Abstract
Electrically conductive polymer composites composed of polycaprolactone fumarate and polypyrrole (PCLF-PPy) have been developed for nerve regeneration applications. Here we report the synthesis and characterization of PCLF-PPy and in vitro studies showing PCLF-PPy materials support both PC12 cell and dorsal root ganglia (DRG) neurite extension. PCLF-PPy composite materials were synthesized by polymerizing pyrrole in pre-formed PCLF scaffolds (Mn 7,000 or 18,000 g mol−1) resulting in interpenetrating networks of PCLF-PPy. Chemical compositions and thermal properties were characterized by ATR-FTIR, XPS, DSC, and TGA. PCLF-PPy materials were synthesized with five different anions (naphthalene-2-sulfonic acid sodium salt (NSA), dodecylbenzenesulfonic acid sodium salt (DBSA), dioctyl sulfosuccinate sodium salt (DOSS), potassium iodide (I), and lysine) to investigate effects on electrical conductivity and to optimize chemical composition for cellular compatibility. PCLF-PPy materials have variable electrical conductivity up to 6 mS cm−1 with bulk compositions ranging from 5 to 13.5 percent polypyrrole. AFM and SEM characterization show microstructures with a root mean squared (RMS) roughness of 1195 nm and nanostructures with RMS roughness of 8 nm. In vitro studies using PC12 cells and DRG show PCLF-PPy materials synthesized with NSA or DBSA support cell attachment, proliferation, neurite extension, and are promising materials for future studies involving electrical stimulation.
Keywords: Electrically Conductive, Polypyrrole, Nerve, PCLF
1. Introduction
Traumatic injuries resulting in neurological damage to either the central or peripheral nervous system occur frequently. Spinal cord injuries (SCI) affect over 250,000 individuals in the U.S. with 12,000 new cases occurring every year [1]. Peripheral nerve injuries (PNI) are more common, with estimates as high as 5 percent of all patients admitted to level 1 trauma [2]. The frequency and disability associated with PNI injury necessitates the need for therapies to restore the loss of function. The current clinical standard for the treatment of PNI with segmental nerve loss is the use of nerve autografts, which remove a piece of non-critical nerve from a secondary site on the body to replace the missing nerve section. This technique has significant drawbacks including donor site morbidity, insufficient donor nerve length, mismatch of diameter between donor nerve and recipient site, misaligned endoneurial tubes, and mismatched regenerating axons.
The drawbacks associated with autografts motivate the search for alternate treatment options. Synthetic materials have great potential for applications as nerve guidance conduits because they can be fabricated with various dimensions, degradation rates, chemical compositions, mechanical properties, micro-architectures, and external geometries [3–8]. In addition, therapeutic drugs can be loaded into the scaffolds for controlled release over days or weeks, and cellular therapies, such as stem cells [9, 10], adipose derived stromal cells [11], or Schwann cells can be cultured on the scaffolds before implantation [12, 13].
Regeneration of damaged nerves faces another obstacle in addition to the above mentioned challenges. As time passes and nerves extend from the proximal to the distal stump, regenerating axons and the target organs or muscle increasingly lose their regenerative capacity [14–16]. Therefore, increasing the rate of nerve regeneration through stimulation may be a critical step to realizing full functional recovery after segmental nerve loss. Electrical stimulation as a therapeutic treatment is a rapidly expanding area in the field of tissue engineering, especially for nerve applications, with numerous reports showing electrical stimulation increases neurite and axon extension in vitro and nerve regeneration in vivo. Electrical stimulation by either direct exposure to electrical current (AC or DC) or via an electrical field has been shown to effect stem cell differentiation [17, 18], neurite extension [19, 20], and influence directionality of growing axons [21].
Techniques to incorporate electrically conductive materials into biomaterials have included attachment of metal electrodes to proximal and distal nerve stumps [22, 23], scaffolds coated with gold nanoparticles [24], and electrically conductive polymers such as polypyrrole [25–36] or polyaniline [37, 38]. Schmidt et al. was one of the first researchers to demonstrate that using the conductive polymer polypyrrole and applying an electrical current through the material has a positive effect on neurite extension from PC12 cells [20]. Since then, numerous groups have thoroughly investigated many aspects of polypyrrole including in vitro and in vivo biocompatibility, stability, conductivity, incorporation of the cell adhesive polypeptide RGD, and more [20, 27, 29–31, 36]. However, most of this work focuses on thin films of polypyrrole.
Although polypyrrole could be very useful for tissue engineering applications, materials composed solely of polypyrrole are not acceptable as biomaterials. PPy has very low solubility in most solvents that makes it difficult to process into complex three-dimensional structures, poor mechanical properties that make the materials brittle and weak, and is non-biodegradable. Different approaches have been attempted to overcome these limitations and incorporate electrically conductive polymers into biomaterials. Some examples include blending polypyrrole with poly(lactic-co-glycolic acid) [34, 39–42] , block copolymers of polylactide and polyaniline [37, 38], nanoparticles composed of polypyrrole-polyethyleneglycol-polylactic acid [35], and the templated synthesis of polypyrrole [26].
Here we report the synthetic method to produce composite materials composed of polycaprolactone fumarate (PCLF) and polypyrrole (PPy). PCLF (chemical structure shown in Figure 1) is a chemical or photo-cross-linkable derivative of polycaprolactone that can be easily processed into complex three-dimensional structures by injection molding or solid freeform fabrication. PCLF has been shown to exhibit biocompatibility, good mechanical properties, and tunable degradation rates that make it a promising material for application as nerve guidance conduits[8, 43]. PCLF has previously been shown to direct nerve regeneration in the rat sciatic nerve defect model [7] and is currently under in vivo study as nerve guidance conduits in conjunction with therapeutic drugs, Schwann cells, and adipose-derived stem cells. However, a major issue with polymeric nerve conduits in general is that regenerating nerve tissue grows through the polymer as a cable and is surrounded by a thick wall of fibrous tissue that does not make any contact with the polymer walls[7]. This significantly restricts the available space for regenerating tissue. Therefore, the development of materials that promote neural cell attachment and decrease fibrous tissue ingrowth into the scaffold would represent an attractive improvement to these scaffolds.
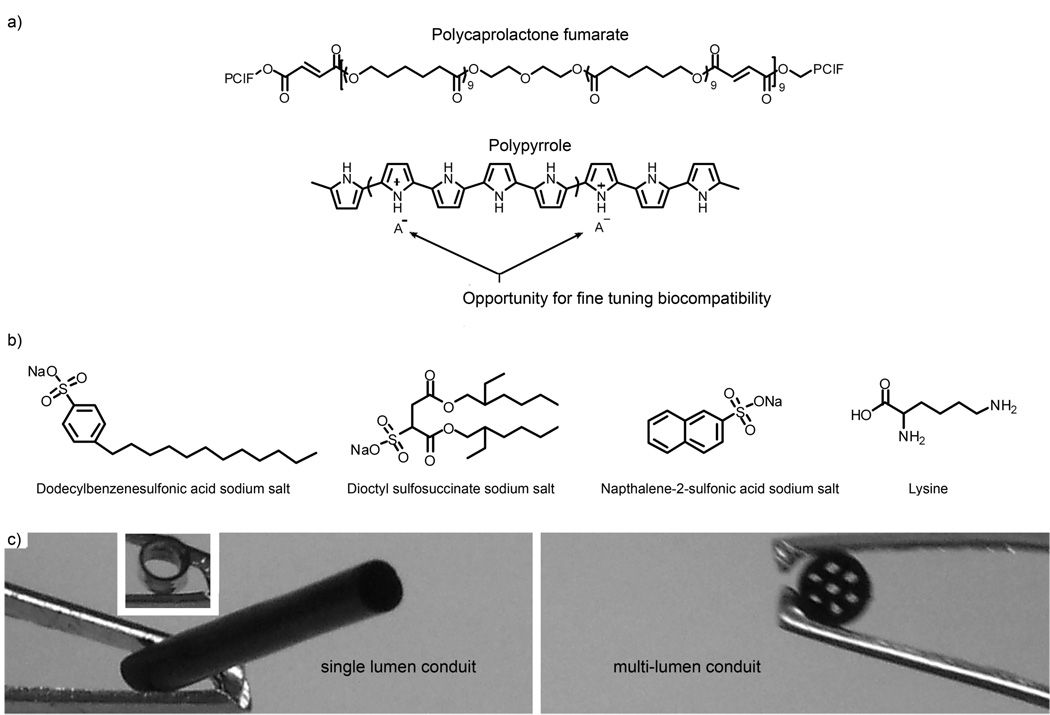
Figure 1.
A) Chemical structures of polycaprolactone fumarate and polypyrrole. B) Anions used in the synthesis of polypyrrole to modify the chemical composition of the resulting PCLF-PPy scaffolds. C) Single lumen and multi-lumen nerve conduits composed of PCLF-PPy illustrating that these materials can be easily fabricated into three-dimensional structures.
To increase cellular compatibility and stimulate nerve regeneration, PCLF was extended to the electrically conductive PCLF-PPy composite materials. PCLF-PPy polymer composites can be easily fabricated into complex three-dimensional structures, such as single lumen and multi-lumen nerve conduits shown in Figure 1, and overcome the limitations associated with processing polypyrrole into complex three-dimensional structures. PCLF-PPy materials maintained the physical properties of the host polymer PCLF. This alleviates the poor mechanical properties associated with using PPy and incorporates the property of electrical conductivity into the scaffold. Herein we describe the synthesis and characterization of PCLF-PPy electrically conductive composite polymeric materials. We also report the cellular response of PC12 cells and DRG explants when cultured on these materials, and determine the chemical compositions that promote the most favorably cellular responses for future work involving electrical stimulation.
2. Materials and Methods
2.1 Materials
All chemicals were purchased from Aldrich or Fisher Chemicals and used as received unless noted otherwise. Polycaprolactone fumarate (PCLF) was synthesized by previously reported procedures[8] from PCL diol with a number average molecular weight (Mn) of 2,000 g mol−1. The resulting PCLF had a Mn of 7, 000 or 18,000 g mol−1 and a polydispersity index (PDI) of 1.96 or 1.82, respectively. Different molecular weight PCLF will be distinguished in this manuscript by the following nomenclature PCLF7000 or PCLF18000. The Mn and PDI were characterized by gel permeation chromatography (GPC) using polystyrene standards. The GPC system consists of a Waters 2410 refractive index detector, 515 HPLC pump, and 717 Plus autosampler, and a Styragel HR4E column. THF was used as the eluent at 1 mL/min.
2.2 Synthesis of PCLF-PPy composite materials
2.2.1 PCLF scaffold fabrication
The photo-initiator phenyl bis(2,4,6-trimethylbenzoyl) phosphine oxide (BAPO) (300 mg, 72 µmol) was dissolved in 3 mL methylene chloride (MeCl2). Three hundred µL of BAPO solution was added to PCLF7000 (3.0 g), and MeCl2 (0.6 mL). The mixture was gently heated and vortexed to form a viscous homogenous liquid that was poured into glass molds consisting of two glass slides separated by a Teflon spacer with thicknesses of 0.5 mm. These molds containing the PCLF mixture were then placed in a UV chamber and irradiated for 1 h at λ = 315–380 nm to cross-link the PCLF material. The cross-linked PCLF sheets were removed from the molds and submerged in MeCl2 to remove uncross-linked oligomers and then dried under vacuum. Scaffolds using PCLF18000 were fabricated under the same conditions. Single and multi-lumen nerve conduits of PCLF were fabricated by injection molding as described previously[3, 7].
2.2.2 Synthesis of PCLF18000PPyNSA composite materials
Benzoyl peroxide was dissolved in MeCl2 at concentrations ranging from 12.5 to 100 mg mL−1. PCLF scaffolds fabricated above (0.1 g) were submerged in the benzoyl peroxide solution for times ranging from 30 to 600 seconds. Variation of concentration and time were used to control the amount of benzoyl peroxide occluded within the PCLF scaffold. The swollen PCLF scaffold containing benzoyl peroxide was removed from the benzoyl peroxide solution and then dried under vacuum for 10 minutes to remove residual MeCl2. Freshly distilled pyrrole (0.56 g, 8.4 mmol) and naphthalene-2-sulfonic acid sodium salt (NSA) (0.4 g, 1.7 mmol) were dissolved in 20 mL of deionized distilled water and cooled to 0 °C. For anion variation the molar equivalent of NSA was used. The dry PCLF scaffolds containing benzoyl peroxide were submerged in the aqueous pyrrole/anion solution and stirred for 12 h. The scaffold was removed, washed with acetone and excessive amounts of water to remove excess dopant and pyrrole, and then swelled sequentially in methylene chloride, acetone, and ethanol to remove residual impurities before drying under vacuum. The nomenclature for PCLF-PPy materials used from here on is PCLFmolecular weightPPyanion when addressing a specific composition, and PCLF-PPy when making general statements about the materials.
2.3 Characterization of PCLF-PPy composite materials
2.3.1 X-ray Photoelectron Spectroscopy (XPS)
The surface elemental composition was characterized on a custom-designed Kratos Axis Ultra X-ray photoelectron spectroscopy system. A complete description of the instrument is given elsewhere [44]. Briefly, the surface analysis chamber is equipped with a monochromated 1486.6 eV aluminum Kα source having a 500 mm Rowland circle silicon single crystal monochromator. The typical X-ray gun settings were 15 mA emission current at an accelerating voltage of 15 kV. Low energy electrons were used for charge compensation to neutralize the sample. Survey scans were collected using the following instrument parameters: an energy scan range of 1200 to −5 eV; pass energy of 160 eV; step size of 1 eV; dwell time of 200 ms and an X-ray spot size of 700×300 µm. High resolution spectra were acquired in the region of interest using the following experimental parameters: 20 to 40 eV energy window; pass energy of 20 eV, step size of 0.1 eV and dwell time of 1000 ms. The absolute energy scale was calibrated to the Cu 2p2/3 peak binding energy of 932.6 eV using an etched copper plate. A magnetic lens, mounted below the sample, combined with the electrostatic lenses, was used to focus the scattered electron beam from the surface. A hemispherical sector analyzer (HSA) was used to analyze the electron kinetic energy, while a delay-line detector measured the electron count.
All spectra were calibrated using the adventitious carbon 1s peak at 285.0 eV. A Shirley-type background was subtracted from each spectrum to account for inelastically scattered electrons that contribute to the broad background. Commercially available CasaXPS software was used to process the XPS data [45]. Transmission corrected relative sensitivity factor (RSF) values from the Kratos library were used for elemental quantification, as implemented into CasaXPS. The components of the peaks contain a Gaussian/Lorentzian product with 30% Lorentzian and 70% Gaussian character. An error of ±0.2 eV is reported for all the peak binding energies.
2.3.2 Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
The chemical compositions of cross-linked polymeric materials were characterized on a Nicollet 8700 FTIR spectrometer. A Germanium ATR crystal was used at a resolution of 4 cm−1 at 1000 cm−1. Spectra were obtained with a minimum of 64 scans.
2.3.3 Scanning electron microscopy (SEM)
Surface topographies were imaged using a Hitachi 4700 field emission spectrometer. PCLF polymer disks were sputter coated with Au/Pd to prevent sample charging during imaging. PCLF-PPy composite materials were imaged uncoated because the materials are electrically conductive and do not require coating to prevent charging. The samples were imaged at an accelerating voltage of 5 kV.
2.3.4 Atomic Force Microscopy (AFM)
Atomic force microscopy images were taken using Asylum Research MFP-3D instrument. NSC15-AlBS cantilevers from MikroMasch with typical resonant frequency of 315 kHz and typical force constant of 40 N/m were used in all experiments. Samples were fixed to a glass slide with epoxy glue. Images were acquired in air using alternating current (AC) method.
2.3.5 Thermal Analysis
Thermogravimetric analysis (TGA) was performed on a TA Instruments Q500 thermal analyzer. Samples were heated from room temperature to 800°C at a rate of 1°C min−1 under flowing nitrogen. Dynamic scanning calorimetry (DSC) was performed on a TA Instruments Q1000 differential scanning calorimeter. Under a nitrogen atmosphere the sample underwent a heat-cool-heat cycle to ensure the same thermal history between samples. Samples were heated from room temperature to 100°C, then cooled to −80°C, and then heated to 150°C at a rate of 5°C min−1.
2.3.6 Electrical conductivity
The resistance of polymer films was measured by the 4-point probe method. The 4-point probe was fabricated based on previous literature reports using the parallel plate model [46]. Gold electrodes used for the measurement were purchased from Case Western Reserve Electronics Design Center. An electrophoresis DC current source (Hoeffer PS 3000) was used to supply the current. The voltage and current were measured using a Fluke 73 multimeter. The resistance was calculated using the following equation: ρ = 4.53Vwh/IL. 4.53 is the geometric correction factor for samples with h < 0.5L, V is volts measured, I is current measured, w is width of sample, h is sample thickness, and L is sample length between electrodes[46–48]. Conductivity is equal to 1/ρ.
2.4 In vitro cell culture studies
2.4.1 PC12 cellular response to PCLF-PPy composite materials
Dubelco’s Modification of Eagle’s Medium (DMEM) (Mediatech) media supplemented with 10% heat inactivated horse serum, 5% heated inactivated fetal bovine serum, and 0.5% penicillin/streptomycin was used for PC12 (ATCC) cell culture. PCLF-PPy composite materials were fabricated into disks of diameters 1.0 cm as described above, sterilized with 70 % ethanol and used as is. Toxicity of residual starting materials leaching from PCLF-PPy scaffolds was evaluated using a non contact method. PC12 cells were seeded in 12-well plates at a density of 20,000 cells cm−2 for 24 h prior to the addition of the polymeric material contained in transwells. PC12 cells were cultured in the presence of polymeric materials for 1 day, and then the cell numbers were quantified with an MTS assay (CellTiter 96, Promega, Madison WI). The transwells were transferred to fresh wells containing cells and cultured for another 3 and 7 days.
To investigate PC12 cell response when cultured on different polymeric materials, 1.0 cm disks were placed in 24-well plates. The scaffolds were sterilized in 70% aqueous ethanol for 30 minutes and then rinsed with sterile phosphate buffered saline (PBS). Autoclaved medical grade silicon tubing was inserted into the well to limit the surface area of the polymer disk to a diameter of 0.95 cm with a surface area of 0.71 cm2. The well was filled with media and incubated for 12 hours to remove any remaining impurities. PC12 cells were plated at a density of 30,000 cells cm−2. Experiments were performed with 50 ng mL−1 nerve growth factor (Harlan) supplemented media.
Cell viability was determined using MTS (CellTiter 96, Promega, Madison, WI) assays. First, 0.5 mL trypsin was added to each well, aspirated, and put in the incubator for 10 min. Then 0.5 mL media was added to each well, and cells were gently dislodged from the surface with a cell scraper. Media and cells were then transferred to a new well and 0.1 mL of MTS reagent was added to each well then and incubated for 2 h at 37°C. The absorbance was measured at 490 nm on a Molecular Devices spectra max plate reader.
Cell morphologies were imaged by fluorescence microscopy. PC12 cells on polymer scaffolds were fixed in 2% paraformaldehyde in PBS for 25 min, and then washed with PBS three times. Cells were permeablized in 0.1% Triton 100X for 3 min and then incubated in 10% horse serum in PBS for 1 h. Cells were stained in 1% rhodium phalloidin (Invitrogen, CA) in 5% horse serum in PBS for 1 h and then washed with PBS three times. Nuclei were stained with DAPI (Vector) just prior to mounting on a glass cover slip. Samples were imaged on an LSM 510 inverted confocal microscope and imaged at excitation wavelengths of 368 and 488 nm.
2.4.2 Dorsal root ganglia (DRG) explants
The animal studies were approved by the Institutional Animal Use and Care Committee at the Mayo Clinic College of Medicine. Between 10–15 DRG explants were plucked from the dissected rat E15 spinal cords in L15 media and transferred onto each of the test polymer discs with a minimum volume of MEM, 10% FCS, 33mM glucose, 5ngml NGF (nerve growth factor) media. This minimum volume allowed the DRG explants to be covered, but not to float. This enabled the DRG explants to attach for one hour in a humid 5%CO2 incubator. Additional media was then added and the DRG explants were cultured for 48 – 72 hours, before fixation in 3% paraformaldehyde, and staining with phalloidin as previously described. Fluorescence microscopy was performed with a Zeiss Axio Image Z1 microscope with a Nikon CCD camera with the longest neurite measured using Zeiss imaging software. The data was reported as means +/− standard error of the mean (sem)
2.4.3 Statistics
Experiments were performed with triplicate specimens and results are reported as mean ± standard deviation. Single factorial analysis of variance (ANOVA) was performed to determine statistical significance of the data. When a global F-test showed a significant difference at the p < 0.05 level, a paired t-test was used to determine significant differences between treatments.
3. Results
Electrically conductive PCLF-PPy composite materials were synthesized by polymerizing pyrrole in preformed cross-linked PCLF scaffolds. The resulting interpenetrating networks (IPN) of PPy and PCLF resulted in scaffolds that appeared to be macroscopically homogenous and were colored black characteristic of PPy. Because subtle differences in chemical composition have large impacts on scaffold properties, PPy was synthesized with five different anions to investigate the effect anions have on the electrical conductivity and cell attachment. The anions chosen (shown in Figure 1) include dodecylbenzenesulfonic acid sodium salt, naphthalene-2-sulfonic acid sodium salt, dioctyl sulfosuccinate sodium salt, lysine and potassium iodide. The effect of anion choice proved to be important factors for both electrical conductivity and cell attachment to PCLF-PPy materials.
3.1 Characterization of PCLF-PPy composite materials
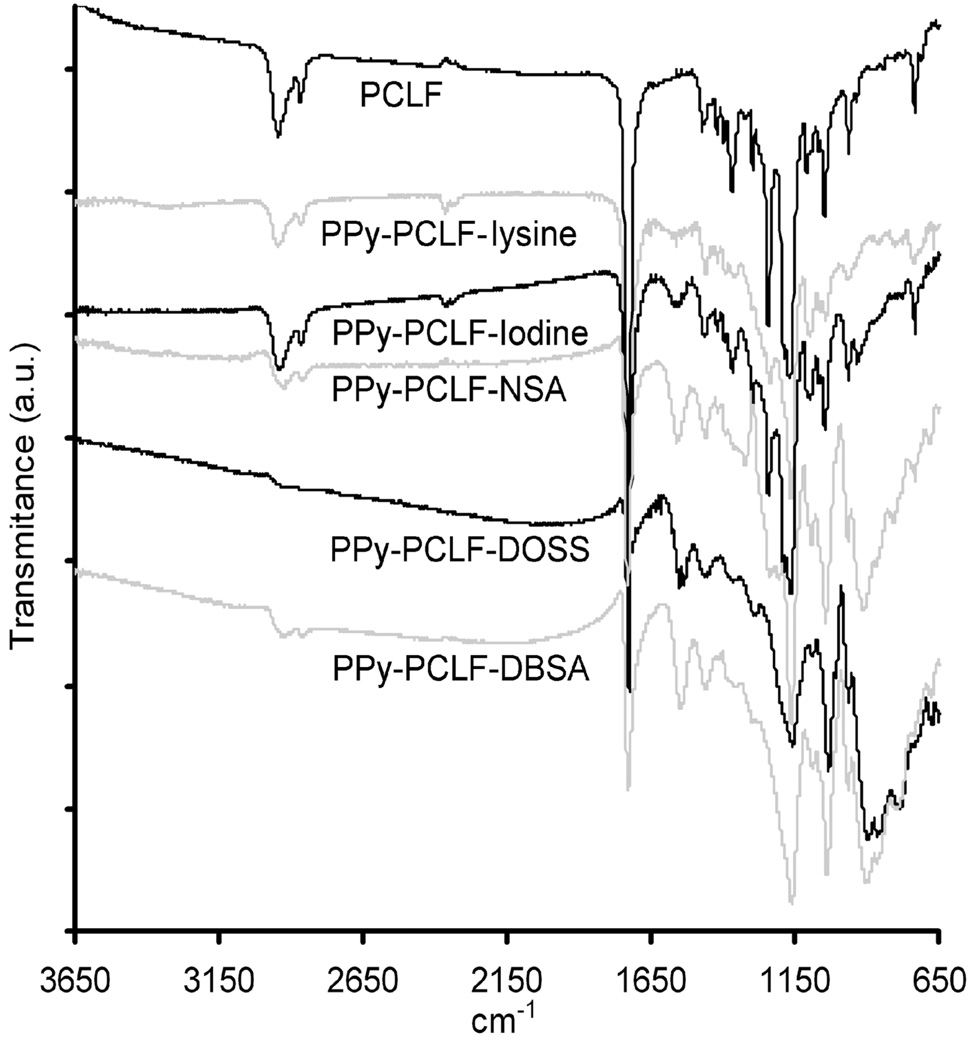
PCLF-PPy scaffolds were characterized by ATR-FTIR to confirm the presence of polypyrrole. Figure 2 shows the ATR-FTIR spectra of PCLF and PCLF-PPy composite materials synthesized with different anionic dopants. The appearance of a strong band from 1520–1610 cm−1, present in all PCLF-PPy spectra and clearly absent in the PCLF spectra, is characteristic of skeletal C-C stretches from the pyrrole ring. PCLF-PPy materials doped with sulfonic acid anions also show absorption bands corresponding to the sulfonate group at 1020–1050 cm−1 and 1140–1210 from S=O symmetric and asymmetric stretches, respectively, and 770–940 cm−1 for S-O stretch.
Figure 2.
ATR-FTIR spectra of PCLF and PCLF-PPy polymer composite materials. Absorption band from 1520–1610 cm−1 is C-C stretches from pyrrole, 1020–1050 cm−1 and 1140–1210 are S=O symmetric and asymmetric stretches respectively and 770–940 cm−1 for S-O stretches of the sulfonate anions.
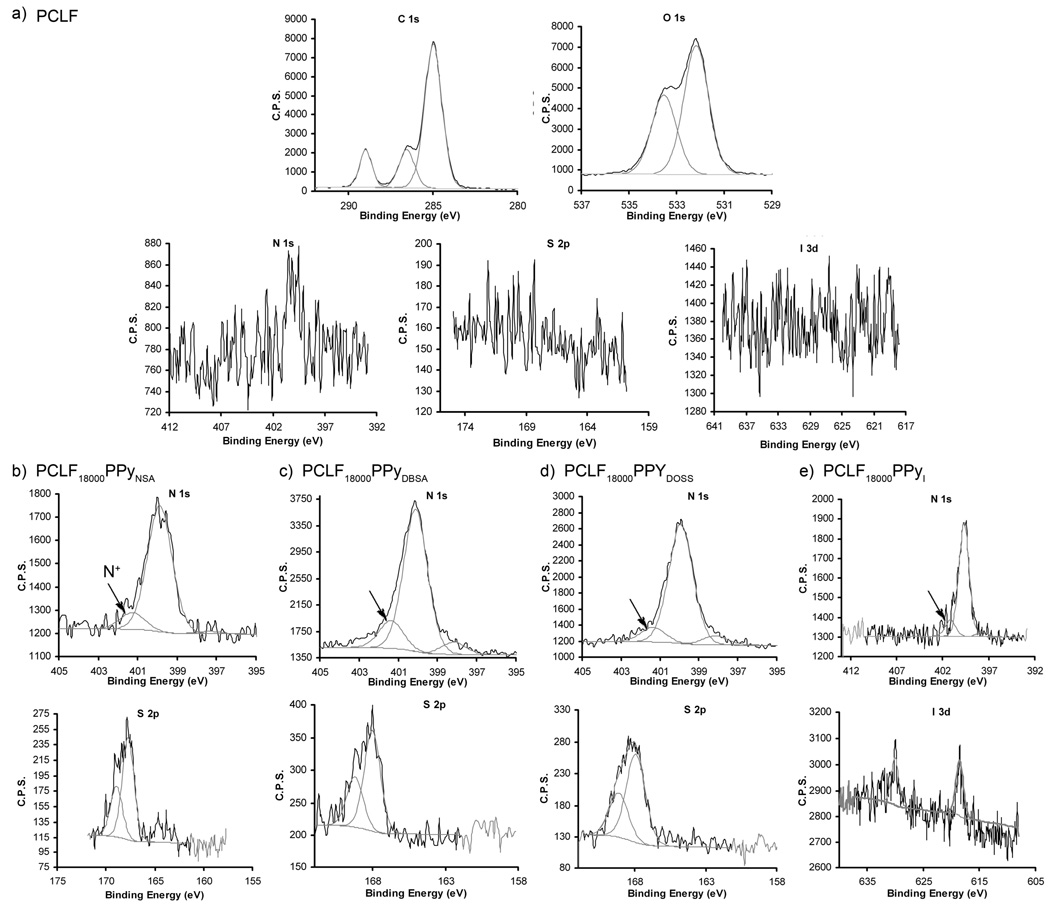
The surface elemental composition of PCLF and PCLF-PPy composite materials were characterized by XPS to quantify the amount of polypyrrole incorporated onto the scaffold surface, and the percent polypyrrole oxidized with the corresponding anion to the conductive polypyrrole species. XPS spectra of PCLF (Figure 3a) show no detectable amounts of nitrogen, sulfur, or iodide. Figures 3b, 3c, 3d, and 3e show regional scans for N and S or I corresponding to the different PCLF-PPy composites materials. Table 1 presents the PCLF-PPy scaffolds elemental compositions quantified by XPS, their corresponding electrical conductivity, the bulk composition determined by TGA, and benzoyl peroxide solution concentrations and times used to synthesize the PCLF-PPy scaffolds in this study. All PCLF-PPy composite materials show the presence of nitrogen. The nitrogen composition as determined by XPS ranged from 0.6 to 6.2 atomic percent. PCLF18000PPylysine had 0.6 atomic percent nitrogen and was not conductive. Several attempts to synthesize an electrically conductive composite polymer using this anion was attempted but was never successful. The conductive PCLF-PPy scaffolds had compositions ranging from 1.5 to 6.2 percent nitrogen. These correspond to 7.5 to 31 mol percent polypyrrole incorporate into the scaffolds. The percent of cationic nitrogen of the total nitrogen incorporated into PCLF-PPy scaffolds range from 12.2 to 23.2 percent. Cationic nitrogen is used gauge the percent of polypyrrole in the conductive form. The fully conductive bipolaron should have close to 25% cationic nitrogen and the corresponding amounts of the anion. The percent S and I measured for the PCLF-PPy samples agree well with the theoretical amounts expected based on the cationic nitrogen measured. One sample that did not have the expected anion content was PCLF18000PPyI with the anion corresponding to only 5% of the measured nitrogen. This sample also had a conductivity of 0.1 mS cm−1 which is an order of magnitude lower than other samples.
Figure 3.
a) XPS characterization of PCLF showing the presence of C1s and O1s peaks, and absence of N 1s,S 2p and I 2p peaks. b) PCLF18000PPyNSA, c) PCLF18000PPyDBSA, d) PCLF18000PPyDOSS, and e) PCLF18000PPyI regional scans of N from polypyrrole (top) and S or I from the anionic dopant (bottom).
Table 1. Quantification of polymer composition and conductivity.
XPS data showing atomic percent scaffold composition for the top 10 nm and the electrical conductivity related to the scaffold compositions.
| Polymer Composite | XPS Atomic Composition |
Conductivity | TGA Bulk Compositionb |
BP Concc | PCLF time in BP solnd |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | N | aN+ | S | I | mS cm−1 | % weight PPy | (mg mL−1) | (seconds) | |
| PCLF | 76.0 | 24.0 | 0 | - | - | - | - | - | - | - |
| PCLF7000PPyDBSA | 75.0 | 19.0 | 4.9 | 15.9 | 1.1 | - | 1.70 | 11.8 | 50 | 300 |
| PCLF7000PPyDOSS | 71.9 | 24.0 | 3.4 | 14.9 | 0.7 | - | 1.00 | 12.9 | 50 | 300 |
| PCLF7000PPyNSA | 77.8 | 19.5 | 1.5 | 12.6 | 1.2 | - | 0.01 | 5.7 | 12.5 | 90 |
| PCLF7000PPyNSA | 75.1 | 22.6 | 1.7 | 12.2 | 0.6 | - | 0.30 | 9.4 | 37.5 | 60 |
| PCLF7000PPyNSA | 80.0 | 15.9 | 3.2 | 15.7 | 0.9 | - | 1.60 | 13.5 | 100 | 90 |
| PCLF7000PPyNSA | 76.5 | 15.9 | 6.2 | 16.5 | 1.4 | - | 1.20 | 8.6 | 50 | 300 |
| PCLF18000PPyDBSA | 77.5 | 16.0 | 5.6 | 18.1 | 1.0 | - | 0.80 | 8.0 | 50 | 600 |
| PCLF18000PPyDOSS | 75.6 | 18.3 | 5.2 | 23.0 | 0.9 | - | 1.50 | 6.5 | 50 | 600 |
| PCLF18000PPyI | 77.6 | 18.3 | 3.9 | 23.2 | - | 0.2 | 0.10 | 8.0 | 50 | 600 |
| PCLF18000PPylysine | 76.8 | 22.6 | .6 | 0 | - | - | - | 7.9 | 50 | 600 |
| PCLF18000PPyNSA | 76.9 | 19.2 | 3.4 | 20.8 | .5 | - | 6.00 | 8.1 | 50 | 600 |
N+ percent of total N species.
Percent polypyrrole determined by thermogravimetric analysis.
Concentration of benzoyl peroxide in methylene chloride used to occlude benzoyl peroxide in PCLF scaffolds.
Time that PCLF scaffolds are submerged in benzoyl peroxide/methylene chloride solutions to allow diffusion of benzoyl peroxide into the PCLF scaffolds.
Generally the differences in scaffolds electrical conductivity were observed with the anionic dopant selection. The highest conductivity was measured for PCLF18000PPyNSA at 6 mS cm−1. All except one PCLF-PPy material synthesized with sulfonic acid anions and containing greater than 3% N had conductivities greater than 1 mS cm−1 as shown in Table 1. Materials synthesized with sulfonic acid anions did not exhibit large differences in conductivity even with fluctuating PPy compositions ranging from 3.4 to 6.2 atomic percent N. This maybe due to similarities of NSA, DBSA, and DOSS. PCLF18000PPyI conductivity that was an order of magnitude lower at 0.1 mS cm−1 and PCLF18000PPylysine had no measureable conductivity. PCLF18000PPylysine contained only 0.6% N in the composition, and PCLF18000PPyI contained only five percent of the anion, both are likely contributors to the low conductivity of those two scaffolds. The fluctuation in composition shown in Table 1 illustrates the difficulty of precisely controlling the final composition. Many scaffolds were synthesized under nearly identical conditions. The composition and conductivity of the material is undoubtedly influenced by a number of intimately connected factors including loading of benzoyl peroxide into the scaffold, anion diffusion into the polymer, pyrrole diffusion, polymer molecular weight, cross-linking density, and scaffold swelling ratio. It is believed that difference in ability of the different anions to diffuse into the polymeric scaffold is likely one of the critical parameters for the resulting compositions and conductivity.
Scaffolds with varying percents of polypyrrole were fabricated to investigate the effect of composition on electrical conductivity. PCLF7000PPyNSA was synthesized with 1.5 to 6.2% nitrogen incorporated into the scaffold as shown in Table 1 and the electrical conductivity ranged from 0.1 to 1.6 S cm−1. The amount of polypyrrole incorporated was influenced by varying the amount of benzoyl peroxide occluded within the PCLF scaffold by controlling benzoyl peroxide concentrations and times that PCLF scaffolds were submerged in these solutions. PCLF-PPy composite materials with compositions having greater than 3% N exhibit conductivities around 1 mS cm−1, however significant increases in nitrogen composition greater than 3% did not result in substantial increases in the electrical conductivity.
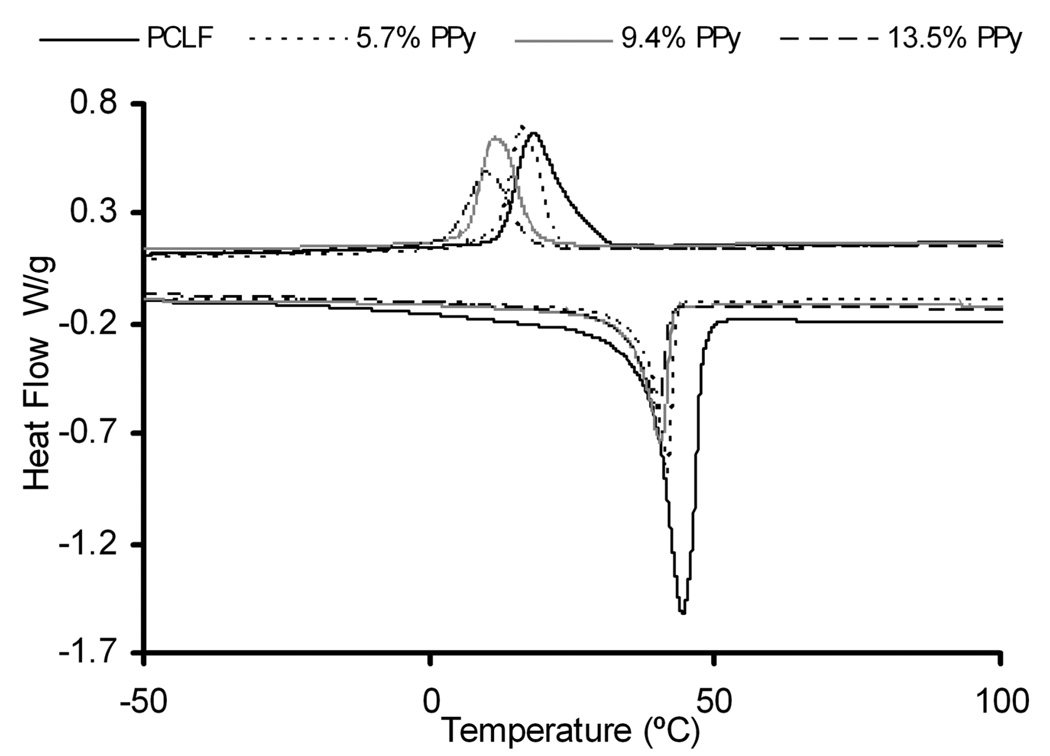
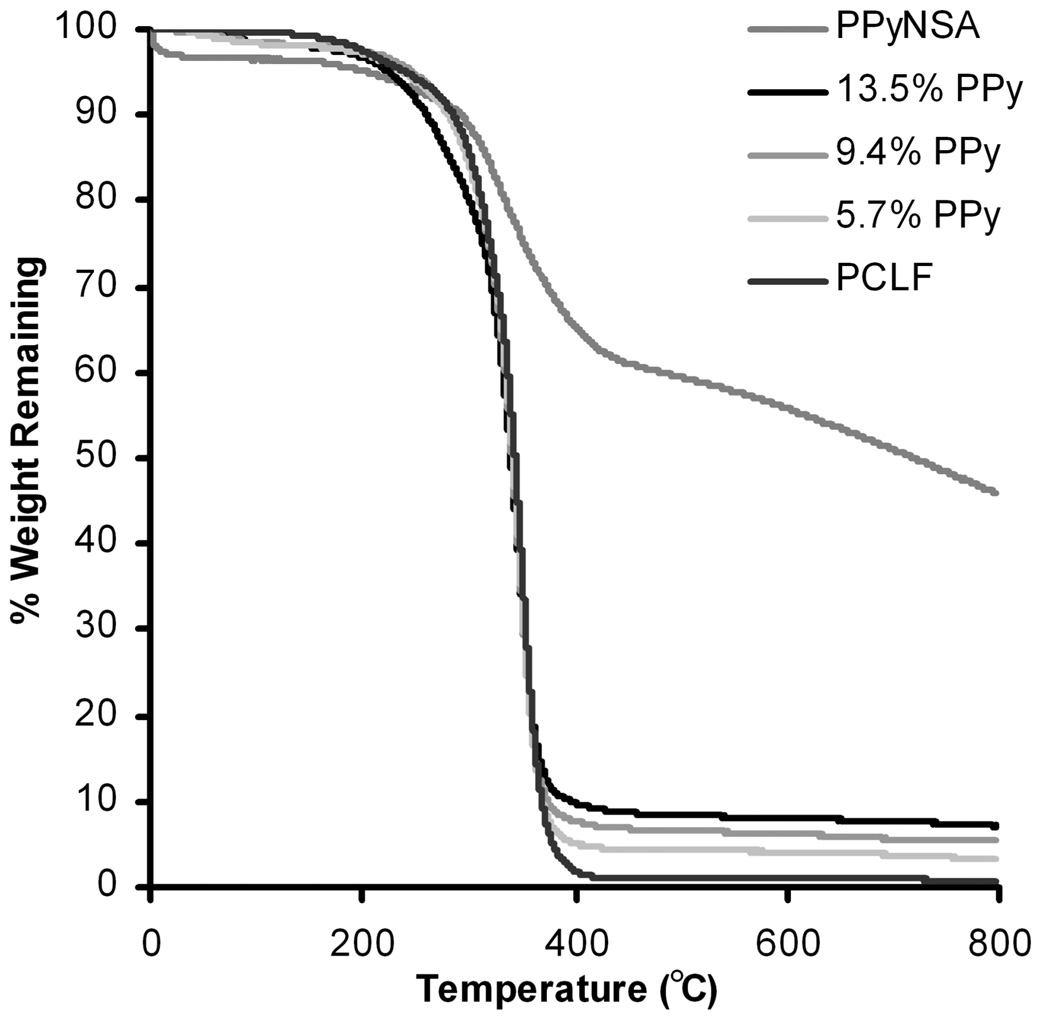
In addition to controlling electrical conductivity, the percent polypyrrole incorporated into the scaffolds can be used to tune the physical properties of PCLF-PPy scaffolds. The thermal transitions of PCLF and PCLF7000PPyNSA composites were investigated by DSC. PCLF is a semi-crystalline material with Tm and Tc transitions that straddle the 37°C body temperature. Figure 4 shows the DSC traces for PCLF7000PPyNSA as a function of bulk composition. DSC indicates that PCLF7000 has a Tm of 45°C that lowers to 42, 41, and 40°C with increasing polypyrrole composition. PCLF also has a Tc of 18°C that decreases to 16, 11, and 9°C with increases in polypyrrole. Depression of both the Tm and Tc temperatures translates to an amorphous material at physiological temperatures. The material after transitioning from crystalline to amorphous has increased flexibility that will be especially advantageous for application as nerve guidance conduits. Figure 5 shows select TGA runs of PCLF7000PPyNSA showing up to 13.5% polypyrrole successfully incorporated into the scaffold bulk material. The percent composition of the bulk material is influenced by the molecular weight of the preformed PCLF scaffolds. Because PCLF7000 an increased swelling ratio compared to PCLF18000, PCLF7000 is able to incorporate a higher amount of benzoyl peroxide into the PCLF scaffold resulting in higher PPy compositions of the final PCLF-PPy scaffolds compared to PCLF18000.
Figure 4.
DSC of PCLF7000PPyNSA as a function of percent polypyrrole composition determined by TGA.
Figure 5.
TGA of varying compositions of PCLF7000PPyNSA
3.2 Surface characterization by SEM and AFM
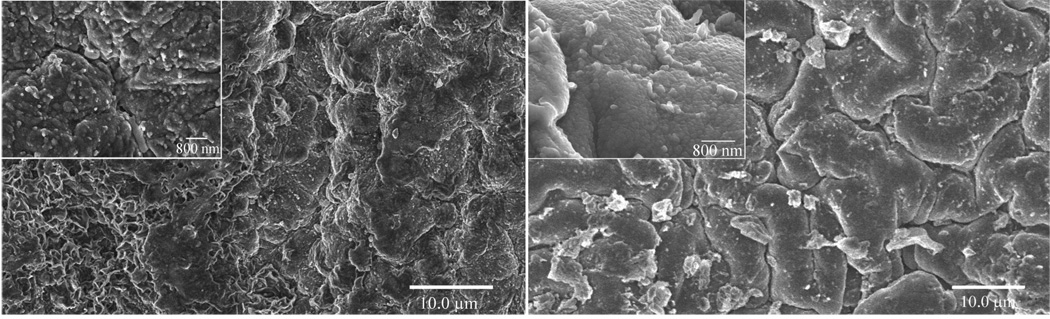
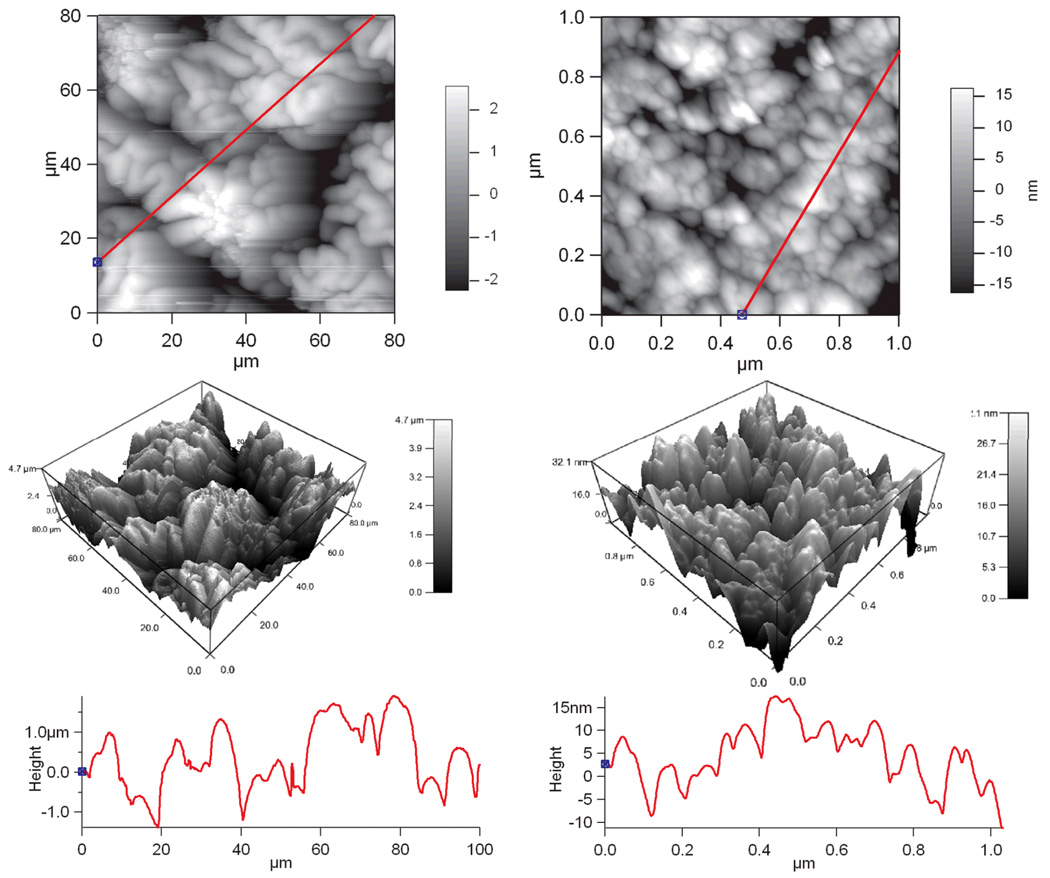
Scaffold surface topography is an important factor influencing cell attachment that can either hinder or promote cell attachment. It is generally known that rougher surfaces promote cell attachment, and it is important to be able to control this scaffold property. The scale size typically believed to be an important factor for cell attachment ranges from 10 to 1000 nm. Figure 6 contains scanning electron micrographs showing the differences in surface topography between PCLF7000PPyNSA and PCLF18000PPyNSA . The SEM shows that PCLF7000PPyNSA has a rougher surface than PCLF18000PPyNSA. This is believed to be influenced by differences in the cross-link density and sol-gel fraction of the different molecular weight PCLF polymers. PCLF18000 had increased cross-linking as indicated by the decrease in its swelling ratio to 8 from 13 for PCLF7000. This result is not surprising as PCLF18000 has approximately three times the number of potential cross-linking sites per polymer chain, and increased cross-linking density would be expected. The SEM of PCLF18000PPyNSA shows the topographical features that are tens of microns in size. This size is much larger than the nanometer size roughness that is believed to be important for increasing cell attachment. Therefore PCLF18000PPyDBSA was analyzed by AFM. The AFM micrographs of PCLF18000PPyDBSA are shown in Figure 7. Figure 7 confirms the large micron size structures seen in SEM for PCLF18000PPyNSA. The AFM quantification shows this size scale has root mean squared roughness (RMS) of 1195 nm when analyzed over tens of microns. However, when the same sample is analyzed over a 1 micron to eliminate the large structures a RMS of 8 nm is observed. The granular 100–200 nm structures observed in the Figures 6 and 7 are characteristic of polypyrrole.
Figure 6.
SEM micrographs showing surface topographies of PCLF7000PPyNSA (left), PCLF18000PPyNSA (right).
Figure 7.
AFM micrographs of PCLF18000PPyDBSA surface microstructures (left) with root mean squared roughness (RMS) of 1195 nm and nanostructure with an RMS roughness of 8 nm (right).
3.3 In vitro evaluation and comparison of materials
The PC12 cell line was used for in vitro evaluation of cytotoxicity due to leaching materials from the scaffolds, and cellular attachment, proliferation, and morphology when cultured directly PCLF-PPy scaffolds. Because of toxicity associated with starting materials from the synthesis of polypyrrole, residual monomers were extracted from PCLF-PPy scaffolds by swelling in methylene chloride followed by acetone prior to cell culture experiments. Initial cytotoxicity evaluations were performed, by plating cells 24 h prior to the addition of PCLF-PPy materials suspended within a transwell. PC12 cells remain viable for the duration of the experiment, which totaled 11 days. No decrease in cell viability was observed for cells cultured on tissue culture plates in the presence of PCLF or PCLF-PPy scaffolds indicating no toxicity of leaching materials from PCLF-PPy scaffolds.
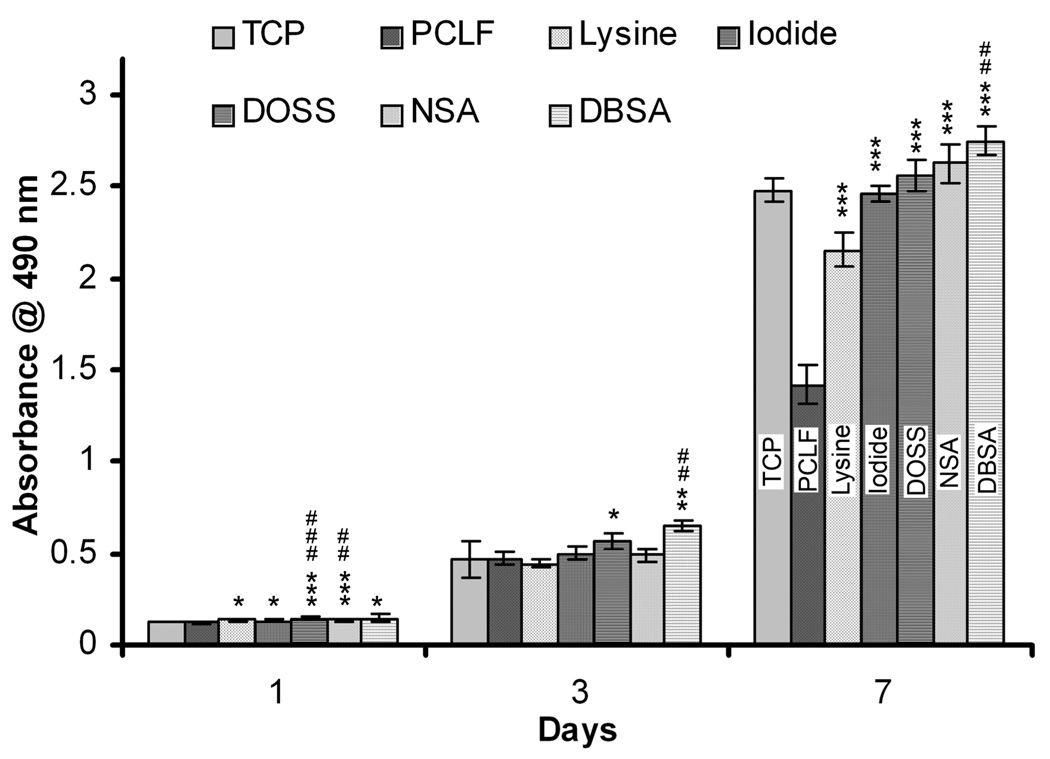
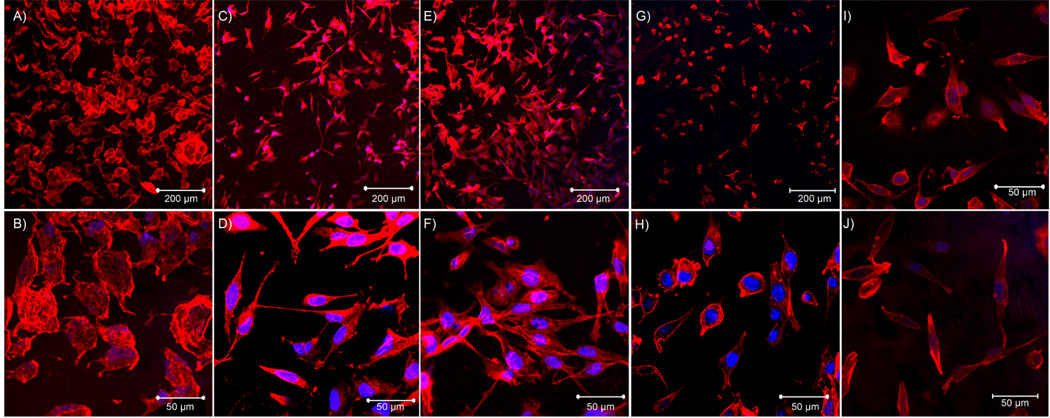
PC12 cells were plated on the surface of PCLF and PCLF-PPY scaffolds at a density of 30,000 cells cm−2. Cell attachment and proliferation were characterized by MTS assay at days 1, 3, and 7. Polystyrene tissue culture plates were used as a positive control. In order to quantify cell numbers with the MTS reagent, the PC12 cells were trypsinized from the polymer scaffolds and transferred to a new well without the polymeric material. This was necessary because interference due to interactions between the MTS dye or resulting formazan product and the PCLF-PPy scaffolds results in artificially low absorbance values. This interaction is not unexpected as the MTS dye contains a sulfonate functional group and may coordinate to the positively charged polypyrrole. Figure 8 shows that incorporation of polypyrrole into PCLF scaffolds enhances PC12 cell attachment and proliferation on PCLF-PPy over PCLF scaffolds. Significant differences were observed at day 1 for the initial PC12 cell attachment. All PCLF-PPy material showed significant increases in cell attachment (p < 0.05) over PCLF. Significant differences between PCLF and all PCLF-PPy composite materials could also be seen at day 7 (p<0.001). This could indicate that PC12 cells have a greater proliferation rate on the surface of PCLF-PPY scaffolds or that they are better attached and are not removed during media changes compared to being cultured on PCLF. PC12 cells were significantly higher PCLF18000PPyNSA and PCLF18000PPyDOSS composite materials than on the tissue culture plates after Day 1. PC12 cell morphology is an important indicator of neuronal differentiation, and can be influenced by subtle chemical cues from the scaffolds. Morphologies of PC12 cells cultured on PCLF or PCLF-PPy scaffolds show distinct differences between the materials. Cell morphologies shown in Figure 9 were imaged after 24 h by fluorescence microscopy after staining with a rhodium phalloidin stain for F-actin and DAPI stain for nuclei. PC12 cells cultured on PCLF have round morphologies with virtually no neurite extension from the cell (Figure 9A,B). However, PC12 cells cultured on PCLF18000PPyNSA (Figure 9C,D) and PCLF18000PPyDBSA (Figure 9E,F) exhibit the expected morphology for differentiating PC12 cells extending neurites. PC12 cells cultured on PCLF18000PPyDOSS (Figure 9G,H), PCLF18000PPyI (Figure 9I) and PCLF18000PPylysine (Figure 9J) have extending cell bodies but not good neurite extension.
Figure 8.
MTS assay showing differences in cell viability of PC12 on polystyrene tissue culture plates (TCP), PCLF and PCLF18000PPy composite materials doped with lysine, iodide, DOSS, NSA, and DBSA. Materials that showed results significantly greater than TCP are #p < 0.05, ##p < 0.01, and ###p < 0.001. PCLF-PPy treatments were compared to PCLF. PCLF-PPY materials with significantly higher cell numbers than PCLF are denoted as *p < 0.05, **p < 0.01, and ***p < 0.001.
Figure 9.
Confocal microscopy micrographs at 24 h of PC12 cells cultured on different polymeric materials. The different scaffolds are A & B) PCLF18000, C & D) PCLF18000PPyNSA, E & F) PCLF18000PPyDBSA, G & H) PCLF18000PPyDOSS, I) PCLF18000PPyI, J) PCLF18000PPylysine.
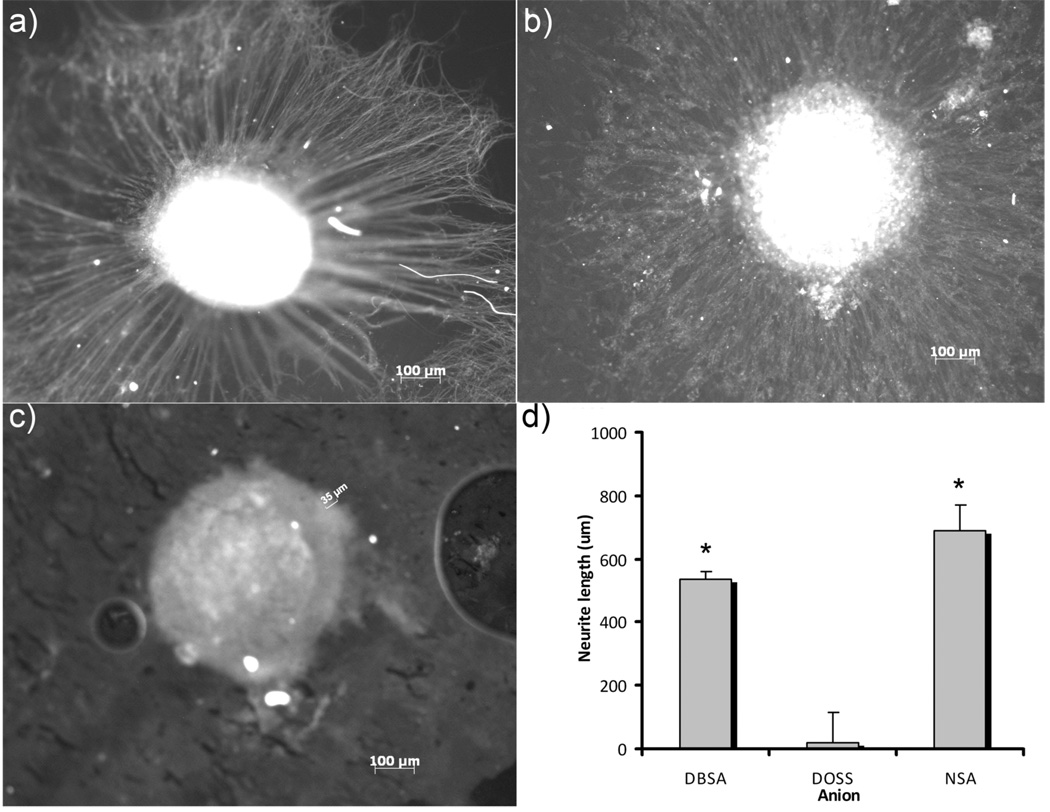
Dorsal root ganglia (DRG) were extracted from rat embryos and cultured on PCLF-PPy materials doped with DOSS, NSA, and DBSA. DRG explants include nerve cells and supporting cells such as Schwann and Glial cells that play important roles in neuron extension. Figure 10 shows that PCLF-PPy materials can support DRG attachment and neurite extension, and that the DRG response is influenced by the dopant used in the composite material. This result matches the effect of dopant on cell morphology seen with PC 12 cells with PCLF18000PPyNSA and PCLF18000PPyDBSA materials performing the best. Figure 10 shows fluorescence microscopy images of a representative DRG explants cultured on each of the different scaffolds. The images show the striking differences in neurite extension. DRG on both PCLF18000PPyNSA and PCLF18000PPyDBSA materials have neurites, while DRG on PCLF18000PPyDOSS show almost none. The quantification of the neurite extension is reported as mean ± sem, and shows that PCLF18000PPyNSA has a neurite extension of 690 ± 80 um, PCLF18000PPyDBSA has 536 ± 99 um, and PCLF18000PPyDOSS shows 17 ± 23 um. Statistical analysis indicates that both PCLF18000PPyNSA and PCLF18000PPyDBSA are significantly greater than PCLF18000PPyDOSS at the p < 0.05 level, but no significant difference was observed for DRG neurite extension on PCLF18000PPyNSA and PCLF18000PPyDBSA scaffolds.
Figure 10.
Fluorescence microscopy images of neurite extension from DRG explants cultured on a) PCLF18000PPyNSA, b) PCLF18000PPyDBSA, and c) PCLF18000PPyDOSS. d) Quantification of neurite extension from DRG explants. *Statistically significant greater neurite extension than PCLF18000PPyDOSS at p < 0.05. No other statistically significant differences were observed.
4. Discussion
Polypyrrole has gained increasing interest in biomaterials over the last decade because of the positive effect electrical stimulation has been shown to have on tissue regeneration. However polypyrrole has poor mechanical properties resulting in weak and brittle materials and is non-degradable making it unsuitable for applications in peripheral nerve regeneration. Composite materials that incorporate a small amount of polypyrrole with another polymer that has suitable material properties can overcome these limitations. This approach was motivated by our interest in studying electrical stimulation for its benefits on nerve regeneration in future work. PCLF has been proven to be a promising material for nerve regeneration because of its material properties and in vitro performance. Because of this we extended PCLF to the electrically conductive PCLF-PPy composite materials. PCLF-PPy was synthesized by polymerizing pyrrole in preformed cross-linked PCLF scaffolds. This allows the fabrication of complex three-dimensional scaffolds that avoids processing difficulties often associated with PPy. Cross-linked polymeric materials have advantages over non-cross-linked materials because they swell in organic solvents, but do not dissolve. This results in materials that are more robust for post scaffold fabrication modification by allowing the occlusion of small molecules within the cross-linked polymer matrix, while maintaining the original geometric shape of the scaffolds. Benzoyl peroxide, the initiator for polymerization of pyrrole, was occluded within cross-linked PCLF scaffolds by submerging scaffolds in a solution of benzoyl peroxide in methylene chloride. The cross-linked scaffold swells as methylene chloride and benzoyl peroxide diffuse in. Subsequent removal of methylene chloride by evaporation leaves benzoyl peroxide occluded within the PCLF scaffold. PCLF scaffolds containing benzoyl peroxide are then submerged in aqueous solutions of pyrrole. Pyrrole diffuses into the scaffold and is rapidly polymerized resulting in an interpenetrating network (IPN) of PCLF and PPy. This methodology for creating IPNs is robust and should be able to be applied to many different types of cross-linked polymeric materials.
PCLF was synthesized from PCL diol with a Mn of 2000 g mol−1 and fumaryl chloride. Two different molecular weight PCLF polymers were synthesized with Mn of 7,000 or 18,000 g mol−1. Initially PCLF7000 was used to synthesize PCLF-PPy scaffolds. However, due to large degrees of swelling, it was difficult to handle during processing because the polymer was weak and easily broke when swollen. Therefore PCLF18000 was synthesized in order to increase the cross-linking density and lower the swelling ratio in an effort to increase the processing ease. The actual cross-linking density was not measured for this work, however a 30% decrease in the volume change was observed for PCLF18000 from PCLF7000, indicating increased cross-link density of PCLF18000. This decrease in swelling made PCLF18000 much easier to handle during processing. The two different molecular weight PCLF polymers synthesized were used investigate the properties of PCLF-PPy composite materials. PCLF-PPy composite materials synthesized from PCLF7000 and PCLF18000 did not have substantially different electrically conductivity, both had conductivities on the order of 1mS cm−1 when synthesized with sulfonic acid anions. The compositions varied with the synthetic procedure with higher concentrations of benzoyl peroxide and longer PCLF submersion times resulting in higher PPy content. However it seems that significant increases in conductivity were not observed after roughly 3% nitrogen or 15% polypyrrole content was reached. The thermal transitions studied include Tc and Tm. Cross-linked PCLF7000 has Tc and Tm that straddle the 37°C. Polymerizing PPy in PCLF scaffolds lowers the Tc and Tm transitions resulting in amorphous materials and increased flexibility under physiological conditions. Surface topographies of PCLF-PPy materials were visually rough and appear favorable for cell attachment. PCLF18000 had RMS of 1195 nm when imaged over 100 µm and 8 nm when imaged over 1 µm.
The synthesis of PPy requires selection of an anionic dopant that stabilizes the positive charges formed along the conjugated pi system that is responsible for the conductivity of PPy. The anion selection allows for variation in chemical composition and material properties. This initial study used five different anions to investigate obvious differences between materials. Three sulfonic acid analogs and iodide were chosen because they have been previously used to dope PPy and resulting materials had high electrical conductivity. The fifth, lysine, was chosen because it is zwitter ionic and an amino acid. The five anions were investigated to fine tune the electrical and biological properties of the scaffolds. The sulfonic acid derivatives (NSA, DBSA, and DOSS) had the highest conductivity on the order of 1–6 mS cm−1, PCLF18000PPyI measured 0.1 mS cm−1, and PCLF18000PPylysine had no measurable conductivity. Differences in conductivity maybe attributed to the ability of the different anionic dopants to diffuse into the PCLF polymer matrix during polymerization of pyrrole. If diffusion of the anionic dopant into PCLF is limited, PPy will stay in the reduced non-conductive form.
PC12 is a cell line derived from a pheochromocytoma of the rat adrenal medulla. These cells stop dividing and differentiate into the neurons when treated with nerve growth factor. In this study, we used PC12 cells as a model system for neuronal differentiation, and investigation of their cellular response when cultured directly on PCLF-PPy composite materials. PCLF is currently being investigated in vitro for peripheral nerve regeneration [7]. The goal of these experiments was at minimum to find an electrically conductive PCLF-PPy composition that performs well as the PCLF, but also possessed the electrically conductive properties. Therefore, five anionic dopants were investigated for PC12 cell response. PC12 cells cultured on PCLF-PPy materials exhibited significantly higher cell numbers than on PCLF at days 1 and 7 indicating better cell attachment. After 7 days PC12 cells have dramatically lower cell numbers on PCLF than on any other material. This is caused by poor cell attachment on PCLF resulting in the removal of cells during media changes. PC12 cell morphologies cultured on the different materials are shown in Figure 9. The PC12 cells are stained with a rhodium phalloidin dye that stains F-actin. The F-actin is a critical protein for attachment and cytoskeleton organization of the cells. They can be seen as the red strands with in the cell bodies after staining. PC12 cells cultured on PCLF18000 (Figure 9 A , B) show very little F-actin staining compared to PC12 cells on PCLF-PPy materials. Lack of F-actin expression can be an indicator of poor attachment[49]. Fluorescence microscopy images show obvious differences between the materials. Cells cultured on PCLF18000PPyNSA and PCLF18000PPyDBSA show a typical differentiating morphology in the presence of NGF. No round cell morphologies were observed, as is seen with the other materials. These cells exhibit extended cell bodies with multiple long straight neurites extending from the cell.
DRG explants are an excellent model that utilizes nerve cells in combination with Schwann and supporting cells to investigate a treatment’s ability to support neurite outgrowth. In this study DRG explants were used to confirm the observations of neurite extension with PC12 cells. PCLF18000PPyDBSA, PCLF18000PPyNSA, and PCLF18000PPyDOSS were chosen because they had superior properties for electrical conductivity and PC12 cell response. These experiments show that PCLF18000PPyNSA and PCLF18000PPyDBSA are able to support DRG attachment and neurite extension. Significant increases at the p < 0.05 level was observed for DRG neurite extension when cultured on PCLF18000PPyDBSA and PCLF18000PPyNSA scaffolds compared to being cultured on PCLF18000PPyDOSS. This corroborated observations of seen with PC12 cellular responses.
Conclusions
Electrically conductive composite materials were synthesized by polymerizing PPy in preformed cross-linked scaffolds of PCLF resulting in interpenetrating networks of PCLF-PPy. This fabrication technique overcomes the challenges associated with using PPy in biomaterial applications such as poor mechanical properties, processing difficulties, and non-biodegradability. PCLF-PPy materials were synthesized with five different anionic dopants to determine the optimal composition for both the electrical and biological properties. PCLF18000PPyNSA and PCLF18000PPyDBSA materials exhibited conductivity up to 6 mS cm−1. Surface analysis by XPS indicates the scaffolds contain up to 30 mol percent of polypyrrole in the surface 10 nm. The TGA shows that the bulk material incorporates up to 13.5 percent polypyrrole by weight showing that the majority of the scaffold is composed of biodegradable PCLF. Cellular studies show PC12 cells cultured on PCLF-PPy materials perform better than on PCLF. However, not all PCLF-PPy materials are equal; PCLF18000PPyNSA and PCLF18000PPyDBSA consistently show better cell morphologies indicated by elongated cell bodies and long neurites extending straight out from the cell in addition to higher cell numbers than on other PCLF18000PPylysine or PCLF18000PPyDOSS materials. PCLF-PPy is a promising new material for incorporating electrically conductive materials into tissue engineering. This methodology for producing composites of polypyrrole incorporated into preformed cross-linked materials is robust and will be extended to hydrogels and collagen based materials currently being investigated for nerve regeneration applications.
Acknowledgements
We thank NIH training grant 1T32AR056950-01, the NIH Loan Repayment Program, and the Armed Forces Institute of Regenerative Medicine by DOD activity contract # W81XWH-08-2-0034 for their generous support. We would also like to acknowledge the University of Iowa Central Microscopy Research Facility for XPS characterization.
Footnotes
Conflicts of Interest
A provisional patent has been filed on polycaprolactone fumarate-polypyrrole based materials, and this technology has been licensed to BonWrx.
References
- 1.Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21(10):1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 2.Taylor CA, Braza D, Rice JB, Dillingham T. The Incidence of Peripheral Nerve Injury Extremity Trauma. Am J Phys Med Rehabil. 2008;87(5):381–385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- 3.Ruiter GCd, Onyeneho IA, Liang ET, Moore MJ, Knight AM, Malessy MJA, et al. Methods for in vitro characterization of multichannel nerve tubes. J Biomed Mater Res A. 2007;84(3):643–651. doi: 10.1002/jbm.a.31298. [DOI] [PubMed] [Google Scholar]
- 4.Ruiter GCWd, Malessy MJA, Alaid AO, Spinner RJ, Engelstad JK, Sorenson EJ, et al. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211(2):339–350. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiter GCd, Spinner RJ, Malessy MJA, Moore MJ, Sorenson EJ, Currier BL, et al. Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly(lactic-co-glycolic acid) nerve tubes. Neurosurgery. 2008;63(1):144–155. doi: 10.1227/01.NEU.0000335081.47352.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore MJ, Friedman JA, Lewellyn EB, Mantilla SM, Krych AJ, Ameenuddin S, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27(3):419–429. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Yaszemski MJ, Knight AM, Gruetzmacher JA, Windebank AJ, Lu L. Photo-crosslinked poly(caprolactone fumarate) networks for guided peripheral nerve regeneration: Material properties and preliminary biological evaluations. Acta Biomater. 2009;5(5):1531–1542. doi: 10.1016/j.actbio.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Lu L, Gruetzmacher JA, currier BL, Yaszemski MJ. Synthesis and characterizations of biodegradable and crosslinkable poly(e-caprolactone fumarate), poly(ethylene glycol fumarate), and their amphiphilic copolymer. Biomaterials. 2006;27(6):832–841. doi: 10.1016/j.biomaterials.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Kemp SWP, Walsh SK, Midha R. Growth factor and stem cell enhanced conduits in peripheral nerve regeneration and repair. Neurol Res. 2008;30(10):1030–1038. doi: 10.1179/174313208X362505. [DOI] [PubMed] [Google Scholar]
- 10.Cui G-X, Li Y-Z, Yue S-W. Advances in stem cell transplantation for spinal cord injury. J Clin Rehabil Tiss Eng Res. 2008;12(47):9335–9338. [Google Scholar]
- 11.Sago K, Tamahara S, Tomihari M, Matsuki N, Asahara Y, Takei A, et al. In vitro differentiation of canine celiac adipose tissue-derived stromal cells into neuronal cells. J Vet Med Sci. 2008;70(4):353–357. doi: 10.1292/jvms.70.353. [DOI] [PubMed] [Google Scholar]
- 12.Tabesh H, Amoabediny G, Nik NS, Heydari M, Yosefifard M, Siadat SOR, et al. The role of biodegradable engineered scaffolds seeded with schwann cells for spinal cord regeneration. Neurochem Int. 2009;54(2):73–83. doi: 10.1016/j.neuint.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Arino H, Brandt J, Dahlin LB. Implantation of schwann cells in rat tendon autografts as a model for peripheral nerve repair: long term effects on functional recovery. Scand J Plast Reconstr Surg Hand Surg. 2008;42(6):281–285. doi: 10.1080/02844310802393966. [DOI] [PubMed] [Google Scholar]
- 14.Ashley Z, Sutherland H, Russold MF, Lanmuller H, Mayr W, Jarvis JC, et al. Therapeutic stimulation of denervated muscles: the influence of pattern. Muscle Nerve. 2008;38(1):875–886. doi: 10.1002/mus.21020. [DOI] [PubMed] [Google Scholar]
- 15.Vivo M, Puigdemasa A, Casals L, Asensio E, Udina E, Navarro X. Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Experimental Neurology. 2008;211(1):180–193. doi: 10.1016/j.expneurol.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Song JW, Yang LJ, Russell SM. Peripheral nerve: what's new in basic science laboratories. Neurosurg Clin N Am. 2009;20(1):121–131. doi: 10.1016/j.nec.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Kam NWS, Jan E, Kotov NA. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2009;9(1):273–278. doi: 10.1021/nl802859a. [DOI] [PubMed] [Google Scholar]
- 18.Li L, El-Hayek YH, Liu B, Chen Y, Gomez E, Wu X, et al. Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells. 2008;26(8):2193–2200. doi: 10.1634/stemcells.2007-1022. [DOI] [PubMed] [Google Scholar]
- 19.Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22(10):1055–1064. doi: 10.1016/s0142-9612(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conductive polymer. Proc Natl Acad Sci USA. 1997;94(17):8948–8953. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao L, Shanley L, Mccaig C, Zhao M. Small applied electric fields guide migration of hippocampal neurons. J Cell Physiol. 2008;216(2):527–535. doi: 10.1002/jcp.21431. [DOI] [PubMed] [Google Scholar]
- 22.Ahlborn P, Schachner M, Irintchev A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol. 2007;208(1):137–144. doi: 10.1016/j.expneurol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VMK. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205(2):347–359. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Park JS, Park K, Moon HT, Woo DG, Yang HN, Park K-H. Electrical pulsed stimulation of surfaces homogeneously coated with gold nanoparticles to induce neurite outgrowth of PC12 cells. Langmuir. 2009;25(1):451–457. doi: 10.1021/la8025683. [DOI] [PubMed] [Google Scholar]
- 25.Ateh DD, Navsaria HA, Vadgama P. Polypyrrole-based conducting polymers and interactions with biological tissues. J R Soc Interface. 2006;3(11):741–752. doi: 10.1098/rsif.2006.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen SJ, Wand DY, Yuan CW, Wany XD, Zhang PY, Gu XS. Template synthesis of the polypyrrole tube and its bridging in vivo sciatic nerve regeneration. J Mater Sci Lett. 2000;19(2):157–159. [Google Scholar]
- 27.Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2007;81(1):135–149. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez N, Lee JY, Nickels JD, Schmidt CE. Micropatterned polypyrrole: a combination of electrical and topographical characteristics for the stimulation of cells. Adv Funct Mater. 2007;17(10):1645–1653. doi: 10.1002/adfm.200600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J-W, Serna F, Nickels J, Schmidt CE. Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules. 2006;7(6):1692–1695. doi: 10.1021/bm060220q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J-W, Serna F, Schmidt CE. Carboxy-endcapped conductive polypyrrole:biomimetic conducting polymer for cell scaffolds and electrodes. Langmuir. 2006;22(24):9816–9819. doi: 10.1021/la062129d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao C, Zhu A, Wu Q, Chen X, Kim J, Shen J. New biocompatible polypyrrole-based films with good blood compatibility and high electrical conductivity. Colloids Surf B Biointerfaces. 2008;67(1):41–45. doi: 10.1016/j.colsurfb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Neoh KG, Lau KKS, Wang VVT, Kang ET, Tan KL. Structure and degradation behavior of polypyrrole doped with sulfonate anions and different sizes subject to undoping-redoping cycles. Chem Mater. 1996;8(1):167–172. [Google Scholar]
- 33.Olayo R, Rios C, Salgado-Ceballos H, Cruz GJ, Morales J, Olayo MG, et al. Tissue spinal cord response in rats after implants of polypyrrole and polyethylene glycol obtained by plasma. J Mater Sci Mater Med. 2008;19(2):817–826. doi: 10.1007/s10856-007-3080-z. [DOI] [PubMed] [Google Scholar]
- 34.Shi G, Zhang Z, Rouabhia M. The regulation of cell functions electrically using biodegradable polypyrrole-polylactide conductors. Biomaterials. 2008;29(28):3792–3798. doi: 10.1016/j.biomaterials.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Shustak G, Gadzinowski M, Slomkowski S, Domb AJ, Mandler D. A novel electrochemically synthesized biodegradable thin film of polypyrrole-polyethyleneglycol-polylactic acid nanoparticles. New J Chem. 2007;31(1):163–168. [Google Scholar]
- 36.Wang X, Gu X, Yuan C, Chen S, Zhang P, Zhang T, et al. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J Biomed Mater Res A. 2003;68(3):411–422. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 37.Huang L, Hu J, Lang L, Wang X, Zhang P, Jing X, et al. Synthesis and characterization of electroactive and biodegradable ABA block copolymer of polylactide and aniline pentamer. Biomaterials. 2007;28(10):1741–1751. doi: 10.1016/j.biomaterials.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Zhuang X, Hu J, Lang L, Zhang P, Wang Y, et al. Synthesis of biodegradable and electroactive multiblock polylactide and aniline pentamer copolymer for tissue engineering applications. Biomacromolecules. 2008;9(3):850–858. doi: 10.1021/bm7011828. [DOI] [PubMed] [Google Scholar]
- 39.Shi G, Rouabhia M, Meng S, Zhang Z. Electrical stimulation enhances viability of human cutaneous fibroblasts on conductive biodegradable substrates. J Biomed Mater Res A. 2007;84(4):1026–1036. doi: 10.1002/jbm.a.31337. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Rouabhia M, Wang Z, Roberge C, Shi G, Roche P, et al. Electrically conductive biodegradable polymer composite for nerve regeneration: electricity-stimulated neurite outgrowth and axon regeneration. Artif Organs. 2007;31(1):13–22. doi: 10.1111/j.1525-1594.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 41.Shi G, Rouabhia M, Wang Z, Dao LH, Zhang Z. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterials. 2004;25(13):2477–2488. doi: 10.1016/j.biomaterials.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Roberge C, Dao LH, Wan Y, Shi G, Rouabhia M, et al. In vivo evaluation of a novel electrically conductive polypyrrole/poly(D,L- lactide) composite and polypyrrole-coated poly(D,L-lactide-co-glycolide) membranes. J Biomed Mater Res A. 2004;70(1):28–38. doi: 10.1002/jbm.a.30047. [DOI] [PubMed] [Google Scholar]
- 43.Jabbari E, Wang S, Lu L, Gruetzmacher JA, Ameenuddin S, Hefferan TE, et al. Synthesis, material properties, and biocompatibility of a novel self-cross-linkable poly(caprolactone fumarate) as an injectable tissue engineering scaffold. Biomacromolecules. 2005;6(5):2503–2511. doi: 10.1021/bm050206y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baltrusaitis J, Usher CR, Grassian V. Reactions of sulfur dioxide on calcium carbonate single crystal and particle surfaces at the adsorbed water carbonate interface. Phys Chem Chem Phys. 2007;9(23):3011–3024. doi: 10.1039/b617697f. [DOI] [PubMed] [Google Scholar]
- 45.Fairley N. 2.3.14. CV. 1999–2008 [Google Scholar]
- 46.Hiremath RK, Rabinal MK, Mulimani BG. Simple setup to measure electrical properties of polymeric films. Rev Sci Instrum. 2006;77(12) 126106-1-26106-3. [Google Scholar]
- 47.Smits FM. Measurements of Sheet Resistivity with the Four-Point Probe. BSTJ. 1958;37:371. [Google Scholar]
- 48.Valdes LB. Resistivity Measurements on Germanium for Transistors. Proc IRE. 1954;42(2):420–427. [Google Scholar]
- 49.Dadsetan M, Jones JA, Hiltner A, Anderson JM. Surface chemistry mediates adhesive structure, cytoskeletal organization, and fusion of macrophages. J Biomed Mater Res A. 2004;71(3):439–448. doi: 10.1002/jbm.a.30165. [DOI] [PubMed] [Google Scholar]