Abstract
Attempts to explain the associations among metabolic syndrome (MetS) features using factor analysis to identify unobserved potential causes have resulted in inconsistent findings. We examined whether an unobserved categorical factor explains the associations among MetS features using latent class analysis. A cross-sectional analysis of 499 nondiabetic Japanese-Americans who underwent measurements of fasting blood, waist circumference (WC) and CT-measured intra-abdominal fat (IAF) area was conducted. MetS components were defined by IDF criteria. IAF and fasting serum insulin (FI) were dichotomized at the 75th percentile. Latent two- and three-class models were fit that included hypertension, dyslipidemia, hyperglycemia, and either WC, IAF, or FI for a total of six models. A three-class latent model fit the data well, while a two-class model did not. In the three-class model, one latent class was strongly associated with all MetS components, while another was associated with hyperglycemia and hypertension only. IAF was associated with only one latent class. Latent class analysis supports the presence of an unobserved factor linked to the co-occurrence of MetS features. One class of this factor was associated with hypertension and hyperglycemia but not central adiposity or FI, suggesting another pathway for observed MetS features.
Keywords: metabolic syndrome, latent class analysis, intra-abdominal fat, waist circumference, Japanese American
The metabolic syndrome refers to the co-occurrence of multiple conditions associated with cardiovascular disease beyond what would be expected by chance alone [1]. There has been much discussion and debate on the underlying cause of the syndrome mainly focusing on the presence of insulin resistance or central adiposity [2; 3]. To assist with the identification of a cause or causes of the metabolic syndrome, statistical methods have been employed to identify whether unobserved factors also known as latent variables might explain observed associations among metabolic syndrome features (Figure 1). The methods employed to date include factor analysis, confirmatory factor analysis, and structural equation modeling [4-6]. These methods have in common the ability to identify whether unobserved factors underlie the associations among metabolic syndrome components, but make the assumption that all observed conditions and unobserved factors are measured on a continuous scale. What is not clear is whether the assumption of continuous associations between observed and unobserved metabolic syndrome features is correct, or whether an underlying categorical condition may predispose to the appearance of the multiple manifestations of the metabolic syndrome, such as, for example, an as yet to be discovered genotype. The clinical definitions of the metabolic syndrome consider the syndrome though to be a categorical state that is either present or absent, but it is not clear whether this approach is valid either [7; 8]. The previously employed methods to identify underlying features of the metabolic syndrome may not be appropriate to address the question of whether a condition that is present or absent explains the observed manifestations of the metabolic syndrome as defined using current criteria based on dichotomization of levels of blood pressure, lipid, glycemia, and central obesity.
Figure.
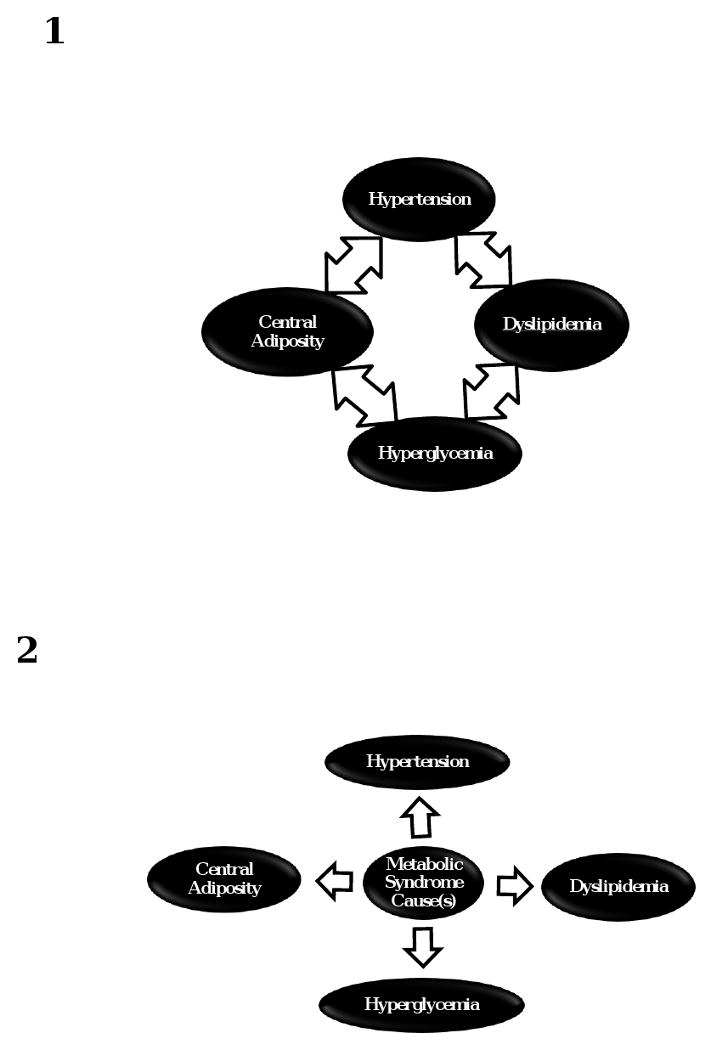
Associations among metabolic syndrome features. Panel 1 illustrates observed associations among several metabolic syndrome features, while panel 2 displays these observed associations due to an underlying unobserved factor.
Latent class analysis permits assessment of whether associations between observed categorical variables can be explained by the existence of an unobserved categorical variable [9]. Latent class analysis has been little used in medical research other than in the area of mental health where clinical diagnoses cannot be confirmed with diagnostic gold standards such as biopsy or microbiologic culture. The metabolic syndrome currently has a similar status since the underlying abnormality associated with its observed manifestations has not been positively identified. To better understand the underlying mechanisms of the metabolic syndrome, we utilized latent class analysis to determine whether an unobserved categorical variable adequately explains its observed features.
Methods
A cross-sectional analysis was performed in a study of Japanese-American men and women of 100% Japanese ancestry. The methods used in this study population have been previously described [10]. Subjects were chosen from volunteers through community-wide recruitment from 1983-88 and were representative of Japanese-American residents of King County, WA, in age distribution, residential distribution, and parental immigration pattern. A comprehensive mailing list and telephone directory that included almost 95% of the Japanese-American population of King County, WA, was used. The study protocol was reviewed and approved by the University of Washington Human Subjects Review Committee, and all subjects provided written informed consent. A total of 658 men and non-pregnant women aged 34-74 years were enrolled and underwent a baseline exam. Subjects were excluded if they met diagnostic criteria for type 2 diabetes as described below (n=138) due to the potential effects of diabetes and/or its treatments to alter body composition and fasting insulin level, or had missing data on any covariate of interest (n=21), leaving 499 for this analysis. Subjects excluded for missing data on covariates were generally similar in age and gender to those included.
Measurements pertinent to this analysis include waist circumference measured with a tape measure at the umbilicus in men and at the narrowest portion of the waist in women. Measurement of intra-abdominal fat area (IAF) in centimeters2 was derived from a single-slice CT of the abdomen at the level of the umbilicus [11]. This measurement correlates highly with directly ascertained total visceral fat volume by CT or magnetic resonance imaging [12; 13]. Blood pressure was measured with a mercury sphygmomanometer in triplicate on participants while supine with the latter two measurements averaged and reported in millimeters of mercury.
Blood was drawn after a 10 hour fast for laboratory analyses. Plasma glucose was assayed by an automated glucose oxidase method. Fasting plasma insulin was measured by radioimmunoassay [14] and triglycerides by enzymatic analytical chemistry. HDL cholesterol was separated by precipitation of the other lipoproteins with dextran-Mg++ and cholesterol was measured enzymatically. Diabetes was diagnosed if participants were taking oral hypoglycemic medication or insulin or if the fasting plasma glucose (FPG) level was ≥ 7.0 mmol/l [15].
Definitions of metabolic syndrome components were taken from current guidelines as follows: hypertension, systolic ≥ 130 mm Hg or diastolic blood pressure ≥ 85 mm Hg or treatment with antihypertensive medication; dyslipidemia, serum triglyceride level ≥ 1.7 mmol/l or HDL-cholesterol level < 1.03 mmol/l for men or 1.29 mmol/l for women or treatment with lipid lowering medication; hyperglycemia, fasting glucose ≥ 5.6 mmol/l; and waist circumference, ≥ 90 cm men or ≥ 80 cm for women [7]. These waist circumference cut-points were those recommended for Asian Americans [7].
We also used alternate measurements and definitions in additional models. Since waist circumference serves as a proxy for the size of the visceral fat depot with far less than perfect accuracy (correlation coefficients between waist circumference and visceral fat: men 0.66, women, 0.68), we also substituted CT measurements of IAF in place of waist circumference to determine whether this more direct and accurate measure led to different results [16]. IAF was dichotomized at the 75th percentile by gender due to the well-known differences in central body fat distribution between men and women. As insulin resistance has been implicated in metabolic syndrome pathogenesis, we performed an additional model using fasting insulin dichotomized at the 75th percentile in place of the central adiposity measurement [17]. Also, we fit additional models that included persons with diabetes to determine if their inclusion altered our results. A fasting insulin model that included persons with diabetes was not fit due to the potential effects of diabetes treatment on insulin levels.
Latent class analysis was used to test whether a single unobserved categorical variable adequately explained the observed associations among the metabolic syndrome features [9]. Latent class analysis was performed using LEM (Log-linear and event history analysis with missing data using the EM algorithm, Jeroen Vermunt, Tilburg University, the Netherlands). A successful model fit was determined by a chi-square Goodness of Fit (GOF) statistic p-value greater than 0.05. Latent categorical variables with two and three classes were evaluated for model fit. This analysis also provides estimates of the conditional probabilities of the observed metabolic syndrome features given the presence or absence of the latent factor, otherwise known as sensitivity and specificity. An assumption of latent class analysis is that the observed conditions are conditionally independent of each other, that is, that the associations do not differ by the presence or absence of another factor. The likelihood of conditional dependence in this analysis is low since we measured clinically distinct entities using different methodologies that are not likely to be independently correlated (e.g., sphygmomanometer for measurement of blood pressure compared to enzymatic chemistry determination of serum triglycerides) as opposed to other measurements which might be correlated independent of the underlying condition, such as different serologic tests for an infectious disease or two questionnaires for the presence of depression that share a number of similar items. We formally tested for the presence of conditional dependence using the log-odds ratio check as modified by Uebersax [18; 19]. Latent class models may yield more than one solution. To lessen this likelihood, model convergence criterion was set at 10-10. Each model was run 5 times with a different seed random value, and if more than one solution was achieved, each model was run for a total of 10 repetitions, with the most frequently occurring solution presented. Comparison across models was performed using the Akaike Information Criterion (AIC), with lower AIC indicating better model fit [20].
Results
Characteristics of the 499 non-diabetic subjects were as follows: men n=260, mean age 52.1 yrs, mean BMI 25.2 kg/m2; women n=239, mean age 51.8 yrs, mean BMI 23.0 kg/m2. Proportions of men and women with each metabolic syndrome component are shown in Table 1. The three most frequent criteria met in order of decreasing frequency by gender were: men – hypertension, hyperglycemia, dyslipidemia; and women – hyperglycemia, hypertension, waist circumference. The fasting insulin cut-point at the 75th percentile among all subjects was 111 pmol/l.
Table 1.
Metabolic Syndrome Features, Intra-Abdominal Fat Area, and Fasting Insulin Level by Sex
| Feature† | Men (n=260) | Women (n=239) |
|---|---|---|
| Hyperglycemia | 139 (53.5%) | 118 (49.4%) |
| Hypertension | 140 (53.9%) | 87 (36.4%) |
| Dyslipidemia | 104 (40.0%) | 55 (23.0%) |
| Waist Circumference | 102 (39.2%) | 70 (29.3%) |
| IAF | 65 (25.0%) | 60 (25.1%) |
| Fasting Insulin | 55 (21.2%) | 76 (31.8%) |
The following cut-points were used: hyperglycemia (fasting plasma glucose ≥ 5.6 mmol/l), hypertension (systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 85 mm Hg), dyslipidemia (triglycerides ≥ 1.7 mmol/l or HDL < 1.03 mmol/l in men, < 1.29 mmol/l in women, or treatment with a lipid-lowering medication), waist circumference (≥ 80 cm women or ≥ 90 cm men), IAF (intra-abdominal fat area upper quartile > 129.8 cm2 men, > 91.3 cm2 women), fasting insulin (upper quartile > 111 pmol/l).
The distribution and cross-classification of metabolic syndrome features is shown in Table 2. This table displays in 3 different models the presence or absence of hyperglycemia, dyslipidemia, and hypertension by waist circumference ≥ 90 cm men or ≥ 80 cm for women (Model A), by intra-abdominal fat area > 129.8 cm2 for men or > 91.3 cm2 for women (Model B), or by fasting insulin level > 111 pmol/l (Model C). The numbers of subjects with 0-4 features can be obtained by summing appropriate cells in Table 2. For example, in Model A, the numbers of subjects with 0, 1, 2, 3, and 4 features are as follows: 108, 139, 126, 86, and 40. Similar counts can be derived from Models B and C. As shown in the footnote to Table 2, the overall chi-square test of association for each Model was highly statistically significant, demonstrating that associations among these components of the metabolic syndrome occur at a far greater frequency than would be expected by chance alone. The greatest chi-square values were seen in Model B where the frequency of dyslipidemia, hypertension, and hyperglycemia were cross-classified with intra-abdominal fat area.
Table 2.
Cross-classification of metabolic syndrome features (hypertension, dyslipidemia, hyperglycemia) by A) waist circumference, B) CT-measured intra-abdominal fat area, and C) fasting insulin level
| Model | Metabolic Syndrome Feature† | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A‡ | |||||||||
| Waist circumference | - | - | - | - | + | + | + | + | |
| Dyslipidemia | - | - | + | + | - | - | + | + | |
| Hypertension | - | + | - | + | - | + | - | + | |
| Hyperglycemia(-), column N | 108 | 44 | 27 | 8 | 20 | 12 | 13 | 16 | |
| Hyperglycemia (+), column N | 48 | 55 | 18 | 19 | 20 | 33 | 18 | 40 | |
| B§ | |||||||||
| IAF | - | - | - | - | + | + | + | + | |
| Dyslipidemia | - | - | + | + | - | - | + | + | |
| Hypertension | - | + | - | + | - | + | - | + | |
| Hyperglycemia (-), column N | 124 | 44 | 35 | 11 | 4 | 12 | 5 | 13 | |
| Hyperglycemia (+), column N | 54 | 58 | 26 | 22 | 14 | 30 | 10 | 37 | |
| C‖ | |||||||||
| Fasting insulin | - | - | - | - | + | + | + | + | |
| Dyslipidemia | - | - | + | + | - | - | + | + | |
| Hypertension | - | + | - | + | - | + | - | + | |
| Hyperglycemia (-), column N | 113 | 46 | 28 | 14 | 15 | 10 | 12 | 10 | |
| Hyperglycemia (+), column N | 53 | 64 | 17 | 33 | 15 | 24 | 19 | 26 |
- or + indicates presence or absence of the metabolic syndrome feature; the numbers of subjects with 1, 2, 3 or 4 metabolic syndrome features can be derived from the column N.
For model A: X2=51.46 df=7 P<0.001; 2 latent classes: GOF X2=19.50 df=6 p=0.0034; 3 latent classes: GOF X2=0.6541 df=1 p=0.4186; AIC=2560
For model B: X2=60.89 df=7 p<0.001; 2 latent classes: GOF X2=13.25 df=6 p=0.0392; 3 latent classes: GOF X2=2.32 df=1 p=0.1278; AIC=2449
For model C: X2 =49.44 df= 7 p<0.001; 2 latent classes: GOF X2=16.77 df=6 p=0.0102; 3 latent classes: GOF X2=0.079 df=1 p=0.7786; AIC=2519
Goodness of fit for Models A-C showed poor fit for the two class latent variable model as judged by the criterion of a GOF p-value > 0.05 for good fit (Table 2 footnote). P-values for the two class models were all < 0.05. The three-class model, however, fit Models A-C well with p-values all > 0.05. The best fitting model overall as judged by the lowest AIC was Model B that included IAF as the measure of central adiposity. In multiple repetitions of the three-class models, the solution presented in Tables 2 and 3 were achieved in 5/5 waist models, 6/10 IAF models, and 4/5 fasting insulin models. The assessment of conditional dependence using the log odds ratio check was negative for all models.
Table 3.
Estimated prevalence of latent classes and the probability of observed metabolic syndrome features for each class. All observed variables are dichotomous as defined in Table 1. Models include either a measure of central adiposity or fasting insulin.
| Latent Classes | 1 | 2 | 3 | |
|---|---|---|---|---|
| Model | ||||
| A | Latent factor | 0.38 | 0.18 | 0.44 |
| Hypertension | 0.00 | 0.85 | 0.69 | |
| Dyslipidemia | 0.20 | 0.003 | 0.55 | |
| Hyperglycemia | 0.26 | 0.51 | 0.71 | |
| Waist circumference | 0.13 | 0.00 | 0.67 | |
| B | ||||
| Latent Factor | 0.47 | 0.12 | 0.41 | |
| Hypertension | 0.07 | 1.00 | 0.73 | |
| Dyslipidemia | 0.22 | 0.00 | 0.52 | |
| Hyperglycemia | 0.29 | 0.55 | 0.73 | |
| IAF | 0.00 | 0.00 | 0.61 | |
| C | ||||
| Latent Factor | 0.58 | 0.25 | 0.17 | |
| Hypertension | 0.26 | 0.86 | 0.49 | |
| Dyslipidemia | 0.19 | 0.37 | 0.67 | |
| Hyperglycemia | 0.29 | 0.89 | 0.66 | |
| Fasting insulin | 0.09 | 0.24 | 0.91 |
As the choice of the upper quartile cut-point for IAF and fasting insulin was arbitrary, we explored additional latent class models with the cut-point set at the median value for these variables. Results were similar to the upper-quartile cut-point models with one exception. The three-class latent model did not fit the fasting insulin median cut-point model (data not shown). The AIC for the three-class upper-quartile models was less than for the median models (IAF upper quartile/median 2449/2549; insulin 2519/2649), indicating that the upper quartile as compared with the median cut-point resulted in a better model fit.
The results of the three-class latent variable models are shown in Table 3. For the waist and IAF models, similar estimates of the latent class prevalences were obtained, with one class occurring less frequently (0.12-0.18) and the other two occurring with generally similar frequency (0.38-0.47). The specific associations with the observed factors are shown by the probabilities of the observed metabolic syndrome feature for each latent factor. We present the latent variables in Table 3 based on the general pattern of associations with observed variables, with latent variables with the fewest associations designated as 1 and more numerous associations designated as 3. The first latent class for the waist and IAF models showed low probabilities for metabolic syndrome features, with hypertension and central adiposity present infrequently, and dyslipidemia and hyperglycemia present less frequently than seen in Table 1. The second latent class for waist circumference and IAF was related mainly to hypertension with a prevalence of this condition of 0.85-1.00 although hyperglycemia was also related but at a frequency near to the expectation from Table 1 (0.51-0.55). The third waist and IAF latent classes were associated with all four components of the metabolic syndrome, with higher frequencies of the components than would have been predicted in the absence of an unknown factor. When the analysis was repeated for IAF and waist circumference in all subjects including those with diabetes, we obtained generally similar results for the three-class models compared to the results shown in Tables 2 and 3 that excluded persons with diabetes (data not shown).
The three-class insulin latent model differs somewhat from those that included waist and IAF (Table 3, C). Class 3 again showed higher frequencies of all components but its frequency is lower (0.17) than in the waist (0.44) and IAF (0.41) models. Class 2 mainly showed higher frequencies of hypertension and hyperglycemia, while class 1 showed lower than expected frequencies of all metabolic syndrome components and a higher prevalence of this class (0.58) compared to the waist (0.38) and IAF (0.47) models.
The probabilities of high waist, IAF, or fasting insulin differ by class of the three-class model (Table 3). The probability of high waist or IAF in classes 1 and 2 is zero in class 2 for both measures of central adiposity, 0.13 and zero in class 1, respectively, and 0.67 and 0.61 in class 3. The pattern for high fasting insulin also shows a low probability in class 1 (0.09) but differs somewhat in showing a high probability in class 3 (0.91) and a probability greater than zero in class 2 (0.24).
Discussion
This is to our knowledge the first attempt to apply latent class analysis to better understand the metabolic syndrome. The results are consistent with a three-class unobserved factor explaining associations among the syndrome's observed components. The associations between this factor and observed metabolic syndrome components were fairly consistent regardless of whether IAF or waist was used as the measure of central adiposity or if fasting insulin was used to reflect insulin resistance. One class was consistently associated with all metabolic syndrome features (Table 3, Models A-C, class 3). This class may represent a pathophysiologic state producing the observed features of the metabolic syndrome. Since the unobserved factor this analysis identified cannot be directly observed at this time, it is not possible to better define its nature as a mechanistic pathway or a correlate of other causes of the metabolic syndrome.
A second class was associated mainly with hypertension and hyperglycemia (Table 3, Models A-C, class 2). Of note, the second class was not strongly related to either IAF or fasting insulin, suggesting that hyperglycemia and hypertension may correlate via a physiologic pathway independent of visceral adiposity or insulin resistance. To our knowledge none of the multiple factor analyses of the metabolic syndrome that have been performed has identified a factor on which hyperglycemia and blood pressure only load. One analysis did demonstrate glucose and blood pressure loading on one factor, but in addition other metabolic syndrome features loaded, including waist-to-hip ratio and lipid concentrations [21].
The latent class analysis method produces estimates of conditional probabilities that represent the strength of associations between the observed measures and the latent factor (Table 3), similar to factor loadings in factor analysis. These estimates revealed a prevalence of 0 for IAF in classes one and two (Table 3, Model B) indicating a very high specificity for IAF for these classes, which would imply that its presence effectively rules in the presence of the latent class 3 and potentially the metabolic syndrome as well [22]. The next highest specificity was seen for waist circumference (class 2 - 0, class 1 - 0.13). That waist circumference and IAF should differ is not surprising given the less than perfect correlation between these two measures [16]. Fasting insulin similarly showed a low probability of occurrence with classes one and two. Whether the high specificity for fasting insulin depends on its association with IAF or insulin resistance or both cannot be determined from this analysis. Sensitivity measures for class 3 overall tended to be lower, and demonstrated that waist circumference or IAF above the cut-points we used do not effectively rule out the presence of class 3. Fasting insulin on the other hand showed higher sensitivity for class 3 and would therefore better rule out class 3 if absent than IAF or waist circumference above the cut-points defined for this analysis [22].
Both insulin resistance and IAF have been proposed as the underlying abnormality responsible for the manifestations of the metabolic syndrome. One could argue that as such they would not be appropriate observed measurements to consider in conducting a latent class analysis. The results of this analysis, however, do not justify this argument as shown by the imperfect sensitivity of fasting insulin, IAF, and waist circumference, which indicates that the latent factor class 3 may still be present even if these values are in the lower end of the defined range. Cut-points for IAF and fasting insulin were defined based on a chosen percentile but the waist circumference cut-points were based on published international criteria for the definition of the metabolic syndrome [7]. Similar results were seen whether the 75th percentile or median value was used to define elevated IAF and fasting insulin, although the 75th percentile models showed better fit as judged by the AIC.
This analysis has several limitations. A latent class model could not be fit using all 5 observed metabolic syndrome features due to sparse data in the cross-classification of these variables. It is possible to fit more complex latent class models (e.g., ≥ four classes or ≥ one latent variable) if the number of degrees of freedom in the cross-classification of metabolic syndrome components exceeds the number of parameters to be estimated. These more complex models were not fit as they would have required the estimation of more parameters than would have been possible given the 15 degrees of freedom available (Table 2). Whether more complex latent variables would produce a better model fit will require additional investigation. In addition, the issue of local dependence was explored in this analysis and found to be unlikely. We took pre-emptive measures to avoid this problem by entering composite variables for hypertension and dyslipidemia in the models, as opposed to the individual components of these variables, such as systolic and diastolic blood pressure, which are likely to be correlated independent of underlying pathophysiology [23]. Fasting insulin represents a surrogate measure for insulin sensitivity, with insulin resistance having been implicated in metabolic syndrome pathogenesis and of highest interest for assessment in a latent class model. A more direct and accurate measure of insulin sensitivity may have led to different results. Despite this problem, a strong correlation exists between fasting insulin and insulin sensitivity measured by clamp and minimal model [24; 25]. Lastly, this analysis focused on a population of Japanese-Americans of 100% Japanese ancestry. Whether these results would apply to other ethnic and racial groups is not know.
In summary, latent class analysis shows promise in furthering our understanding of the metabolic syndrome. These analyses found that one three-class latent factor best explains associations among observed features of the metabolic syndrome. One latent factor class is associated with all metabolic syndrome features, while another reflects mainly hypertension and hyperglycemia but not central adiposity or fasting insulin level. The results support a sufficient but not necessary role for IAF in this syndrome due to its high specificity and differ from previously conducted factor analyses that were not entirely suited to identify latent factors measured on a categorical scale. In addition, these results suggest that the clustering of hyperglycemia and hypertension may be due to mechanisms independent of central adiposity or insulin resistance. Further investigations are needed in larger populations with direct measures of insulin sensitivity to better define the nature of the latent factor underlying this syndrome.
Acknowledgments
This work was supported by National Institutes of Health Grants DK-31170, HL-49293, and DK-02654; by facilities and services provided by the Diabetes and Endocrinology Research Center (DK-17047), Clinical Nutrition Research Unit (DK-35816), and the General Clinical Research Center (RR-00037) at the University of Washington, and by resources from the VA Puget Sound Health Care System, Seattle, Washington. We are grateful to the King County Japanese-American Community for support and cooperation.
Grant Support: National Institutes of Health Grants DK-31170, HL-49293, DK-02654, DK-17047, DK-35816, and RR-00037
Footnotes
Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149:1514–1520. doi: 10.1001/archinte.149.7.1514. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Chan JC, Cheung JC, Stehouwer CD, Emeis JJ, Tong PC, Ko GT, et al. The central roles of obesity-associated dyslipidaemia, endothelial activation and cytokines in the Metabolic Syndrome--an analysis by structural equation modelling. Int J Obes Relat Metab Disord. 2002;26:994–1008. doi: 10.1038/sj.ijo.0802017. [DOI] [PubMed] [Google Scholar]
- 5.Meigs JB. Invited commentary: insulin resistance syndrome? Syndrome X? Multiple metabolic syndrome? A syndrome at all? Factor analysis reveals patterns in the fabric of correlated metabolic risk factors. Am J Epidemiol. 2000;152:908–911. doi: 10.1093/aje/152.10.908. discussion 912. [DOI] [PubMed] [Google Scholar]
- 6.Shen BJ, Todaro JF, Niaura R, McCaffery JM, Zhang J, Spiro A, 3rd, et al. Are metabolic risk factors one unified syndrome? Modeling the structure of the metabolic syndrome X. Am J Epidemiol. 2003;157:701–711. doi: 10.1093/aje/kwg045. [DOI] [PubMed] [Google Scholar]
- 7.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 9.Formann AK, Kohlmann T. Latent class analysis in medical research. Stat Methods Med Res. 1996;5:179–211. doi: 10.1177/096228029600500205. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto WY, Leonetti DL, Kinyoun JL, Newell-Morris L, Shuman WP, Stolov WC, et al. Prevalence of diabetes mellitus and impaired glucose tolerance among second-generation Japanese-American men. Diabetes. 1987;36:721–729. doi: 10.2337/diab.36.6.721. [DOI] [PubMed] [Google Scholar]
- 11.Shuman WP, Morris LL, Leonetti DL, Wahl PW, Moceri VM, Moss AA, et al. Abnormal body fat distribution detected by computed tomography in diabetic men. Invest Radiol. 1986;21:483–487. doi: 10.1097/00004424-198606000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Han TS, Kelly IE, Walsh K, Greene RM, Lean ME. Relationship between volumes and areas from single transverse scans of intra-abdominal fat measured by magnetic resonance imaging. Int J Obes Relat Metab Disord. 1997;21:1161–1166. doi: 10.1038/sj.ijo.0800530. [DOI] [PubMed] [Google Scholar]
- 13.Schoen RE, Thaete FL, Sankey SS, Weissfeld JL, Kuller LH. Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes Relat Metab Disord. 1998;22:338–342. doi: 10.1038/sj.ijo.0800591. [DOI] [PubMed] [Google Scholar]
- 14.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 15.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 16.Oka R, Miura K, Sakurai M, Nakamura K, Yagi K, Miyamoto S, et al. Comparison of waist circumference with body mass index for predicting abdominal adipose tissue. Diabetes Res Clin Pract. 2009;83:100–105. doi: 10.1016/j.diabres.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91:1063–1077. viii. doi: 10.1016/j.mcna.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Garrett ES, Zeger SL. Latent class model diagnosis. Biometrics. 2000;56:1055–1067. doi: 10.1111/j.0006-341x.2000.01055.x. [DOI] [PubMed] [Google Scholar]
- 19.Uebersax JS. A Practical Guide to Local Dependence in Latent Class Models. [July 7, 2009]; at http://ourworld.compuserve.com/homepages/jsuebersax/condep.htm.
- 20.Vermunt JK, Magidson J. Latent Class Analysis. [July 7, 2009]; at www.statisticalinnovations.com/articles/Latclass.pdf.
- 21.Ferrannini E, Balkau B, Coppack SW, Dekker JM, Mari A, Nolan J, et al. Insulin resistance, insulin response, and obesity as indicators of metabolic risk. J Clin Endocrinol Metab. 2007;92:2885–2892. doi: 10.1210/jc.2007-0334. [DOI] [PubMed] [Google Scholar]
- 22.Boyko EJ. Ruling out or ruling in disease with the most sensitive or specific diagnostic test: short cut or wrong turn? Med Decis Making. 1994;14:175–179. doi: 10.1177/0272989X9401400210. [DOI] [PubMed] [Google Scholar]
- 23.Lawlor DA, Ebrahim S, May M, Davey Smith G. (Mis)use of factor analysis in the study of insulin resistance syndrome. Am J Epidemiol. 2004;159:1013–1018. doi: 10.1093/aje/kwh150. [DOI] [PubMed] [Google Scholar]
- 24.Laakso M. How good a marker is insulin level for insulin resistance? Am J Epidemiol. 1993;137:959–965. doi: 10.1093/oxfordjournals.aje.a116768. [DOI] [PubMed] [Google Scholar]
- 25.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]


