Abstract
Insects transmit millions of cases of disease each year, and cost millions of dollars in agricultural losses. The control of insect-borne diseases is vital for numerous developing countries, and the management of agricultural insect pests is a very serious business for developed countries. Control methods should target insect-specific traits in order to avoid non-target effects, especially in mammals. Since insect cells have had a billion years of evolutionary divergence from those of vertebrates, they differ in many ways that might be promising for the insect control field—especially, in iron metabolism because current studies have indicated that significant differences exist between insect and mammalian systems. Insect iron metabolism differs from that of vertebrates in the following respects. Insect ferritins have a heavier mass than mammalian ferritins. Unlike their mammalian counterparts, the insect ferritin subunits are often glycosylated and are synthesized with a signal peptide. The crystal structure of insect ferritin also shows a tetrahedral symmetry consisting of 12 heavy chain and 12 light chain subunits in contrast to that of mammalian ferritin that exhibits an octahedral symmetry made of 24 heavy chain and 24 light chain subunits. Insect ferritins associate primarily with the vacuolar system and serve as iron transporters—quite the opposite of the mammalian ferritins, which are mainly cytoplasmic and serve as iron storage proteins. This review will discuss these differences.
Keywords: ferritin, infection, iron transporter, insect, oxidative stress, secreted
1. Introduction
Iron plays a major role in cell viability because it is a co-factor in numerous cellular processes, such as nucleotide synthesis, oxygen metabolism, the tricarboxylic acid cycle, steroid production, and amino acid production [1]. Since Fe+3 can catalyze oxidative damage to biomolecules, all living organisms have proteins that bind, transport and sequester this ion [2]. For mammals, extracellular iron is transported by transferrin, while cellular iron is stored in ferritin [1]. Mammalian ferritins are divided into three subgroups (Table 1): cytoplasmic, serum and mitochondrial ferritins [2]. Cytoplasmic ferritins functions as iron storage proteins and are involved in iron regulation. Serum ferritins are secreted and are involved in iron transfer/donor activities [3, 4]. Mitochondrial ferritin serves as an antioxidant and protector against iron toxicity [5, 6].
Table 1.
| Location | Function | Size (kDa) | Crystal symmetry | Subunit | Ferroxidase center | Leader peptide | |
|---|---|---|---|---|---|---|---|
| Vertebrat e | Cytoplasm | Iron storage | ∼440 | Octahedral | H | Yes | No |
| Mitochondri a | Antioxidant | ||||||
| Serum | Iron transfer/donor | L | No | No | |||
| Insect | ER | Iron transport | 400-600 | Tetrahedral | HCH | Yes | Yes |
| Mitochondri a | Antioxidant | ||||||
| Hemolymph | Iron transport/antioxidant | LCH | No | Yes |
Vertebrate cytoplasmic ferritin is a ubiquitous iron storage protein with a molecular mass of ∼440 kDa [2]. It is a hetero-multimer, consisting of 24 subunits, made of heavy and light chains (H and L), which are encoded by different genes. The ratio of H to L varies according to the need for iron uptake or storage. The H chain is characterized by amino acid residues that make up the “ferroxidase center” responsible for the oxidation of Fe+2 to Fe+3, and tyrosine residues responsible for the rapid biomineralization of iron; while the L chain has the amino acid residues that induce nucleation of iron. Ferritin influences iron availability and responses to oxidative stress [7], and the loss of cytosolic ferritin expression is associated with increased susceptibility to iron overload, iron-dependent toxicity and increased oxidative stress [8, 9]. Cytosolic ferritin overexpression is associated with augmented iron deposition, changes in cell growth and improved response to oxidative challenge [10-13].
Mammalian H and L lack leader signal sequences that target the subunits to the secretory pathway, yet secreted ferritin is found in body fluids such as serum, cerebrospinal, and synovial fluids [2]. Mammalian secreted ferritin counts for a minor proportion of total body ferritin, but it is clinically important, since its level is often used as an index of body iron status—specifically, for the diagnosis of iron deficiency and overload [14]. Serum ferritin concentration also reflects different degrees of inflammation, infection, liver function, cancer and oxidative stress.
Current data in insects suggest that, unlike their mammalian counterparts, both transferrin and ferritin play key roles in iron transport [15-17], and insect ferritins are mostly secreted proteins [15-25]. Like mammalian ferritins, insect ferritins are composed of two types of subunits. However, most insect ferritins are of greater mass (400-600 kDa) than vertebrate ferritins, and have heavier subunits (21-36 kDa). The subunits are often glycosylated and found in the endoplasmic reticulum (ER) in insect cells as well as in hemolymph (blood). Insect ferritins are synthesized with a signal peptide that presumably directs the mature subunit to the secretory pathway. For many years, insect ferritins have been hypothesized to function as iron transport proteins, and recently they have been shown to play such role [26]. In addition to a role in iron metabolism, insect ferritins may also have significant roles in the immune response, as ferritin is induced in proteomic studies with different pathogenic challenges [27, 28].
Work on insect ferritins has been reviewed previously [29, 30]. This review will focus on progress made in the last five years. Although the Class Insecta has thirty two orders, the focal point of the review will be on the two orders where the most work has been done: Lepidoptera (moths and butterflies) and Diptera (flies and mosquitoes). This review will cover the structure of insect ferritins, the role of secreted ferritins, the involvement of ferritin in insect iron absorption, and putative roles of insect ferritin expression based on data obatained in response to different chemical and pathogenic treatments. The unique problems (specifically, assembly and iron loading) faced by the secreted insect ferritins will be discussed in depth.
2. Structure comparison
Insect ferritin subunits with greatest amino acid sequence similarity to the heavy chain of vertebrate ferritin subunits hereafter will be referred to as heavy chain homologue (HCH) subunits. All of the HCH are considered such because they have a conserved ferroxidase site. Although similarity to the vertebrate light chain is variable, insect sequences that lack the ferroxidase site are considered as a light chain homologue (LCH), and this nomenclature is used throughout this review.
In insects, the crystal structure of ferritin is determined only for the lepidopteran Trichoplusia ni (cabbage looper). The crystal structure of ferritin from T. ni [31] consists of 12 heavy chain and 12 light chain subunits configured in tetrahedral (32) symmetry. This is in contrast to homopolymers of the recombinant mammalian heavy chain or heteropolymers of horse spleen ferritin that consist of 24 subunits configured in octahedral (432) symmetry. Ferritin symmetry allows the formation of pores in the molecule where iron can enter; iron is subsequently oxidized, and nucleation of ferrihyrdrite mineral occurs in the core of the molecule and in coordination with phosphate and oxygen.
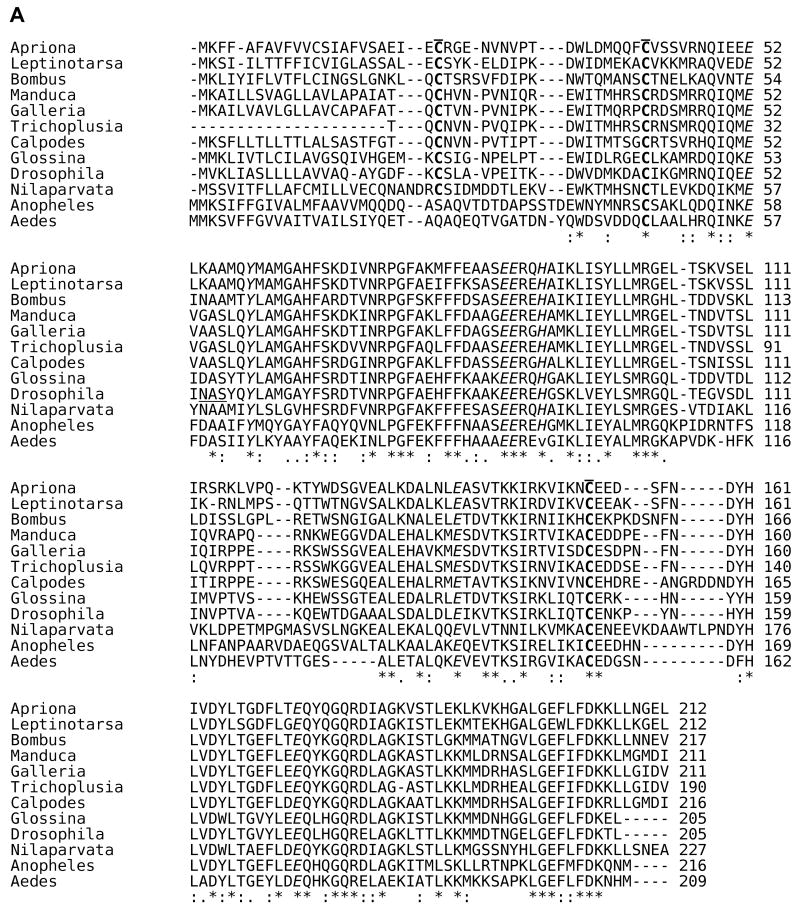
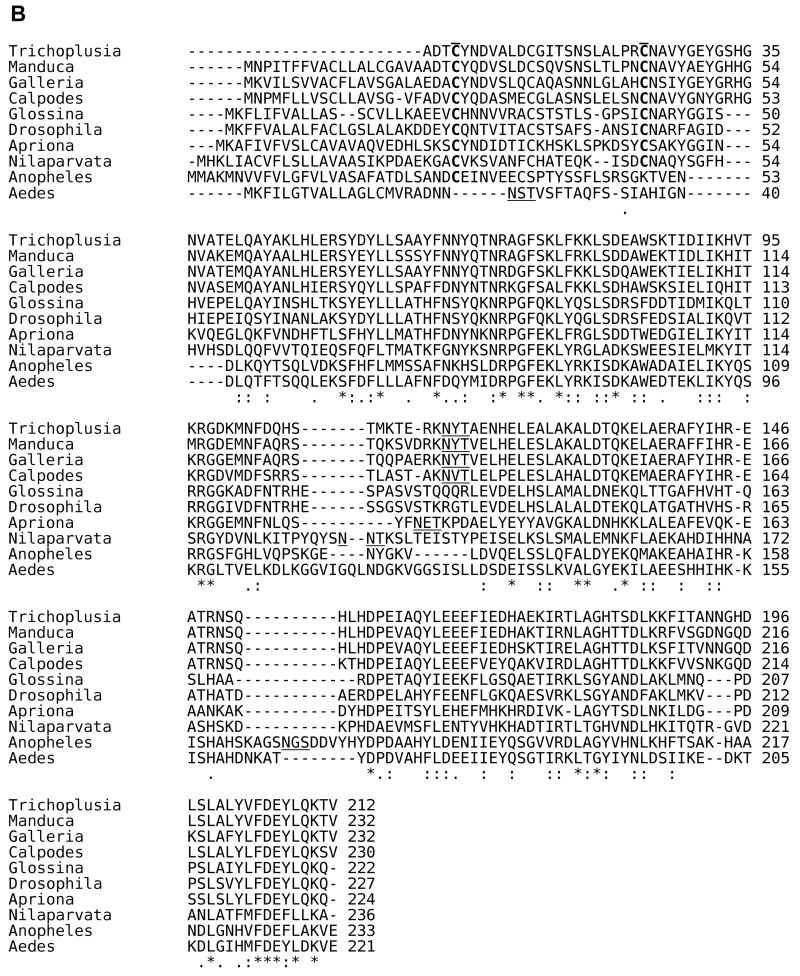
An analysis of the primary structure of the known insect ferritin subunits is shown in Fig. 1. Structural analysis indicates that C groups present on each of the subunits allow for the formation of a tetrahedral structure with equal numbers of HCH and LCH. Generally, these C residues are well conserved among insect ferritin subunits. C21 and C130 in the HCH and C4 and C24 in the LCH form intra-subunit disulfide bridges, while HCH-C3 and LCH-C12 form inter-subunit disulfide bridges. (The amino acid numbers refer to those for the T. ni ferritin subunits.) These bridges allow folding of the molecules into the more stable tetrahedral structure. T. ni ferritin consists of 22 kDa HCH and 27 kDa LCH subunits [31]. The disulfide bridges were confirmed by SDS-PAGE in T. ni by the presence of a 50 kDa constituent, validating that assembly of the ferritin molecule is initiated by bridges forming between HCH and LCH subunits. Notably, some of the C residues required for this configuration are not conserved in the mosquito ferritins, suggesting that the structure of these molecules in mosquitoes could differ from that of other insects. Insect subunits show the characteristic 5 〈-helices (A-E) of the vertebrate ferritin subunits. However, the insect subunits show an extended N-terminal region forming loops that bridge adjacent subunits on the surface of the shell. The loop between the B and C helices in T.ni ferritin LCH chain is also longer and relatively disordered, which suggests that this region could be glycosylated at the putative N-linked glycosylation site at N115 [31]. Even though most other insect LCH show a putative N-glycosylation site (N-X-S/T), only the LCH subunits of Calpodes ethlius (skipper butterfly) [21] and Aedes aegypti (yellow fever mosquito) [32] have been confirmed to be glycosylated. Although the HCH appears to be processed post-translationally as the subunit mass determined from the deduced amino acid sequence is less than that indicated by migration on SDS-PAGE, this does not appear to be due to glycosylation [18, 22, 33].
Fig. 1. Amino acid sequence alignment for insect HCH and LCH subunits.
The signal sequences for both the HCH and LCH T. ni subunits are removed to maintain the same numerical assignments as cited in the original structural work [31]. * = identical residues; : = conserved residues; . = semi-conserved residues. The sequence alignments were performed using Clustal 2.0.11 multiple sequence alignment at http://www.ebi.ac.uk/Tools/clustalw2/index.html with known insect HCH and LCH sequences deposited at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). A. Sequence alignment for insect HCH subunits. C residues involved in inter- and intrasubunit disulside bonds are bolded and overlined; amino acid residues in ferroxidase center are italicized; amino acid residues engaged in the salt bridges and pi-cation interactions are shaded in grey. Sequences are from Manduca sexta (hawkmoth, Lepidoptera) [19], Calpodes ethlius (skipper butterfly, Lepidoptera) [21], Galleria mellonella (wax moth, Lepidoptera) [23], Nilaparvata lugens (plant hopper, Hemiptera) [22], Aedes aegypti (yellow fever mosquito, Diptera) [18], Drosophila melanogaster (fruit fly, Diptera) [20], Anopheles gambiae (malaria mosquito, Diptera) [80], Apriona germari (long horned beetle, Coleoptera) [24], Leptinotarsa decemlineata (Colorado potato beetle, Coleoptera [25], Glossina morsitans morsitans (tsetse fly, Diptera) [15], Bombus ignitus (bumble bee, Hymenoptera), [16, 17] and Trichoplusia ni (cabbage looper, Lepidoptera) [31]. B. Sequence alignment for insect LCH subunits. C residues and amino acid residues engaged in the salt bridges and pi-cation interactions are represented as described in (A). Putative N-glycosylation sites (N-X-S/T) are underlined. LCH subunit sequences are from the same species and references cited above; no LCH sequence was reported for B. ignitus.
The secondary and tertiary structures of the T. ni HCH chain appears similar to the mammalian H chain as it retains all five ordered α-helices (Fig. 2). In contrast, although the A. aegypti HCH subunit preserves all five α-helix domains, it shows more disorder in the fourth helix (D; yellow; Fig. 2A). Lepidopteran ferritin polymers have been observed to crystalize with ease [31, 34]; this phenomenom, however, has not been reported for dipterans. This disparity in crystalization tendency or formation of polymers might be defined by the difference in secondary and tertiary structures of the ferritin subunits in the two orders. Previous work in mammals indicates that the H/L ratio in ferritin polymers can be used to explain their redox activity [35]. The slight divergence in secondary and tertiary structures of the ferritin subunits could explain the variation in the eventual HCH/LCH ratio in ferritin polymers of the two orders in time of stress as they face very different oxidative stress challenge in their nutrional needs (plant diet versus blood meal; see below).
Fig. 2. Molecular modeling for insect HCH and LCH subunits.
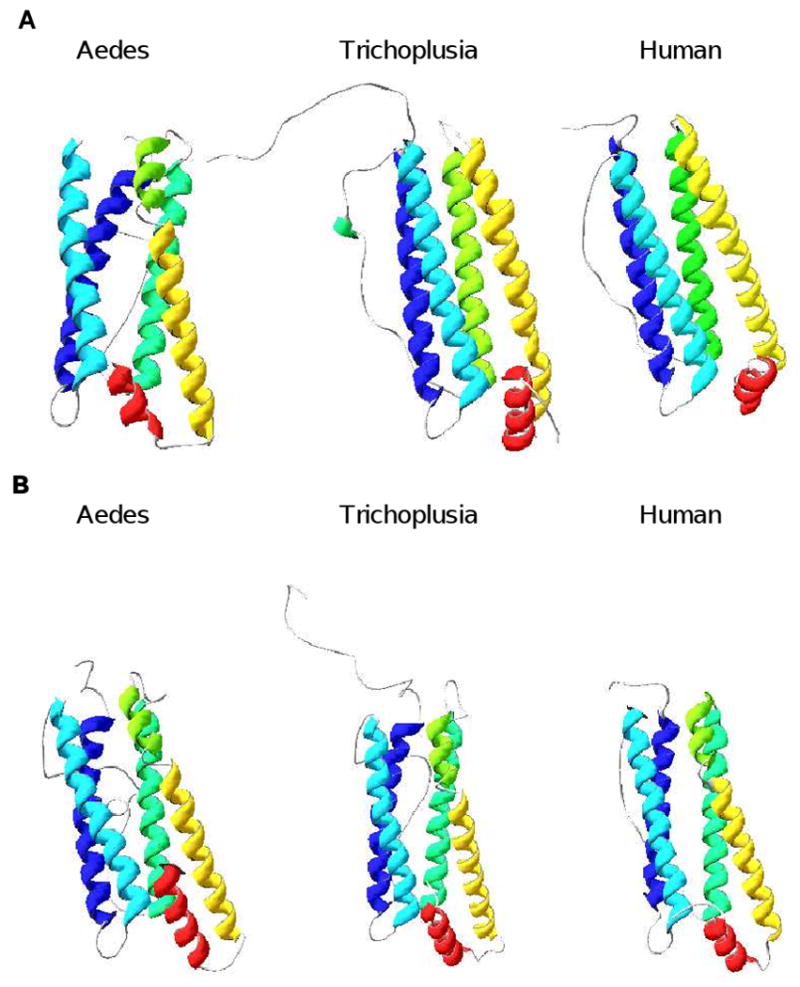
The molecular modeling [81-83] was performed at Swiss-Model website (http://swissmodel.expasy.org). A. Structures of heavy chains. Aedes = A. aegypti; Trichoplusia = T. ni; human = Homo sapiens. Helix A = blue; helix B = magenta; helix C = green; helix D = yellow; helix E = red. Color coding: blue = N-terminus; red = C-terminus. Arrows = C-terminus of domain. B. Structures of light chains. Abbreviations and colors are as described in A.
3. Secreted ferritins
While ferritins are generally sinks for iron, their functions in iron metabolism differ within the animal kingdom. In mammals, ferritin is found mainly in the cytoplasm of cells and serves as an iron storage protein, with the liver as the primary iron storage tissue. In insects, ferritin occurs primarily in association within the vacuolar system and in hemolymph, and serves a role in iron transport. Homopterans are an exception; these xylem-feeding insects, such as Philaenus spumarius, have abundant cytosolic and nuclear holoferritin in addition to vacuolar holoferritin [36], and their cytosolic ferritin is an HCH homopolymer [37].
Although where ferritin assembly and iron loading occur remains unknown, early electron microscopy work indicates that iron-loaded ferritin is present in the Golgi, as well as in the rough endoplasmic reticulum (RER) [38-40]. Insects appear to store iron in ferritin found in the ER and nuclear envelope as well as associated with the Golgi [41]. This suggests that ferritin assembles in RER, but is not immediately secreted. In dictyopterans (cockroaches and termites), dipterans, coleopterans (beetles), hemipterans (true bugs) and lepidopterans, intracellular holoferritin is rarely seen in the cytosol or the nuclear compartment, but is consistently observed in the secretory pathway [36, 42]. Only in P. spumarius are similar levels of cytosolic and nuclear holoferritin observed [36].
Insect ferritin expression is increased in fat body in response to iron [19, 21, 39, 43]. In lepidopterans, hemolymph ferritin appears to originate from fat body [21, 39, 42], while in Apis mellifera (honey bees, Hymenoptera [44]), as well as several other insects, holoferritin is also found in the secretory pathway of fat body tissue [39-42]. Interestingly, both transferrin and ferritin are found in droplets and not diffuse in cytoplasm in bumblebees, suggesting that both are sequestered to the vacuolar compartments and not associated with other cellular organelles [17]. Drosophila melanogaster (fruit fly) ferritin is also found in intracellular membrane-bound compartments of the vacuolar system [45]. Work in D. melanogaster showed that GFP-tagged HCH co-assembled in vivo with endogenous HCH and LCH subunits into mature heteropolymers that contained iron. Iron fed to Drosophila larvae stimulated the formation of ferritin that co-localized with the Golgi organelle in “iron cells” of the gut. Two other larval cell types that accumulated ferritin in response to iron were the Garland cells of the sub-esophageal body and the pericardial cells; both serve a nephrocytic function and filter hemolymph. Since ferritin is found in hemolymph, the ferritin in these cells could come from the hemolymph or be expressed in these cells and receive iron from the hemolymph. In the Drosophila larval brain, high ferritin levels were found in the Golgi and appeared unaltered by dietary iron treatment. In mosquitoes, when ferritin synthesis is stimulated by dietary iron, ferritin is iron loaded, traffics through the Golgi, and is secreted into hemoplymph [46, 47].
In eukaryotes, all proteins that enter the secretory pathway contain an ER signal sequence, generally at the N-terminus [48]. This sequence directs the ribosomes to the RER. At the RER, the protein crosses the ER membrane co-translationally, and is sorted to the lysosome or in vesicles to be secreted from the cell. Proteins to be secreted are incorporated into small transport vesicles, which either fuse with the cis-Golgi or with each other to form the membrane stacks known as the cis-Golgi reticulum. In the process called cisternal migration, a new cis-Golgi stack moves from the cis position (closest the ER) to the trans position (farthest from the ER), becoming first a medial-Golgi cisterna and then a trans-Golgi cisterna. From there, a secretory protein is sorted into one of two types of vesicles: continuous or regulated. Most modifications, including disulfide formation and oligomerization, occur in the RER or Golgi.
Work on flies, moths, and mosquitoes [47, 49, 50] has indicated that the iron-loaded ferritin travels through the Golgi and is then secreted into the hemolymph. In non-blood-feeding insects such as fruit flies and moths, secreted ferritin consists of equal numbers of HCH and LCH subunits [31, 49]. How do insect ferritins assemble into a multimer with equal numbers of HCH and LCH? Are the HCH and LCH subunits signaled for simultaneous expression so both types of subunits can arrive at the RER or Golgi in tandem for assembly? If not, how are they held in the RER until complete assembly occurs? Even more puzzlingly, how is the multimeric unit loaded with iron?
Current views deem that the mature ferritin polypeptide translocates to the ER followed with cleavage of the signal sequence. From there, the particle enters the Golgi. How do 24 subunits come together in an orderly manner in the lumen of the RER and why are they not secreted prior to complete assembly? Possibly, the constant ratio of HCH and LCH subunits is established through a strict post-transcriptional regulation where similar expression of the HCH and LCH subunits allow an in tandem arrival of the two types of ferritin units in the RER or Golgi for assembly [49]. Recent crystollography data [31] for the cabbage looper T. ni suggest that the interface interactions, including salt bridges and cation pi interactions, are vital to the rapid assembly of the HCH/LCH hetorodimer, which in turn serves as the nucleation site for subsequent oligomerization. These data also confirm the existence of inter- and intra-subunit disulfide bonds, which may serve as knobs on the surface of insect ferritins that could bind to an ER-resident receptor, and thus allow the oligomer to be retained in the ER for iron loading.
The hypothesis that the knobs formed by disulfide bonds serve as recognition sites for an internal ER receptor, however, cannot be extended to mosquitoes, as mosquito ferritins do not have most of the C residues identified for this process (Fig. 1). (This exception may be unique to mosquitoes and not necessary to other dipterans—as both Drosphila [51] and Glossina (tsetse fly) [15] retain these C residues.) Mosquitoes could use a different post-translational modification, such as acylation or myristoylation, to retain the subunits in the ER as has been speculated for the lepidopterans Calpodes and Galleria (wax moth) [29, 52]. On the other hand, since secreted ferritins from mosquitoes consist mainly of HCH chains [46, 47], the oligomers do not have to be retained within the ER or Golgi to await the incorporation of the LCH subunits. Thus, problems faced by the other insects regarding assembly of the ferritin shell with equal numbers of LCH and HCH may not be pertinent to mosquitoes. Surprisingly, although the blood-feeding Glossina retain all the C residues, their secreted ferritins also contain much higher HCH content [15]. These data together suggest that, under iron overload conditions such as the blood meal, synthesizing ferritins with higher oxidative capabilities to meet the urgent need for detoxification surpasses the requirement for ferritins to remain in the lumen to obtain a constant ratio of LCH to HCH.
In vivo iron loading and release mechanisms from insect ferritins, however, remain unknown. Ferritin has the potential to store up to 4500 atoms of iron in a single molecule. In Drosophila, considerable iron storage of dietary iron occurs even with lowered levels of ferritin. Specifically, dietary iron administration increases iron loading of GFP-tagged ferritin in vivo even if ferritin levels are limited [49]. These data indicate that, if adequate ferritin is present, ferritin levels do not determine the levels of iron stores. This agrees with the work in mosquito larval epithelial cells where iron levels correlate with the types of ferritin isoforms and not the levels of ferritin expression—specifically, vesicular holoferritin consisting of both the LCH and HCH subunits is maximal at low levels of iron, while secreted holoferritin consisting primarily of HCH subunits increases in direct linear relationship to high iron dose [46].
4. Iron absorption
Animals in general control iron homeostasis primarily by limiting dietary iron absorption. Iron enters the diet in two forms, as heme and as ionic iron released from proteins during digestion. In mammals, ferric is reduced at the enterocyte apical border and absorbed via an apical membrane protein (Divalent Metal Transporter 1 (DMT1), [1]). In contrast, heme is absorbed intact and degraded within the cell, thus releasing the iron. Iron can be stored inside enterocyte ferritin that is lost in the feces when the cells slough off, or it can be transported into blood via a basolateral membrane protein, ferroportin (reviewed by [53-55]). Ferroportin levels are controlled by hepcidin, which is secreted from the liver in response to elevated hepatic iron stores and signals a reduction in iron delivery to blood by increasing degradation of ferroportin [56]. Hepcidin synthesis, in turn, is controlled by several other proteins. In the absence or mutation of these proteins, hepcidin secretion is reduced and iron absorption is unhindered, resulting in conditions of hemochromatosis. Hepcidin is increased also in infection by the induction of hemojuvelin. Although DMT1 is found in insects [57], the role of this protein is not yet clear. Hepcidin and ferroportin are well conserved among mammals; however, these proteins have not been reported in insects.
For aquatic insects, dietary iron would be primarily in the forms found in bacteria because ferric is insoluble at the pH of most fresh waters. For adult insects, iron could be obtained from soils and plants in a variety of forms, including as plant ferritin [58, 59]. The blood meal of hematophagous insects constitutes a high iron load in the forms of heme and ferric, which well exceeds the iron exposure of non-blood-feeding insects. How insects absorb iron and maintain iron homeostasis is unknown.
Lepidopterans express high levels of ferritin messages in gut tissues [19, 21, 23, 33]. Iron administration increases gut message expression and, in Calpodes larvae, provokes secretion of holoferritin into the posterior midgut lumen [40], presumably for excretion in the feces [29, 40].
Like the lepidopterans, ferritin message and protein are increased in the gut tissues of the dipterans—D. melanogaster and Musca domestica (housefly) in response to iron [60, 61] as well as in Glossina mortisans and A. aegypti females following a blood meal [62]. As noted above, feeding iron to Drosophila increases iron-loaded ferritins in the “iron cells” of the anterior midgut, Garland cells, pericardial cells and larval brain [45, 63]. The normal laboratory diet for Drosophila larvae allows sufficient iron and ferritin to accumulate in the “iron cells” such that ferritin levels are not further increased with additional dietary iron. Although limiting dietary iron by the inclusion of an iron-chelating agent in the diet mobilizes iron from the ferritin of the “iron cells,” ferritin levels in these animals are maintained on this regimen. This is similar to findings in larval mosquito cells, where ferritin from the membrane fractions is maintained over time in the presence of treatment with the chelating agent desferrioxamine [46]. These data suggest that iron is not the only stimulus for the maintainance of ferritin levels in insects.
In Drosophila, inactivation of either of the ferritin subunits by mutation results in developmental arrest and fly embryonic lethality [49]. A mutation in the open reading frame of the LCH or in an intron sequence of the HCH with P-element insertions is lethal if homozygous regardless of the mutation site, indicating that both subunits are essential to maintain iron in non-toxic, bioavailable form. The heterozygous mutants for either the HCH or LCH subunit show lowered endogenous expression of the counter subunit. The subunits do not share redundant function in iron uptake and storage because, although overexpression of the normal subunit rescues the viability of mutant of the same subunit, it does not restore viability of the mutants of the counter subunit. The overexpression of HCH that lacks the ferroxidase center in flies with the HCH mutation also will not rescue lethality of the HCH mutant indicating that the ferroxidase site is required for function of the HCH, probably for iron loading.
Iron absorption in hematophagous insects could differ somewhat from that of other insects (Fig. 3A). In A. aegypti, substantial amounts of heme from the blood meal are bound to the peritrophic matrix and lost when the matrix is shed into at the end of blood digestion [64]. The majority of iron provided as heme in a blood meal is found in waste (87%) [26]. Of heme iron absorbed from the meal, about half is delivered to the ovaries and eggs, and the remainder is distributed among the tissues. Iron distribution to the fat body, head and midgut does increase significantly 72 hours post blood feeding. In contrast to heme, 92% of iron delivered in a blood meal as ferric-transferrin is absorbed, and 77% of the absorbed iron is delivered to the ovaries and eggs. Radiolabeled dietary iron is found in secreted ferritin in hemolymph, leaving little doubt that, in these animals, hemolymph ferritin serves to transport meal iron into the hemolymph from the gut (Fig. 3B). Furthermore, this radiolabeled iron is delivered to ovaries and eggs where it is found in association with ferritin. A different mechanism limiting iron absorption is found in Rhodnius prolixus (kissing bug, Hemiptera), where heme from the blood meal is sequestered in the gut as hemozoin [65].
Fig. 3. Iron metabolism in mosquitoes. A. Main route for iron absorption.
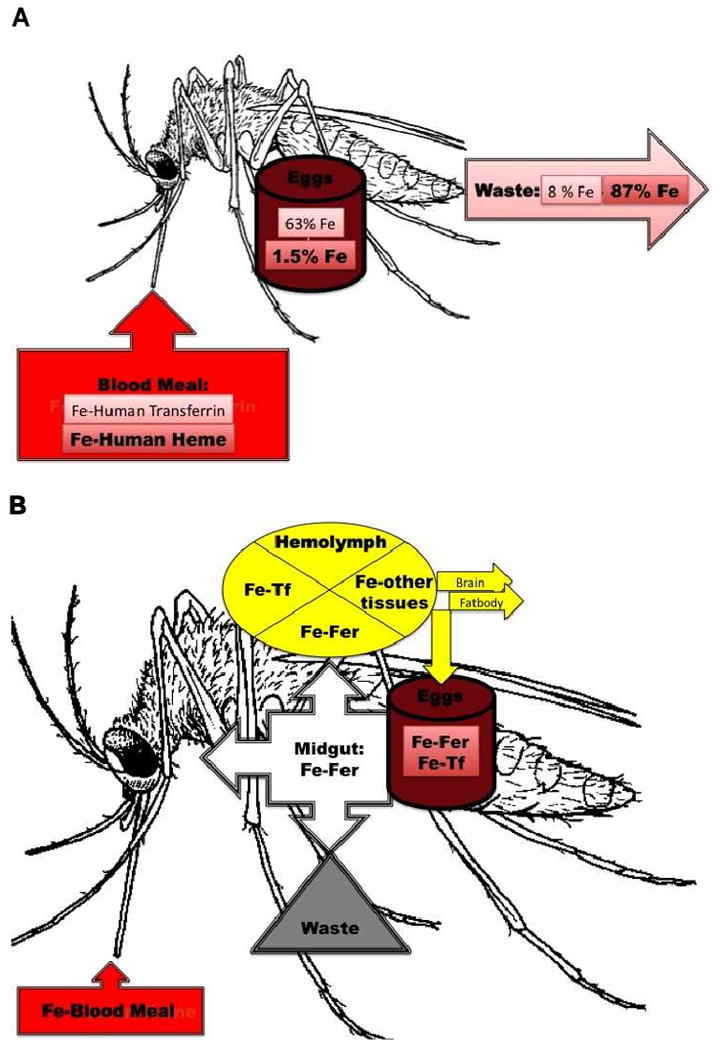
Fe-Human Transferrin = iron from human transferrin in the blood meal; Fe-Human Heme = iron from human hemoglobin in the blood meal. Fe (not bolded) = % of Fe-Human Transferrin deposited in eggs or going to waste; Fe (bolded) = % of Fe-Human Heme deposited in eggs or going to waste. B. Proteins involved in iron transfer to different tissues. Fe = iron; Fer = insect ferritin; Tf = insect transferrin.
Given differences in the primary structure of other insect ferritins from mosquito ferritins, as well as the levels of dietary iron to which hematophageous insects are normally exposed, disparities among insects with regards to iron metabolism are to be expected. However, the secretion of holoferritin into the hindgut, the upregulation of ferritin expression in gut tissues and the sequestering of heme as hemozoin, all represent mechanisms that would protect insects against iron overload. Thus, insects appear to have developed a variety of mechanisms for maintaining iron homeostasis by limiting iron uptake or by enhancing iron excretion.
6. Functions of insect ferritins
a. Iron transport
It has been long known that insect ferritins are secreted proteins and that they circulate to different tissues through the hemolymphatic system (see previous reviews [29, 30]). These observations have led to early speculation that they serve as iron transport protein. This role, nonetheless, has only been recently confirmed [26]. Work in mosquitoes showed that iron provided as 59Fe transferrin in a blood meal was identified in hemolymph and egg ferritins—indicating that secreted ferritin is an iron transporter that carries iron first through the hemolymph and subsequently delivers iron to other tissues (Fig. 3B). Current data suggest that all insect tissues make ferritin as all tissues examined show expression of both the LCH and HCH messages (Table 2), though at different expression levels [15, 17, 19, 21-25, 33, 60, 62, 66, 67]. However, as stated earlier, where or how insect ferritin is iron loaded or unloaded remains unknown.
Table 2. HCH and LCH expression in different tissues.
| Subunit | Tissue | Common name | Reference |
|---|---|---|---|
| HCH | Gut cells | Bumblebee | [17] |
| Colorado Potato Beetle | [25] | ||
| Honey Bee | [44] | ||
| Tobacco Hornworm | [19] | ||
| Skipper Butterfly | [21, 40] | ||
| California Lopper | [23, 33] | ||
| House Fly | [61] | ||
| Fruit Fly | [49, 60] | ||
| Mosquito | [62] | ||
| Hemolymph | Skipper Butterfly | [21, 41] | |
| Tobacco Hornworm | [50, 84] | ||
| Mosquito | [26, 62] | ||
| Fruit Fly | [60] | ||
| Fat Body | Skipper Butterfly | [21, 38, 41] | |
| Tobacco Hornworm | [19] | ||
| Fruit Fly | [45] | ||
| Pericardial cells | Fruit Fly | [45] | |
| Garland cells | Fruit Fly | [45] | |
| Brain/Head | Fruit Fly | [45] | |
| Ovaries | Fruit Fly | [60] | |
| Mosquito | [62] | ||
| Testis | Mosquito | [62] | |
| LCH | Gut cells | Colorado Potato Beetle | [25] |
| Honey Bee | [44] | ||
| Tobacco Hornworm | [19] | ||
| Skipper Butterfly | [21, 40] | ||
| California Looper | [23, 33] | ||
| House Fly | [61] | ||
| Fruit Fly | [49, 60] | ||
| Mosquito | [62] | ||
| Hemolymph | Skipper Butterfly | [21, 41] | |
| Tobacco Hornworm | [50, 84] | ||
| Mosquito | [26, 62] | ||
| Fruit Fly | [60] | ||
| Fat Body | Skipper Butterfly | [21, 38, 41] | |
| Tobacco Hornworm | [19] | ||
| Fruit Fly | [45] | ||
| Brain/Head | Fruit Fly | [45] | |
| Ovaries | Fruit Fly | [60] |
Iron is released from mammalian ferritin mainly by proteolytic lysosomal degradation of the protein [68, 69]. Release of 59Fe from ferritin, studied in three different cell lines, was blocked by inhibitors of lysosomal activity (leupeptin, chymostatin and chloroquine), but not by the proteosomal inhibitor (lactacystin) [69, 70]. In a similar manner, the insect ferritins may enter the cell and release iron through lysosomal degradation of ferritin and/or lysosomal autophagy in tissues such as ovaries that require high levels of iron for growth and cell differentiation.
b. Roles in oxidative stress and infection
Mammalian cytosolic ferritin is considered an important inhibitor of free radical production, and seems to be the only cytosolic protein carrying ferroxidase activity [2]. Mammalian cytosolic ferritins that are H-rich are often found in tissues with more pronounced anti-oxidant activity, such as the heart and brain, whereas those that are L-rich are located mostly in tissues with a more elevated iron storage function, such as the spleen and liver. Just as their mammalian counterparts, insect ferritins also play a role as a cytotoxic protector against oxidative challenges. In non-hemaphagous insects, such as Drosophila, overexpression of both the HCH and LCH gene is required to confer protection against oxidative stress [49]. Likewise, in hematophogous insects, expression of both LCH and HCH genes in mosquito cultured cells increases with iron, H2O2 or hemin treatment, and the temporal expression of the genes is very similar; in addition, expression of both LCH and HCH genes in whole animals are induced with a blood meal [71]. These results suggest that ferritin could serve as the cytotoxic protector in mosquitoes against the oxidative challenge of the blood meal. Hematophagous insects face a much higher iron load than non-hematophagous insects. In both tsetse flies and mosquitoes, secreted ferritins are HCH-rich conceivably to deal with the antioxidant needs demanded by the nature of their diets, comparable to H-rich cytosolic ferritin isoforms found in mammalian tissues that must cope with high anti-oxidant activities [15, 47].
In addition to cytosolic ferritin, mammals also have mitochondrial ferritin, which has a limited tissue distribution and functions probably as a protector against mitochondrial iron toxicity and oxidative damage [72]. In the same way, insect mitochondrial ferritin expression is limited mainly to the testis [63]. Overexpression of insect mitochondrial ferritin has diminutive effects on development and total iron stores, but significantly improves resistance to oxidative stress [63]. These results support an antioxidant role for insect mitochondrial ferritin parallel to that of the mammalian mitochondrial ferritin.
c. Role in immunity
In bumblebees, HCH is upregulated in response to iron, wounding and bacterial infection [16]. In the beetle Tribolium castaneum, when suppression subtractive hybridization was used to identify genes that are transcriptionally induced in response to injection of crude lipopolysaccharide (LPS), ferritin genes were identified as potential antimicrobial effector genes [27]. In the malaria mosquito, the LCH and HCH chains were among a subset of proteins with decreased expression following bacterial injections [28]. These observations of an altered expression in ferritin genes with different pathogenic exposure suggest a role for these proteins in insect immunity.
d. Reciprocal function with transferrin?
In Bombus ignitus (bumble bee), injection of FeCl3 into worker bees causes a nearly linear increase in HCH mRNA in epidemis, gut, fat body and muscle until 15 h post iron administration [17]. Interference with HCH using dsRNA also increases transferrin mRNA and vice versa. These preliminary data suggest that ferritin and transferrin may share a reciprocal role under iron overload conditions. However, these data are somewhat difficult to interpret because the controls do not include a scrambled dsRNA, and the levels of upregulation, although significant, are low.
7. Conclusion and future directions
Significant progress has been made in the field of iron metabolism in insects since the initial work in the eighties and nineties [40, 43, 73]. Current data indicate that insect ferritins not only serve as iron storage proteins, but also as an iron transport vehicle [26]. They also appear to be involved in the oxidative stress and immune responses. A total knock-out of either insect HCH or LCH chain proves to be lethal [49], suggesting that, as in mammals [74], there is no functional redundancy between the two subunits and that neither homopolymer is able to maintain iron in a nontoxic form.
In fruit flies, overexpression of ferritin shows no significant change in total iron. These results differ from those observed in mammals where ferritin overexpression causes iron deficiency due to increased iron retention in ferritin [75, 76]. Currently, no data on the relationship between cellular iron and ferritin levels in hematophogous insects is available. As transgenic mosquitoes become a reality, and genetic manipulation of mosquitoes becomes a routine procedure, transgenic mosquitoes that overexpress ferritin could be used in the future to determine: (1) whether there is a relationship between ferritin and cellular iron levels in mosquitoes, and (2) whether ferritin protects these animals against chemicals known to cause oxidative stress.
In addition, repression of the subunits through transgenic mosquitoes or the use of RNA interference (RNAi) in future studies will resolve the effects of the ferritin subunits on growth and development. In the hematophagous arthropod Ixodes ricinus (sheep tick), silencing of the HCH (FER2) subunit through the use of RNAi adversely affects the hatching rate of eggs, decreases the weight of female ticks and dramatically impairs the ability of ticks to feed [77]. Similar results to those of the sheep ticks are expected for hematophagous insects, as the HCH subunit for the insects plays a similar iron transport role to that of the ticks. In the near future, RNAi techniques could be used to inhibit expression of ferritin and to determine the effects of subunit loss on growth and fitness, as well as the extent of oxidative damage following blood feeding or exposure to strong oxidants. The repression of the ferritin subunits will probably produce significant phenotypic changes in the cell growth and maturation of ovaries and eggs, as well as interfere with feeding due to the potential for high oxidative stress caused by the intake of a blood meal.
Insects are responsible for numerous human diseases (e.g., malaria, trypanosomiasis, dengue, etc.; www.who.org and www.cdc.gov) and cause serious agricultural losses [78, 79]. As elaborated throughout this review, significant differences have been found between mammalian and insect iron metabolism (Table 1). These differences may be used as potential strategies for insect control. Currently, a major obstacle in controlling disease vector insects, such as mosquitoes and tsetse flies, is that the genome of the pathogen as well as the genome of the host insect is quite plastic. Hence, to control insect disease vectors, a multi-faceted approach must be used. A simple blockage of the pathogen invasion pathway will not be sufficient to prevent disease transmission because pathogens are well known for their ability to evolve resistance to new environments/obstacles. Thus, preventing the insect hosts from becoming efficient carriers is also necessary, but there again, the genetics shift of insects creates serious problems. Further, agriculturally important insect pests have analogous genetic plasticity, and to manage these latter insects, comparable issues must be addressed.
Thus, a thorough understanding of the biology of the host insect, including its iron biology, is essential for the development of effective control strategies. Fundamental problems regarding the assembly of the ferritin subunits, iron loading of ferritin multimers, and iron usage in insects remain to be resolved. In solving these problems, a fascinating and distinctive pathway may be revealed for insects that differs from their mammalian counterparts (Precambian divergence) and from each other (Paleozoic divergence) by hundreds of millions of years—as has already been established in some of the current work reviewed here.
Acknowledgments
We are grateful to Prof. G. C. Mayer for reading this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daphne Q. D. Pham, Email: daphne.pham@uwp.edu, Department of Biological Sciences, University of Wisconsin—Parkside, Kenosha, WI 53141-2000.
Joy J. Winzerling, Email: jwinzerl@Ag.arizona.edu, Department of Nutritional Sciences, University of Arizona, Tucson, AZ 85721-0038.
References
- 1.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arosio P, Ingrassia R, Cavadini P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2009;1790:589–599. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Leimberg JM, Prus E, Link G, Fibach E, Konijn AM. Iron-chelator complexes as iron sources for early developing human erythroid precursors. Transl Res. 2008;151:88–96. doi: 10.1016/j.trsl.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Fisher J, Devraj K, Ingram J, Slagle-Webb B, Madhankumar AB, Liu X, Klinger M, Simpson IA, Connor JR. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am J Physiol Cell Physiol. 2007;293:C641–649. doi: 10.1152/ajpcell.00599.2006. [DOI] [PubMed] [Google Scholar]
- 5.Levi S, Arosio P. Mitochondrial ferritin. Int J Biochem Cell Biol. 2004;36:1887–1889. doi: 10.1016/j.biocel.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Cazzola M, Invernizzi R, Bergamaschi G, Levi S, Corsi B, Travaglino E, Rolandi V, Biasiotto G, Drysdale J, Arosio P. Mitochondrial ferritin expression in erythroid cells from patients with sideroblastic anemia. Blood. 2003;101:1996–2000. doi: 10.1182/blood-2002-07-2006. [DOI] [PubMed] [Google Scholar]
- 7.Aisen P, Enns C, Wessling-Resnik M. Chemistry and biology of eukaryotinc iron metabolism. Int J Biochem Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 8.DeRusso PA, Philpott CC, Iwai K, Mostowski HS, Klausner RD, Rouault TA. Expression of a constitutive mutant of iron regulatory protein 1 abolishes iron homeostasis in mammalian cells. J Biol Chem. 1995;270:15451–15454. doi: 10.1074/jbc.270.26.15451. [DOI] [PubMed] [Google Scholar]
- 9.Kakhlon O, Gruenbaum Y, Cabantchik ZI. Repression of ferritin expression increases the labile iron pool, oxidative stress, and short-term growth of human erytholeukemia cells. Blood. 2001;97:2863–2871. doi: 10.1182/blood.v97.9.2863. [DOI] [PubMed] [Google Scholar]
- 10.Kato J, Niitsu Y. Recent advance in molecular iron metabolism: translational disorders of ferritin. Int J Hematol. 2002;76:208–212. doi: 10.1007/BF02982789. [DOI] [PubMed] [Google Scholar]
- 11.Kakhlon O, Gruenbaum Y, Cabantchik ZI. Ferritin expression modulates cell cycle dynamics and cell responsiveness to H-ras-induced growth via expansion of the labile iron pool. Biochem J. 2002;363:431–436. doi: 10.1042/0264-6021:3630431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epsztejn S, Glickstein H, Picard V, Slotki IN, Breuer W, Beaumont C, Cabantchik ZI. H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood. 1999;94:3593–3603. [PubMed] [Google Scholar]
- 13.Corsi B, Perrone F, Bourgeois M, Beaumont C, Panzeri MC, Cozzi A, Sangregorio R, Santambrogio P, Albertini A, Arosio P, Levi S. Transient overexpression of human H- and L-ferritin chains in COS cells. Biochem J. 1998;330(Pt 1):315–320. doi: 10.1042/bj3300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann MB. Methods to assess iron and iodine status. Br J Nutr. 2008;99 3:S2–9. doi: 10.1017/S000711450800679X. [DOI] [PubMed] [Google Scholar]
- 15.Strickler-Dinglasan PM, Guz N, Attardo G, Aksoy S. Molecular characterization of iron binding proteins from Glossina morsitans morsitans (Diptera: Glossinidae) Insect Biochem Mol Biol. 2006;36:921–933. doi: 10.1016/j.ibmb.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Kim BY, Lee KS, Yoon HJ, Cui Z, Lu W, Jia JM, Kim DH, Sohn HD, Jin BR. Molecular characterization of iron binding proteins, transferrin and ferritin heavy chain subunit, from the bumblebee Bombus ignitus. Comp Biochem Physiol B Biochem Mol Biol. 2009;152:20–27. doi: 10.1016/j.cbpb.2008.09.082. [DOI] [PubMed] [Google Scholar]
- 17.Kim BY, Lee KS, Yoon HJ, Kim I, Li J, Sohn HD, Jin BR. Expression profile of the iron-binding proteins transferrin and ferritin heavy chain subunit in the bumblebee Bombus ignitus. Comp Biochem Physiol B Biochem Mol Biol. 2009;153:165–170. doi: 10.1016/j.cbpb.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Dunkov BC, Zhang D, Choumarov K, Winzerling JJ, Law JH. Isolation and characterization of mosquito ferritin and cloning of a cDNA that encodes one subunit. Arch Insect Biochem Physiol. 1995;29:293–307. doi: 10.1002/arch.940290307. [DOI] [PubMed] [Google Scholar]
- 19.Pham DQD, Zhang D, Hufnagel DH, Winzerling JJ. Manduca sexta hemolymph ferritin: cDNA sequence and mRNA expression. Gene. 1996;172:255–259. doi: 10.1016/0378-1119(96)00012-1. [DOI] [PubMed] [Google Scholar]
- 20.Charlesworth A, Georgieva T, Gospodov I, Law JH, Dunkov BC, Ralcheva N, Barillas-Mury C, Ralchev K, Kafatos FC. Drosophila melanogaster ferritin. Isolation and properties, molecular cloning of a cDNA encoding one subunit, and localization of the gene on the third chromosome. Eur J Biochem. 1997;247:470–475. doi: 10.1111/j.1432-1033.1997.00470.x. [DOI] [PubMed] [Google Scholar]
- 21.Nichol H, Locke M. Secreted ferritin subunits are of two kinds in insects: molecular cloning of cDNAs encoding two major subunits of secreted ferritin from Calpodes ethlius. Insect Biochem Mol Biol. 1999;29:999–1013. doi: 10.1016/s0965-1748(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 22.Du J, Foissac X, Carss A, Gatehouse AMR, Gatehouse JA. Ferritin acts as the most abundant binding protein for snowdrop lectin in the midgut of rice brown planthoppers (Nilaparvata lugens) Insect Biochem Mol Biol. 2000;30:297–305. doi: 10.1016/s0965-1748(99)00130-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim BS, Yun CY, Yeo SM, Lee HJ, Kim HR. Cloning and expression of a ferritin subunit for Galleria mellonella. Arch Insect Biochem Physiol. 2001;47:8–17. doi: 10.1002/arch.1030. [DOI] [PubMed] [Google Scholar]
- 24.Kim SR, Lee KS, Yoon HJ, Park NS, Lee SM, Kim I, Seo SJ, Sohn HD, Jin BR. Molecular cloning, expression and characterization of cDNAs encoding the ferritin subunits from the beetle, Apriona germari. Comp Biochem Physiol B Biochem Mol Biol. 2004;138:423–433. doi: 10.1016/j.cbpc.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Qiu L, Gao JR, Clark JM. Sequencing and characterization of a cDNA encoding a ferritin subunit of Colorado potato beetle, Leptinotarsa decemlineata. Arch Insect Biochem Physiol. 2005;60:140–150. doi: 10.1002/arch.20089. [DOI] [PubMed] [Google Scholar]
- 26.Zhou G, Kohlhepp P, Geiser DL, Frasquillo MD, Vazquez-Moreno L, Winzerling JJ. Fate of blood meal iron in mosquitoes. J Insect Physiol. 2007;53:1169–1178. doi: 10.1016/j.jinsphys.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altincicek B, Knorr E, Vilcinskas A. Beetle immunity: Identification of immune-inducible genes from the model insect Tribolium castaneum. Dev Comp Immunol. 2008;32:585–595. doi: 10.1016/j.dci.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Paskewitz SM, Shi L. The hemolymph proteome of Anopheles gambiae. Insect Biochem Mol Biol. 2005;35:815–824. doi: 10.1016/j.ibmb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Nichol H, Law JH, Winzerling JJ. Iron metabolism in insects. Ann Rev Entomol. 2002;47:535–559. doi: 10.1146/annurev.ento.47.091201.145237. [DOI] [PubMed] [Google Scholar]
- 30.Winzerling JJ, Pham DQD. Ferritin. In: Gilbert LI, Iatrou K, Gill SS, editors. Com Insect Physio Biochem Pharm and Mol Biol. Elsevier; 2004. [Google Scholar]
- 31.Hamburger AE, West AP, Jr, Hamburger ZA, Hamburger P, Bjorkman PJ. Crystal structure of a secreted insect ferritin reveals a symmetrical arrangement of heavy and light chains. J Mol Biol. 2005;349:558–569. doi: 10.1016/j.jmb.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 32.Geiser DL, Chavez CA, Flores-Munguia R, Winzerling JJ, Pham DDQ. Aedes aegypti ferritin: a cytotoxic protector against iron and oxidative challenge? Eur J Biochem. 2003;270:3667–3674. doi: 10.1046/j.1432-1033.2003.03709.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim BS, Lee CS, Seol JY, Yun CY, Kim HR. Cloning and expression of 32 kDa ferritin from Galleria mellonella. Arch Insect Biochem Physiol. 2002;51:80–90. doi: 10.1002/arch.10050. [DOI] [PubMed] [Google Scholar]
- 34.Larsen WJ. Cell remodeling in the fat body of an insect. Tissue Cell. 1976;8:73–92. doi: 10.1016/0040-8166(76)90021-5. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JL, Norcross DC, Arosio P, Frankel RB, Watt GD. Redox reactivity of animal apoferritins and apoheteropolymers assembled from recombinant heavy and light human chain ferritins. Biochemistry. 1999;38:4089–4096. doi: 10.1021/bi982690d. [DOI] [PubMed] [Google Scholar]
- 36.Nichol H, Locke M. The localization of ferritin in insects. Tissue & Cell. 1990;22:767–777. [Google Scholar]
- 37.Collin O, Thomas D, Flifla M, Quintan C, Gouranton J. Characterization of a ferritin isolated from the midgut epithelial cell of a homopteran insect, Philaenus spumarius L. Biol Cell. 1988;63:297–305. [PubMed] [Google Scholar]
- 38.Locke M. Apoferritin in the vacuolar system of insect hemocytes. Tissue & Cell. 1991;23:367–375. doi: 10.1016/0040-8166(91)90054-w. [DOI] [PubMed] [Google Scholar]
- 39.Locke M, Ketola-Pirie C, Leung H, Nichol H. Vacuolar apoferritin synthesis by the fat body of an insect. J Insect Physiol. 1991;37:297–309. [Google Scholar]
- 40.Locke M, Leung H. The induction and distribution of an insect ferritin—a new function for the endoplasmic reticulum. Tissue & Cell. 1984;16:739–766. doi: 10.1016/0040-8166(84)90007-7. [DOI] [PubMed] [Google Scholar]
- 41.Locke M. Surface membranes, Golgi complexes, and vacuolar systems. Annu Rev Entomol. 2003;48:1–27. doi: 10.1146/annurev.ento.48.091801.112543. [DOI] [PubMed] [Google Scholar]
- 42.Locke M, Nichol H. Iron economy in insects: transport, metabolism, and storage. Annu Rev Entomol. 1992;37:195–215. [Google Scholar]
- 43.Huebers HA, Huebers E, Finch CA, Webb BA, Truman JW, Riddiford LM. Iron binding proteins and their roles in the tobacco hornworm, Manduca sexta (L.) J Comp Physiol B. 1988;158:291–300. doi: 10.1007/BF00695327. [DOI] [PubMed] [Google Scholar]
- 44.Keim CN, Cruz-Landim C, Carneiro FG, Farina M. Ferritin in iron containing granules from the fat body of the honeybees Apis mellifera and Scaptotrigona postica. Micron. 2002;33:53–59. doi: 10.1016/s0968-4328(00)00071-8. [DOI] [PubMed] [Google Scholar]
- 45.Mehta A, Deshpande A, Bettedi L, Missirlis F. Ferritin accumulation under iron scarcity in Drosophila iron cells. Biochimie. 2009;91:1331–1334. doi: 10.1016/j.biochi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Geiser DL, Mayo JJ, Winzerling JJ. The unique regulation of Aedes aegypti larval cell ferritin. Insect Biochem Mol Biol. 2007;37:418–429. doi: 10.1016/j.ibmb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Geiser DL, Zhang D, Winzerling JJ. Secreted ferritin: mosquito defense against iron overload? Insect Biochem Mol Biol. 2006;36:177–187. doi: 10.1016/j.ibmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Lodish H, Berk A, Kaiser CA, Krieger M, Scott MP, Bretscher A, Ploegh H, Matsudaira P. Molecular Cell Biol ogy. Sixth. W. H. Freeman; 2008. [Google Scholar]
- 49.Missirlis F, Kosmidis S, Brody T, Mavrakis M, Holmberg S, Odenwald WF, Skoulakis EM, Rouault TA. Homeostatic mechanisms for iron storage revealed by genetic manipulations and live imaging of Drosophila ferritin. Genetics. 2007;177:89–100. doi: 10.1534/genetics.107.075150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winzerling JJ, Nez P, Porath J, Law JH. Rapid and efficient isolation of transferrin and ferritin from Manduca sexta. Insect Biochem Mol Biol. 1995;25:217–224. doi: 10.1016/0965-1748(94)00058-p. [DOI] [PubMed] [Google Scholar]
- 51.Dunkov BC, Georgieva T. Organization of the ferritin genes in Drosophila melanogaster. DNA and Cell Biol. 1999;18:937–944. doi: 10.1089/104454999314791. [DOI] [PubMed] [Google Scholar]
- 52.Nichol H, Locke M. The characterization of ferritin in an insect. Insect Biochem Mol Biol. 1989;19:587–602. [Google Scholar]
- 53.Kemna EH, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93:90–97. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- 54.Zhang AS, Enns CA. Iron homeostasis: recently identified proteins provide insight into novel control mechanisms. J Biol Chem. 2009;284:711–715. doi: 10.1074/jbc.R800017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EM, Beutler E, Beutler B. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winzerling JJ, Pham DQ. Iron metabolism in insect disease vectors: mining the Anopheles gambiae translated protein database. Insect Biochem Mol Biol. 2006;36:310–321. doi: 10.1016/j.ibmb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- 59.Andrews SC, Arosio P, Bottke W, Briat JF, Von Darl M, Harrison PM, Laulhere HP, Levi S, Lobreaux S, Yewdall SJ. Structure, function and evolution of ferritin. J Inorg Biochem. 1992;47:161–174. doi: 10.1016/0162-0134(92)84062-r. [DOI] [PubMed] [Google Scholar]
- 60.Georgieva T, Dunkov BC, Dimov S, Ralchev K, Law JH. Drosophila melanogaster ferritin: cDNA encoding a light chain homologue, temporal and tissue specific expression of both subunit types. Insect Biochem Mol Biol. 2002;32:295–302. doi: 10.1016/s0965-1748(01)00090-x. [DOI] [PubMed] [Google Scholar]
- 61.Capurro MD, Iughetti P, Ribolla PEM, DeBianchi AG. Musca domestica hemolymph ferritin. Archs Insect Biochem Physiol. 1996;32:197–207. doi: 10.1002/(SICI)1520-6327(1996)32:2<197::AID-ARCH4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 62.Dunkov BC, Georgieva T, Yoshiga T, Hall M, Law JH. Aedes aegypti ferritin heavy chain homologue: feeding of iron or blood influences message levels, lengths and subunit abundance. J Insect Science. 2002;2:7. doi: 10.1093/jis/2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Missirlis F, Holmberg S, Georgieva T, Dunkov BC, Rouault TA, Law JH. Characterization of mitochondrial ferritin in Drosophila. Proc Natl Acad Sci U S A. 2006;103:5893–5898. doi: 10.1073/pnas.0601471103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pascoa V, Oliveira PL, Dansa-Petretski M, Silva JR, Alvarenga PH, Jacobs-Lorena M, Lemos FJ. Aedes aegypti peritrophic matrix and its interaction with heme during blood digestion. Insect Biochem Mol Biol. 2002;32:517–523. doi: 10.1016/s0965-1748(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 65.Oliveira MF, Silva JR, Dansa-Petretski M, de Souza W, Braga CMS, Masuda H, Oliveira PL. Haemozoin formation in the midgut of the blood-sucking insect Rhodnius prolixus. FEBS Let. 2000;477:95–98. doi: 10.1016/s0014-5793(00)01786-5. [DOI] [PubMed] [Google Scholar]
- 66.Georgieva T, Dunkov B, Law JH. Anopheles gambia secreted ferritin: characterization of cDNAs encoding two subunits, mRNA expression patterns, and gene organization. Fourth International Symposium on Molecular Insect Science; Tucson, AZ. 2002. [Google Scholar]
- 67.Kim HJ, Yun CY, Cheon HM, Chae B, Lee IH, Park SJ, Kang YJ, Seo SJ. Hyphantria cunea ferritin heavy chain homologue: cDNA sequence and mRNA expression. Arch Insect Biochem Physiol. 2004;56:21–33. doi: 10.1002/arch.10141. [DOI] [PubMed] [Google Scholar]
- 68.Konijn AM, Glickstein H, Vaisman B, Meyron-Holtz EG, Slotki IN, Cabantchik ZI. The cellular labile iron pool and intracellular ferritin in K562 cells. Blood. 1999;94:2128–2134. [PubMed] [Google Scholar]
- 69.Kidane TZ, Sauble E, Linder MC. Release of iron from ferritin requires lysosomal activity. Am J Physiol Cell Physiol. 2006;291:C445–455. doi: 10.1152/ajpcell.00505.2005. [DOI] [PubMed] [Google Scholar]
- 70.Truty J, Malpe R, Linder MC. Iron prevents ferritin turnover in hepatic cells. J Biol Chem. 2001;276:48775–48780. doi: 10.1074/jbc.M105392200. [DOI] [PubMed] [Google Scholar]
- 71.Geiser DL, Chavez C, Pham DQD, Zhang DZ, Winzerling JJ. Response of Aedes aegypti larval cells to iron. FASEB J. 2003;17:A174–A174. [Google Scholar]
- 72.Levi S, Corsi B, Bosisio M, Invernizzi R, Volz A, Sanford D, Arosio P, Drysdale J. A human mitochondrial ferritin encoded by an intronless gene. J Biol Chem. 2001;276:24437–24440. doi: 10.1074/jbc.C100141200. [DOI] [PubMed] [Google Scholar]
- 73.Bartfeld NS, Law JH. Isolation amd molecular cloning of transferrin from the tobacco hornworm Manduca sexta. J Biol Chem. 1990;265:21684–21691. [PubMed] [Google Scholar]
- 74.Ferreira C, Bucchini D, Martin ME, Levi S, Arosio P, Grandchamp B, Beaumont C. Early embryonic lethality of H ferritin gene deletion in mice. J Biol Chem. 2000;275:3021–3024. doi: 10.1074/jbc.275.5.3021. [DOI] [PubMed] [Google Scholar]
- 75.Wilkinson J, Di X, Schonig K, Buss JL, Kock ND, Cline JM, Saunders TL, Bujard H, Torti SV, Torti FM. Tissue-specific expression of ferritin H regulates cellular iron homoeostasis in vivo. Biochem J. 2006;395:501–507. doi: 10.1042/BJ20060063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cozzi A, Corsi B, Levi S, Santambrogio P, Albertini A, Arosio P. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J Biol Chem. 2000;275:25122–25129. doi: 10.1074/jbc.M003797200. [DOI] [PubMed] [Google Scholar]
- 77.Hajdusek O, Sojka D, Kopacek P, Buresova V, Franta Z, Sauman I, Winzerling J, Grubhoffer L. Knockdown of proteins involved in iron metabolism limits tick reproduction and development. Proc Natl Acad Sci U S A. 2009;106:1033–1038. doi: 10.1073/pnas.0807961106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacob M. Scientists target return of infectious diseases. Gen Eng News. 2001;21:11–80. [Google Scholar]
- 79.Marshall E. A renewed assault on an old and deadly foe. Science. 2000;290:428–441. doi: 10.1126/science.290.5491.428. [DOI] [PubMed] [Google Scholar]
- 80.Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides G, Chrystal M, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans C, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer M, Hladun S, Hogan J, Hong Y, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O'Brochta DA, Pfannkoch C, Qi R, Regier M, Remington K, Shao H, Sharakhova M, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton L, Topalis P, Tu Z, Unger M, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu S, Zhimulev I, Coluzzi M, della Torre A, Roth C, Louis C, Kalush F, Mural R, Myers E, Adams M, Smith H, Broder S, Gardner M, Fraser C, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos F, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambia. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 81.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 82.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 83.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang D, Ferris C, Kohlhepp P, Winzerling JJ. Manduca sexta IRP1: Molecular characterization and in vivo IRP1/IRE binding activity in response to iron. Insect Biochem Mol Biol. 2001;32:85–96. doi: 10.1016/s0965-1748(01)00083-2. [DOI] [PubMed] [Google Scholar]