Abstract
Background
Salicylate and quinine have been shown to reliably induce short-term tinnitus when administered at high doses. The present study compared salicylate and quinine induced tinnitus in rats using the gap prepulse inhibition of acoustic startle (GPIAS).
Methods
Twenty-four rats were divided into 2 groups; the first group (n=12) was injected with salicylate (300 mg/kg/d), the second (n=12) was treated with quinine orally at a dose of 200 mg/kg/d. Animals were treated daily for 4 consecutive days. All rats were tested for tinnitus and hearing loss before and 2, 24, 48, 72, 96 hours after the first drug administration. Tinnitus was assessed using GPIAS; hearing function was measured with DPOAEs and ABR.
Results
Salicylate treatment induced transient tinnitus with a pitch near 16 kHz starting 2 h post-treatment, persisting over the 4-day treatment period and disappearing 24 h later. Animals in the quinine group showed GPIAS changes at a higher pitch (20 kHz); however, changes were more variable among animals and the mean data were not statistically significant. Hearing function varied across treatments. In the salicylate group, high-level DPOAEs were slightly affected; most changes occurred 2 h post-treatment. Low-level DPOAEs were affected at all frequencies with a progressive dose-dependent effect. In the quinine group, only high-level DPOAEs were affected, mainly at 16 kHz.
Conclusion
The present study highlights the similarities and differences in the frequency and the time course of tinnitus and hypoacusis induced by salicylate and quinine. Transient tinnitus was reliably induced pharmacologically with salicylate while hearing loss remained subclinical with only minor changes in DPOAEs.
1. Introduction
Tinnitus, defined as the perception of a sound when no external stimulation is present, is a common condition affecting 7-14% of Europeans and over 40 million Americans with severe consequences on daily activities, productivity and the overall quality of life in a subpopulation of these individuals (1). Tinnitus has been studied in different animal models using multiple induction methods such as salicylate, quinine, cisplatin and noise overexposure. Sodium salicylate, the active component in aspirin, is a widely used drug for its analgesic, antipyretic and anti-inflammatory effects; the mechanism that underlies most of its effects is the inhibition of prostaglandin synthesis and the consequential blockade of the pyretic and inflammatory processes that are mediated by prostaglandins (2). The main effect of high doses of salicylate on the auditory system is a reversible, dose-dependent hearing loss and tinnitus. Several studies have focused on the molecular mechanisms underlying salicylate ototoxicity. These include the inhibition of cyclooxygenase (COX) activity resulting in the blockage of arachidonic acid conversion to prostaglandin H2 by cyclooxygenase (3-6). The increased levels of arachidonic acid act on NMDA receptor currents inducing an increase in spontaneous activity in single units of the auditory nerve. Salicylates also impair outer hair cell (OHC) electromotility resulting in hearing loss (7-10). These peripheral changes are thought to give rise to phantom auditory perception following salicylate administration; additional changes that take place in the auditory system following salicylate administration include a decrease in cochlear blood flow (11). More recent evidence indicates that salicylate also influences activity in the central auditory pathway and induces hyperactivity in the auditory cortex (12, 13). Quinine and its derivatives continue to be used in humans to treat malaria particularly in sub-Saharan Africa and until 1997 for night cramps in the US; hearing loss and tinnitus are among the numerous reported side-effects (14). Previous studies have also demonstrated the effects of quinine on the hearing system in animal models (15, 16). Although the clinical manifestations of quinine and salicylate on tinnitus induction are similar, different mechanisms of ototoxicity may be present. Many of quinine’s effects have been reported to induce tinnitus; these include vasoconstriction in the cochlea through an alteration of the cochlear blood flow and the interaction with calcium channels and calcium-dependent potassium channels (17-19).
Salicylate and quinine have been used by researchers to develop and test a number of animal models of tinnitus and to investigate its pitch, loudness and time course. Many behavioral models are based on the association of a specific behavioral response with the presence of sound. Consequently, if the animal perceives the phantom sound of tinnitus on a trial in which the sound is absent (silent interval), the assumption is that the animal will respond to the phantom sound. Many of these conditioned-training paradigms depend on dietary manipulations (i.e., food or water deprivation), memory and motivation. In addition, these models are difficult to implement in old animals, from which there could be a reduced compliance to the training paradigm. Moreover, data collection times are sometime too lengthy to study acute tinnitus and, lastly, some procedures require lengthy training making it difficult to test large numbers of animals (20-28).
A possible solution to the aforementioned limitations of current tinnitus models was proposed by Turner and colleagues who introduced a paradigm based on a reflex instead of on an overt behavioral response (29, 30). This model takes advantage of the fact that the acoustic startle can be modulated by the presence of a preceding signal, such as a tone burst in quiet or a gap embedded in a background sound. The assessment of tinnitus is accomplished by using a silent gap in an otherwise continuous background noise; the silent gap serves as the prepulse to inhibit the startle reflex evoked by the startle stimulus. This paradigm is referred to as gap prepulse inhibition of the acoustic startle (GPIAS). The advantages of the GPIAS model include increased objectivity in animal’s response made possible by the analysis of a reflex instead of a conditioned response, the possibility to use the same reflex repeatedly over time, and the ability to study acute tinnitus and monitor its time course with little or no habituation. Lastly, this method significantly reduces the training and testing time allowing a larger number of animals to be evaluated.
The GPIAS paradigm has not yet been used to evaluate quinine-induced tinnitus. GPIAS was originally used to evaluate salicylate-induced tinnitus with 150 and 250 mg/kg doses of salicylate (30); this study produced behavioral evidence of tinnitus near 16 kHz consistent with an operant behavioral paradigm. However, a more recent study using three different doses of salicylate (150, 250, 300 mg/kg) found evidence of a noise-like tinnitus only with the 300 mg/kg dose; there was no evidence of tinnitus with the lower doses and no evidence of tonal tinnitus in disagreement with previous GPIAS results that had been cross validated with a second behavioral measure (31). None of the earlier GPIAS studies have investigated the temporal onset and recovery of salicylate-induced tinnitus in details. Therefore, the goals of the present study were to compare salicylate and quinine-induced tinnitus in rats using GPIAS, to explore the results of a longer drug administration schedule than used in the past and to investigate the temporal onset and recovery of tinnitus assessed by GPIAS in more detail. Finally, to gain a better understanding of the role of hearing loss in quinine and salicylate-induced tinnitus, distortion product otoacoustic emissions (DPOAE) and auditory brainstem responses (ABR) were measured.
2. Materials and Methods
2.1 Subjects
Twenty-four adult male Sprague Dawley rats (3-5 months, 250-450g) were used for this study. Rats were divided into two groups; twelve animals were used to evaluate salicylate-induced tinnitus and twelve were used to assess quinine-induced tinnitus. The experimental protocol was approved by the University at Buffalo Institutional Animal Care and Use Committee. Animals were housed in a colony with a 12 h light-dark cycle; food and water were available ad-lib. A background masking noise ranging from 315 to 2000 Hz with a peak at 1000 Hz and an intensity of 45-55 dB SPL intensity (HoMedics Natural Sleep Aid SS-200) was continuously played in the colony to prevent the rats from adapting to or becoming familiar with the sound of their tinnitus.
2.2 Sodium Salicylate and Quinine
Sodium Salicylate has been shown to reliably induce short-term tinnitus in different animal models when administered at high doses. Rats in the salicylate-induced tinnitus group received 300 mg/kg/d of sodium salicylate (IP, diluted in bacteriostatic saline, 50 mg/ml, Sigma) for four consecutive days.
Animals in the quinine experimental group were treated with quinine via gavage at a dose of 200 mg/kg/d (diluted in bacteriostatic saline, 50 mg/ml, Sigma) for four consecutive days. Drug administration was performed in each group 2 h before testing animals for tinnitus.
2.3 Gap and noise burst prepulse inhibition of acoustic startle (GPIAS)
Tinnitus was assessed using GPIAS as described previously (29-31). Each rat was placed in an acoustically transparent wire mesh cage (7 cm W, 20 cm L, 6 cm H) located in a dark, soundproof chamber. The cage was placed on a plexiglass platform (20 cm × 10 cm) that rested on a modified 50 mm piezoelectric transducer (MCM 28-745) beneath the platform. A Fostex FT17H tweeter was placed on the chamber’s ceiling, 15 cm above and centered on the rat’s head. Acoustic stimuli were calibrated with a Larson Davis sound level meter (SLM 824), and ½ inch condenser microphone. The continuous background noise, the gap in the background noise, the prepulse noise burst and the acoustic startle stimuli were generated using a digital-to-analog converter at ~100 kHz sampling rate (Tucker Davis Technologies, RP2.1, PA5, SA1). Startle amplitude measured by the piezoelectric transducer was amplified (10-100x) and low-pass filtered at 1000 Hz; WPI, USA) and fed to the analog-to-digital converter on a separate data acquisition module (TDT, RP2.1) using custom software. Data were analyzed using Microsoft Excel 2007.
GPIAS sessions were composed of 100 gap trials (gap) and 100 no-gap trials (nogap), 20 measurements were made at each noise- band center frequency (narrow band noise centered at 6, 12, 16, 20, and 24 kHz). Gap and nogap trials were presented in random pairs. Trials were separated by a variable quiet period ranging from 7 to 15 s long. A 2-min acclimation period occurred at the beginning of each session during which no gaps or startle sounds occurred. Gap trials were composed of a 60 dB SPL continuous narrow band background noise (1000 Hz wide, centered at 6, 12, 16, 20 and 24 kHz), and a 50 ms startle stimulus (single broadband noise burst, 117dB SPL, 50 ms length, 5 ms rise/fall time) preceded by a 100 ms gap ending 100 ms before the onset of the startle stimulus. In nogap trials, the background sound was continuous without a silent period preceding the startle stimulus. When a gap was present, the root-mean-square (RMS) power of the startle reflex signal was reduced compared to nogap trials.
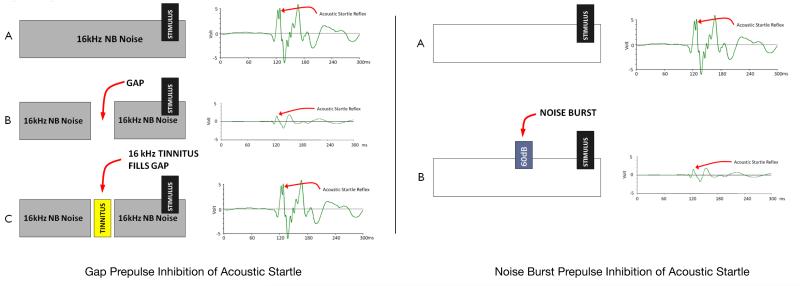
Noise burst prepulse inhibition of the acoustic startle reflex (NBPIAS) was used to assess hearing function at different frequencies and time intervals. NBPIAS was used to determine the audibility of the GPIAS background sound. NBPIAS was recorded using the same equipment as GPIAS: the startle stimulus was presented in a quiet or was preceded by a 60 dB SPL narrowband noise burst (noiseburst) (1000 Hz wide, 100 ms, 5 ms rise/fall time) centered at 6, 12, 16, 20 or 24 kHz; 200 randomized trials were performed during each session (100 quiet, 100 noiseburst). When the startle stimulus was preceded by a noise burst, the startle reflex value was reduced demonstrating that the noise burst was audible to the rat. GPIAS and NBPIAS were calculated for each frequency as a percent using the formulas: 1-(gap/nogap) for GPIAS; 1-(noiseburst/quiet) for NBPIAS. A significant GPIAS reduction at a specific frequency was indicative of tinnitus because the silent gap was no longer perceived by the animal. A significant reduction of NBPIAS indicated that the animal was not able to hear the background sound used for the GPIAS protocol. Therefore, GPIAS tinnitus assessment during drug treatment would only be valid if NBPIAS was unchanged from baseline. Prepulse inhibition of acoustic startle is schematized in Fig. 1. Note the large reduction of the startle reflex when a prepulse stimulus is present. Animals were tested daily with GPIAS and NBPIAS before and 2 h after each drug administration; measurements were obtained for five consecutive days. GPIAS and NBPIAS tests were performed only once per day.
Fig 1.
(Left) Gap prepulse inhibition of acoustic startle: (A) Note the large amplitude startle reflex following the startle stimulus when no prepulse (gap) is present. (B) When a 50 ms gap (silent interval) prepulse was inserted prior to the startle stimulus, the startle reflex response was significantly reduced. (C) When tinnitus is present, the tinnitus fills in the gap and the animal can no longer hear the silent gap, consequently the startle response is reduced similar to the no-gap condition. (Right) (A) Noise burst prepulse inhibition of acoustic startle: NBPIAS is used to measure the ability of rats to hear the background noise used in the GPIAS paradigm. In NBPIAS, the startle stimulus is presented in a quite environment; this results in a large startle response. (B) When a 60 dB noise burst pre-pulse precedes the startle stimulus, the startle response is significantly reduced.
2.4 Auditory Brainstem Response (ABR)
Hearing function was also evaluated physiologically using the ABR; measurements were obtained before and 14 days after drug treatment. ABR testing was performed at 6, 12, 16, 24 and 32 kHz in all animals to confirm that the drug treatments did not cause any permanent effects. Rats were mildly anaesthetized (1.5% isoflurane) and placed in a sound-attenuating booth. Three stainless steel recording electrodes were inserted subcutaneously; one posterior to the tested pinna (active), one at the vertex (reference) and one at the contralateral pinna (ground). A computer-controlled TDT System 3 (BioSigRP, Tucker–Davis Technologies, Alachua, Florida, USA) data acquisition system was used to record the ABR and generate the auditory stimulus. Tone bursts ranging from 6 to 32 kHz (rise/fall time, 2 ms; total duration, 2 ms; repetition rate, 21/s) were presented monaurally in an open field using a horn tweeter (Fostex, TD28D, USA). Responses were filtered (100-3000 Hz bandpass), digitized and averaged over 1000 samples at each frequency-level combination. Thresholds were determined by reducing the intensity of the tone bursts in 10 dB steps until no ABR response was detected. The stimulus intensity was then increased in 5 dB steps until the response could be detected again. Threshold was defined as the lowest intensity able to evoke an ABR response with clear morphology.
2.5 DPOAE
DPOAEs were measured from both ears of all animals using a commercial otoacoustic emission system (Intelligent Hearing System, Miami, FL, USA) as described previously (32). Briefly, DPOAEs were measured using two primary tones, f1 and f2, using an f2/f1 ratio of 1.2. The f1 intensity (L1) was 10 dB higher than the f2 intensity (L2). Six primary tone frequencies were evaluated from 4 to 32 kHz. Animals were mildly anaesthetized (1% isoflurane) and placed in a sound-attenuating booth. The DPOAE probe assembly containing a microphone and two sound delivery tubes coupled to two loudspeakers. The assembly was placed in the animal’s external ear canal. Input/output functions were obtained by increasing L1 intensity from 25 to 70 dB SPL at f2 frequencies of 8, 12, 16 and 20 kHz (32 sweeps per frequency pair). DPOAEs were recorded before and 2 h after each drug treatment for 5 consecutive days.
2.6 Statistical Analysis
GPIAS and NBPIAS data were analyzed using a two-way repeated measures analysis of variance (RM-ANOVA, α < 0.05) to determine main effects, interactions among treatments and time course; post-hoc testing was performed using Tukey’s test for type I errors associated with multiple comparisons. Significant differences between frequency-specific data recorded at each time point and baseline values were analyzed using one-way ANOVA at a confidence level of P<0.05. This type of test is robust enough to compare paired values for different variables. Statistical analysis of ABR and DPOAE measurements were performed using a one-way ANOVA with post-hoc Student-Newman-Keuls method analysis. All results were presented as mean +/− SEM.
3. Results
3.1 Tinnitus assessment
All rats were evaluated for tinnitus and hearing loss at 6, 12, 16, 20 and 24 kHz using GPIAS and NBPIAS. During a five-day period preceding the induction of tinnitus with salicylate or quinine, animals underwent five separate GPIAS and NBPIAS sessions (one/day) to assess their baseline values at each frequency.
Salicylate or quinine was administered for four consecutive days; animals were tested before, 2 h after each drug treatment and 24 h after the last administration (wash-out period) with GPIAS and NBPIAS. Both drugs are known to induce transient tinnitus that lasts for the duration of the treatment period and for a short period of time afterwards (~24-48 h). Previous studies suggest that the pitch of salicylate-induced tinnitus occurs between 10 and 16 kHz (16, 30); quinine has been reported to induce a lower-pitch tinnitus (15, 16).
GPIAS
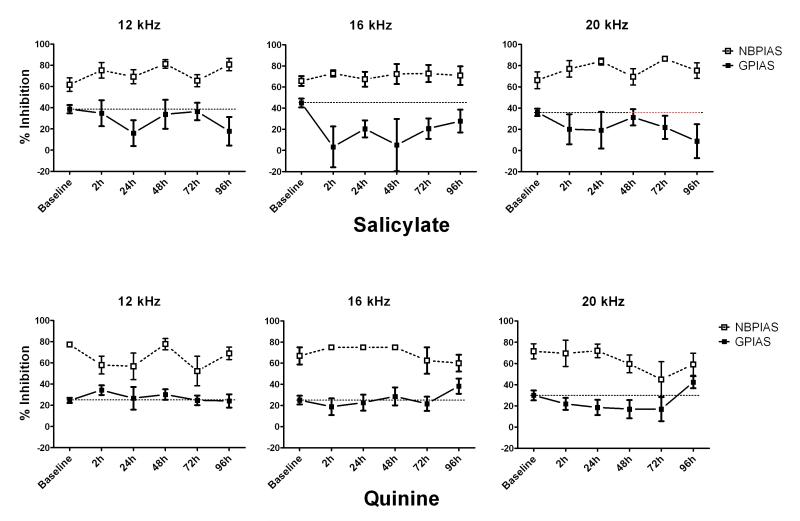
During baseline sessions, animals showed a mean GPIAS of 36% among the tested frequencies in the salicylate group and 33% in the quinine group, there were no statistically significant differences across sessions or frequencies. In the salicylate group, mean GPIAS values recorded at 2, 24, 48 and 72 h after the first injection were 38.14% at 6 kHz, 33.64% at 12 kHz, 7.70% at 16 kHz, 27.94% at 20 kHz and 26.81% at 24 kHz. During the entire duration of salicylate treatment (2 h-96 h), a statistically significant decrease in mean GPIAS was observed at 16 kHz at 2 h (P=0.026), 24 h (P=0.046), 48 h (P=0.015) and 72 h (P=0.039) after the first injection. No significant changes were observed at 6 kHz (P=0.755), 12 kHz (P=0.961), 20 kHz (P=0.872) and 24 kHz (P=0.631) over the entire testing period. Twenty-four hours after the last salicylate injection (96 h after first injection) all GPIAS values returned near to baseline values (29.69%). In the quinine group, no significant changes were observed at 6 kHz (28.80%), 12 kHz (28.23%), 16 kHz (21.19%), 20 kHz (15.18%) or 24 kHz (24.54%) for the entire length of the treatment although a small, non significant reduction of GPIAS was observed at 20 kHz from 2 to 72 h after the first injection.
The data for both salicylate and quinine have been plotted and compared in Fig. 2. In the salicylate group, a large GPIAS reduction was found at 16 kHz while other frequencies were not much affected; this could indicate the presence of tinnitus with a pitch near 16 kHz. In the quinine group, GPIAS was not significantly affected during the treatment period; however, a minor change (not statistically significant) occurred at 20 kHz possibly suggestive of a low-level tinnitus with this particular dose of quinine.
Fig 2.
Salicylate Top row) Figure showing NBPIAS and GPIAS results at 12, 16 and 20 kHz. Salicylate treatment (300 mg/kg) induced a significant reduction in GPIAS (black continuous line) with a pitch near 16 kHz starting 2 h post-treatment, persisting over the 4 day treatment period and disappearing 24 h after the last day of treatment. No significant changes were observed at 12 and 20 kHz over the entire testing period (similar results at 6 and 24 kHz, data not shown). NBPIAS (white dotted line) did not change. Quinine (Bottom row) Figure showing NBPIAS and GPIAS results at 12, 16 and 20 kHz. Animals in the quinine group only showed a minor reduction in GPIAS at 20 kHz; however none of the GPIAS changes were statistical significant (similar results at 6 and 24 kHz, data not shown). No significant change in NBPIAS after quinine treatment (similar results at 6 and 24 kHz, data not shown).
In the salicylate group, a two-way repeated measures analysis of variance (RM-ANOVA) showed a statistically significant main effect for frequency (P<0.014; F=6.761); no significant effect was found for time interval (P=0.362; F=1.147. No significant interaction effect between frequency and time interval was found (P=0.195; F=1.143). In the quinine group no significant effect was found for frequency (P=0.271; F=1.490), time interval (P=0.670; F=0.642), or frequency × time interval (P=0.906; F=0.462).
NBPIAS
No significant changes in NBPIAS were observed before and after salicylate or quinine treatment over the entire testing period at all the frequencies evaluated. Before treatment, the average noise-burst pre pulse inhibition was 74.51% among all frequencies. No significant change was observed from 2 h to 96 h after the first injection or thereafter. Only one animal in the salicylate group showed a decrease in NBPIAS at 16 kHz 2 h after injection (40.62%; P=0.61; F=0.283). Another rat in the same group experienced a lower NBPIAS in the low-middle frequencies (6-16 kHz) 2 h after injection (42.11%; P=0.79; F=0.073). Both changes were not statistically significant.
3.2 ABR
The ABR was recorded in each animal before and 14 days after treatment to evaluate whether salicylate or quinine treatment could induce a permanent auditory threshold shift. Baseline threshold values recorded before salicylate or quinine treatment did not show a statistically significant difference among animals (Two-Way ANOVA, P=0.28). In both the salicylate and quinine groups, no significant changes could be observed in ABR thresholds 14 days post-treatment (P=0.79).
3.3 DPOAEs
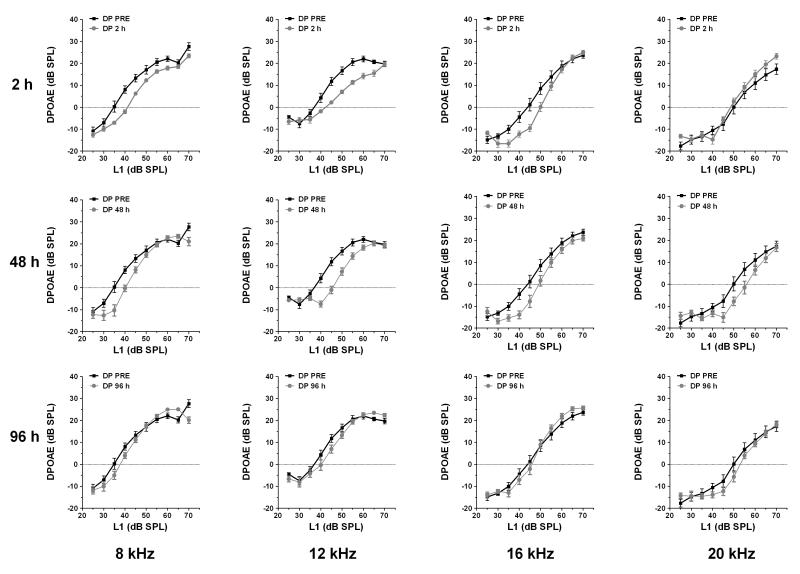
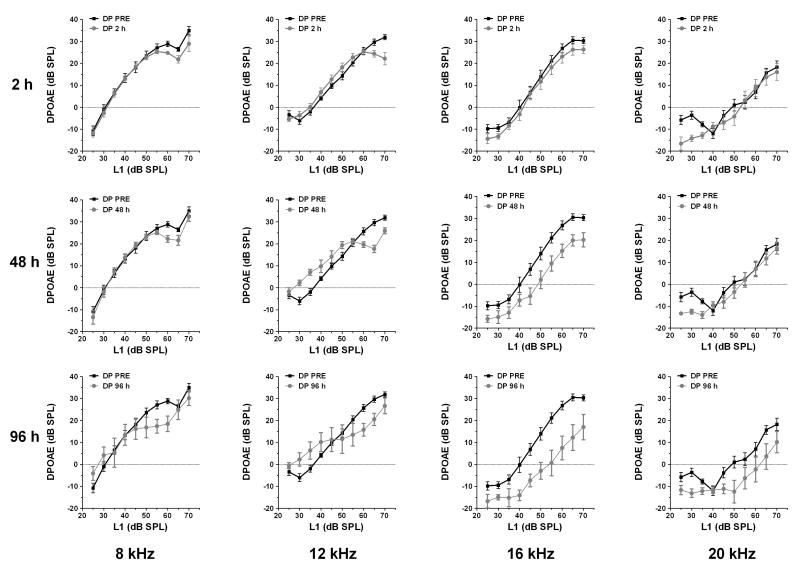
DPOAEs were tested at 4, 8, 12, 16 and 20 kHz for five consecutive days. In the salicylate group, an amplitude reduction was observed mainly on low-level DPOAEs at 8, 12 and 16 kHz. High level DPOAEs were slightly affected; most changes could only be seen 2 h after injection at 8 and 12 kHz. DPOAEs completely recovered 1 day post-treatment. In the quinine group, high-level DPOAEs were affected mainly at 16 kHz, starting 48 h after the first injection with no spontaneous recovery after suspension of the treatment. Although DPOAEs were reduced near 16 kHz, this reduction was insufficient to alter the ABR or NBPIAS. The changes in DPOAE over time for both groups are plotted in Fig. 3 (salicylate) and Fig. 4 (quinine). Note the complete recovery of DPOAE in the salicylate group compared to incomplete recovery in the quinine group 24 h.
Fig 3.
DPOAEs in the salicylate group at 8, 12, 16 and 20 kHz at 2, 48 and 96 h after the first salicylate injection. Note decrease in DPOAE mainly on low-level DPOAEs at 8, 12 and 16 kHz. High level DPOAEs were slightly affected; most changes could only be seen 2 h after injection at 8 and 12 kHz.
Fig 4.
DPOAEs for animals in the quinine group tested at 8, 12, 16 and 20 kHz at 2, 48 and 96 h after the first quinine administration. High-level DPOAEs were affected, mainly at 16 kHz, starting 48 hours after the first injection, with no recovery post-treatment.
4. Discussion
The animal models used so far for the study of tinnitus require the use of electric shock, food or water deprivation, long training and other elements that may limit their use in specific conditions. The model adopted in this study is based on the startle reflex, an unconditioned reflex evoked by a short and intense sound, and its inhibition by a prepulse stimulus presented shortly before the startle sound. The neural basis of the startle reflex and the modulation of the reflex by prepulse stimuli have been extensively studied (33-37). Molecular factors underling the reflex and its alterations with specific diseases or treatments have also been reported (38, 39). Furthermore, prepulse inhibition of the startle reflex has been highlighted as one of the most reliable paradigms for evaluating sensorimotor gating (40). The use of startle reflex in the study of tinnitus has several advantages including an objective response that is independent of other behavioral factors. The startle reflex does not require food or water deprivation and, since behavioral habituation is not a significant factor, this paradigm can be used to evaluate tinnitus and hearing loss over an extended time. Finally, this model is suitable for high-throughput screening of potential therapeutic compounds.
The present results confirm that salicylate can induce transient, reversible tinnitus when administered at high doses. Tinnitus-like behavior was observed starting 2 h after the first administration, lasting for the entire length of the salicylate treatment and disappearing 24 h after the last salicylate dose. This model also identified the pitch of tinnitus as being near 16 kHz with no substantial evidence of tinnitus at other frequencies. Such results are consistent with what has been reported in humans after high-dose salicylate administration (41, 42). Our GPIAS data confirms the 16 kHz tonal nature of tinnitus reported previously with GPIAS and other behavioral paradigms (30). The 16 kHz pitch observed in the present study is slightly higher than the 10 kHz pitch estimated from a conditioned lick suppression paradigm (42). In contrast to previous studies, a more recent GPIAS study found evidence of noise-like tinnitus only with the 300 mg/kg dose of salicylate (31); they found no evidence of tinnitus with the 150 or 250 mg/kg doses of salicylate in contrast previous behavioral studies using a variety of behavioral testing paradigms (27, 28). Surprisingly, this GPIAS study found evidence of increased behavioral salience (greater prepulse inhibition) of gaps embedded in narrow band stimuli which was interpreted as evidence of hyperacusis (31).
In the quinine group, no changes were observed at 6, 12, 16 or 24 kHz, and only a minor reduction of GPIAS was observed at 20 kHz; however, this was not statistically significant. Our results differ from those of previous studies that found evidence of tinnitus in rats treated with 200 mg/kg of quinine given subcutaneously (15). The differences between our results and those of previous studies could be explained by the route of quinine administration or attributed to the differences in behavioral paradigms used to assess tinnitus. Also, the quinine GPIAS data varied across animals to a much larger extent than in the salicylate group. Therefore, larger quinine doses and different administration methods should be tested in future experiments with GPIAS to determine if tinnitus-like behavior can be induced.
Previous behavioral studies indicate that high doses of sodium salicylate cause a hearing loss that tends to be greatest around 10-20 kHz (43). In this study, the 300 mg/kg dose of salicylate elevated behavioral thresholds to approximately 20 dB SPL (<15 dB threshold shift). Based on these results, the 60 dB background noise used in our GPIAS study would be ~40 dB above thresholds. Since gap detection performance is near its optimal level 40 dB above thresholds (44), it seems unlikely that the slight hearing loss induced by salicylate would impair gap detection performance. Moreover, auditory function was monitored in the current study with NBPIAS, ABR and DPOAEs. No significant changes were observed in the NBPIAS for animals in both groups indicating that the changes in the auditory function after drug administration were not sufficient to prevent the rats from hearing the background sound used to assess GPIAS. Collectively, these findings suggest that the slight changes in threshold induced by salicylate are not sufficient to account for the changes in GPIAS. However, we cannot not completely rule out the possibility that salicylate has an effect on the gap-modulated startle response.
DPOAEs showed a reduction indicative of an outer hair cell dysfunction; however, DPOAEs returned to normal, baseline values after the end of the treatment. Salicylate’s effects on DPOAEs are likely explained by the action of salicylate on the OHC electromotile protein, prestin. Salicylate has been shown to reduce OHC motility following acute administration (45-47). Quinine has been shown to effect OHC morphology and function; (48) however no recovery of DPOAEs was observed in this group after the suspension of quinine treatment. Despite the fact that DPOAE had not fully recovered following quinine treatment, the ABR showed no changes in threshold 14 days after quinine or salicylate treatments. These results indicate that neither drug caused a permanent threshold shift as measured by the ABR.
In summary, GPIAS appears to be a reliable method for assessing the presence of salicylate induced tinnitus and its pitch. Our GPIAS data suggest that the 300 mg/kg/d dose of sodium salicylate induced tinnitus with a pitch near 16 kHz that lasted for the entire length of the treatment and disappeared 24 h after the last drug injection. In contrast, the 200 mg/kg/d dose of quinine failed to induce tinnitus-like behavior; however, further testing with higher doses of quinine may yield more significant changes.
Acknowledgments
Supported in part by grant from NIH (R01DC009091 & R01DC009219) and TRI
Abbreviations
- ABR
auditory brainstem response
- GPIAS
gap prepulse inhibition of acoustic startle
- NBPIAS
noise burst prepulse inhibition of acoustic startle
- COX
cyclooxygenase
- DPOAE
distortion product otoacoustic emission
- IHC
inner hair cell
- I/O
input/output
- OHC
outer hair cell
- ROS
reactive oxygen species
- SD
Sprague–Dawley
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erlandsson SI, Hallberg LR, Axelsson A. Psychological and audiological correlates of perceived tinnitus severity. Audiology. 1992;31:168–79. doi: 10.3109/00206099209072912. [DOI] [PubMed] [Google Scholar]
- 2.Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol. 2000;62:583–631. doi: 10.1016/s0301-0082(00)00027-7. [DOI] [PubMed] [Google Scholar]
- 3.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 4.Vane JR, Botting RM. Mechanism of action of antiinflammatory drugs. Int J Tissue React. 1998;20:3–15. [PubMed] [Google Scholar]
- 5.Mitchell JA, Akarasereenont P, Thiemermann C, et al. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90:11693–7. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiemermann C. Biosynthesis and interaction of endothelium-derived vasoactive mediators. Eicosanoids. 1991;4:187–202. [PubMed] [Google Scholar]
- 7.Guitton MJ, Caston J, Ruel J, et al. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003;23:3944–52. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald JJ, Robertson D, Johnstone BM. Effects of intra-cochlear perfusion of salicylates on cochlear microphonic and other auditory responses in the guinea pig. Hear Res. 1993;67:147–56. doi: 10.1016/0378-5955(93)90242-s. [DOI] [PubMed] [Google Scholar]
- 9.McMahon CM, Patuzzi RB. The origin of the 900 Hz spectral peak in spontaneous and sound-evoked round-window electrical activity. Hear Res. 2002;173:134–52. doi: 10.1016/s0378-5955(02)00281-2. [DOI] [PubMed] [Google Scholar]
- 10.Martin WH, Schwegler JW, Scheibelhoffer J, et al. Salicylate-induced changes in cat auditory nerve activity. Laryngoscope. 1993;103:600–4. doi: 10.1288/00005537-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ochi K, Kinoshita H, Kenmochi M, et al. Effects of nimodipine on salicylate ototoxicity. Ann Otol Rhinol Laryngol. 2002;111:1092–6. doi: 10.1177/000348940211101206. [DOI] [PubMed] [Google Scholar]
- 12.Su YY, Luo B, Wang HT, et al. Differential effects of sodium salicylate on current-evoked firing of pyramidal neurons and fast-spiking interneurons in slices of rat auditory cortex. Hear Res. 2009;253:60–6. doi: 10.1016/j.heares.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Wang HT, Luo B, Zhou KQ, et al. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res. 2006;215:77–83. doi: 10.1016/j.heares.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Rybak LP. Drug ototoxicity. Annu Rev Pharmacol Toxicol. 1986;26:79–99. doi: 10.1146/annurev.pa.26.040186.000455. [DOI] [PubMed] [Google Scholar]
- 15.Jastreboff PJ, Brennan JF, Sasaki CT. Quinine-induced tinnitus in rats. Arch Otolaryngol Head Neck Surg. 1991;117:1162–6. doi: 10.1001/archotol.1991.01870220110020. [DOI] [PubMed] [Google Scholar]
- 16.Lobarinas E, Yang G, Sun W, et al. Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol Suppl. 2006:13–9. doi: 10.1080/03655230600895408. [DOI] [PubMed] [Google Scholar]
- 17.Eleno N, Botana L, Espinosa J. K-channel blocking drugs induce histamine release and 45Ca uptake in isolated mast cells. Int Arch Allergy Appl Immunol. 1990;92:162–7. doi: 10.1159/000235208. [DOI] [PubMed] [Google Scholar]
- 18.Hermann A, Gorman AL. Action of quinidine on ionic currents of molluscan pacemaker neurons. J Gen Physiol. 1984;83:919–40. doi: 10.1085/jgp.83.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenmochi M, Eggermont JJ. Salicylate and quinine affect the central nervous system. Hear Res. 1997;113:110–6. doi: 10.1016/s0378-5955(97)00137-8. [DOI] [PubMed] [Google Scholar]
- 20.Jastreboff PJ, Brennan JF, Coleman JK, et al. Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci. 1988;102:811–22. doi: 10.1037//0735-7044.102.6.811. [DOI] [PubMed] [Google Scholar]
- 21.Jastreboff PJ, Brennan JF, Sasaki CT. An animal model for tinnitus. Laryngoscope. 1988;98:280–6. doi: 10.1288/00005537-198803000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Bauer CA, Brozoski TJ, Rojas R, et al. Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg. 1999;121:457–62. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- 23.Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol. 2001;2:54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guitton MJ, Caston J, Ruel J, et al. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003;23:3944–52. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hear Res. 2002;170:83–95. doi: 10.1016/s0378-5955(02)00343-x. [DOI] [PubMed] [Google Scholar]
- 26.HE, Koay G. Tinnitus and hearing loss in hamsters (Mesocricetus auratus) exposed to loud sound. Behav Neurosci. 2005;119:734–42. doi: 10.1037/0735-7044.119.3.734. [DOI] [PubMed] [Google Scholar]
- 27.Lobarinas E, Sun W, Cushing R, et al. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear Res. 2004;190:109–14. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- 28.Ruttiger L, Ciuffani J, Zenner HP, et al. A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: a new approach for an animal model on tinnitus. Hear Res. 2003;180:39–50. doi: 10.1016/s0378-5955(03)00075-3. [DOI] [PubMed] [Google Scholar]
- 29.Turner JG, Brozoski TJ, Bauer CA, et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–95. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Lobarinas E, Zhang L, et al. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–53. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol. 2008;17:S185–92. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- 32.Jamesdaniel S, Ding D, Kermany MH, et al. Analysis of cochlear protein profiles of Wistar, Sprague-Dawley, and Fischer 344 rats with normal hearing function. J Proteome Res. 2009;8:3520–8. doi: 10.1021/pr900222c. [DOI] [PubMed] [Google Scholar]
- 33.Roskam S, Koch M. Enhanced prepulse inhibition of startle using salient prepulses in rats. Int J Psychophysiol. 2006;60:10–4. doi: 10.1016/j.ijpsycho.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- 35.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–28. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 36.Schicatano EJ, Peshori KR, Gopalaswamy R, et al. Reflex excitability regulates prepulse inhibition. J Neurosci. 2000;20:4240–7. doi: 10.1523/JNEUROSCI.20-11-04240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis M, Antoniadis EA, Amaral DG, et al. Acoustic startle reflex in rhesus monkeys: a review. Rev Neurosci. 2008;19:171–85. doi: 10.1515/revneuro.2008.19.2-3.171. [DOI] [PubMed] [Google Scholar]
- 38.Moriwaki M, Kishi T, Takahashi H, et al. Prepulse inhibition of the startle response with chronic schizophrenia: a replication study. Neurosci Res. 2009;65:259–62. doi: 10.1016/j.neures.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Dieckmann M, Freudenberg F, Klein S, et al. Disturbed social behavior and motivation in rats selectively bred for deficient sensorimotor gating. Schizophr Res. 2007;97:250–3. doi: 10.1016/j.schres.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Yee BK, Feldon J. Distinct forms of prepulse inhibition disruption distinguishable by the associated changes in prepulse-elicited reaction. Behav Brain Res. 2009;204:387–95. doi: 10.1016/j.bbr.2008.11.049. [DOI] [PubMed] [Google Scholar]
- 41.Jung TT, Rhee CK, Lee CS, et al. Ototoxicity of salicylate, nonsteroidal antiinflammatory drugs, and quinine. Otolaryngol Clin North Am. 1993;26:791–810. [PubMed] [Google Scholar]
- 42.Jastreboff PJ, Sasaki CT. An animal model of tinnitus: a decade of development. Am J Otol. 1994;15:19–27. [PubMed] [Google Scholar]
- 43.Brennan JF, Brown CA, Jastreboff PJ. Salicylate-induced changes in auditory thresholds of adolescent and adult rats. Dev Psychobiol. 1996;29:69–86. doi: 10.1002/(SICI)1098-2302(199601)29:1<69::AID-DEV4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 44.Syka J, Rybalko N, Mazelova J, et al. Gap detection threshold in the rat before and after auditory cortex ablation. Hear Res. 2002;172:151–9. doi: 10.1016/s0378-5955(02)00578-6. [DOI] [PubMed] [Google Scholar]
- 45.Peleg U, Perez R, Freeman S, et al. Salicylate ototoxicity and its implications for cochlear microphonic potential generation. J Basic Clin Physiol Pharmacol. 2007;18:173–88. doi: 10.1515/jbcpp.2007.18.3.173. [DOI] [PubMed] [Google Scholar]
- 46.Yang K, Huang ZW, Liu ZQ, et al. Long-term administration of salicylate enhances prestin expression in rat cochlea. Int J Audiol. 2009;48:18–23. doi: 10.1080/14992020802327998. [DOI] [PubMed] [Google Scholar]
- 47.Yu N, Zhu ML, Johnson B, et al. Prestin up-regulation in chronic salicylate (aspirin) administration: an implication of functional dependence of prestin expression. Cell Mol Life Sci. 2008;65:2407–18. doi: 10.1007/s00018-008-8195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarboe JK, Hallworth R. The effect of quinine on outer hair cell shape, compliance and force. Hear Res. 1999;132:43–50. doi: 10.1016/s0378-5955(99)00031-3. [DOI] [PubMed] [Google Scholar]