Abstract
The goal of the present study was to investigate hepato-protective effects of growth factor (GF) arrays during alcohol injury. Hepatocyte growth factor (HGF) and bone morphogenetic protein (BMP)7 were mixed with collagen (I) and robotically printed onto standard glass slides to create arrays of 500 μm diameter spots. Primary rat hepatocytes were seeded on top of the arrays forming clusters corresponding in size to the underlying protein spots. Cell arrays were then injured in culture by exposure to 100 mM ethanol for 48h. Hepatocytes residing on GF spots were found to have less apoptosis then cells cultured on collagen-only spots. Least apoptosis (0.3 % as estimated by TUNEL assay) was observed on HGF/BMP7/collagen spots whereas most apoptosis (17.3%) was seen on collagen-only arrays. Interestingly, the extent of alcohol-induced apoptosis in hepatocytes varied based on the concentration of printed GF. In addition to preventing apoptosis, printed GFs contributed to maintenance of epithelial phenotype during alcohol injury as evidenced by higher levels of E-cadherin expression in HGF-protected hepatocytes. Importantly, GF microarrays could be used to investigate heterotypic interactions in the context of liver injury. To highlight this, stellate cells - nonparenchymal liver cells involved in fibrosis - were added to hepatocytes residing on arrays of either HGF/collagen or collagen-only spots. Exposure of these cocultures to ethanol followed by RT-PCR analysis revealed that stellate cells residing alongside HGF-protected hepatocytes were significantly less activated (less fibrotic) compared to controls. Overall, our results demonstrate that GF microarray format can be used to screen anti-fibrotic and anti-apoptotic effects of growth factors as well as to investigate how signals delivered to a specific cell type modulate heterotypic cellular interactions.
Keywords: Protein microarrays, Micropatterned cocultures, Hepatocytes, Growth factor microarrays, Apoptosis, Fibrosis, Ethanol injury
Introduction
Alcohol abuse is a prevalent etiological factor of fibrosis and cirrhosis of the liver. Ethanol injury leads to apoptosis of hepatocytes and to an increase in production of fibrogenic cytokines such as transforming growth factor (TGF)-β1 [1]. The pathogenesis of alcohol injury is complex, involving several signal transduction pathways and several liver cell types. Stellate cells - nonparecnhymal cells in the liver - become activated during liver injury by alcohol and initiate programs of TGF-β production and extracellular matrix (ECM) remodeling that contribute to apoptosis of hepatocytes [1-3]. TGF-β has also been shown to induce de-differentiation and epithelial-mesenchymal transition (EMT) in hepatocytes [4, 5].
Identifying TGF- β antagonists is an important strategy in preventing fibrosis of the liver as well as other tissues. Hepatocyte growth factor (HGF) is a signaling molecule central to liver development and regeneration [6] and that has been reported to antagonize TGF-β and prevent fibrosis [7-9]. BMP7 is another important growth factor that has been shown to counteract pro-fibrogenic effects of TGF- β1 [10, 11].
Several studies have reported that systemic administration of HGF in laboratory animals provided protection against liver injury[12, 13]. However, these studies were only able to compare two or three concentrations of HGF because of the cost of animals and reagents. Determining optimal concentration is critical for both HGF, which has been implicated in cancer metastasis [14, 15] and BMP7, which has been shown to induce apoptosis at higher concentrations [16]. While it is beneficial to develop in vitro assays to determine liver protective concentrations and combinations of GFs, traditional cell culture approaches of adding soluble GFs to culture media require large amounts of expensive growth factors and are suboptimal for high-throughput screening.
Increasingly, the attention has turned to surface immobilization and solid-phase presentation of GFs to cells [17-20]. A number of studies reported covalent immobilization of GFs on cell culture surfaces [18, 21, 22]. However, in vivo, GF molecules interact with components of extracellular matrix (ECM) via secondary bonds and are dynamically released upon cell-initiated proteolysis. Moreover, association with ECM components has been shown to stabilize and enhance stimulatory effects of GFs [17, 23]. Recently, we described the use of HGF/ECM protein arrays for cultivating primary rat hepatocytes [24] where HGF was mixed in solution with ECM proteins without chemical modification of GF molecules and was printed in an array format onto glass slides. Primary rat hepatocytes cultured on HGF/ECM protein spots were found to maintain differentiated hepatic phenotype for ten days at levels comparable to or better than hepatocytes receiving daily doses of soluble HGF in the media [24]. Experiments with printed HGF/ECM arrays required 200 times less of the expensive growth factor.
The present study builds on the concept of GF arrays to explore how hepatocytes residing on HGF and BMP7 arrays are impacted by exposure to ethanol – a model of acute alcohol injury. While protective effects of soluble HGF and BMP7 in the context of in vitro and in vivo liver injury have been reported [8, 25, 26], these effects have not been studied for solid-phase presented GFs. The microarray format has shown utility for a range of cell-surface interaction studies [27-32] and this paper highlights a new application of arrays for screening protective effects of GFs or other signaling molecule during cell injury. A combination of localized GF signaling and micropatterned cocultures described in this study may also be used to investigate heterotypic paracrine signaling underlying fibrosis.
Materials and Methods
Chemicals and Materials
Glass slides (75 × 25 mm2) were obtained from VWR (West Chester, PA). (3-acryloxypropyl)trichlorosilane was purchased from Gelest, Inc. (Morrisville, PA). Sulfuric acid, hydrogen peroxide, ethanol, collagenase, collagen from rat tail (type I), streptavidin-conjugated Alexa 546, AlexaFluor 488 anti-mouse IgG, hepatocyte growth factor (HGF), bone morphogenetic protein 7 (BMP7), and transforming growth factor-β1 (TGF-β1) were obtained from Sigma–Aldrich (St. Louis, MO). Mouse anti-E-cadherin antibody was purchased from BD Biosciences. Concentrated phosphate-buffered saline (10× PBS) was purchased from Lonza (Walkersville, MD). Minimal essential medium (MEM), sodium pyruvate, nonessential amino acids, fetal bovine serum (FBS), Superscript III, RNaseOut (RNase inhibitor), dNTPs and biotinylated anti-HGF antibodies were purchased from Invitrogen (Carlsbad, CA). 384-well polypropylene microarray plates were obtained from Genetix (New Milton, Hampshire). Goat anti-rat cross-adsorbed albumin antibody was obtained from Bethyl Laboratories (Montgomery, TX). Formalin was purchased from Fisher (Pittsburgh, PA). ApopTag Red in situ Apoptosis Detection kit was obtained from Chemicon (Temecula, CA). DAPI stain mounting media was purchased from Vectorshield (Burlingame, CA).
Animals
Adult female Lewis rats weighing 125–200 g were purchased from Charles River Laboratories (Boston, MA) and fed with a commercial diet and water. All animal experiments were performed according to the National Institutes of Health (NIH) guidelines for the ethical care and use of laboratory animals and the experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Davis.
Printing collagen and GF arrays
Glass slides were modified with acryloxypropyl trichlorosilane using protocols described previously [33]. Collagen was dissolved in 1× PBS + 0.005% Tween-20 at 0.2 mg/mL concentration. GFs were mixed with the ECM solution to the desired final concentration of 50, 125, 250 or 500 ng/mL and allowed to bind to the ECM protein for 30 min at room temperature prior to printing. Protein microarrays were contact-printed under ambient conditions on silane-modified 75 × 25 mm2 glass slides using a hand-held MicroCaster. The pins collected protein from a 382-well plate, dispensing 20–70 nL of solution onto the glass slide and forming circular spots ∼500 μm in diameter. Pins were cleaned in acetone via sonication for 5 min and subsequently washed with pin-cleaning solution (DI water and isopropanol) between each change in growth factor. Pins were dried with nitrogen before protein printing. Protein arrays were kept in a refrigerator prior to cell cultivation.
Characterization of HGF retention on collagen arrays
Immunofluorescent staining was used to determine retention of HGF on printed protein arrays. Solution containing 100, 250 and 500 ng/mL HGF and 0.2 mg/mL collagen (I) was printed onto silanized glass slides and the resultant microarrays were incubated for 2 h in cell culture media at 37 °C. Substrates with microarrays were then removed from media and incubated with 1 μg/ml (in 1× PBS) of anti-human HGF-biotin conjugate at 37° for 2 h followed by incubation in 10 μg/ml of streptavidin-Alexa 546 for 1 h at room temperature. Samples were washed between each staining step with 1× PBS + 0.05% Tween-20. In order to quantify fluorescence signal emanating from the array, the laser microarray scanner (Agilent G2565BA fluorescent scanner) was employed to scan the glass slides at a spot pixel resolution of 5 μm. The fluorescence intensity of each array element was determined using GenePix Pro 6.0 data analysis software (Molecular Devices, Downingtown, PA).
Cultivation of primary hepatocytes on GF microarrays
Primary hepatocytes were isolated from adult female Lewis rats using a standard two-step collagenase perfusion procedure[34]. Typically, 100–200 million hepatocytes were obtained with viability >90% as determined by trypan blue exclusion. Primary hepatocytes were maintained in DMEM supplemented with epidermal growth factor (EGF), glucagon, hydrocortisone sodium succinate, recombinant human insulin, 200 units/mL penicillin, 200 μg/mL streptomycin and 10% FBS.
For cell seeding experiments, a glass slide containing printed arrays of ECM + GF was cut into 1 in. × 1 in. squares to fit into a 6-well plate. The design of different GF arrays tested in our studies is shown in Figure 1. Typically GFs were printed into arrays of 500 μm diameter spots where different rows contained different concentration of GFs. The distance between rows was 250 μm and the center-to-center spacing of the spots was 1 mm. HGF, BMP7 and TGF-β arrays were constructed in this manner. Hepatocytes were seeded to form cellular arrays using protocols described earlier [35]. In brief, glass slides containing printed ECM/GF spots were first exposed to 3 mL of hepatocytes suspended in culture medium at a concentration of 1 × 106 cells/mL. After 1 h of incubation at 37 °C, hepatocytes bound on ECM/GF domains, but did not attach on the surrounding silane-modified surface. The samples were then washed twice in PBS to remove unbound hepatocytes and fresh media was added to the sample well.
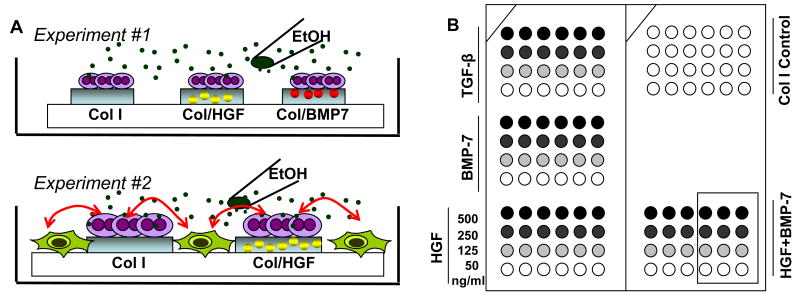
Figure 1.
Schematic description of studies carried out in this paper. (A) In Experiment 1, primary rat hepatocytes cultured on printed arrays of collagen I (Col) or Col/GF mixture were insulted with alcohol. Apoptosis and fibrogenic signals in hepatocytes residing on protein spots were assessed. In Experiment 2, stellate cells (green) were added to clusters of hepatocytes creating micropatterned cocultures. Activation of stellate cells as a function of protective GF signals delivered to neighboring hepatocytes was assessed. (B) The layout of printed GF arrays. Different concentrations of GFs were mixed in solution with Col and printed on the surface. HGF and BMP7 were tested for hepato-protective effects whereas TGF-β was printed as a pro-apoptotic positive control.
Alcohol injury of primary hepatocytes
In our experiments, hepatocytes cultured on GF/collagen microarrays were compared with cells cultured on collagen (I) arrays without GF (see Figure 1 for description of experiments). Cells were allowed to recover after isolation for 1 day and were then exposed to 100 mM ethanol (6 μL/mL). This concentration of ethanol was chosen to mimic acute alcohol liver injury.(ref) Hepatocytes were kept in ethanol-containing culture media for 48 h. To analyze alcohol-induced apoptosis we employed TUNEL (TdT-mediated dUTP nick end labeling) assay using ApopTag Red in situ Apoptosis Detection kit (Chemicon, Temecula, CA) according to the manufacturer's instructions. DAPI was used to stain cell nucleus. TUNEL-positive cells were scored as apoptotic and were evaluated with a confocal microscope.
RT-PCR analysis of apoptotic and fibrotic markers
For real-time RT-PCR experiments, 6 × 12 arrays of each GF condition were created and cultured in a separate culture well. Cells (∼20,000) were collected from microarrays using trypsin for 10 min at 37°. Extracted cell contents were stabilized in 100 μL of lysis buffer and stored at −20 °C. Total RNA was extracted from the cell lysates using absolute total mRNA isolation microprep kit (Stratagene) according to the manufacturer's instructions. cDNA was synthesized using QuantiTect Reverse Transcription kit (Qiagen) according to the manufacturer's instructions using 12 μL of DNAse pretreated total mRNA. Quantitative real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems). Primers for rat caspase-6, TGF-β1 and GAPDH genes were selected from a database http://medgen.ugent.be/rtprimerdb (Table 1). Primer (Sigma Genosys) concentrations were optimized before use. SYBR Green Master Mix (1×) was used with 1 μl of forward and reverse primers in a total volume of 12 μl that also included 1 μl of cDNA. All PCR reactions were done in duplicate. PCR amplification was performed as follows: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 60 °C for 10 s and 68 °C for 1 min on Mastercycler Realplex (Eppendorf). The comparative Ct value method, using housekeeping gene (GAPDH) as an internal standard, was employed to determine relative levels of caspase-6 and TGF-β1.
Table 1.
RT-PCR primers for detecting markers of hepatic apoptosis (caspase-6) and fibrosis (TGF-β1). GAPDH is a housekeeping gene.
| Gene | Sequence 5′ to 3′ | Conc/μM |
|---|---|---|
| Rat GAPDH | F: ATGATTCTACCCACGGCAAG | 1 |
| R: CTGGAAGATGGTGATGGGTT | 1 | |
| Rat Caspase-6 | F: GGCAGTTCCCTGGAGTTCAC | 1 |
| R: GACCTTCCTGTTCACCAGCG | 1 | |
| Rat TGF-β1 | F: AGGACCTGGGTTGGAAGTGG | 1 |
| R: AGTTGGCATGGTAGCCCTTG | 1 | |
Immunofluorescent staining for E-cadherin
For immunostaining, cells were fixed in 4% formalin+0.3% Triton-X 100 in PBS for 15 min. The cells were then incubated in blocking solution (1% bovine serum albumin (BSA)+0.3% Triton-X 100 in 1× PBS) for 1 h at room temperature and exposed to 1:100 diluted anti-E-cadherin antibody (in 1%BSA+0.1% Triton-X 100 in PBS) for 2 h at 37 °C. Finally, cells were incubated in 1:200 diluted anti-mouse IgG conjugated with Alexa 488 (in 1%BSA+0.1% Triton-X 100 in PBS) for visualization. Cells were washed between each step with 1× PBS three times for 5 min. Slides were mounted onto cover slips using DAPI stain mounting media. All incubations were performed at room temperature if not specified otherwsie. Stained cells were visualized and imaged using a confocal microscope (Zeiss LSM Pascal). Intensity of E-cadherin staining was quantified using ImageJ software.
Formation of micropatterned hepatocyte-stellate cell cocultures
A human stellate cell line, created by the Zern laboratory [36], was maintained in DMEM supplemented with 10% FBS, 200 units/mL penicillin, and 200 μg/mL streptomycin at 37 °C in a humidified 5% CO2 atmosphere. Cells were cultured until 90% confluence and then passaged. Construction of the cocultures was enabled by silane treatment that rendered glass regions non-adhesive to hepatocytes and adhesive to stellate cells. Hepatocytes, seeded first, attached exclusively on printed protein spots (6 × 12 array of each protein array type) and were allowed to spread out overnight. The following day stellate cells were resuspended at 0.25 × 106 cells/mL in DMEM-based media described above and were seeded on the surface containing arrays of hepatic islands. After 30 min of incubation stellate cells became adherent to the glass substrate around hepatocyte clusters. Nonadherent cells were removed from the surface and cocultures were subsequently cultured in hepatocyte medium described in the preceding section.
Alcohol injury of micropatterned cocultures and analysis of stellate cell activation
The goal of these experiments was to compare how GF signaling to the hepatocytes affected activation of neighboring stellate cells in the context of alcohol injury. Ethanol (100 mM) was added for 48 hours into culture medium bathing the cocultures. Activation of stellate cells was quantified by real-time RT-PCR analysis of collagen I, tissue inhibitor of metalloproteinases (TIMP), α-smooth muscle actin (α - SMA1), and TGF-β1 gene expression (see Table 2 for primers). House keeping gene used in these studies was β-actin In these experiments cocultures were trypsinized and nucleic acids were processed according to the protocols described in the preceding section. Importantly, we checked to make sure that primers used for human stellate cells were not cross-reactive with rat hepatocytes.
Table 2.
RT-PCR primers for detecting stellate cell activation. β-actin was used as a housekeeping gene.
| Gene | Sequence 5′ to 3′ | Conc/μM |
|---|---|---|
| Human β-actin | F: ACGGCCAGGTCATCACTATTG | 1 |
| R: ATACCCAAGAAGGAAGGCTGGA | 0.5 | |
| Human Collagen type I α1 | F: GAACGCGTGTCATCCCTTGT | 1 |
| R: GAACGAGGTAGTCTTTCAGCAACA | 1 | |
| Human TIMP | F: CTTCTGGCATCCTGTTGTTG | 1 |
| R: AGAAGGCCGTCTGTGGGT | 1 | |
| Human α -SMA1 | F: CAGCCAAGCACTGTCAGG | 1 |
| R: CCAGAGCCATTGTCACACAC | 1 | |
| Human TGF-β1 | F: CCCTGGACACCAACTATTGC | 1 |
| R: CTTCCAGCCGAGGTCCTT | 1 | |
Micropatterned cocultures were stained for albumin to assess extent of de-differentiation of hepatocytes after alcohol insut. Albumin immunostaining was carried out using the protocol similar to E-cadherin staining described above. Cell morphology and cell motility was observed daily via brightfield microscopy.
Results and Discussion
This study investigated hepato-protective effects of two growth factors HGF and BMP7 co-printed with collagen (I) in a microarray format. Hepatocytes exposed to ethanol while receiving signals bottom-up signals from GF arrays were significantly better protected against apoptosis and fibrosis compared to hepatocytes residing on collagen-only spots. Furthermore, protection of hepatocytes on GF arrays prevented activation of adjacent stellate cells in a heterotypic coculture. Our study underscores the potential of microarrays for in vitro screening of therapeutic effects of GFs and for investigating heterotypic cellular interactions underlying fibrosis.
Printing and characterization of GF microarrays
We have previously described forming cellular monocultures and cocultures on GF/ECM arrays[24]. The arrays were made using a hand-held contact arrayer printing 500 μm diameter protein spots. As shown in Figure 1, the arrays consisted of 6 by 12 members with center-to-center distance between the spots of 1 mm. In a typical experiment, four different solution concentration of a given GF were printed on a 1in. × 1in. glass substrate. Prior to printing of protein arrays, glass substrates were treated with acrylated silane. The silanization procedure enhanced the quality of the printed microarray spots by rendering the surface somewhat hydrophobic (53 ± 2° contact angle). More importantly, the silanization created moderately cell adhesive glass substrates that did not support attachment of primary hepatocytes and could be used to guide/confine attachment of these cells onto protein arrays. As described later in this paper, the silanized surfaces did permit attachment of more “adhesive” nonparenchymal cells (e.g. stellate cells) and allowed for sequential assembly of micropatterned hepatocyte–stellate cell cocultures on protein microarrays.
When creating GF microarrays, a solution of collagen was mixed with either HGF and/or BMP7 or TGF-β1 in a microtiter plate and then printed onto a silanized glass slide. To verify presence of HGF after printing, arrays were incubated under cell culture conditions for 2 h, stained with fluorescently-labeled anti-HGF and visualized with a fluorescence microscopy or a microarray scanner. Figure 2A shows fluorescence images and fluorescence intensity of arrays printed from solution with HGF concentration ranging from 100 to 500 ng/ml. As seen from these data, higher HGF solution concentration translated into higher signal of surface immobilized HGF. In our previous paper we demonstrated that ECM protein microarrays retained over 50% printed HGF molecules after five days under cell culture conditions[24]. Therefore, GF molecules were expected to be available for cell stimulation during alcohol exposure experiments that typically lasted for 48 h. While immunostaining of printed arrays of BMP7 and TGF-β was not carried, cellular responses to these arrays described in the next section clearly indicate presence of functional GF molecules on the surface.
Figure 2.
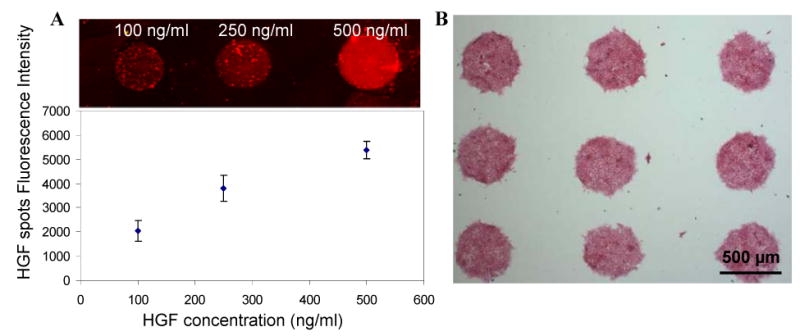
Characterization of HGF retention and cell patterning on protein microarrays. (A) Col/GF arrays printed from different solution concentrations of HGF were incubated for 2h in cell culture media at 37°C, immunofluorescently stained and visualized using a microarray scanner. Fluorescent signal due to presence of HGF on protein spots varied as a function of HGF solution concentration. (B) Primary rat hepatocytes adhered onto printed protein spots (500 μm diameter) creating cell arrays. Minimal attachment of hepatocytes was observed on silane-modified glass regions surrounding protein islands.
Quantification of hepatocyte apoptosis on GF arrays during alcohol injury
Hepatocytes were cultured on HGF- and BMP7-containing collagen (I) spots to characterize protective effects of bottom-up GF signaling during alcohol insult. As shown in Figure 2B, hepatocytes selectively attached on protein domains, each 500 μm diameter spot containing ∼250 cells. The number of cells adherent to the spots was defined by presence of collagen (I) molecules and was consistent on arrays with and without GF molecules. Cells were allowed to spread out overnight before ethanol treatment.
We investigated anti-apoptotic effects of solid-phase presented HGF and BMP7 during alcohol injury of primary rat hepatocytes in vitro. Markers for apoptosis such as internucleosome DNA degradation and caspase-6 activation were analyzed after 48h incubation of hepatocytes in 100mM ethanol. This ethanol concentration is physiologically relevant and has been used to mimic acute alcohol injury in vitro [37]. TUNEL staining was used to visualize apoptotic hepatocytes after ethanol exposure. Representative images shown in Figure 3A highlight that a significant fraction of hepatocytes cultured on collagen spots underwent apoptosis whereas majority of hepatocytes cultured on GF arrays were not apoptotic. TUNEL staining of hepatocytes residing on GF arrays of varying concentrations and combinations (see Figure 1B for experiment design) was used to quantify apoptosis as a function of the underlying substrate.
Figure 3.
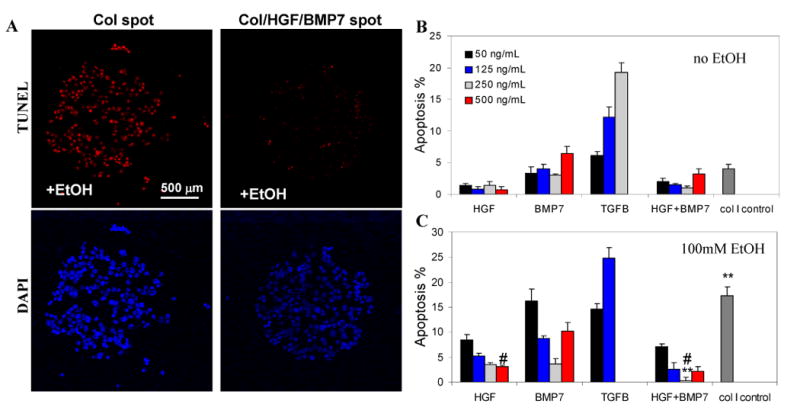
Anti-apoptotic effects of HGF- and BMP7-containing protein arrays. (A) Hepatocytes cultured on either Col or Col/GF spots were exposed to 100 mM EtOH. The extent of apoptosis was assessed using TUNEL assay (stains nucleus red). DAPI (blue) was used to stain nuclei of hepatocytes. Representative images show that alcohol exposure caused significant apoptosis in hepatocytes on Col spots (17.3%) and limited apoptosis in hepatocytes cultured on Col/HGF/BMP7 spots (0.3%). (B-C) Quantifying apoptotic hepatocytes by TUNEL assay in the absence (B) and presence (C) of alcohol. Arrays of pro-apoptotic TGF-β were used as positive control. Hepatocytes cultured on HGF or BMP7 were protected from alcohol-induced apoptosis. Anti-apoptotic effects of printed GFs could be titrated in a concentration-dependent manner. Apoptotic nuclei were counted in 6 spots per experiment and the percentage of apoptosis was calculated as apoptotic cells to total cells in spot (#p < 0.05, **p < 0.01; n = 6).
Figure 3(B,C) quantify hepatic apoptosis after alcohol injury. The purpose of experiments detailed in Figure 3B was to verify that printed GF arrays did not cause apoptosis in the absence of alcohol. These results compiled in Figure 3B show that HGF/collagen printed in varying concentrations caused only minimal hepatic apoptosis whereas higher concentration of BMP7 did result in significantly higher number of apoptotic hepatocytes (4.0% vs. 6.4% for 250 ng/ml and 500 ng/ml respectively) compared to collagen-only spots. TGF-β1 – an apoptotic and pro-fibrotic factor – was co-printed with collagen (I) to serve as a positive control. As seen from Figure 3B, TGF-β signaling promoted hepatic apoptosis in concentration-dependent fashion with 250 ng/ml solution concentration of this morphogen corresponding to 19.3% apoptosis.
The next set of experiments (Figure 3C) investigated the relationship between protein array composition and the extent of hepatic apoptosis after exposure to alcohol. These data reveal that hepatocytes cultured on collagen-only spots were susceptible to alcohol-induced apoptosis (17.3 %) whereas hepatocytes on GF spots were protected from alcohol injury. Significantly, as shown in Figure 3C, printed HGF had a dose-dependent anti-apoptotic effect with highest solution concentration of HGF (500 ng/ml) leading to lowest apoptosis (3.1% +/- 0.2). Interestingly, the most protective concentration of BMP7 was 250 ng/mL (3.6% +/- 1.0) whereas higher concentration (500 ng/mL) resulted in significantly more apoptosis (10.2% +/- 1.8). Another significant finding was that the maximal anti-apototic effect was achieved with a combination of HGF and BMP7 (0.3% +/- 0.5), an over 50-fold reduction in apoptosis compared to hepatocytes on collagen spots (17.3% apoptosis).
Findings described in Figure 3 clearly demonstrate that hepatic apoptosis observed after alcohol exposure was a strong function of printed GF concentration and could be titrated up or down based on the type and concentration of GF presented on a protein array. Another finding is that with BMP7 arrays, higher concentration was not necessarily better and that an intermediate concentration was found to be most protective against apoptosis. This observation is consistent with reports of pro-apoptotic activity of BMP7 at elevated concentrations [38]. Finally, we demonstrate that lowest levels of alcohol-induced apoptosis in hepatocytes were observed by presenting both HGF and BMP7 on collagen (I) arrays. This points to benefits of combining different GF types on microarrays. All of the findings underscore the need for screening of GF-cell interactions in order to identify optimal concentrations and combinations of GFs.
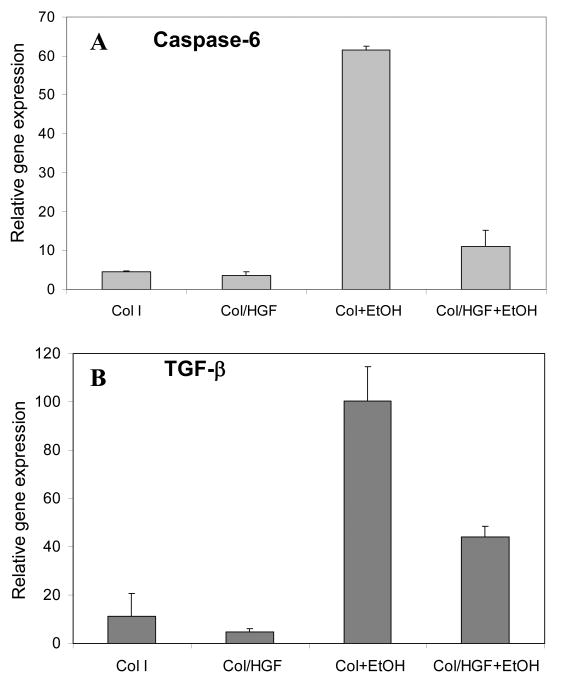
To corroborate TUNEL apoptosis results, we also carried out RT-PCR analysis of caspase-6 - a cysteine-aspartic protease that plays an essential role in apoptosis. As shown in Figure 4A, ethanol treatment induced a 13.5-fold increase in caspase-6 gene expression in hepatocytes residing on collagen spots compared to untreated cells residing on the same surface. The caspase-6 gene expression was reduced by ∼6 fold in hepatocytes residing on HGF spots during alcohol treatment. This once again supported the notion that hepatocytes cultured on top of printed GF molecules were protected against apoptosis.
Figure 4.
Quantitative real-time RT-PCR analysis of apoptotic marker caspace-6 (A) and firbogenic cytokine TGF-β1 (B) in rat hepatocytes cultured on Col and Col/GF arrays during alcohol insult. Gene expression was normalized to a housekeeping gene GAPDH. These data show that hepatocytes residing on Col/HGF spots had significantly lower expression of apoptotic and fibrotic markers.
Maintenance of epithelial phenotype in hepatocytes cultured on HGF arrays during alcohol injury
The production of fibrogenic TGF-β molecules in the liver has historically been assigned to nonparenchymal cells (e.g. activated stellate cells), however, more recently TGF-β has been detected in primary hepatocytes cultured in vitro [4]. These recent findings are part of an emerging paradigm with hepatocytes assuming a more prominent role in initiating/promoting liver fibrosis and contributing to epithelial-mesenchymal transition (EMT) during injury.[5] Given the importance of TGF-β signaling in promoting fibrosis and mesenchymal phenotype, the hepatic expression of this cytokine after alcohol injury was assessed by RT-PCR. As shown in Figure 4B, TGF-β gene expression of ethanol-treated hepatocytes on collagen spots was 9 fold higher compared to untreated hepatocytes. Hepatocytes residing on HGF/collagen spots during alcohol insult produced significantly less TGF-β transcripts (3.9-fold increase over untreated controls). These data corroborate previous reports of pro-fiborogenic cytokine production by injured hepatocytes[4] and also demonstrate anti-fibrogenic effects of solid-phase presented HGF in our system.
We hypothesized that attenuation of pro-fibrogenic and mesenchymal signaling on HGF arrays should occur concurrently with maintenance or promotion of epithelial phenotype. Expression of E-cadherin, a cell-cell adhesion molecule, is one marker of epithelial phenotype. As shown in Figure 5, immunofluorescence staining revealed considerably stronger E-cadherin signals in hepatocytes residing on HGF/collagen spots compared to collagen-only spots. Importantly, strong E-cadherin expression was observed in hepatocytes cultured on HGF arrays during exposure to ethanol. Antagonizing TGF-β expression with surface-bound HGF in our experiments delayed or prevented EMT in primary rat hepatocytes.
Figure 5.
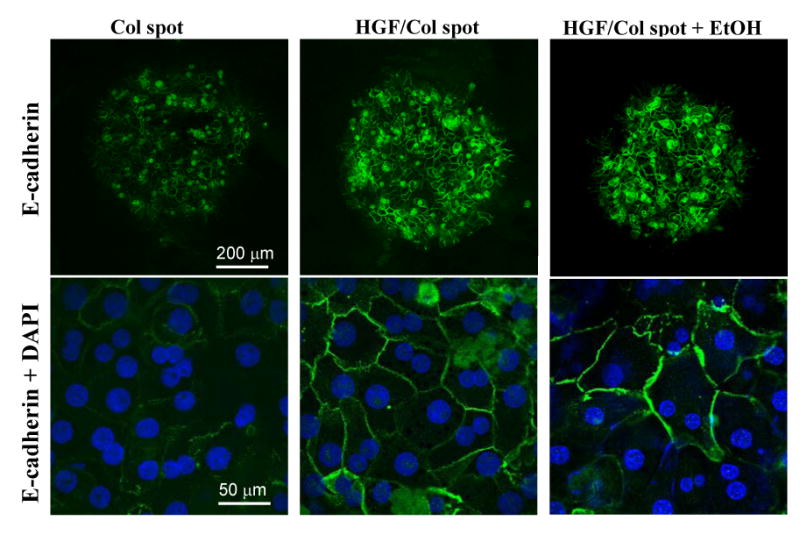
Immunofluorescent staining for E-cadherin in hepatocytes residing on Col or Col/HGF spots in the absence or presence of alcohol. These images show that E-cadherin expression was significantly stronger in hepatocytes cultured on HGF-containing protein spots and was unaffected by exposure to ethanol.
Alcohol injury of micropatterned hepatocyte-stellate cell cocultures
Chronic liver injury, such as alcohol consumption, leads to an inflammatory response that includes activation of stellate cells – mesenchymal cells of the liver. These cells are central players in fibrosis and are responsible for secreting TGF-β, depositing collagen I and other ECM proteins in the fibrotic liver [1]. However, liver is a complex organ where multiple cell types residing in close proximity may contribute to fibrosis via paracrine signaling. We were intrigued by our data suggesting TGF-β expression in injured hepatocytes and by the possibility that injured hepatocytes can initiate fiborosis [4, 5]. Therefore, we wanted to create a simplified in vitro mimic of the liver where hepatocytes and stellate cells reside in close proximity during alcohol injury. Micropatterned cocultures, described by us and others previously [39-42] are particularly suited for these studies because the behavior of one cell type with respect to the other is easier to monitor and characterize. Another significant advantage of the micropatterned coculture format is the possibility of delivering GF signals to a specific cell type during injury [24].
As mentioned previously in this paper, printing GF/collagen arrays on silanized glass substrates created surfaces with highly cell-adhesive protein islands surrounded by moderately adhesive silane regions. When seeded first, primary rat hepatocytes selectively adhered on protein spots forming 500 μm cell islands. Subsequent seeding of stellate cells resulted in a micropatterned co-culture with stellate cells adhering around the hepatic islands. Figure 6A shows images of micropatterned cocultures where an island of hepatocytes is surrounded by stellate cells. We compared cocultures where hepatocytes were residing either on HGF/collagen or collagen-only spots during alcohol injury. To assess hepatic phenotype cocultures were stained for albumin – a hallmark liver product. As seen from Figure 6A (left panel), hepatocytes receiving bottom-up HGF signaling during alcohol insult maintained cell-cell contacts within the cluster, synthesized a lot of albumin (green fluorescence signal) and kept stellate cells on the periphery. In contrast, hepatocytes residing on collagen-only spots during alcohol injury expressed considerably less albumin and the hepatic cluster were infiltrated by stellate cells (Figure 6A, right panel). The results of Figure 6A are in line with other results presented in this paper suggesting that HGF signaling from the bottom-up promotes E-cadherin expression in hepatocytes, and helps maintain differentiated hepatic (epithelial) phenotype.
Figure 6.
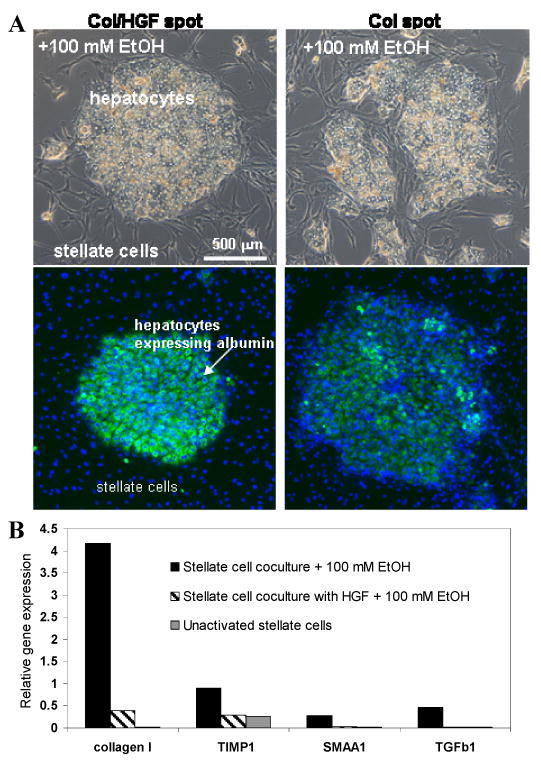
Alcohol insult of micropatterned hepatocyte-stellate cell cocultures. (A) Left panel demonstrates clusters of hepatocytes formed on Col/HGF spots and surrounded by stellate cells. After alcohol injury hepatocytes expressed high levels of intracellular albumin (green fluorescence) while stellate cells remained on the periphery of hepatocyte islands. Right panel shows that hepatocyte clusters formed on Col spots synthesized less albumin and were invaded by stellate cells. (B) RT-PCR analysis revealed that exposure of cocultures to alcohol resulted in significant activation of stellate cells in the case where neighboring hepatocyte were cultured on Col spots. Conversely, expression of stellate cell activation markers was significantly lower when hepatocytes were cultured on Col/HGF spots during alcohol injury. Markers of stellate cell activation were tissue inhibitor of metalloproteinases (TIMP), α -smooth muscle actin1 (α -SMA1) and transforming growth factor-β1(TGF-β1).
Invasion of hepatocyte clusters by the stellate cells during alcohol injury (see Figure 6A, right panel) suggested activation of these cells. We therefore wanted to assess the extent of stellate cell activation in cocultures in the presence or absence of bottom-up HGF signals delivered to the hepatocytes. These experiments were designed to study the role hepatocytes may play in activating neighboring nonparenchymal cells during liver injury. To investigate this, micropatterned cocultures were exposed to 100 mM ethanol for 48 h, trypsinized and analyzed by RT-PCR for expression of genes associated with activated stellate cell phenotype. It should be noted that stellate cells and hepatocytes were from different species (human and rat respectively) which allowed us to design species-specific PCR assays. RT-PCR data presented in Figure 6B offer compelling evidence that HGF protection delivered to hepatocytes significantly diminished expression of markers associated with activation of proximal stellate cells. Compared to cocultures created on collagen spots, stellate cells cultured next to HGF-protected hepatocytes had 10.4-fold lower level of collagen type I α1 transcripts, 3.1-fold lower tissue inhibitor of metalloproteinases (TIMP) and 11.7-fold lower α -smooth muscle actin1 (α -SMA1) gene expression.
Results presented in Figure 6 demonstrate that printed HGF contributed to maintenance of hepatic phenotype in micropatterned cocultures undergoing alcohol injury. In addition, HGF signaling to the hepatocytes had dramatic effects in attenuating fibrogenic response of neighboring stellate cells. The latter result, in concert with the findings of GF-induced protection against hepatic apoptosis and fibrosis, points to an important role of injured hepatocytes in triggering/initiating fibrogenic signaling and stellate cell activation.
One should note that stellate cells as well as hepatocytes express HGF receptor c-met and that HGF, while protecting hepatocytes, has been shown to activate stellate cells[43]. Therefore, HGF introduced in soluble form to hepatocyte-stellate cell coculture would have complex and divergent effects on the two cell types. Providing HGF signal from the bottom-up to a specific cell type within the coculture offers a much cleaner way of modulating heterotypic interactions.
Growth factors are increasingly being considered as therapeutics to prevent or reverse fibrosis of the liver or other tissues [25, 44-46]. However, given the pleiotropic nature of most growth factors, there is a need to select an optimal therapeutic concentration and/or combination of these molecules. The use of GF microarrays offers the advantage of multiplexing cell-GF interactions while conserving expensive reagents. It is also likely that systemic administration of GFs will not suffice and that tissue- and cell-specific targeting of GFs will be required in the future. Delivery of GF signals to a specific cell type in heterotypic cocultures demonstrated in our study may serve as a simplified model of cell-targeted GF delivery in vivo. As highlighted by our results, combining heterotypic micropatterned cocultures with targeted GF delivery will also be useful for delineating complex heterotypic signaling underlying early events in inflammation and fibrosis.
Conclusions
The present paper explored protective effects of GF arrays during in vitro liver injury. Primary rat hepatocytes cultured on HGF- and BMP7-containing collagen arrays during exposure to ethanol were effectively protected against apoptosis. The number of apoptotic hepatocytes decreased with an increasing concentration of printed GFs. Protein spots containing both HGF and BMP7 provided best protection to hepatocytes undergoing alcohol exposure. Additional studies pointed to decrease in TGF-β gene expression and maintenance of epithelial hepatic phenotype in HGF-protected hepatocytes. These experiments strongly support the notion that bottom-up GF signals delivered from the microarrays protect hepatocytes against alcohol-induced apoptosis and fibrosis. In addition, we employed micropatterned cocultures to demonstrate that hepatic apoptosis and fibrosis cause activation of stellate cells, likely through the release of paracrine factors such as TGF-β. Cultivation of hepatocytes on HGF arrays resulted in attenuation of pro-fibrogenic gene expression in neighboring stellate cells. The cell microarray format combined with an in vitro injury model may be used in the future for high-throughput screening of other prospective anti-fibrotic agents. In addition, GF stimulation of specific cells within micropatterned cocultures may help parse out heterotypic signaling in the context of liver injury and may represent an interesting in vitro model of cell type-targeted GF delivery in vivo.
Acknowledgments
We thank Ms. Dipali Patel for the assistance with primary hepatocyte isolation. We also thank Dr. Jian Wu in the Department of Internal Medicine at UC Davis for helpful discussions and suggestions. NT was supported in part by a fellowship from the National Center for Biotechnology, Republic of Kazakhstan. Financial support for this work was provided by NIH grants (DK073901 and EB010131) awarded to AR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuman MG. Apoptosis in diseases of the liver. Crit Rev Clin Lab Sci. 2001;38(2):109–66. doi: 10.1080/20014091084182. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32(2):247–55. doi: 10.1053/jhep.2000.9109. [DOI] [PubMed] [Google Scholar]
- 4.Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem. 2007;282(30):22089–101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 5.Meindl-Beinker NM, Dooley S. Transforming growth fator-beta and hepatocyte transdifferentiation in liver fibrosis. J Gastro Hepatol. 2008:S1, S122–S7. doi: 10.1111/j.1440-1746.2007.05297.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, et al. Scatter Factor/Hepatocyte Growth-Factor Is Essential For Liver Development. Nature. 1995;373(6516):699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 7.Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2001;98(1):247–52. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang D, Jiang Z, Han F, Zhang Y, Li Z. HGF suppresses the production of collagen type III and alpha-SMA induced by TGF-beta1 in healing fibroblasts. Eur J Appl Physiol. 2008;103(5):489–93. doi: 10.1007/s00421-008-0733-7. [DOI] [PubMed] [Google Scholar]
- 9.Nishino M, Iimuro Y, Ueki T, Hirano T, Fujimoto J. Hepatocyte growth factor improves survival after partial hepatectomy in cirrhotic rats suppressing apoptosis of hepatocytes. Surgery. 2008;144(3):374–84. doi: 10.1016/j.surg.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9(7):964–8. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 11.Yu MA, Shin KS, Kim JH, Kim YI, Chung SS, Park SH, et al. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrol. 2009;20(3):567–81. doi: 10.1681/ASN.2008040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuda Y, Matsumoto K, Ichida T, Nakamura T. Hepatocyte growth factor suppresses the onset of liver cirrhosis and abrogates lethal hepatic dysfunction in rats. J Biochem(Tokyo) 1995;118(3):643–9. doi: 10.1093/oxfordjournals.jbchem.a124958. [DOI] [PubMed] [Google Scholar]
- 13.Kosai K, Matsumoto K, Nagata S, Tsujimoto Y, Nakamura T. Abrogation of Fas-induced fulminant hepatic failure in mice by hepatocyte growth factor. Biochem Biophysl Res Com. 1998;244(3):683–90. doi: 10.1006/bbrc.1998.8293. [DOI] [PubMed] [Google Scholar]
- 14.Navab R, Liu J, Seiden-Long I, Shih W, Li M, Bandarchi B, et al. Co-overexpression of Met and hepatocyte growth factor promotes systemic metastasis in NCI-H460 non-small cell lung carcinoma cells. Neoplasia. 2009;11(12):1292–300. doi: 10.1593/neo.09622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S, et al. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res. 2007;67(18):8615–23. doi: 10.1158/0008-5472.CAN-07-0232. [DOI] [PubMed] [Google Scholar]
- 16.Tacke F, Gabele E, Bataille F, Schwabe RF, Hellerbrand C, Klebl F, et al. Bone morphogenetic protein 7 is elevated in patients with chronic liver disease and exerts fibrogenic effects on human hepatic stellate cells. Dig Dis Sci. 2007;52(12):3404–15. doi: 10.1007/s10620-007-9758-8. [DOI] [PubMed] [Google Scholar]
- 17.Kato S, Ishii T, Hara H, Sugiura N, Kimata K, Akamatsu N. Hepatocyte growth factor immobilized onto culture substrates through heparin and matrigel enhances DNA synthesis in primary rat hepatocytes. Exp Cell Res. 1994;211(1):53–8. doi: 10.1006/excr.1994.1058. [DOI] [PubMed] [Google Scholar]
- 18.Alberti K, Davey RE, Onishi K, George S, Salchert K, Seib FP, et al. Functional immobilization of signaling proteins enables control of stem cell fate. Nat Methods. 2008;5(7):645–50. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- 19.Marcantonio NA, Boehm CA, Rozic RJ, Au A, Wells A, Muschler GF, et al. The influence of tethered epidermal growth factor on connective tissue progenitor colony formation. Biomaterials. 2009;30(27):4629–38. doi: 10.1016/j.biomaterials.2009.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22(5):439–44. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 21.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26(16):3227–34. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25(5):1241–51. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 23.Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor-beta binds collagen IV of basement membrane matrix: Implications for development. Dev Biol. 1991:143303–8. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- 24.Jones CN, Tuleuova N, Lee JY, Ramanculov E, Reddi AH, Zern MA, et al. Cultivating liver cells on printed arrays of hepatocyte growth factor. Biomaterials. 2009;30(22):3733–41. doi: 10.1016/j.biomaterials.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168(5):1500–12. doi: 10.2353/ajpath.2006.050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motazed R, Colville-Nash P, Kwan JT, Dockrell ME. BMP-7 and proximal tubule epithelial cells: activation of multiple signaling pathways reveals a novel anti-fibrotic mechanism. Pharm Res. 2008 doi: 10.1007/s11095-008-9551-1. [DOI] [PubMed] [Google Scholar]
- 27.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nature Methods. 2005;3119 doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 28.Soen Y, Mori A, Palmer TD, Brown PO. Exploring the regulation of human neural precursor cell differentiation using arrays of signaling microenvironments. Mol Syst Biol. 2006 doi: 10.1038/msb4100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilkhanizadeh S, Teixeira AI, Hermanson O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials. 2007;28(27):3936–43. doi: 10.1016/j.biomaterials.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 30.LaBarge MA, Nelson CM, Villadsen R, Fridriksdottir A, Ruth JR, Stampfer MR, et al. Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integrative Biology. 2009;1(1):70–9. doi: 10.1039/b816472j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brafman DA, de Minicis S, Seki E, Shah KD, Teng DY, Brenner D, et al. Investigating the role of the extracellular environment in modulating hepatic stellate cell biology with arrayed combinatorial microenvironments. Integrative Biology. 2009;1(8-9):513–24. doi: 10.1039/b912926j. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Tuleuova N, Jones CN, Ramanculov E, Zern MA, Revzin A. Directing hepatic differentiation of embryonic stem cells with protein microarray-based co-cultures. Integrative Biology. 2009:1460–8. doi: 10.1039/b905757a. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Jones C, Zern M, Revzin A. Analysis of local tissue-specific gene expression in cellular micropatterns. Anal Chem. 2006;78(24):8305–12. doi: 10.1021/ac0613333. [DOI] [PubMed] [Google Scholar]
- 34.Dunn JCY, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989:3174–9. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 35.Revzin A, Rajagopalan P, Tilles A, Berthiaume F, Yarmush M, Toner M. Designing a hepatocellular microenvironment with protein microarraying and poly(ethylene glycol) photolithography. Langmuir. 2004;20(8):2999–3005. doi: 10.1021/la035827w. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Wu J, Frizell E, Liu SL, Bashey R, Rubin R, et al. Rapamycin inhibits hepatic stellate cell proliferation in vitro and limits fibrogenesis in an in vivo model of liver fibrosis. Gastroenterology. 1999;117(5):1198–204. doi: 10.1016/s0016-5085(99)70406-3. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Venugopal SK, He S, Liu P, Wu J, Zern MA. Ethanol induces apoptosis in hepatocytes by a pathway involving novel protein kinase C isoforms. Cell Signal. 2007;19(11):2339–50. doi: 10.1016/j.cellsig.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Piscione TD, Phan T, Rosenblum ND. BMP7 controls collecting tubule cell proliferation and apoptosis via Smad1-dependent and -independent pathways. Am J Physiol Renal Physiol. 2001;280(1):F19–33. doi: 10.1152/ajprenal.2001.280.1.F19. [DOI] [PubMed] [Google Scholar]
- 39.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Probing heterotypic cell interactions: hepatocyte function in microfabricated co-cultures. J Biomater Sci Polymer Ed. 1998:91137–60. doi: 10.1163/156856298x00695. [DOI] [PubMed] [Google Scholar]
- 40.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of heptocytes and nonparenchymal cells. FASEB J. 1999:131883–900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 41.Revzin A, Rajagopalan P, Tilles AW, Berthiaume F, Yarmush ML, Toner M. Designing a hepatocellular microenvironment with protein microarraying and poly(ethylene glycol) photolithography. Langmuir. 2004:202999–3005. doi: 10.1021/la035827w. [DOI] [PubMed] [Google Scholar]
- 42.Lee JY, Shah SS, Yan J, Howland MC, Parikh AN, Pan TR, et al. Integrating sensing hydrogel microstructures into micropatterned hepatocellular cocultures. Langmuir. 2009;25(6):3880–6. doi: 10.1021/la803635r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikeda H, Nagoshi S, Ohno A, Yanase M, Maekawa H, Fujiwara K. Activated rat stellate cells express c-met and respond to hepatocyte growth factor to enhance transforming growth factor beta1 expression and DNA synthesis. Biochem Biophys Res Comm. 1998:250769–75. doi: 10.1006/bbrc.1998.9387. [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto H, Yang C, LeBleu VS, Soubasakos MA, Giraldo M, Zeisberg M, et al. BMP-7 functions as a novel hormone to facilitate liver regeneration. Faseb J. 2007;21(1):256–64. doi: 10.1096/fj.06-6837com. [DOI] [PubMed] [Google Scholar]
- 45.Yu Y, Lv L, Qian X, Chen N, Yao A, Pu LY, et al. Anti-fibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem Cells Dev. 2009 doi: 10.1089/scd.2009.0254. [DOI] [PubMed] [Google Scholar]
- 46.Wang ZX, Wang ZG, Ran HT, Ren JL, Zhang Y, Li Q, et al. The treatment of liver fibrosis induced by hepatocyte growth factor-directed, ultrasound-targeted microbubble destruction in rats. Clin Imaging. 2009;33(6):454–61. doi: 10.1016/j.clinimag.2009.07.001. [DOI] [PubMed] [Google Scholar]