have been based on death certificate data. Aluli et al. [15] compared state health data in Native Hawaiians with non-Native Hawaiians in the state of Hawaii and in the general United States; CHD mortality rates in Native Hawaiians were 1.35/1000 person years and CVD mortality rates were 3.13/1000 person-years, both higher than the rates for non-Hawaiians in the state or among the general U.S. population and nearly equal to the present analysis.
Henderson et al. [16] reported CHD mortality rates in the Multiethnic Cohort Study (MCS). The MCS cohort was assembled from drivers' license and Health Care Financing Administration files that included data on more than 9000 Native Hawaiians in Hawaii and California who were free of self-reported CHD at baseline. The MCS study evaluated mortality in Native Hawaiians in Hawaii and California identified from drivers licenses and a cancer registry. Age standardized rates of MI and other heart disease deaths as determined by death certificate data were 1.25 and 2.59 per 1000 person-year, respectively, in non-Native vs. Native Hawaiian men and 0.44 and 1.11 per 1000 person-years, respectively, in non-Native vs. Native Hawaiian women. These rates are somewhat lower than those indicated by state health data, probably because they were incidence data. In the MCS cohort, mortality rates from MI and other heart disease in Native Hawaiians were higher than those for whites, Japanese Americans, and Latinos. Thus, the current study verifies, using systematic methodology, the previous reports of high CHD and CVD mortality in Native Hawaiians and validates the need for public health attention to the problem.
Of interest, therefore, are our data on the relation of CVD to established risk factors. As in all other studies, CVD mortality increased with age; no deaths in those younger than age 35 were attributable to CVD. The data showed that, except for stroke and other CVD, the rates were higher in men than in women. This pattern was also seen in state health data and in the MCS [16]. Although MCS did not report rates for stroke mortality, state health data and our data both indicated stroke mortality rates that were 2- to 5-fold lower than those for CHD; this may reflect the relatively younger age distribution of Native Hawaiians. Stroke incidence may increase with aging and/or diabetes duration.
CVD mortality rates were nearly 4-fold higher in individuals with diabetes compared with those without diabetes. We are not aware of any other data comparing CHD or CVD incidence by diabetes status in Native Hawaiians; however, this ratio is consistent with the 2- to 6-fold higher rates of CVD reported when comparing diabetic and nondiabetic individuals in other populations [17]. As in several other ethnic groups, diabetes rates are high in Native Hawaiians, and thus diabetes is responsible for a large proportion of the CVD. The prevalence of diabetes found in this study is identical with diabetes prevalence found among Native Hawaiian enrolled in the Native Hawaiian Health Research Project and supports the idea that the sample population in this study is representative of the general Native Hawaiian population. A number of prevention strategies for diabetes have been established and are being implemented in Native Hawaiian communities, particularly following the results of the Diabetes Prevention Program and other translation research studies that have enrolled Native Hawaiians with or at risk for diabetes [18, 19, 20]. These public health efforts, if sustained, will likely have the greatest impact on reversing the tide of CVD events. In the meantime, aggressive control of the standard CVD risk factors in diabetic patients is warranted.
Hypertension and high LDL-C were the other two risk factors that appeared to have major impact on CVD mortality. Hypertension is a major risk factor for CVD in all populations, and risk prediction equations, such as Framingham [21, 22] show large effects of rising blood pressure on CHD incidence; several studies show that the increasing risk begins with only mild increases in blood pressure [23]. In our previous report on Native Hawaiians [24], hypertension rates were 32% and 40% in nondiabetic women and men, respectively, and 60% in both genders with diabetes [15]. In MCS, 49% of Native Hawaiian men and 50% of women self-reported hypertension. Recent reports [25] have shown rates of 52% (≥140/90 mmHg) in people who reported 75–99% degree Hawaiian ancestry (DHA) and a significant linear relationship between DHA and increased blood pressure. Curb et al. [26] analyzed data collected on Molokai in 1985 and found 24% of women and 26% of men were hypertensive when the definition was ≥160/90 mmHg. Hypertension appears to be an important determinant of CVD in Native Hawaiians, and because the CVD rate increases with diabetes, attention should be given to BP control in individuals with diabetes. Studies have shown that BP lowering reduces CVD incidence in individuals with and without diabetes [27].
A striking finding in our previous analysis [24] of risk factors in this cohort was the high level of LDL-C, especially in diabetic men (155 mg/dL) and women (157 mg/dL). LDL-C in our analysis was correlated with CVD, although the relationship was attenuated after adjustment for diabetes. Thus the data suggest that some of the diabetes-associated CVD appears attributable to the markedly elevated LDL-C in diabetic individuals in this population [8]. Curb et al. [26] and Mau et al. [28] also suggested that LDL-C levels are above acceptable targets in a large proportion of Native Hawaiians. Aggressive LDL-C lowering is achievable given the availability of LDL-C lowering agents, and this strategy has been shown to significantly lower rates of CVD in nondiabetic and diabetic individuals in many populations [29, 30]. An apparent negative association was observed between CVD and smoking and obesity. This association may be due to the lack of ability to adjust for relevant covariates, or more likely to the fact that most deaths occur in older diabetic individuals, in whom weight loss is common, and who are more likely to quit smoking.
This study has a number of strengths. This study provides the first assessment of cardiovascular mortality rates and cause of death in a representative sample of Native Hawaiians determined by medical record review using standardized criteria. Because of the high participation rate, the data are likely to represent most Native Hawaiians. The data were collected using standardized methods by trained personnel on a wide range of physiologic and behavioral risk factors. All deaths were reviewed and adjudicated using standardized criteria, allowing comparisons with other longitudinal studies. Finally, this study provides the first prospective data on the strength of CVD risk factors and thus offers information on which to base treatment and prevention strategies.
This study was limited by several factors. This sample was not systematically population-based and, therefore, the data may be biased. However, the recruitment by community members, who were familiar with the Molokai population, allowed for increased yield of the eligible population and probably increased the range of individuals who attended the exam despite co-morbidities or other barriers that often accompany similar studies. Although the laboratory measures were not made by a CVD core lab, all measures were made by the same commercial laboratory using longitudinal standardization procedures throughout the exam period. The data evaluating the relations of CVD risk factors are limited because the small number of events did not allow for systematic multivariate analyses.
In summary, this report verifies, using systematic criteria, that mortality rates for CVD, particularly for MI and coronary death, are high in Native Hawaiian men and women. The risk factor data indicate that diabetes is a major determinant of CVD and that most of the CVD is occurring in those with diabetes. Hypertension and high LDL-C are also important determinants. Research on the most effective strategies for diabetes prevention, along with blood pressure and lipid management, are needed to reduce CVD rates.
ACKNOWLEDGEMENTS
FUNDING This work was supported by grants [P20 MD 000173, P20 MD00174] from the National Center for Minority Health and Health Disparities (NCMHD), National Institutes of Health (NIH). This study was also supported in part by the Myron Pinky Thompson Endowed Chair [S21 MD000228] and a grant from the National Heart, Lung and Blood Institute [U01 HL079163]. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of NCMHD, NLHBI, or NIH.
POTENTIAL CONFLICTS OF INTEREST Dr. B.V. Howard has served on the advisory boards of Merck, Schering Plough, and the Egg Nutrition Council and has received research support from Merck and Pfizer. Dr. Wm. J. Howard has received research support from Pfizer, AstraZeneca, Merck, and Schering-Plough; has served as a consultant for Merck, Schering-Plough, Pfizer, and Reliant; and has served on the Speakers' Bureaus for Merck, Schering-Plough, Pfizer, AstraZeneca, Abbott, and Daiichi Sankyo. Dr. M. K. Mau has served as a consultant for Sanofi-Aventis and Merck, and also has served on the Speaker's Bureau for both companies. The other authors have nothing to declare.
The authors gratefully acknowledge the Molokài community; Hua Kanawao Ka Liko's Community Council; Na Pùuwai Native Hawaiian Health Care System; Queen Lilìuokalani Children's Center – Molokài Unit; and Papa Ola Lōkahi for their commitment to CVD research that adhered to community-based participatory research principles. We thank Rachel Schaperow, MedStar Research Institute, for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
An abstract of this data was presented at the American Heart Association Epi/NPAM meeting, March 2009, Palm Harbor, FL.
REFERENCES
- 1.Centers for Disease Control and Prevention Available online at: http://www.state.hi.us/doh/stats/surveys/hhs/hhs00.htm. Retrieved [10/24/2007]
- 2.Asian American and Pacific Islander Workshops . Summary Report on Cardiovascular Health. U.S. Department of Health and Human Services. Public Health Service. National Institutes of Health, National Heart, Lung, and Blood Institute; Mar, 2000. NIH Publication No. 00-3793. [Google Scholar]
- 3.Mau MK, Glanz K, Severino R, Grove J, Johnson B, Curb JD. Mediators of Lifestyle Behavior Change in Native Hawaiians: Initial Findings from the Native Hawaiian Diabetes Intervention Program. Diabetes Care. 24:1770–1775. doi: 10.2337/diacare.24.10.1770. [DOI] [PubMed] [Google Scholar]
- 4.Ravakhah K. Death certificates are not reliable: revivification of the autopsy. South Med J. 2006;99:728–733. doi: 10.1097/01.smj.0000224337.77074.57. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Coady SA, Sorlie PD, Cooper LS, Folsom AR, Rosamond WD, Conwill DE. Validation of death certificate diagnosis for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Clin Epidemiol. 2001;54:40–50. doi: 10.1016/s0895-4356(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 7.Tavora F, Crowder C, Kutys R, Burke A. Discrepancies in initial death certificate diagnoses in sudden unexpected out-of-hospital deaths: the role of cardiovascular autopsy. Cardiovasc Pathol. 2008;17:178–182. doi: 10.1016/j.carpath.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Aluli NE, Jones KL, Reyes PW, Brady SK, Tsark JU, Howard BV. Diabetes and Cardiovascular Risk Factors in Native Hawaiians. Hawaii Medical Journal. 2009;68:148–153. [PMC free article] [PubMed] [Google Scholar]
- 9. [(Accessed May 12, 2009)]; http://www.napuuwai.com/
- 10. [(Accessed May 12, 2009)]; http://uhfamily.hawaii.edu/Cof_Data/profiles/indicatorResults.asp?page=latest&geo=11500910865,11500900000,11500000000.
- 11.U.S. Census Bureau [(Accessed May 12, 2009)];Census 2000. 2000 (May 8, 2009) [Online:] http://www.census.gov/census2000/states/hi.html.
- 12.Greenlee RT. Measuring disease frequency in the Marshfield Epidemiologic Study Area (MESA) Clin Med Res. 2003;1:273–280. doi: 10.3121/cmr.1.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke definitions for use in a multicenter clinical trial. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.American Heart Association, Inc Case definitions for acute coronary heart disease in epidemiology and clinical research studies. Circulation. 2003;108:2543. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 15.Aluli NE, Reyes PW, Tsark JU. Cardiovascular disease disparities in Native Hawaiians. Journal of the CardioMetabolic Syndrome. 2007;2:250–253. doi: 10.1111/j.1559-4564.2007.07560.x. [DOI] [PubMed] [Google Scholar]
- 16.Henderson SO, Haiman CA, Wilkens LR, Kolonel LN, Wan P, Pike MC. Established risk factors account for most of the racial differences in cardiovascular disease mortality. PLoS ONE. 2007;2:e377. doi: 10.1371/journal.pone.0000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard BV, Best LG, Galloway JM, Howard WJ, Jones K, Lee ET, et al. Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors. Diabetes Care. 2006;29:391–397. doi: 10.2337/diacare.29.02.06.dc05-1299. [DOI] [PubMed] [Google Scholar]
- 18.Gilliland SS, Carter JS, Perez GE, Two Feathers J, Kenui CK, Mau MK. Recommendations for Development and Adaptation of Culturally Competent Community Health Interventions in Minority Populations with Type 2 Diabetes Mellitus. Diabetes Spectrum. 1998;11:166–174. [Google Scholar]
- 19.Diabetes mellitus and heart disease risk factors in Hawaiians: the Native Hawaiian Health Research Project, RCMI Program. Hawaii Med J. 1994;53:340–343. 364. [PubMed] [Google Scholar]
- 20.Nacapoy AH, Kaholokula JK, West MR, Dillard AY, Leake A, Kekauoha BP, et al. Partnerships to Address Obesity Disparities in Hawai'i: The PILI `Ohana Project. HMJ. 2008;67:237–241. [PMC free article] [PubMed] [Google Scholar]
- 21.Buse JB, Ginsberg HN, Bakris HL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular disease in people with diabetes: A scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 22.Cappuccio FP, Oakeshotte P, Stazullo P, Kerry SM. Application of the Framingham risk estimates to ethnic minorities in the United Kingdom and implications for primary prevention of heart disease in general practice: cross sectional population based study. BMJ. 2002;325:1271. doi: 10.1136/bmj.325.7375.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Lee ET, Devereux RB, Yeh J, Best LG, Fabsitz RR, et al. Prehypertension, diabetes and cardiovascular disease risk in a population-based sample: the Strong Heart Study. Hypertension. 2006;47:410–414. doi: 10.1161/01.HYP.0000205119.19804.08. [DOI] [PubMed] [Google Scholar]
- 24.Aluli NE, Jones KL, Reyes PW, Brady SK, Tsark JU, Howard BV. Diabetes and cardiovascular risk factors in Native Hawaiians. Hawaii Med J. 2009;68:152–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Grandinetti A, Chen R, Kaholokula JK, Yano K, Rodriguez BL, Chang HK, et al. Relationship of blood pressure with degree of Hawaiian ancestry. Ethn Dis. 2002;12:221–228. [PubMed] [Google Scholar]
- 26.Curb JD, Aluli NE, Kautz JA, Petrovitch H, Knutsen SF, Knutsen R, et al. Cardiovascular risk factor levels in ethnic Hawaiians. Am J Public Health. 1991;81:164–167. doi: 10.2105/ajph.81.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 009;338:b1665. doi: 10.1136/bmj.b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mau MK, Grandinetti A, Arakaki RF, Chang HK, Kinney EK, Curb JD. The insulin resistance syndrome in Native Hawaiians. Diabetes Care. 1997;20:1376–1380. doi: 10.2337/diacare.20.9.1376. [DOI] [PubMed] [Google Scholar]
- 29.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: The Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]


