Abstract
Purpose
The tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib are effective in non-small-cell lung cancers (NSCLCs) with epidermal growth factor receptor (EGFR) gene mutations. The usual clinical dose of gefitinib (250 mg/day) is only one third of its maximum tolerated dose (MTD), while the dose of erlotinib (150 mg/day) is at its MTD. In NSCLC cell lines both TKIs have similar micromolar (μM) inhibitory concentrations. We explored if erlotinib at 25 mg/day (trough serum concentration similar to gefitinib 250 mg/day) would be efficacious in EGFR mutated NSCLC.
Methods
To study inhibitory concentrations of gefitinib and erlotinib, we exposed EGFR mutated cell lines (HCC827, H3255, PC-9, H1975) to increasing concentrations of these TKIs. Further on, we performed a retrospective evaluation of seven patients with advanced EGFR mutated (exon 19 deletions and L858R) NSCLC that were given erlotinib at 25 mg/day as their first EGFR TKI.
Results
Gefitinib and erlotinib generated similar inhibitory curves across our panel of EGFR mutated NSCLC cell lines with overlapping mean IC50 95% confidence intervals (CI) for HCC827, PC-9 and H1975. Both drugs also displayed a high degree of correlation in mean IC50 (Pearson’s r = 0.99, p = 0.0417). Of the 7 patients, 5 (71.5%) had partial responses to erlotinib 25 mg/day. Median progression-free survival (PFS) was 17 months (95% CI, 6 - 35 months). Toxicities were minimal with only 2 (28.5 %) patients having a rash and none experiencing (0%) diarrhea.
Conclusions
In NSCLC cell lines, gefitinib and erlotinib have similar inhibitory profiles. In patients with NSCLC and EGFR activating mutations, a dose of erlotinib 25 mg/day (equivalent to gefitinib 250 mg/day) leads to impressive response rates and PFS similar to the growing experience with the approved doses of gefitinib (250 mg/day) and erlotinib (150 mg/day). Identifying prospectively the lowest and clinically active dose ranges of erlotinib and gefitinib will help further personalize care for patients with tumors harboring EGFR mutations.
Keywords: Epidermal growth factor receptor, EGFR, mutation, tyrosine kinase inhibitors, gefitinib, erlotinib, L858R, exon 19 deletions, lung cancer, non-small cell lung cancer
INTRODUCTION
Somatic mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) gene were identified in patients with non-small-cell lung cancer (NSCLC) in 2004 (1-3). These mutations are more prevalent in women, never/light smokers, patients with East Asian ancestry and patients whose tumors demonstrate adenocarcinoma histology (4). Most clinically-relevant EGFR mutations consist of a single point mutation (L858R) in exon 21 and inframe deletions around the conserved LREA motif of exon 19 (residues 747-750) (5). EGFR mutations are oncogenic, alter the tyrosine kinase pocket of EGFR to a degree that enhances the sensitivity to ATP-competitive EGFR inhibitors, and make lung cancers with EGFR mutations dependent on EGFR signaling for their survival and proliferation (6).
Two EGFR tyrosine kinase inhibitors (TKIs) have reached regulatory approval for NSCLC: gefitinib and erlotinib (7-9). When given as first, second or subsequent lines of therapies for patients with EGFR mutations these agents lead to significant clinical and radiographic responses in most patients (10). The reported response rates (RRs) exceed 70%, with median progression-free survival (PFS) intervals of approximately 6-12 months and overall survival times beyond 20-24 months (11;12). Gefitinib improves RR and PFS when compared to standard platinum-doublet chemotherapy in therapy-naïve patients with metastatic EGFR mutated NSCLC (13). Erlotinib is equally efficacious in EGFR mutated tumors when given as first line therapy but has not been compared head-to-head with chemotherapy in published reports (14).
NSCLCs that harbor activating EGFR mutations are sensitive to gefitinib or erlotinib in equivalent sub micromolar (μM) concentrations (6). Despite the similar in vitro spectrum of action in NSCLC cells, the clinical development and therapeutic doses used in the clinic for these two TKIs, which were initially developed to target wild-type EGFR, differs. The dose of gefitinib (250 mg/day) that entered late-stage clinical trials for NSCLC is only one third of its maximum tolerated dose (MTD), whereas the dose used for erlotinib (150 mg/day) is its MTD (15;16). Data from phase I dose-escalation trials show that the expected mean trough steady state serum concentration of gefitinib at 250 mg/day is about 0.5 μM (17), whereas erlotinib at 150 mg/day has mean trough steady state concentrations that exceed 2.5 μM (18). This knowledge led us to hypothesize that erlotinib at 25 mg/day (its lowest available tablet size with presumed trough serum concentration around 0.5 μM) would be active in EGFR mutated NSCLC.
Here, we confirm that both gefitinib and erlotinib have similar in vitro inhibitory concentrations in NSCLC cells with EGFR mutations, and demonstrate that erlotinib 25 mg/day leads to a high response rate and PFS in patients with NSCLC harboring EGFR activating mutations.
MATERIAL AND METHODS
Reagents
Gefitinib and erlotinib were purchased from a commercial supplier. Stock solutions for gefitinib and erlotinib were prepared as previously described (19).
Cell culture
The human lung cancer derived cells lines NCI-H1975 (H1975), NCI-H3255 (H3255), PC-9 and HCC827 were maintained in RPMI supplemented with 10% fetal bovine serum.
Cell proliferation Assay
Growth inhibition was assessed by CellTiter 96 AQueous One solution proliferation kit (Promega, Madison, WI) as previously described (20;21). Briefly, cells were transferred to wells at 1,500 to 5,000 cells/ well (for H1975 1,500, for HCC827 2,500, for PC-9 3,500, for H3255 5,000) in 96-well flat-bottom plates with various concentrations of inhibitors and incubated for 72 hours prior to photometric analysis. Experiments were repeated three times.
Patient selection
Patients were identified retrospectively from the clinical databases of two academic medical centers: 1) Beth Israel Deaconess Medical Center and 2) Memorial Sloan-Kettering Cancer Center. Both centers have institutional approved protocols for chart review and genomic analysis of stored tumor tissues. Inclusion criteria to use the patient’s data included a diagnosis of stage IV metastatic NSCLC with a proven EGFR mutation and exposure to erlotinib at a dose of 25 mg daily. Data was collected from the patients’ medical records for clinical, demographic and pathologic characteristics as well as toxicity. Radiographic data was reviewed by each center. Treatment decisions were made by each treating physician.
EGFR genotype in the identified patients
DNA isolation from paraffin-embedded tissue and EGFR genotype followed protocols described previously (15). In all cases either exons 18 to 21 of the EGFR gene were sequenced or sensitive polymerase chain reaction (PCR) amplification techniques identified L858R and deletions in exon 19.
Treatment schedules, response, progression-free survival assessment and statistical analysis in the identified patients
All retrospectively identified patients had the same initial treatment schedule for erlotinib. This medication was given orally at a dose of 25 mg/day, and erlotinib was used until tumor progression, unacceptable toxicity or at the physician’s discretion. Since the patients identified had been treated as part of routine clinical care, the investigators had no control of the dosing schedules and follow-up proposed by each treating physician. Objective tumor response was determined retrospectively by Response Evaluation Criteria in Solid Tumors (RECIST) v1.0 (22). PFS was calculated from the date of starting erlotinib until the date of radiographic tumor progression. All patients had adverse reactions determined, by chart extraction, while receiving erlotinib according to the Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0. The data cut off for analysis of PFS and other endpoints was February 1, 2010.
Statistical Analysis
Inhibitory proliferation curves and the 50% inhibitory concentration (IC50) in the aforementioned proliferation assays were generated and calculated, respectively, using the GraphPad Prism 5 software (GraphPad Software, Inc, La Jolla, CA). Pearson’s correlation coefficient (r) was used as a measure of association between IC50s for gefitinib and erlotinib. A log transformation was applied to the dose data, and the exact two-sided p-value was calculated using StatXact (Cytel Software Corporation, Cambridge, MA). PFS estimates were made using the Kaplan-Meier method (23), and the 95% confidence interval (CI) for the median was based on the sign test. Analysis was conducted with SAS 9.1 (SAS Institute, Cary, NC)
RESULTS
In vitro inhibitory concentration of gefitinib and erlotinib in NSCLC cells with EGFR activating mutations
We selected a set of NSCLC cell lines to identify the sensitivity of EGFR mutated cells to the effects of increasing concentrations of gefitinib and erlotinib. HCC827 and PC-9 have an identical exon 19 deletion (delE746_A750) (20). H3255 carries the L858R EGFR exon 21 point mutation (2). H1975 carries the L858R mutation in association with the T790M EGFR TKI-resistant mutation (24).
After exposure to increasing concentrations of gefitinib or erlotinib, HCC827, PC-9 and H3255 had their proliferation inhibited by these EGFR TKIs in sub 0.5 μM concentrations (Figure 1). H1975 cells were not significantly inhibited up to 5 μM of exposure (Figure 1).
Figure 1.
Proliferation assays of NSCLC cells exposed to gefitinib (—) and erlotinib (---) for 72 hours. A) HCC827 (n=3). B) PC-9 (n=3). C) H3255 (n=3). D) H1975 (n=3).
As indicated in Figure 1, both gefitinib and erlotinib led to similar inhibitory curves across our sample of EGFR mutated cells. At concentrations between 0.001 and 1 μM the curves overlapped for HCC827, PC-9 and H1975.
The calculated mean IC50 for gefitinib was: 0.002686 μM (95% CI, 0.001649 to 0.004376) for HCC827, 0.02632 μM (95% CI, 0.01748 to 0.03963) for PC-9, 0.03843 μM (95% CI, 0.03149 to 0.04690) for H3255, and 11.580 μM (95% CI, 9.827 to 13.660) for H1975. The mean IC50 for erlotinib was: 0.002142 μM (95% CI, 0.001307 to 0.003510) for HCC827, 0.03136 μM (95% CI, 0.02082 to 0.04724) for PC-9, 0.08898 μM (95% CI, 0.07294 to 0.1085) for H3255, and 9.183 μM (95% CI, 7.640 to 11.040) for H1975.
The mean IC50 values for both gefitinib and erlotinib in 3 out of 4 EGFR mutated cell lines had overlapping 95% CIs (HCC827, PC-9 and H1975). Gefitinib and erlotinib also had a high degree of correlation in mean IC50 (Pearson’s r = 0.99, p = 0.0417).
These results indicate that both EGFR inhibitors have similar inhibitory patterns in EGFR mutated cells and that they are very strongly correlated.
Patient characteristics
Records of EGFR genotyped patients from our centers during the periods of 2004 to 2010 were reviewed. We retrospectively identified 7 EGFR mutated patients that had received erlotinib at a dose of 25 mg daily as their first EGFR TKI.
Clinical, demographic, pathologic and molecular characteristics of this cohort are displayed in Table 1. Medical record extraction and direct confirmation with the treating physician identified that in all cases the treating physician offered the dose of 25 mg daily of erlotinib due to its supposed equivalence to gefitinib 250 mg daily and that patients were informed the 25 mg dose was not the standard dose of erlotinib for NSCLC. In one of the cases (patient 4, Table 2), notes from the treating physician also reported a prior history of treated hepatitis B as a reason to offer erlotinib at 25 mg daily.
Table 1.
Clinical, pathologic, demographic and molecular characteristics of the studied EGFR mutated patients
| Characteristic | no. of patients | % | |
|---|---|---|---|
| Age (years) | |||
| Median | 65 | ||
| Range | 55-86 | ||
| Sex | |||
| Female | 5 | 71.5 | |
| Male | 2 | 28.5 | |
| Smoking history | |||
| Never smoker | 1 | 14.2 | |
| Former smoker | 5 | 71.5 | |
| Current smoker | 1 | 14.2 | |
| Histology | |||
| Adenocarcinoma | 6 | 85.7 | |
| NSCLC – NOS | 1 | 14.2 | |
| EGFR mutation | |||
| Exon 19 deletion* | 5 | 71.5 | |
| L858R | 2 | 28.5 | |
| Therapy prior to erlotinib | |||
| Platinum-based chemotherapy | 2 | 28.5 | |
| No prior therapy | 5 | 71.5 | |
no., number; NSCLC-NOS, non-small cell lung cancer-non otherwise specified;
specific EGFR sequences of the exon 19 deletions are detailed in Table 2.
Table 2.
Clinical, pathologic, demographic, molecular characteristics, response to therapy, and progression-free survival in the studied patients
| Clinical, Pathologic and Molecular Characteristics | Efficacy | Toxicity (grade -CTCAE) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| patient | age (years) | sex | Smoking history | histology | EGFR mutation | daily dose erlotinib (line of therapy) | response (RECIST) | PFS (months) | rash | diarrhea | other |
| 1 | 55 | M | former (60 py) | adenocarcinoma | delE746_A750 | 25 mg (1st line) | PR | 6 | none (0) | none (0) | none |
| 2 | 64 | F | never | adenocarcinoma | delE746_A750 | 25 mg (1st line) | PR | 8+ | none (0) | none (0) | none |
| 3 | 65 | F | former (30 py) | adenocarcinoma | delL747_T751 + R776S | 25 mg (1st line) ## | PR | 11+ | none (0) | none (0) | LFT (2) |
| 4 | 46 | M | former (30 py) | NSCLC, poorly differentiated | L858R | 25 mg (2nd line) | SD | 4 | yes (1) | none (0) | none |
| 5 | 67 | F | current (1.5 py) | adenocarcinoma | exon 19 deletion * | 25 mg (1st line) | PR | 10 | yes (1) | none (0) | none |
| 6 | 69 | F | former (0.5 py) | adenocarcinoma | exon 19 deletion * | 25 mg (2nd line) | SD | 17 | none (0) | none (0) | none |
| 7 | 86 | F | former (30 py) | adenocarcinoma | L858R | 25 mg # (1st line) | PR | 35 | none (0) | none (0) | none |
EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; PFS, progression-free survival; py, pack-years; CTCAE, common terminology criteria for adverse events v3.0; M, male; F, female; RECIST, response evaluation criteria in solid tumors v1.0; PR, partial response; SD, stable disease;
ongoing response; LFT, liver function test abnormalities;
exon 19 deletions were detected using length analysis of fluorescently labeled PCR in patients 5 and 6 and no further characterization of the mutation by sequencing was performed;
patient 6 had her dose of erlotinib increased to 50 mg/day after initial response to erlotinib 25 mg/day;
patient 3 had her dose of erlotinib reduced to 25 mg every other day after LFT abnormalities were noted. All other patients remained on erlotinib 25 mg/day until disease progression. Patients 1-4 were followed at BIDMC and patients 5-7 at MSKCC.
Response and PFS to erlotinib 25 mg/day
5 out of the 7 patients (71.5%) had partial radiographic responses as defined by RECIST to erlotinib (Table 2), a number that is compatible with retrospective and prospective data for EGFR mutated patients treated with gefitinib 250 mg/day or erlotinib 150 mg/day (25). 2 patients (28.5%) had stable disease (SD), and none of the patients had de novo resistance to erlotinib with progressive disease (PD) as best response.
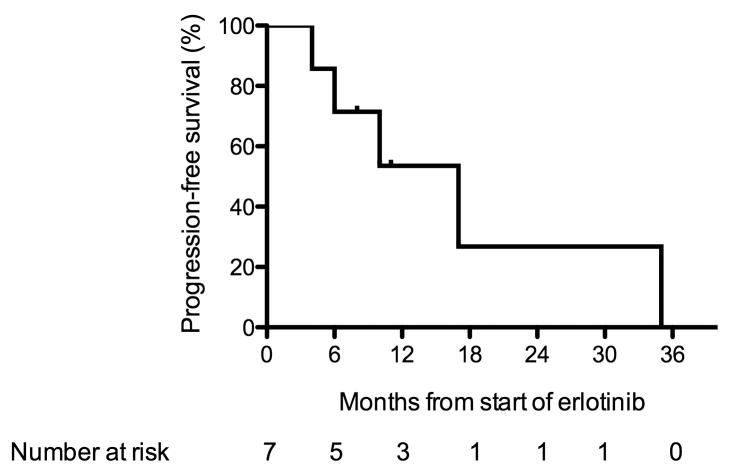
The median PFS calculated by the Kaplan-Meier method was 17 months (95% CI, 6 months – 35 months) as shown in Figure 2. Individual PFS and the patients’ dose schedules while using erlotinib 25 mg/day are detailed in Table 2.
Figure 2.
Kaplan-Meier progression-free survival curve for EGFR mutated patients treated with erlotinib 25 mg/day.
Adverse reactions to erlotinib 25 mg/day
There were no grade 3 or 4 adverse events recorded during the treatment period, and no patients discontinued erlotinib due to unacceptable toxicity. Only 2/7 (29%) patients developed a grade 1 rash while on erlotinib, and none (0%) developed diarrhea (Table 2). The only other toxicity recorded was related to liver test abnormalities in one of the patients (Table 2) that improved with use of erlotinib at 25 mg every other day. Clinical or radiographic signs of interstitial pulmonary fibrosis/pneumonia were not seen in these patients.
DISCUSSION
Gefitinib and erlotinib are orally available anilinoquinazoline small molecule ATP-mimetic TKIs most specific against EGFR. The molecular weight of gefitinib is 446.90 and gefitinib’s clinical dose for NSCLC of 250 mg/day is far less than its MTD of 700-1000 mg/day (16;17;26;27). Based on a phase I dose-escalation trial, the mean steady state trough serum concentration of gefitinib following 225 mg/day averaged 0.16 μg/mL or a calculated 0.358 μM (17). The mean trough concentration increased to 0.24 μg/mL or 0.537 μM at 300 mg/day, and to 1.1 μg/mL or 2.461 μM at 1000 mg/day of gefitinib (17). Erlotinib’s molecular weight is 429.90 and its clinical dose of 150 mg/day is its MTD (7;18). In a phase I dose-escalation trial, erlotinib’s steady state trough concentrations at 150 mg/day ranged from 0.33 to 2.64 μg/mL (18) with a median of 1.26 μg/mL or the equivalent to 2.930 μM. In the same trial, a dose of 25 mg/day led to a median trough below 0.22 μg/mL or the equivalent to less than 0.511 μM (18). Therefore, erlotinib is given at a higher biologically active dose than gefitinib in routine clinical practice (16). In agreement with the pharmacokinetic data, in the larger phase III randomized trials of these TKIs versus placebo for NSCLC (7;9) the rates of rash and diarrhea (the two most common and dose-dependent side-effects of these drugs) were higher (almost double) with erlotinib 150 mg/day (7) than gefitinib 250 mg/day (9).
Despite their different clinical development paths for unselected NSCLCs, both gefitinib and erlotinib have a very similar spectrum of activity in vitro against EGFR mutant proteins or EGFR mutated tumors (2;28;29). Gefitinib 250 mg/day has proven highly effective is prospective and retrospective series of patients with EGFR activating mutations consistently yielding response rates that exceed 70% is chemotherapy-naive patients and PFS times that can reach more than 9 months (12;13). Erlotinib 150 mg/day is equally effective in EGFR mutated NSCLC (5;14). However, both drugs have never been compared head-to-head in a clinical trial of patients with EGFR mutations and until now it was unknown if a “lower” dose of erlotinib could generate similar responses to gefitinib 250 mg/day. Based on the pharmacokinetic data of phase I trials for both compounds and their similar in vitro inhibitory profile, a dose of erlotinib 25 mg/day seemed to best represent a dose of gefitinib 250 mg/day (17;18). At these doses both drugs are expected to have average trough concentrations around 0.5 μM, a concentration that far exceeds the IC50 of most EGFR mutated cells lines treated with these EGFR TKIs. Serum trough concentrations are only surrogate markers of doses of these EGFR TKIs. Gefitinib has a higher distribution volume than erlotinib and drug concentrations in the tumor may be higher with gefitinib than erlotinib (16). We and others (16;30) were able to determine that gefitinib and erlotinib inhibit EGFR mutated cell lines with similar inhibitory curves and remarkable correlation in IC50 values. Therefore, at similar molar concentrations both EGFR inhibitors are expected to have a comparable spectrum of activity. This hypothesis drove our rationale to evaluate the clinical outcomes obtained with erlotinib 25 mg/day in patients with advanced NSCLC and EGFR mutations.
Our compiled retrospective clinical data of 7 patients treated with erlotinib at its lowest available tablet size of 25 mg/day reinforces our assumptions. The radiographic response rate was extraordinarily high at 71.5% in this small cohort of patients. Despite the retrospective nature of the analysis, the PFS values observed are very similar to those reported previously with gefitinib 250 mg/day (12) based on a lower bound of 6 months for the 95% confidence interval for median PFS in our cohort. The lack of significant adverse reactions in most of our treated patients is also consistent with a lower trough concentration of erlotinib when this drug is given at 25 mg instead of 150 mg. Rash and diarrhea, hallmark and dose-dependent side-effects of EGFR inhibitors, were not frequent in our patients. Indeed, only 2 patients (28.5%) had a minor rash to erlotinib 25 mg/day. None of our patients experienced interstitial pulmonary fibrosis, a known and serious side-effect of EGFR TKIs (31). The only other toxicity noted in one patient was liver function test abnormalities, which are well-described idiosyncratic side-effects of EGFR TKIs (7;9).
EGFR mutated patients treated with gefitinib or erlotinib invariably develop acquired resistance to TKI therapy (25). The EGFR-T790M mutation (19;24) accounts for half of cases, other secondary resistance mutations (L747S, D761Y, T854A) have been reported infrequently (20;32;33), and MET amplification (34;35) or expression of hepatocyte growth factor (36) are detected in more than one fifth of patients with EGFR mutated TKI-resistant NSCLC. The pattern of resistance seems very similar between patients treated with gefitinib 250 mg/day or erlotinib 150 mg/day (25;37). Cells with EGFR-T790M and/or MET amplification cannot be inhibited with the use of gefitinib or erlotinib by in vitro doses equivalent to their MTDs (21;34;35). In patient 1 (Table 2) of this study an increase of erlotinib to 150 mg/day after progression on erlotinib 25 mg/day was attempted without changing the progressive nature of the tumor’s radiographic disease (data not shown). Dose escalations to 150 mg/day were not made by treating physicians in the other 6 patients analyzed. Therefore, it is difficult to speculate if erlotinib at 25 mg/day or at other dose ranges below 150 mg/day (38) will alter the course and type of acquired resistance that treated patients will experience.
Our small retrospective study is hypothesis generating and will need to be confirmed in larger prospective series of NSCLC patients. Future prospective clinical trials of erlotinib at 25 mg daily as well as randomized trials of 25 mg versus 150 mg daily doses of erlotinib or of gefitinib 250 mg versus erlotinib 150 mg daily in advanced EGFR mutated NSCLC may shed light on the differential efficacy, time to acquisition of resistance, safety and quality of life attained by these different dosing approaches of approved EGFR TKIs.
In summary, our data indicates that EGFR mutated NSCLC lines with activating sensitive mutations are inhibited by similar concentrations of gefitinib and erlotinib in vitro (below 0.5 μM), and that erlotinib 25 mg/day (a concentration expected to be similar to gefitinib 250 mg/day) may be an alternative dosing scheme for patients with EGFR mutations. Future identification of the lowest and clinically active dose ranges of erlotinib and gefitinib in patients with tumors harboring EGFR mutations will help further personalize their care.
Acknowledgments
Funding/Grant Support: This work was funded in part by grants from the National Institutes of Health (NIH) R00CA126026-03 (SK) and 2PA50-CA090578-07 (DBC, DGT, BYY); the American Association for Cancer Research 07-40-12-COST (DBC); a Career Development Award by the American Society of Clinical Oncology Cancer Foundation CDA-15431 (DBC); and a Research Fellowship Award by the National Medical Research Council, Ministry of Health, Singapore (WY).
Footnotes
Conflict of interest: Consulting fees from Astra-Zeneca (WP, GJR, MGK), Boehringer Ingelheim (GJR, MGK), Pfizer (MGK) and Novartis (MGK). EGFR T790M patent licensed to MolecularMD (WP). No other conflict of interest is stated.
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 5.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 6.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 9.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 10.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 11.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 12.Costa DB, Kobayashi S, Tenen DG, Huberman MS. Pooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non-small cell lung cancers. Lung Cancer. 2007;58:95–103. doi: 10.1016/j.lungcan.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 14.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 15.Costa DB, Nguyen KS, Cho BC, et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res. 2008;14:7060–7067. doi: 10.1158/1078-0432.CCR-08-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rukazenkov Y, Speake G, Marshall G, et al. Epidermal growth factor receptor tyrosine kinase inhibitors: similar but different? Anticancer Drugs. 2009;20:856–866. doi: 10.1097/CAD.0b013e32833034e1. [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20:4292–4302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 20.Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–1679. doi: 10.1371/journal.pmed.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi S, Ji H, Yuza Y, et al. An alternative inhibitor overcomes resistance caused by a mutation of the epidermal growth factor receptor. Cancer Res. 2005;65:7096–7101. doi: 10.1158/0008-5472.CAN-05-1346. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–289. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranson M, Hammond LA, Ferry D, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002;20:2240–2250. doi: 10.1200/JCO.2002.10.112. [DOI] [PubMed] [Google Scholar]
- 27.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 28.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji H, Li D, Chen L, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Gandhi J, Zhang J, Xie Y, et al. Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS One. 2009;4:e4576. doi: 10.1371/journal.pone.0004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–2556. doi: 10.1200/JCO.2005.04.9866. [DOI] [PubMed] [Google Scholar]
- 32.Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 33.Bean J, Riely GJ, Balak M, et al. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin Cancer Res. 2008;14:7519–7525. doi: 10.1158/1078-0432.CCR-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 36.Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 37.Kaira K, Naito T, Takahashi T, et al. Pooled analysis of the reports of erlotinib after failure of gefitinib for non-small cell lung cancer. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Lind JS, Postmus PE, Heideman DA, Thunnissen EB, Bekers O, Smitt EF. Dramatic response to low-dose erlotinib of epidermal growth factor receptor mutation-positive recurrent non-small cell lung cancer after severe cutaneous toxicity. J Thorac Oncol. 2009;4:1585–1586. doi: 10.1097/JTO.0b013e3181bbb2b9. [DOI] [PubMed] [Google Scholar]