Abstract
Stimulation of double-stranded (ds)RNA receptors can increase the effectiveness of cancer vaccines, but the underlying mechanisms are not completely elucidated. In this study, we sought to determine critical roles of host IFN-α and IFN-γ pathways in the enhanced therapeutic efficacy mediated by peptide vaccines and polyinosinic-polycytidylic acid [poly(I:C)] stabilized by lysine and carboxymethylcellulose (poly-ICLC) in the murine central nervous system (CNS) GL261 glioma. C57BL/6-background wild type (WT), IFN-α receptor-1 (IFN-αR1)−/− or IFN-γ −/− mice bearing syngeneic CNS GL261 glioma received subcutaneous (s.c.) vaccinations with synthetic peptides encoding CTL epitopes with or without intramuscular (i.m.) injections of poly-ICLC. The combinational treatment induced a robust transcription of CXCL10 in the glioma site. Blockade of CXCL10 with a specific monoclonal antibody (mAb) abrogated the efficient CNS homing of antigen-specific type-1 CTL (Tc1). Both IFN-αR −/− and IFN-γ −/− hosts failed to up-regulate the CXCL10 mRNA and recruit Tc1 cells to the tumor site, indicating non-redundant roles of type-1 and type-2 IFNs in the effects of poly-ICLC-assisted vaccines. The efficient trafficking of Tc1 also required Tc1-derived IFN-γ. Our data point to critical roles of the host-IFN-α and IFN-γ pathways in the modulation of CNS glioma microenvironment, and the therapeutic effectiveness of poly-ICLC-assisted glioma vaccines.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0876-3) contains supplementary material, which is available to authorized users.
Keywords: CNS glioma, Poly-ICLC, Glioma vaccine, Type-1 immune response, Chemokine
Introduction
dsRNA, such as poly(I:C), has been demonstrated to be a promising vaccine-adjuvant [1, 2], and the enhanced antigen-specific CD8+ T cell responses appear to depend upon host IFN-α signaling [3–5] and the TLR3 [6] as well as cytoplasmic helicase family proteins, retinoic acid induced protein-I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) [7–9]. Although these studies provide us with in depth understanding how dsRNA promotes the induction phase of adaptive immunity, little is known about the effects of systemic dsRNA administration on the effector phase, especially its effects on the tumor microenvironment.
The central nervous system (CNS) requires rapid, innate immune responses by resident brain cells to effectively fight infectious agents in the CNS, and the critical role of TLR3 in CNS immunity has been demonstrated [10, 11]. We have previously demonstrated that administration of poly-ICLC enhances the therapeutic effects of peripheral vaccinations targeting glioma-associated antigens (GAAs) through improved induction of GAA-specific type-1 CTLs expressing very late activation antigen (VLA)-4, which confers efficient CNS tumor homing of vaccine-induced CTLs [11, 12]. However, the key mechanisms underlying how the combination of GAA vaccines and poly-ICLC impacts the CNS tumor microenvironment were not completely elucidated.
With regard to critical steps for T cell trafficking and extravasation into inflamed tissues, in addition to integrins [13], chemokines have been shown to trigger firm adhesion of leukocytes to vascular endothelium under flow condition by enhancing integrin–ligand-binding affinity [14, 15]. Based on our recent studies [12, 16–18], efficient CNS tumor homing is a characteristic of CTLs with a type-1 phenotype (Tc1) as opposed to ones with the type-2 phenotype (Tc2), and this appears to be related to differential T cell response to the type-1 chemokine, IFN-γ-inducible protein (IP)-10/CXCL10.
As TLR3 stimulation efficiently induces production of pro-inflammatory cytokines and chemokines, including CXCL10/IP-10 from astrocytes and microglia [19–21], we hypothesized that the combination treatment of GAA vaccine and poly-ICLC would enhance CXCL10 expression in the glioma microenvironment as a key mediator for the enhanced therapeutic effects.
In the current study, the combination of GAA vaccine and poly-ICLC, but not poly-ICLC administration alone, induced a robust CXCL10 expression in the tumor site as a critical chemokine for Tc1 homing. The induction of CXCL10 was dependent on host IFN-α as well as effector T cell-derived IFN-γ, suggesting critical and non-redundant roles of both type-1 and type-2 IFNs in effective immunotherapy strategies for gliomas.
Results
GAA vaccines in combination with poly-ICLC treatment prolong survival of mice bearing established GL261 glioma
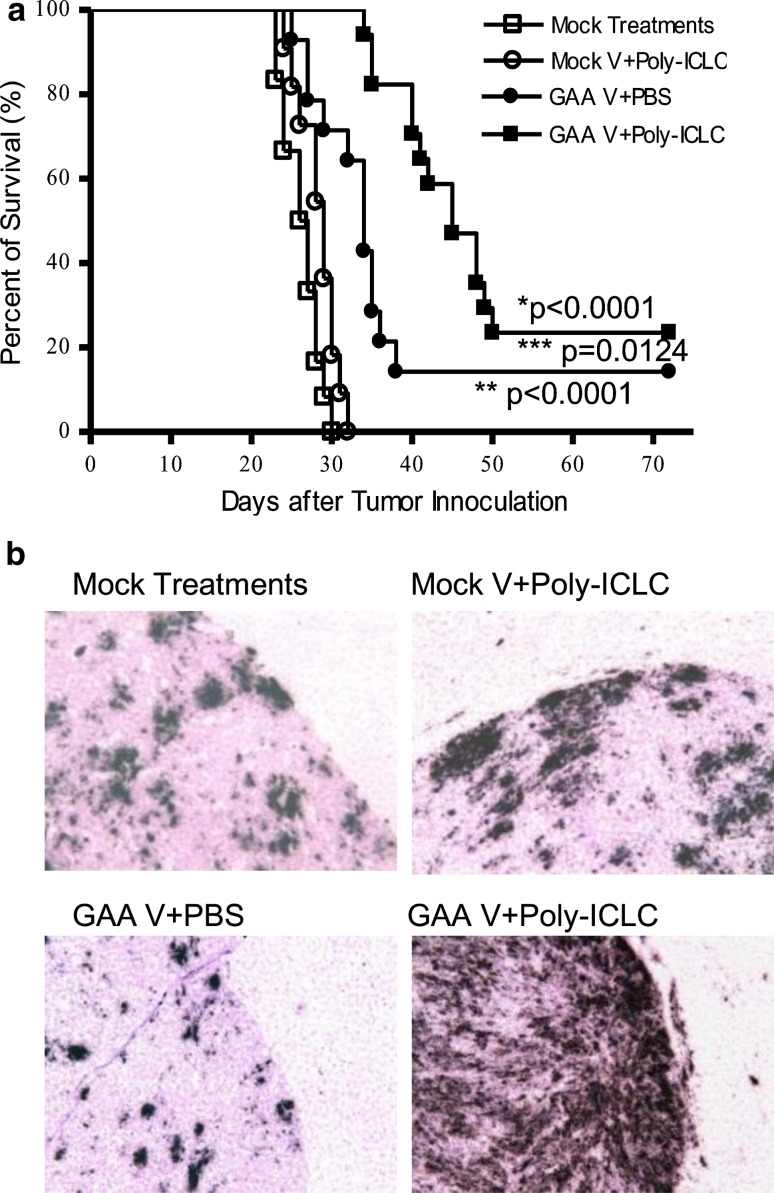
We have previously demonstrated that the combination of GAA vaccines and poly-ICLC administration induce effective protective immunity against i.c. challenge with GL261 [11]. In the current study, we evaluated the therapeutic efficacy of the combination regimen in mice bearing established i.c. glioma. C57BL/6 mice received i.c. inoculation of GL261 glioma on day 0, and were stratified into four groups to receive: (1) control (mock vaccines and i.m. PBS) treatments alone; (2) GAA vaccines and i.m. PBS; (3) i.m. poly-ICLC administrations and mock vaccines, or (4) GAA vaccines combined with i.m. poly-ICLC on days 2, 12 and 22. Although GAA vaccines alone significantly prolonged the survival of mice compared with mice treated with poly-ICLC alone or mock treatments alone (p < 0.0001), addition of poly-ICLC in the GAA vaccine regimen further improved the survival (Fig. 1a) with 4 of 17 mice in this group surviving longer than 72 days (p = 0.0124; the combination group vs. GAA vaccines alone).
Fig. 1.
Poly-ICLC administration significantly improves the therapeutic effects of GAA vaccines in mice bearing GL261 glioma, associated with robust induction of type-1 chemokine transcripts in the CNS glioma microenvironment. a WT C57BL/6 mice bearing i.c. GL261 glioma received s.c. immunizations with each of GAA peptides and HBVcore128 T-helper epitope peptide and/or i.m. poly-ICLC injections as described in “Materials and methods”. The mice were stratified into four treatment groups: (1) GAA vaccine and poly-ICLC (solid squares) (n = 17); (2) GAA vaccine and i.m. PBS (solid circles) (n = 14); (3) Mock vaccines and poly-ICLC (hollow circles) (n = 11) and (4) Mock vaccines and i.m. PBS (hollow squares) (n = 12). Symptom-free survival of mice was monitored. **p < 0.0001 for mice treated with GAA vaccine and poly-ICLC compared with mice treated with mock-treatments only or mock-vaccine plus poly-ICLC, *p < 0.0001 for mice treated with GAA vaccine and PBS compared with the mice treated with mock-treatments only or mock-vaccine plus poly-ICLC, ***p = 0.0124 for mice treated with GAA vaccine and poly-ICLC compared with the mice treated with GAA vaccine alone. b On day 23 following tumor inoculation (1 day following the third s.c and i.m. treatments), brain tissues were collected from WT C57BL/6 mice bearing i.c. GL261 glioma treated with either mock-treatments, mock vaccines plus poly-ICLC, GAA vaccines plus PBS or GAA vaccines plus poly-ICLC. ISH of CXCL10 mRNA in the brain-bearing GL261 glioma. Parallel hybridization of tissue sections with the cognate sense control probe provided no non-specific ISH signals (data not shown) (original magnification, ×100). Results from one of two independent experiments with highly similar results are shown
The combination regimen induces type-1 chemokine CXCL10 in the i.c. glioma environment
Poly-ICLC remarkably enhanced the trafficking of vaccine-induced CTLs [11] to the glioma site, leading us to hypothesize that the combination regimen would also induce type-1 chemokine expression, which our previous studies have shown to be critical for efficient homing of antigen-specific CTLs to i.c. gliomas [17, 18]. To test this, we employed in situ hybridization (ISH) for CXCL10 mRNA with 35S-labeled riboprobes. As shown in Fig. 1b, treatment of mice with GAA vaccines in combination with poly-ICLC resulted in a remarkably high-level expression of CXCL10 mRNA in i.c. GL261 glioma when compared with other groups, including mice treated with mock-vaccine plus PBS, mock vaccines plus poly-ICLC and GAA vaccine plus PBS. It is noteworthy that CXCL10 mRNA-positive cells are mostly localized within the tumor tissue but not seen in surrounding normal brain tissues.
CXCL10 is responsible for recruitment of antigen-specific Tc1 cells
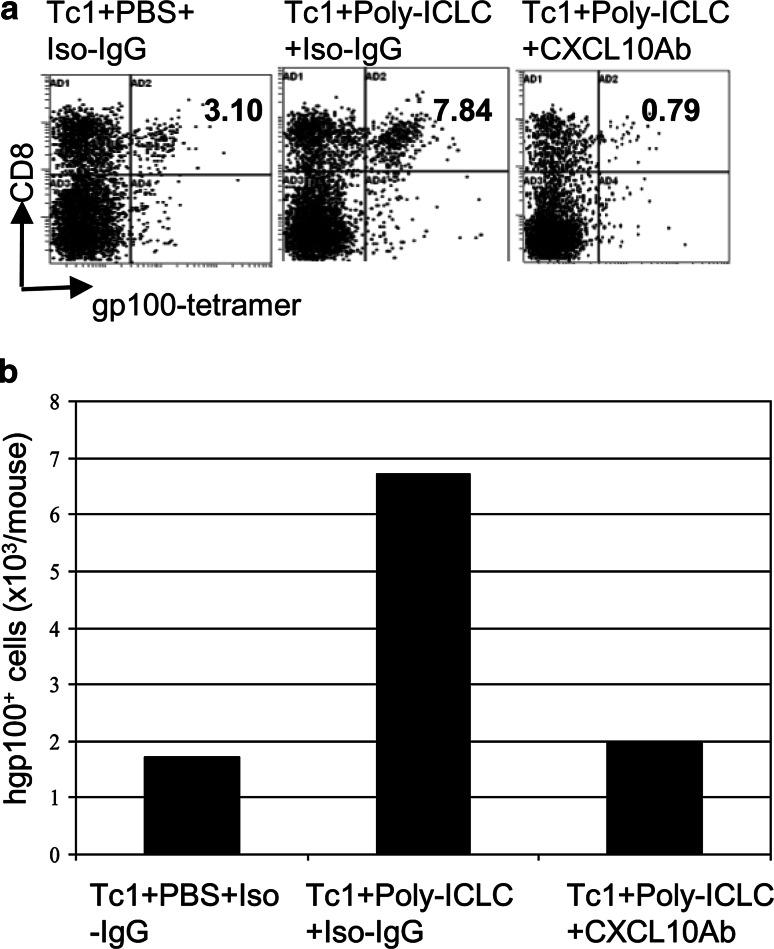
We sought to determine the role of CXCL10 in homing of Tc1 to mouse CNS GL261 glioma. As demonstrated in Fig. 2a, treatment of glioma-bearing mice with i.m. poly-ICLC administration remarkably improved the migration of adoptively transferred CD8+/gp100+-tetramer reactive Tc1 cells to the tumor site. However, administration of anti-CXCL10 mAb sharply diminished the tumor infiltration of CD8+/gp100+-tetramer reactive brain infiltrating lymphocytes (BILs) (Fig. 2a). Consistently, the absolute number of CD8+/gp100+-tetramer reactive BILs per mouse also decreased remarkably after injection of anti-CXCL10 mAb (Fig. 2b). Collectively, these results indicate that CXCL10 plays an essential role in enhancing the recruitment of antigen-specific T cells into brain tumor sites.
Fig. 2.
CXCL10 plays a critical role in CNS glioma homing of Tc1 cells. WT C57BL/6 mice bearing i.c. GL261 tumors received i.m. injections of poly-ICLC (20 μg/dose) or control PBS on days 20 and 23. Mice treated with poly-ICLC received i.p. injections of anti-mouse CXCL10 mAb (250 μg/dose) or the same amounts of hamster IgG on day 20 and 100 µg on days 21, 22, 23, and 24. All mice received a single i.v. infusion of 3 × 106 pmel-1 derived Tc1 cells on day 21. Mice were sacrificed on day 25 and BILs were analyzed by flow cytometry. a Numbers in each dot plot indicate the percentage of CD8+/gp100-tetramer+ T cells in BILs. b Total numbers of CD8+/GP100-tetramer+ T cells in BILs per mouse. Results from one of two experiments with similar results are shown. Due to the small number of BILs obtained per mouse, BILs obtained from all mice in a given group (5 mice/group) were pooled and then evaluated for the relative number/mouse and phenotype of the BILs between groups
Requirements for both the IFN-α and IFN-γ pathways for efficient CXCL10 induction
We sought to determine how the combination regimen promotes the potent CXCL10 induction in the glioma tissue. Because the regimen lead to high-level infiltration of type-1 (i.e. IFN-γ producing) anti-glioma CTLs (i.e. Tc1) [11], it was postulated that IFN-γ produced by the initial group of glioma-infiltrating Tc1s would induce CXCL10 that would allow for further influx of chemokine-reactive Tc1 cells. It was also hypothesized, because poly-ICLC is a potent type-1 IFN inducer, that the host type-1 IFN signaling might play a key role in the observed CXCL10 induction.
Our hypotheses were also supported by previous studies demonstrating that IFN-α and IFN-γ induce CXCL10 from a variety of cells, including astrocytes [22] and dendritic cells (DCs) [23] and that IFN-α up-regulates CXCR3, a receptor for CXCL10, on type-1 T cells [24].
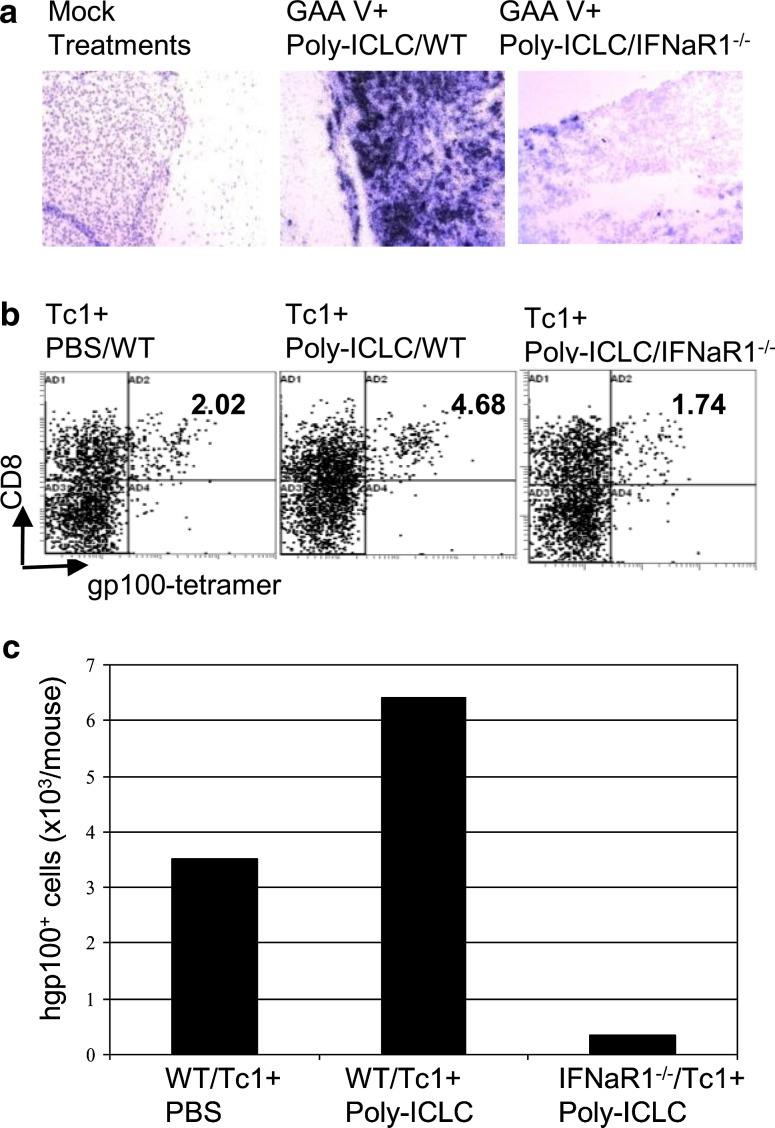
We first investigated the role of the host IFN-α pathway in the induction of CXCL10 and recruitment of antigen-specific T cells using syngeneic IFN-αR1 −/− mice. ISH analyses have revealed that, in striking contrast to WT mice, IFN-αR1−/− mice bearing i.c. GL261 glioma failed to induce CXCL10 mRNA in the tumor site to the same extent as in WT mice following the combination treatment, indicating a critical role of the host type-1 IFN pathway for the induction of CXCL10 mRNA (Fig. 3a).
Fig. 3.
Host IFN-α pathway is critical for induction of CXCL10 and Tc1 homing in CNS gliomas.a WT or IFN-αR1 −/− mice bearing i.c. GL261 glioma were treated with GAA vaccines plus poly-ICLC. Control WT mice received mock vaccines plus PBS. On day 23, brain tissues were collected and ISH for CXCL10 mRNA was conducted (original magnification, ×200). b, c WT or IFN-αR1 −/− mice bearing i.c. GL261 gliomas received i.v. infusion of pmel-1-derived CD8+ Tc1 cells on day 18 and i.m. injections of poly-ICLC on days 17, 18 and 20. Control WT mice received i.v. infusion of pmel-1-derived CD8+ Tc1 cells on day 18 and i.m. injections of PBS. On day 22, BILs were harvested and evaluated. b Numbers in each dot plot indicate the percentage of CD8+/hgp10025–33 tetramer+ cells in lymphocyte-gated BILs. c Total numbers of CD8+/hgp10025–33 tetramer+ cells per mouse brain. Results from one of the two independent experiments with similar results are shown (see Supplementary Fig. S3 for reproducible results from another experiment). Due to the small number of BILs obtained per mouse, BILs obtained from all mice in a given group (5 mice/group) were pooled and then evaluated for the relative number/mouse and phenotype of the BILs between groups
Next, to examine the role of the host type-1 IFN pathway in the tumor trafficking of Tc1 cells, glioma-bearing WT or IFN-αR1 −/− mice received i.v. infusion of ex vivo activated Tc1 cells and i.m. injections of poly-ICLC. BIL analyses in WT mice demonstrated enhanced Tc1 trafficking to i.c. glioma by i.m. poly-ICLC administration, but the absence of intact type-1 IFN pathway in the host almost completely abrogated the Tc1 homing to the glioma lesions (Fig. 3b, c). These data clearly demonstrate a critical role of the host type-1 IFN pathway in effective induction of CXCL10 expression by the combination treatment and the resulting trafficking of antigen-specific Tc1 into i.c. gliomas.
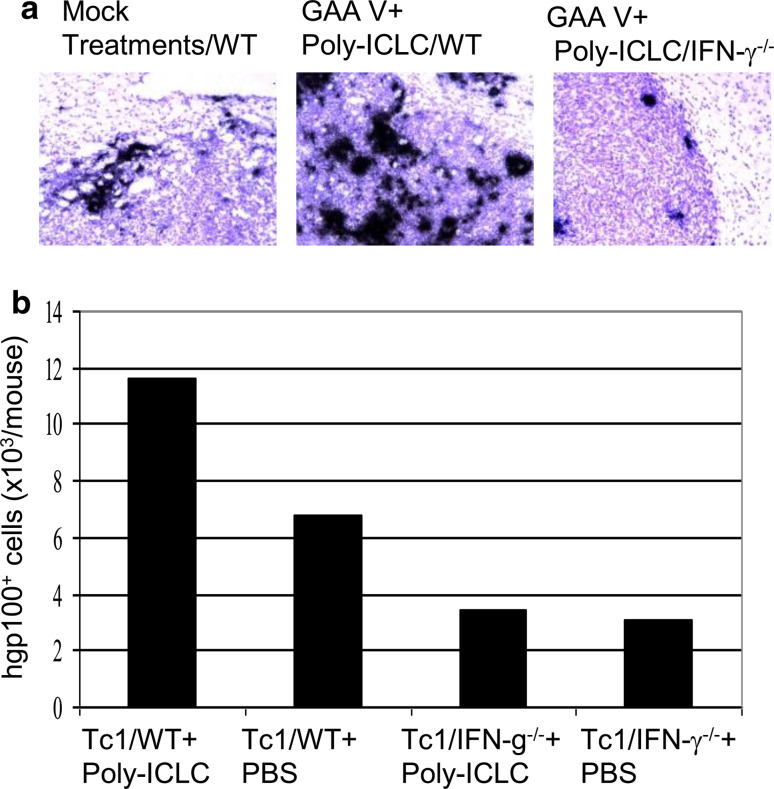
We then investigated the role of the host IFN-γ pathway in the induction of CXCL10 using syngeneic IFN-γ−/− mice. ISH analyses demonstrated that the absence of IFN-γ production attenuated the efficient induction of CXCL10 in the glioma tissue of mice receiving vaccine and poly-ICLC (Fig. 4a). Among the cell types that can produce high-level IFN-γ in the host, as we do not observe significant numbers of NK cells in the glioma environment (data not shown), we specifically evaluated the contribution of T cell-derived IFN-γ by adoptive transfer of Tc1 cells derived from IFN-γ+/+/pmel-1 or IFN-γ−/−/pmel-1 mice. BIL analyses revealed significantly diminished infiltration of antigen-specific IFN-γ−/− Tc1 cells compared with IFN-γ+/+ Tc1 cells (Fig. 4b). These data indicate a critical role of IFN-γ produced by antigen-specific T cells for their efficient infiltration into the glioma lesion.
Fig. 4.
IFN-γ serves as a critical mediator for induction of CXCL10 and Tc1 homing in CNS gliomas. a WT or IFN-γ−/− mice bearing i.c. GL261 glioma were treated with GAA vaccines plus poly-ICLC. Control WT mice received mock treatments (i.e. mock vaccines and PBS) only. On day 23, brain tissues were collected and ISH for CXCL10 mRNA was conducted (original magnification, ×200). b WT mice bearing i.c. GL261 gliomas received i.v. infusion of IFN-γ +/+ /pmel-1- or IFN-γ −/− /pmel-1-derived CD8+ Tc1 cells on day 18 and i.m. injections of poly-ICLC or PBS on days 17, 18 and 20. On day 22, BILs were harvested, counted and enumerated by flow cytometry for the total numbers of CD8+/hgp10025–33 tetramer+ cells per mouse brain. Results from one of two independent experiments with similar results are shown (see Supplementary Fig. S4 for reproducible results from another experiment). Due to the small number of BILs obtained per mouse, BILs obtained from all mice in a given group (5 mice/group) were pooled and then evaluated for the relative number/mouse and phenotype of the BILs between groups
Discussion
In the present study, we demonstrated that the combination of GAA vaccines and poly-ICLC induced high levels of local IP-10/CXCL10 expression in the CNS glioma environment, which was critical for efficient trafficking of GAA-reactive type-1 CTLs. We also found that the host IFN-α signaling and IFN-γ play critical roles in the observed induction of CXCL10 as well as the CNS glioma homing of effector CTLs.
Our ISH studies demonstrated a robust induction of CXCL10 messages in the glioma microenvironment in mice treated with the combination regimen, but not in mice treated by poly-ICLC or GAA vaccines alone (Fig. 1b). Our results in Fig. 2 demonstrated a critical role of CXCL10 in the homing of Tc1 into CNS tumors. These results confirm our previous observations that mAb-mediated depletion of CXCL10 diminishes the infiltration of Tc1 cells to CNS tumor lesions [16, 17]. Type-1 memory/activated T cells express the CXCR3 chemokine receptor, which binds its ligands and allows activated Type-1 T cells to migrate in response to ligand gradients [25, 26]. Although there are at least three CXCR3 ligands, CXCL9, 10 and 11 [27], it is becoming clear that all three exhibit unique expression patterns in vivo. In the CNS, in addition to our own study, Ab-neutralization of CXCL10 has been shown to block the recruitment of autoimmune effector T cells into the CNS in EAE [28] and murine viral-induced neurologic disease [22]. Interestingly, anti-CXCL9 treatment has little effect on disease severity, suggesting that of these two CXCR3 ligands, IP-10 may play a more dominant role in recruiting Type-1 “Ag experienced” T cells into the CNS.
We also attempted to identify populations of cells in the glioma tissue that produce CXCL10 by combining ISH and immunohistochemistry on F4/80+ cells. Our results suggested that populations of both F4/80 positive (microglia and macrophages) and negative (tumor and other stromal cells) cells express high levels of CXCL10 following treatment with vaccines and poly-ICLC (data not shown). These observations support results from previous studies by us and others showing that poly-IC efficiently induces pro-inflammatory cytokines and chemokines, including CXCL10 from glioma cells [11], astrocytes and microglia [19–21].
The robust induction of CXCL10 depended upon the intact type-1 and type-2 IFN pathways of the host (Figs. 3, 4). Furthermore, IFN-γ produced by antigen-specific T cells was found to be critical for their efficient infiltration into the glioma lesion (Fig. 4). In our study, IFN-γ−/−/pmel-1-derived Tc1 cells were able to proliferate and develop into effector cells based on the comparable growth and phenotype as WT cells (Supplementary Fig. S1), indicating that the observed difference is not due to different viability or phenotype of Tc1 cells derived from two strains. Intact response of IFN-γ−/− T cells was also demonstrated following infection with an attenuated strain of Listeria monocytogenes [29].
Based on available information in the literature, we postulate the following possible mechanism. First, systemic administration of poly-ICLC induces type-1 IFN production [11], which activates DCs [30] NK cells [31], as well as vaccine-primed T cells [32] and their survival [32]. Second, the activated DCs further promote IFN-γ production by T cells, thereby enhancing CXCL10 production, especially in the tumor microenvironment. Such local IFN-γ production, in turn, would also induce more CXCL10 production. Thus, this combination treatment strategy offers an IFN-γ-driven positive-feedback loop of the type-1 polarization process in the tumor microenvironment. Although human CD8+ T cells express functional TLR3 [33], it remains controversial as to whether mouse T cells also express TLR3 [1, 34].
The role of the IFN-γ-driven positive-feedback loop may be more significant in CNS tumors than in tumors of other locations as a previous study by Overwijk et al. [35] has demonstrated that vaccine-activated Pmel/IFN-γ−/− mouse-derived CD8+ T cells are able to mediate anti-tumor responses by efficiently trafficking to the subcutaneous tumor lesion in contrast to our study with CNS tumors.
While our previous studies utilized genetic delivery of IFN-α for induction of CXCL10 in the CNS tumor environment [16], our data shown here indicate that the relevant, underlying “prime-boost” regimen can also be achieved in a more clinically feasible manner via administration of a “natural” inducer of IFN-α, poly-ICLC in combination with vaccines. While our current study employed systemic (i.m.) administration of poly-ICLC, it would also be intriguing to evaluate the effect of direct poly-ICLC administration to the i.c. tumor site [34]. With regard to clinically relevant settings, a recent study has demonstrated that poly-IC promotes expansion of DCs and anti-tumor vaccine effects in vivo following cyclophosphamide-induced lymphopenia [36].
Treatment of mice bearing established GL261 glioma in the brain with the combination regimen resulted in a significant improvement of survival compared to mice treated with GAA vaccine alone or poly-ICLC alone (Fig. 1a). However, the therapeutic outcomes are still suboptimal despite the combination regimen induced potent type-1 CTL responses and robust infiltration of the effector cells into the CNS glioma [11]. What are counteracting mechanisms that limit the efficacy of this regimen?—One may be induction of Indoleamine 2,3 dioxygenase (IDO). IFN-α and T cell-derived IFN-γ can induce expression of IDO by non-T cells as the rate-limiting enzyme for tryptophan catabolism-mediated immune regulation [37, 38] which inhibits cell proliferation due to tryptophan depletion by this enzyme [39] and promotes cancer-immune escape by inhibiting the functions of and inducing apoptotic death of anti-tumor T cells within the tumor microenvironment [40]. Human glioma cells produce IDO, and IFN-γ enhances IDO expression by glioma cells [41]. Indeed, we have observed that the combination regimen induces IDO expression in the CNS GL261 glioma microenvironment (Supplementary Fig. S2). Based on these studies, we postulate that IDO may contribute to the negative regulation of treatment-induced anti-glioma T cell responses and that specific inhibition of IDO may further improve the efficacy of the current therapeutic approach [40].
Induction of inducible nitric oxide synthase (iNOS) may represent an important counteractive mechanism because IFN-γ-induced iNOS inhibits anti-tumor CTL responses [42] and glioma-infiltrating APCs [43]. Hence, it is possible that poly-ICLC administration also induces iNOS expression in tumor cells directly through the TLR3, RIG-I or MDA5 pathway and/or through induction of IFNs [44, 45]. The iNOS-mediated local T cell suppression in the tumor site has been documented in human prostate cancers [46]. Evaluation of iNOS-induced suppression is warranted in our future studies.
Thus far, our studies have demonstrated that the combination of GAA vaccines and poly-ICLC promotes type-1 immunological environment within the CNS gliomas, represented by high levels of local IFN-γ [11] and CXCL10. It is this polarized host type-1 status that is responsible for the observed anti-tumor efficacy. The significance of type-1 T cell infiltration in humans has been demonstrated in colorectal cancers [47]. In this referenced study, there was a positive correlation between the frequencies of type-1 helper T (Th1) cells, cytotoxic and memory T cells and a low incidence of tumor recurrence; and such immunological readouts were demonstrated to be better predictors of patient survival than the histopathological methods.
Based on the current study and past studies discussed above, it is likely that patients with gliomas would benefit from promotion of type-1 tumor microenvironment, which would critically depend upon the ability of vaccine-induced T cells to produce IFN-γ and intact host type-1 IFN-signaling. Indeed, in a pilot clinical trial evaluating the safety and efficacy of poly-ICLC as a single agent in patients with malignant glioma, positive clinical response was associated with activation of 2′,5′-oligoadenylate synthetase, as a mediator of type-1 IFN pathway [48]. Development of clinical vaccine studies with poly-ICLC should incorporate adequate monitoring of type-1 and type-2 IFN responses.
Materials and methods
Reagents
The following reagents were purchased: RPMI 1640, FBS, l-glutamine, sodium pyruvate, β-mercaptoethanol, nonessential amino acids, and antibiotics from Invitrogen Life Technologies (Grand Island, NY, USA); recombinant human (rh) interleukin-2 (rhIL-2) from PeproTech (Rocky Hill, NJ, USA); Recombinant mouse (rm) IL-12 from Cell Sciences (Canton, MA, USA); rhIL-2 (rhIL-2) from Peprotech (Rocky Hill, NJ, USA); rm IFN-α from R&D Systems (Minneapolis, MN, USA); rmIFN-γ from PeproTech (Rocky Hill, NJ, USA). Poly-ICLC was kindly provided by Oncovir, Inc. (Washington, D.C.). The following peptides were synthesized by the automated solid-phase peptide synthesizer in University of Pittsburgh Peptide Synthesis Facility: H-2Db-binding mEphA2671–679 (FSHHNIIRL), H-2Db–binding mGARC-177–85 (AALLNKLYA) [49]., H-2Db-binding human gp100 (hgp100)25–33 (KVPRNQDWL), H-2Kb-binding mEphA2682–689 (VVSKYKPM), H-2Kb-binding mTRP2180–188 (SVYDFFVWL) and I-Ab-binding HBV core128–140 (TPPAYRPPNAPIL).
Cell culture
GL261 mouse glioma cell line (H-2b) was kindly provided by Dr. Robert Prins (University of California Los Angeles, LA, USA); GL261 cells express mTRP2, mgp100, mEphA2, and GARC-1 as GAAs [49–51]. All cells were maintained in mouse complete medium (RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, and 10 μM l-glutamine) in a humidified incubator in 5% CO2 at 37°C.
Animals
We obtained WT C57BL/6 mice (H-2 b) from Taconic Farms (Germantown, NY, USA), IFNαR1 −/− mice (H-2 b) from Dr. Murali-Krishna Kaja (University of Washington Seattle), pmel-1 [52] and IFN-γ −/− mice (H-2 b) from The Jackson Laboratory (Bar Harbor, ME, USA). For generation of IFN-γ −/− /pmel-1 mice, heterozygous IFN-γ +/− /pmel-1 F1 mice produced by crossing homozygous female Pmel-1 and male IFN-γ −/− mice were intercrossed to generate IFN-γ −/− /pmel-1 F2 mice. IFN-γ −/− /pmel-1 genotype was screened by PCR of tail DNA. An aliquot of the genomic DNA was amplified in a PCR using primers binding to the mutant IFN-γ gene (MUT, 5′-TCA GCG CAG GGG CGC CCG GTT CTT T-3′; MUT, 5′-ATC GAC AAG ACC GGC TTC CAT CCG-3′) (320 bp product) and the WT IFN-γ gene (WT, 5′-AGA AGT AAG TGG AAG GGC CCA GAA G-3′; WT, 5′-AGG GAA ACT GGG AGA GGA GAA ATA T-3′) (260 bp product). PCR conditions were as follows: one step at 94°C for 3 min; then 12 cycles of 94°C for 20 s, 64°C for 30 s, and 72°C for 35 s; thereafter 25 cycles of 94°C for 20 s, 58°C for 30 s, and 72°C for 35 s. The final step was at 72°C for 2 min. An aliquot of the genomic DNA was also amplified in a PCR using primers for H2-Db-restricted Vα1Vβ13 TCR recognizing hgp100 (25–33): Vα1 chain gene (TCRα, 5′-GGT CCT GTG GCT CCA GTT TAA T-3′; TCRα, 5′-CTG CTT AAC CTG TCC CTC ATG T-3′) (700 bp product) and TCR Vβ13chain gene (TCRβ, 5′-CTG GGC AGT GTT CTG TCT CC-3′; TCRβ, 5′-ACC ATG GTC ATC CAA CAC AG-3′) (600 bp product). PCR conditions were as follows: one step at 94°C for 3 min; then 35 cycles of 94°C for 30 s, 61°C for 1 min, and 72°C for 2 min. The final step was at 72°C for 2 min. Animals were handled in the Animal Facility at the University of Pittsburgh per an Institutional Animal Care and Use Committee-approved protocol.
Antibodies and tetramers
Hamster anti-mouse CXCL10-neutralizing mAb (clone#1F11) was kindly provided by Dr. Andrew D. Luster in Harvard Medical School. Tri-color (TC) conjugated anti-CD3, fluorescein isothiocyanate (FITC) conjugated anti-CD8α, phycoerythrin (PE) conjugated anti-CD8α, TC conjugated anti-CD8, PE-conjugated H-2Db/hgp10025–33 tetramer was produced by the National Institute of Allergy and Infectious Disease tetramer facility within the Emory University Vaccine Center (Atlanta, GA, USA).
Flow cytometry
The procedure used in the current study has been described previously [16]. Briefly, single cell suspensions were surface-stained with fluorescent dye-conjugated antibodies. For cytometric analyses of BILs, because of the small number of BILs obtained per mouse (1.0–4.0 × 105 cells/mouse), BILs obtained from five mice per group were pooled and examined by Coulter EPICS cytometer (Beckman Coulter, Fullerton, CA, USA).
Intracranial injection of GL261 glioma cells
The procedure used in the current study has been described previously [11, 12, 16, 18]. Briefly, using a Hamilton syringe (Hamilton Company, Reno, NV, USA), 1 × 105 GL261 cells in 2 μl PBS were stereotactically injected through an entry site at the bregma, 3 mm to the right of sagittal suture, and 3 mm below the surface of the skull of anesthetized mice by using a stereotactic frame (Kopf, Tujunga, CA, USA).
Treatment of intracranial (i.c.) tumor-bearing mice with s.c. vaccination and i.m. poly-ICLC
The animals received s.c. vaccinations with 100 μg of HBV core128–140 and GAA peptides, 100 μg each of hgp10025–33, mEphA2682–689, mEphA2671–679, mTRP2180–188, and mGARC-177–85, emulsified in incomplete Freund Adjuvant (IFA) (Difco Laboratories, Detroit, Michigan, MI, USA) on days 2, 12 and 22 after tumor inoculation. Negative control mock vaccines consisted of 100 μg of HBV core128–140 but without GAA peptides emulsified in IFA. Poly-ICLC (20 μg/injection in 20 μl) or mock control PBS (20 μl) was i.m. injected twice a week starting on day 2 till day 30 after tumor inoculation. All animals were monitored daily after treatment. In some experiments, symptom-free survival was monitored as the primary endpoint; in other experiments, treated mice were sacrificed on indicated days to evaluate immunological endpoints such as BILs.
Generation of Tc1
Tc1 cells were generated by the methods we described previously with slight modifications [17, 18]. In brief, CD8+ cells were enriched from IFN-γ +/+ /pmel-1 or IFN-γ −/− /pmel-1 mouse-derived splenocytes (SPCs) using CD8-microbeads (Miltenyi Biotec, Auburn, CA, USA), and stimulated with the hgp25–33 peptide (5 μg/ml) in the presence of irradiated (3,000 rad) C57BL/6 SPCs as feeder cells, 2 ng/ml rmIL-12, 1 ng/ml anti-IL-4 mAb, and 100 units/ml rhIL-2. Cells were restimulated under the same conditions at 48 h after the initial stimulation, and were harvested between days 5 and 8.
BIL isolation
BILs were isolated using methods described previously [11, 12]. Briefly, mice were sacrificed by CO2 asphyxia and cervical dislocation, and immediately perfused with PBS through the left cardiac ventricle. Brain tissues were mechanically minced, resuspended in 70% Percoll (Sigma–Aldrich), overlaid with 37 and 30% Percoll, and centrifuged for 20 min at 500×g. Enriched BIL populations were recovered at 70–37% Percoll interface. Due to the small number of BILs obtained per mouse, BILs obtained from all mice in a given group (5 mice/group) were pooled and then evaluated for the relative number and phenotype of the BILs between groups.
In situ hybridization (ISH)
ISH for CXCL10 was conducted following the method reported previously [53]. Briefly, brain tissues were dissected and fixed in 4% PFA for 24 h at 4°C, then dehydrated and embedded in OCT (4583; Sakura Finetek) and frozen in isopentane cooled on dry ice, then cut into 10 μm sagittal sections. Sections were further post-fixed with 4% PFA, microwaved in 0.01 M sodium citrate (pH 6.0), acetylated with acetic anhydride, and dehydrated in graded ethanols. 35S-labeled sense or antisense murine CXCL10 probes were hybridized to the tissue at 50°C overnight. After stringent washing including digestion of unhybridized probe with RNases A and T1, slides were washed in saline-sodium citrate buffer. Slides were dipped in Kodak NTB-2 autoradiographic emulsion, exposed at 4°C for 21 days, and developed.
Statistical analysis
The statistical significance of differences between groups was determined by one-way analysis of variance (ANOVA). Survival data were analyzed by log rank test. We considered differences significant when p < 0.05. All data were analyzed by software GraphPad Prism 4.01.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We appreciate generous funding from Musella Foundation, Pittsburgh Foundation as well as the following funds from the National Institute of Health (NIH): 1R01NS055140 (Hideho Okada), 1P01CA100327 (Hideho Okada), 2P01NS40923 (Hideho Okada) and 1P01 CA132714 (Pawel Kalinski, Todd A. Reinhart and Hideho Okada).
Abbreviations
- BIL
Brain infiltrating lymphocyte
- CNS
Central nervous system
- mAb
Monoclonal antibody
- GAA
Glioma-associated antigen
- Tc1
CTL with type-1 phenotype
References
- 1.Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P. Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin Cancer Res. 2007;13:4565–4574. doi: 10.1158/1078-0432.CCR-07-0274. [DOI] [PubMed] [Google Scholar]
- 2.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28:220–228. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 3.McBride S, Hoebe K, Georgel P, Janssen E. Cell-associated double-stranded RNA enhances antitumor activity through the production of type I IFN. J Immunol. 2006;177:6122–6128. doi: 10.4049/jimmunol.177.9.6122. [DOI] [PubMed] [Google Scholar]
- 4.Ngoi SM, Tovey MG, Vella AT. Targeting poly(I:C) to the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFN-alpha/beta. J Immunol. 2008;181:7670–7680. doi: 10.4049/jimmunol.181.11.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem ML, El-Naggar SA, Kadima A, Gillanders WE, Cole DJ. The adjuvant effects of the toll-like receptor 3 ligand polyinosinic-cytidylic acid poly (I:C) on antigen-specific CD8+ T cell responses are partially dependent on NK cells with the induction of a beneficial cytokine milieu. Vaccine. 2006;24:5119–5132. doi: 10.1016/j.vaccine.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Yoneyama M, Kikuchi M, Matsumoto K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 7.Trumpfheller C, Caskey M, Nchinda G, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Town T, Jeng D, Alexopoulou L, Tan J, Flavell RA. Microglia recognize double-stranded RNA via TLR3. J Immunol. 2006;176:3804–3812. doi: 10.4049/jimmunol.176.6.3804. [DOI] [PubMed] [Google Scholar]
- 9.Gitlin L, Barchet W, Gilfillan S, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X, Nishimura F, Sasaki K, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki K, Zhu X, Vasquez C, et al. Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Res. 2007;67:6451–6458. doi: 10.1158/0008-5472.CAN-06-3280. [DOI] [PubMed] [Google Scholar]
- 13.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 14.Weber C, Alon R, Moser B, Springer TA. Sequential regulation of alpha 4 beta 1 and alpha 5 beta 1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd AR, Oppenheim JJ, Kelvin DJ, Taub DD. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156:932–938. [PubMed] [Google Scholar]
- 16.Nishimura F, Dusak JE, Eguchi J, et al. Adoptive transfer of Type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Res. 2006;66:4478–4487. doi: 10.1158/0008-5472.CAN-05-3825. [DOI] [PubMed] [Google Scholar]
- 17.Fujita M, Zhu X, Ueda R, et al. Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells—significant roles of CXCL10. Cancer Res. 2009;69:1587–1595. doi: 10.1158/0008-5472.CAN-08-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita M, Zhu X, Sasaki K, et al. Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol. 2008;180:2089–2098. doi: 10.4049/jimmunol.180.4.2089. [DOI] [PubMed] [Google Scholar]
- 19.Park C, Lee S, Cho IH, et al. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. GLIA. 2006;53:248–256. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- 20.Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Jack CS, Arbour N, Manusow J, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 22.Liu MT, Chen BP, Oertel P, et al. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol. 2000;165:2327–2330. doi: 10.4049/jimmunol.165.5.2327. [DOI] [PubMed] [Google Scholar]
- 23.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 24.Ogasawara K, Hida S, Weng Y, et al. Requirement of the IFN-alpha/beta-induced CXCR3 chemokine signalling for CD8+ T cell activation. Genes Cells. 2002;7:309–320. doi: 10.1046/j.1365-2443.2002.00515.x. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima C, Mukai T, Yamaguchi N, et al. Induction of the chemokine receptor CXCR3 on TCR-stimulated T cells: dependence on the release from persistent TCR-triggering and requirement for IFN-gamma stimulation. Eur J Immunol. 2002;32:1792–1801. doi: 10.1002/1521-4141(200206)32:6<1792::AID-IMMU1792>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Lazzeri E, Romagnani P. CXCR3-binding chemokines: novel multifunctional therapeutic targets. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:109–118. doi: 10.2174/1568008053174723. [DOI] [PubMed] [Google Scholar]
- 28.Fife BT, Kennedy KJ, Paniagua MC, et al. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2001;166:7617–7624. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- 29.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma 1. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 30.Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 31.Mailliard RB, Son YI, Redlinger R, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 32.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabiasco J, Devevre E, Rufer N, et al. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177:8708–8713. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 34.Salem ML, Diaz-Montero CM, El-Naggar SA, Chen Y, Moussa O, Cole DJ. The TLR3 agonist poly(I:C) targets CD8+ T cells and augments their antigen-specific responses upon their adoptive transfer into naive recipient mice. Vaccine. 2009;27:549–557. doi: 10.1016/j.vaccine.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Overwijk WW, de Visser KE, Tirion FH, et al. Immunological and antitumor effects of IL-23 as a cancer vaccine adjuvant. J Immunol. 2006;176:5213–5222. doi: 10.4049/jimmunol.176.9.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salem ML, az-Montero CM, Al-Khami AA, et al. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J Immunol. 2009;182:2030–2040. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widner B, Fuchs D. Immune activation and degradation of tryptophan. Mod Asp Immunobiol. 2000;1:105–108. [Google Scholar]
- 38.Byrne GI, Lehmann LK, Kirschbaum JG, Borden EC, Lee CM, Brown RR. Induction of tryptophan degradation in vitro and in vivo: a gamma-interferon-stimulated activity. J Interferon Res. 1986;6:389–396. doi: 10.1089/jir.1986.6.389. [DOI] [PubMed] [Google Scholar]
- 39.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 40.Van den EB. A new mechanism of tumor resistance to the immune system, based on tryptophan breakdown by indoleamine 2, 3-dioxygenase. Bull Mem Acad R Med Belg. 2003;158:356–363. [PubMed] [Google Scholar]
- 41.Grant R, Kapoor V. Inhibition of indoleamine 2, 3-dioxygenase activity in IFN-gamma stimulated astroglioma cells decreases intracellular NAD levels. Biochem Pharmacol. 2003;66:1033–1036. doi: 10.1016/S0006-2952(03)00464-7. [DOI] [PubMed] [Google Scholar]
- 42.Nishioka Y, Wen H, Mitani K, et al. Differential effects of IL-12 on the generation of alloreactive CTL mediated by murine and human dendritic cells: a critical role for nitric oxide. J Leukoc Biol. 2003;73:621–629. doi: 10.1189/jlb.0402205. [DOI] [PubMed] [Google Scholar]
- 43.Yang T, Witham TF, Villa L, et al. Glioma-associated hyaluronan induces apoptosis in dendritic cells via inducible nitric oxide synthase: implications for the use of dendritic cells for therapy of gliomas. Cancer Res. 2002;62:2583–2591. [PubMed] [Google Scholar]
- 44.Utaisincharoen P, Anuntagool N, Arjcharoen S, Limposuwan K, Chaisuriya P, Sirisinha S. Induction of iNOS expression and antimicrobial activity by interferon (IFN)-beta is distinct from IFN-gamma in Burkholderia pseudomallei-infected mouse macrophages. Clin Exp Immunol. 2004;136:277–283. doi: 10.1111/j.1365-2249.2004.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utaisincharoen P, Anuntagool N, Limposuwan K, Chaisuriya P, Sirisinha S. Involvement of beta interferon in enhancing inducible nitric oxide synthase production and antimicrobial activity of Burkholderia pseudomallei-infected macrophages. Infect Immun. 2003;71:3053–3057. doi: 10.1128/IAI.71.6.3053-3057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bronte V, Kasic T, Gri G, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 48.Salazar AM, Levy HB, Ondra S, et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery. 1996;38:1096–1103. doi: 10.1097/00006123-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Iizuka Y, Kojima H, Kobata T, Kawase T, Kawakami Y, Toda M. Identification of a glioma antigen, GARC-1, using cytotoxic T lymphocytes induced by HSV cancer vaccine. Int J Cancer. 2006;118:942–949. doi: 10.1002/ijc.21432. [DOI] [PubMed] [Google Scholar]
- 50.Hatano M, Kuwashima N, Tatsumi T, et al. Vaccination with EphA2-derived T cell-epitopes promotes immunity against both EphA2-expressing and EphA2-negative tumors. J Transl Med. 2004;2:40. doi: 10.1186/1479-5876-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prins RM, Odesa SK, Liau LM. Immunotherapeutic targeting of shared melanoma-associated antigens in a murine glioma model. Cancer Res. 2003;63:8487–8491. [PubMed] [Google Scholar]
- 52.Overwijk WW, Tsung A, Irvine KR, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fallert BA, Reinhart TA. Improved detection of simian immunodeficiency virus RNA by in situ hybridization in fixed tissue sections: combined effects of temperatures for tissue fixation and probe hybridization. J Virol Methods. 2002;99:23–32. doi: 10.1016/S0166-0934(01)00378-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.